Featured Application
The effects of a 50 Hz electric field (EF) on brain tissue were evaluated. Juglone antioxidant activity in the prevention of electric field effects were investigated. Biochemical and physiological examinations were performed, and the comet method was applied. It was observed that electric field application caused damage to the brain.
Abstract
The aim of this study was to evaluate the possible effects of a 50 Hz electric field on brain tissue and the positive effects of juglone (5-hydroxy-1,4-naphthoquinone) antioxidant activity, using the immunohistochemical technique on male Wistar-Albino rats. The effects on blood tissue were also examined using biochemical, physiological and comet methods. Animals were randomly divided into three groups (eight in each group): group I: control, group II: electric field, group III: 50 Hz electric field + juglone (5-hydroxy-1,4-naphthoquinone)/300 ppm. Juglone was applied per day by gavage over 30 days. At the end of the experimental procedure, animals were sacrificed and brain tissue was subjected to routine histologic and immunohistochemical processes. As a result of histophatological examination, the brain tissue of rats with 50 Hz electric field exposure showed severe histopathological changes. The differences between groups were statistically significant according to total comet score (p = 0.001). For the antioxidant parameters on the blood, SOD activity in the electric field group was significantly higher among the other groups, although we did not find significant differences in MDA, CAT activity level.
1. Introduction
While electromagnetic waves with a very low frequency, such as 50 Hz originating from electrical networks, they cause damage to living and non-living tissues. The strength of the electric field depends on the proximity to the source. Some devices used currently may disturb the balance between these magnetic fields. It is known that low-frequency and intensity electric fields affect the amount of Na+ and K+ in the brain with the uptake and secretion of Ca++ in cells [1]. Again, it has been proven by experiments that the electric field causes changes in hormones such as LH and FSH [2]. It is known that electric fields cause untimely cataract formation in the eyes, abnormal development of the embryo, rash on the facial skin and weakening of the immune system. Today, intense research has been conducted on the many biological effects of electromagnetic waves. It is known that biological systems give different biological responses to electromagnetic wave applications at different frequencies and intensities [3,4,5,6].
In addition to the differences in the responses of living things to electric fields, it has been stated in many studies that different cell types also respond differently to electric fields in processes such as proliferation and differentiation. Due to the wide spectrum covered by all these different parameters, the effects of EF and the mechanisms of these effects are not yet clearly understood [1,2,3]. Walnut-derived herbal preparations of juglone (5-hydroxy-1,4-naphthoquinone) have been applied topically for the treatment of acne, inflammatory diseases and fungal, bacterial and viral infections. A decrease in cell viability was observed depending on the juglone (1–20 µM) concentration. Juglone has a strong protective effect against various diseases and prevents inflammation and the growth of tumor cells [7,8].
It is thought that the increase in cancer cases in recent years is proportional to the excessive use of mobile phones, the widespread use of high voltage lines, and the increase in the use of electrical household appliances. Our aim in planning this study is to confirm the damaging effects of electric and electromagnetic fields on tissues observed in previous studies. In this study, the effects of a 50 Hz electric field applied to Wistar-Albino male rats on brain tissue, and the positive effect of antioxidant juglone (5-hydroxy-1,4-naphthoquinone) applied to reduce these effects were studied on blood immunohistochemically. The aim was to evaluate these effects on the tissue of the rats by using biochemical, physiological and comet methods.
2. Materials and Methods
2.1. Experimental Animals and Groups
Young Wistar-Albino rats (n = 24, 250–300 g) were used in this study. Ethical permission was obtained from Süleyman Demirel University Experimental Animals Research Unit (decision numbered 21438139-267). All animals were kept under standard conditions in the Experimental Animal Laboratory during the experiment. Rats were randomly divided into three groups as EF, EF + Juglone (EF + JUG) and Control. A 21 h/day electric field was applied to the EF and EF + JUG groups for 30 days. During this period, a juglone (JUG) antioxidant substance was given by gavage method, together with electric field application to the EF + JUG group. The experimental groups were formed as follows.
Group I: Control group. Rats without electric field exposure and gavage (8 rats).
Group II: EF group. Electric field exposure group (8 rats).
Group III: EF + JUG group. The group (8 rats) with exposure to electric field and 1 mL 300 ppm anthoxidant juglone (5-hydroxy-1,4-naphthoquinone) applied by gavage method.
2.2. EF Creation
The methodology used by Anderson et al. was followed for the AC 50 Hz used in the study [9]. The experiment was carried out at Süleyman Demirel University Experimental Animals Research Unit. Power measurement was taken by Süleyman Demirel University, Electronics and Communication Engineering faculty members, and IEEE standards were taken as a basis while measurements were made. Basic parallel plate exposure set up design and restrictions can be found in [10]. No cell phone or any device that might affect the environment was allowed into the room. The control group, on the other hand, was kept in the same room but in an area that could be considered far from the electric field. The electric field (V/m) measured data were taken for both control and exposure groups. Groups were cared and fed under the same environment conditions. Rats were kept in an environment with a constant temperature of 20 ± 2 °C, humidity of 55–60% and a 12/12 h light/dark cycle.
A uniform and homogeneous electric field was used. The faces, which were facing one another, measuring 1 × 0.5 m, were completely smoothed. For perfect conductive plates, which were placed horizontally, and for the area to be smooth enough, they were formed with the help of an AC 50 Hz, 5000 V power transformer, whose ends were welded in the middle of the plates without drilling the plates and connected with stainless-steel bolts. The dimensions of the plates were chosen so that they were large enough to provide a sufficient uniform electric field. The volume of the cage was 40 × 50 × 20 cm3 (w × l × h). The selected plates, in terms of the subject studied, were quite large compared to the material used and researched. Plates of this size can provide sufficiently smooth electric field lines. Corner effects are neglected if the area between the plates is quite smooth. The definition “Smooth” has been used for the noiseless situation. The corner effect is a major problem in the types of parallel plate setups that we used in this study. While the electric field lines are fairly straight in the middle region of the plates, they bend at the corners. For this reason, the plates chosen are relatively large in relation to the test area, and the corners of the plates were physically rounded. Electric field lines should not be curved in such experiments; we therefore had to make sure the electric field lines were not bent. We were monitoring this “smoothness” by utilizing the measurement and instrumentation methodology during the whole experimental period [11].
The cage sizes were calculated for the health and longevity of the animals. The width of the cages was 50 cm, and the irrigation apparatus was also plastic or glass so as not to disturb the homogeneous electric field lines. In order to not disturb the homogeneity of the applied electric field, the cages were cleaned every day and the temperature and humidity of the environment were monitored.
2.3. Histopathological Examinations
Sections of the brain tissue taken at 5–6 μm thickness were first passed through the xylol-alcohol series. They were then passed through distilled water and incubated in Luxol Fast Blue Stain Kit (ScyTek cat. no.: LBC-2 35204, Logan, UT, USA) for 2 h at 60 °C in an oven. The samples were passed through 95% alcohol and washed in distilled water. The samples stayed at room temperature in lithium carbonate solution for 20 min. The samples were kept in 70% alcohol and then placed in distilled water. Afterwards, samples were incubated in Cresyl Violet solution (ScyTek cat. no.: CEA030 33712, Logan, UT, USA) for 2–5 min at room temperature. Preparations that were quickly passed through distilled water were then passed through alcohol and xylol series and closed with entellan [12]. Neuron counting was performed in the preparations examined under a light microscope (Leica DM 500, Wetzler, Germany). The Kolmogrov–Smirnov test was performed to understand whether the number of neurons in the groups had a normal distribution, and it was determined that they had.
2.4. Immunohistochemical Studies
Apoptotic cells in the brain tissue were determined by streptavidin peroxidase immunohistochemical method [13] using tnf-α and caspase-3 antibodies. Firstly, deparaffinized tissues were washed with PBS (Phosphate Buffered Saline) (Sigma cat. no.: P4417, Steinheim, Germany) and treated with hydrogen peroxide solution for 5 min to prevent endogenous peroxidase activity. Tissues washed with PBS for 3 × 5 min were placed in 1 M sodium citrate solution (pH 6.0) for 12 min in a microwave oven to reveal antigenic receptors. Tissue sections were washed several times with PBS and treated with Ultra V Block solution (ScyTek cat. no.: AAA125 69144, Logan, UT, USA) for 5 min to prevent non-specific antibody binding, followed by primary antibodies with tnf-α and caspase-3 for 60 min. Sections were incubated in a humid environment at room temperature. After the primary antibody application, the tissues were washed with PBS for 3 × 5 min and treated with secondary antibody (biotinylated Goat Anti-Polyvalent) (ScyTek cat. no.: ABF125 68813, Logan, UT, USA) for 30 min while kept in a humid environment at room temperature. After the secondary antibody application, the tissues were washed with PBS for 3 × 5 min, incubated with Streptavidin Peroxidase (ScyTek cat. no.: ABG125 69365, Logan, UT, USA) for 30 min in a humid environment at room temperature and then placed into PBS. After the DAB Substrate Kit solution (ScyTek cat. no.: ACH500, Logan, UT, USA) was dripped onto the tissues and the image signal was obtained under the light microscope, they were washed with PBS simultaneously. The prepared preparations were examined and evaluated using a a light microscope (Leica DM 500, Wetzler, Germany) and photographed.
In the evaluation of immunohistochemical staining, the severity and extent of the staining were taken as basis. The severity and extent of cytoplasmic immunostaining were scored semi-quantitatively from 0 to +3 (0: No, +1: Slight, +2: Moderate, +3: Severe) [10]. The Kruskal–Wallis method, one of the non-parametric tests, was used to determine the significant difference between the groups.
2.5. DNA Damage Determination with Comet Assay Method
The Comet Assay technique is a non-invasive, rapid and sensitive fluorescent microscopic method applied to detect and quantify DNA damage at the cellular level [14,15]. Cells embedded in agarose gel on the microscope slide are subjected to lysis to break up the membranes and release the DNA helixes in the nucleus. In an alkaline environment, the DNA helix relaxes and opens, and breaks occur. Then, the DNA strands are broken by electrophoresis migrate to form a comet image. After the lysis step, all procedures were performed in the dark. The slides were heated by adjusting the oven temperature to 500 °C. Agarose was prepared by boiling phosphate buffer (PBS) with 1% normal boiling point agarose (NMA). The prepared agarose tube was placed in a beaker and melted until it became completely transparent. It was kept in hot water to prevent it from polymerizing. An amount of 200 µL of NMA was taken on the slide, and the slides were left to dry. Before cells were embedded in the gel, low boiling point agarose (LMA) was prepared with PBS buffer and placed in a 37 °C water bath. Whole blood samples were removed from −20 °C and placed in a 37 °C water bath. An amount of 20 µL of whole blood was mixed with 150 µL of LMA at 37 °C; 140 µL of the prepared sample was then taken and dropped onto a slide with NMA, covered with a coverslip and kept at +4 °C for 20 min. The slides were immersed in the cold lysis solution, which was prepared at least 30 min in advance and kept in the refrigerator for 1 h. The slides removed from the lysis solution were placed in the electrophoresis tank and the electrophoresis buffer was slowly poured into the tank to cover the slides. The prepared preparations were incubated for 30 min in a highly alkaline (pH > 13) electrophoresis buffer before electrophoresis. Thus, in the alkaline buffer, the double-stranded DNA in the nucleus was opened at the points where the strand breaks were found. After the incubation was completed in alkaline electrophoresis buffer, electrophoresis was performed at 25 V, 300 mA current for 25 min. After electrophoresis in alkaline medium, slides were kept in neutralization buffer with pH 7.5 for 10 min. After neutralization, the slides were stained and left to dry for 1 h at room temperature in the dark. Ethidium bromide was used for dyeing. An amount of 50 µL of ethidium bromide was taken and dropped on the slide; after waiting for 15 min, the samples were examined under fluorescence microscope. One hundred DNA images were analyzed for each sample. One hundred cells were selected from a preparation prepared for each sample and examined under a fluorescence microscope (Olympus BX50, Tokyo, Japan) at 40× magnification. The level of oxidative DNA damage in visually evaluated cells was divided into 5 categories based on their tail lengths; cells with no damage were evaluated as 0, slightly damaged cells as 1, moderately damaged cells as 2, and severely damaged cells as 3. Total comet score was generated for each sample according to the severity of DNA damage.
3. Research Findings
3.1. Biochemical Findings
At the end of our experiment, biochemical evaluations were made in the blood taken from the groups. The Kolmogrov–Smirnov test was performed, and it was observed that the biochemical parameters of the groups were normally distributed. There was no significant difference in the biochemical parameters of Glucose, ALT, Direct Bilirubin or BUN between the groups (p ˃ 0.05). Levels of urea, AST and Indirect bilirubin showed a significant difference between the groups (p ˂ 0.05) (Table 1). It has been determined that a 50 Hz electric field has a negative effect on these parameters.

Table 1.
Statistical evaluation of the biochemical values of the groups by Tukey test method (p ˂ 0.05), (p ˃ 0.05).
3.2. Whole Blood Findings
Whole blood parameters were evaluated in the blood taken from the groups at the end of our 30-day experiment. In order to understand whether the whole blood parameters of the groups were in normal distribution, the Kolmogrov–Smirnov test was performed, and it was determined that they were not in normal distribution. Therefore, the Kruskul–Wallis test, one of the nonparametric tests, was used to determine the significant difference between the following groups: WBC (Amount of Leukocytes), HGB (Amount of Hemoglobin), HCT (volume percent of red blood cells in blood), PLT (Blood platelets), RBC (amount of red blood cells), MCV (size of red blood cells), MCH (average of each red blood cell). It was determined that there was no significant difference between the groups in terms of hemoglobin amount or whole blood parameters, and the p values were p ˃ 0.05. (Table 2). The 50 Hz electric field we used in our study did not have a negative effect on the whole blood parameters.

Table 2.
Statistical evaluation of whole blood values of the groups by Kruskul–Wallis method (p ˃ 0.05).
3.3. Histopathological Findings
Damages that may occur in the brain tissue were scored semi-quantitatively, with numbers ranging from 0 to +3 (0: No, +1: Minor, +2: Moderate, +3: Severe). Groups were compared in terms of degeneration level of neurons, damage to neuroglia cells, cavities and autophagic vacuoles, edema and congestion. As a result, +1 (Less) damage was detected in the EF group, while no damage was detected in the EF + Juglone and Control Groups (0: No). In the EF group, degenerations, vacuolizations and minimal edema were detected in the neurons, especially in the cortical region of the brain, but no congestion was observed. It was determined that the neurons in the cortical region of the brain tissue of the rats in the control and EF + Juglone groups were morphologically normal in number and appearance. In addition, the neurons in the hippocampal region were also found to have a normal histological structure. EF, EF + Juglone and Control groups were compared statistically in terms of neuron numbers, according to Luxol Fast Blue-Cresyl Violet Staining. A significant decrease in the number of neurons was noted in the rats in the EF group (Figure 1a,b). The neuron numbers of the EF + Juglone and Control groups were close to each other (Figure 1c,d). The difference between the control group, EF + Juglone group and the electric field group was statistically significant, and the immune positive cell density decreased in the electric field group (p = 0.001). While this significant difference was due to the EF group (10.583 ± 0.633), it was determined that there was no significant difference between the EF + Juglone (17.500 ± 0.596) group and the Control (17.583 ± 0.652) group (Table 3).
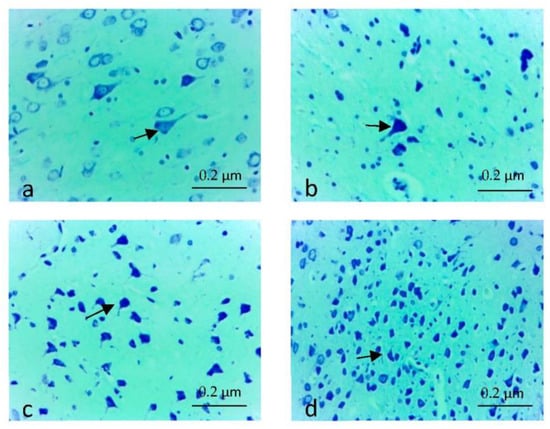
Figure 1.
(a,b) Density of neurons in the electric field group (arrows), decrease in neuron density, Luxol Fast Blue-Cresyl Violet Staining, ×40; (c) Neuron density in the electric field + Juglone group (arrows), increased in neuron density, Luxol Fast Blue-Cresyl Violet, ×40; (d) Neuron density in the control group (arrows), increased in neuron density, Luxol Fast Blue-Cresyl Violet, ×40.

Table 3.
Statistical evaluation of the neuron counts of the groups by Tukey Test method (p = 0.001).
3.4. Immunohistochemical Findings
The severity and extent of cytoplasmic immunostaining were scored semi-quantitatively from 0 to +3 (0: No, +1: Slight, +2: Moderate, +3: Severe). When caspase 3 and tnf-α immune positive cell density were scored individually in the eight rats of each group, +2 (moderate) density was detected in the preparations of two rats in the EF group. While immune positive cell density was detected at the level of +1 (Low) in the brain tissue of the other six rats (Figure 2a,b and Figure 3a,b), in the EF + Juglone and Control groups, immunopositive staining was found to be almost non-existent (Figure 2c,d and Figure 3c,d). In the comparison between the groups, the difference between the Control group, EF + Juglone group and the electric field group was statistically significant, and the immune positive cell density increased in the electric field group (p = 0.001) (Table 4).
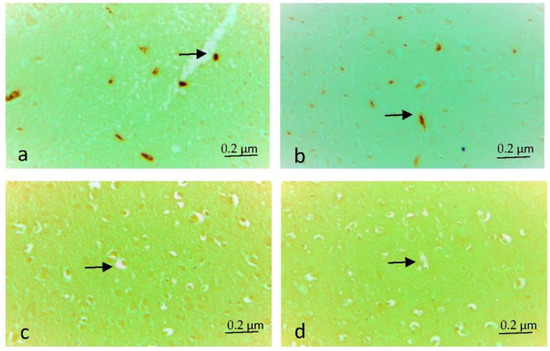
Figure 2.
(a,b) Caspase-3 immune positive cells (arrow) in the electric field group, ×40. (c) Caspase-3 immune negative cells (arrow) in the electric field + Juglone group, ×40 (d) Caspase-3 immune negative cells (arrow) in the Control group, ×40.
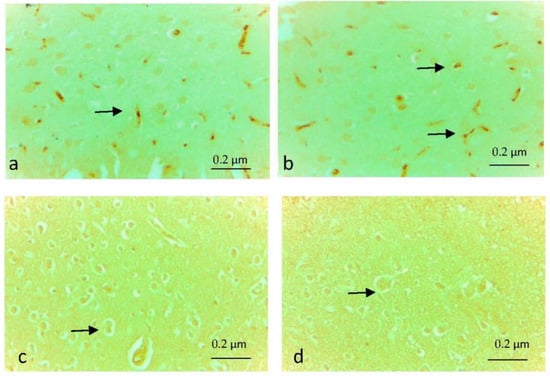
Figure 3.
(a,b) tnf-α immune positive cells (arrows) in the electric field group, ×40; (c) Tnf-α immune negative cells (arrow) in the electric field + Juglone group, ×40; (d) Tnf-α immune negative cells (arrow) in the Control group, ×40.

Table 4.
Statistical evaluation of Caspase-3 and tnf-α immune positive cell density of the groups by Kruskul–Wallis method (p ˃ 0.05).
3.5. Findings of DNA Damage Determination by Comet Assay Method
In the comet method applied to detect DNA breaks as described in the materials and methods section, comets were divided into five categories according to their tail lengths; cells with no damage were evaluated as 0, slightly damaged cell as 1, moderately damaged cell as 2, severely damaged cell as 3, severely damaged cell as 4. Total comet score was generated for each sample according to the severity of DNA damage. As a result of the analysis, no 3rd and 4th degree DNA breaks were found. Scoring was performed over 0, 1st and 2nd degree DNA breaks. The obtained results were expressed as arithmetic mean (X) and standard deviation (SD), and statistical analysis was performed using the SPSS 20.0 program. The conformity of the parameters in all study groups to the normal distribution was evaluated with the Kolmogorov–Smirnov test. The changes observed in the relevant parameters of the study groups were made using One-Way Analysis of Variance (One-Way ANOVA), and multiple comparisons were made according to the Bonferroni test; p < 0.05 values were considered statistically significant. The comet score data obtained and the statistical analysis results are presented in Table 5. The difference between the groups in terms of total comet score was statistically significant (p = 0.001). In the comparison between the groups, the difference between the control group and the electric field group was statistically significant and the comet score increased in the electric field group (p = 0.001) Although the comet score was decreased in the juglone group compared to the control group, the difference was not statistically significant (p > 0.05). The difference between the juglone group and the electric field group was statistically significant, and a decrease in the comet score was detected in the juglone group (p = 0.001). The comet images of the research groups are shown in Figure 4a–c.

Table 5.
Statistical evaluation of comet analysis.
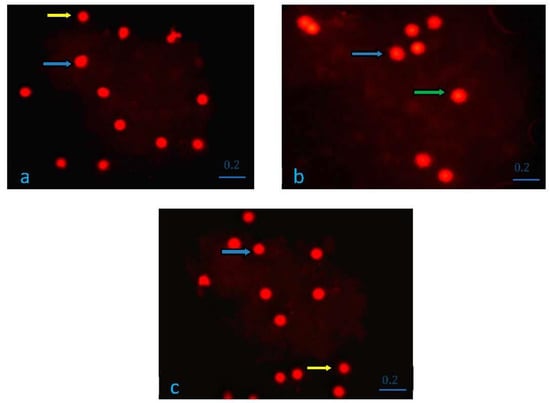
Figure 4.
(a) Group I (K) Comet Analysis. Yellow arrow, Category 0, cell with no damage. Blue arrow, 1st category, less damaged cell; (b) Group II (EF) Comet Analysis. Blue arrow, Category 1, slightly damaged cell. Green arrow, Category 2, heavily damaged cell; (c) Group III (EF + Juglone) Comet Analysis. Yellow arrow, Category 0, cell with no damage. Blue arrow, Category 1, slightly damaged cell.
3.6. Oxidant and Antioxidant Findings
The statistical analysis applied to the malondialdehyde (MDA), catalase (CAT) and superoxide dismutase (SOD) activities of the research groups are shown in Table 6. Although the MDA averages of the research groups were highest in the electric field group, the difference was not statistically significant. In the statistical evaluation applied to the data obtained in terms of CAT values, the difference between the groups was not significant (p > 0.05). Although the CAT activity of the juglone applied group was higher than the electric field applied group, it was determined that the applied dose did not significantly increase the CAT activity. In the statistical evaluation applied to the SOD activities of the research groups, the difference between the groups was found to be significant (p < 0.001). This significant difference is due to the lower mean SOD activity in the juglone group than in the other groups.

Table 6.
Oxidant and antioxidant levels of research groups.
4. Discussion and Conclusions
Among the devices used today, radars, mobile phones, radio and television transmitters, some medical devices, high voltage lines, microwave ovens and various electrical household appliances create electric and electromagnetic fields. Cell phones, which are rapidly increasing day by day in terms of the number of users and duration of use, emit microwave radiation (MWR) in the form of non-ionization electromagnetic radiation. As a result, concerns have increased regarding the possible dangers of electromagnetic field radiation radiated by cell phones affecting human health [16]. Lai et al. [17] revealed that DNA breaks occur in rat brain cells exposed to radiofrequency waves.
Guler et al. [18] found that the increase in serum SOD activity was statistically significant by applying 1.35 kV/m, 1.5 kV/m, 1.8 kV/m vertical and horizontal electric field application for 8 h a day for 3 days, 0.3 kV/m, 1 kV/m. They reported that the increase in SOD activity in the serum of the electric field application was not statistically significant. In our study, the decrease in SOD level was found to be significant in the EF and EF + Juglone groups. In the same study, 1.35 kV/m, 1.5 kV/m, 1.8 kV/m, 0.3 kV/m, 1 kV/m vertical and horizontal electric field application was found in biochemical parameters (total cholesterol, LDL, VLDL, total protein, albumin, GGT, ALT). ALP, LDH, urea, uric acid, glucose, creatine and BUN caused an increase, but this difference was reported to be not statistically significant. In our study, it was determined that there was no significant difference in the biochemical parameters of glucose, ALT, direct bilirubin or BUN between the groups (p > 0.05). It was determined that there was a significant difference between the groups in the biochemical parameters of urea, AST and indirect bilirubin, and this difference was due to the increase in the EF group (p < 0.05).
Rezaei-Tavirani et al. [19] applied an electric field of 50 Hz to the brains of rats and found a change in protein expression related to the cytoskeleton, which contributes to major processes in brain damage. Similarly, in this study, it was determined that the application of a 50 Hz electric field caused damage to neurons and neuroglia cells and edema and caused areas of bleeding. Our immunohistochemical evaluations also support the damage in the EF group in our study.
The effects of a 50 Hz extremely low-frequency EF on lipid peroxidation levels and antioxidant enzyme activities in brain tissue were investigated, and it was reported that there was no difference in lipid peroxidation levels and enzyme activities between groups exposed to electric fields and sham groups [20]. In our study, similar to this study, the changes in MDA and CAT activities were not found to be significant, but the differences between the groups due to the increase in SOD activity in the electric field group were found to be statistically significant.
It has been reported that electromagnetic field applications from 10 mW, 100 mW and 1000 mW mobile phones for 2 h a day causes severe neurological damage in the cerebral cortex, hippocampal region and basal ganglia [21]. In this study, it was determined that although there were decreases in neurons and glial cells in the EF group, very serious damage did not occur.
Uzar et al. [22] found that the MDA and NO levels in the spinal cord tissues of the prenatal + postnatal group were higher than the control group in the prenatal + postnatal group of puppies with a 50 Hz electric field for 24 h during the prenatal and postnatal period. In the postnatal group, there was no significant difference in MDA and NO levels compared to the control group. In this study, similar findings were obtained in terms of MDA level.
It was revealed that in rats exposed to a 50 Hz 01 mT magnetic field continuously for 10 days, young rats developed neurotrophic signaling and anti-oxidative enzymatic defense against a possible increase in oxygen radical species application of ELF-MF. On the contrary, aged rats could not develop anti-oxidative enzymatic defense systems against ELF-MF application. GPX1, GR and GST enzyme activities were also significantly increased compared to young people. It was determined that there was no significant change in SOD1, CAT, GPX1 or GST in young rats. In aged rats, there was a strong decrease in glutathione-induced detoxification enzymatic activity, except for SOD, while a significant decrease was observed in CAT activity. The result of the study showed that exposure to ELF-MF is a risk factor for age-related oxidative stress-based nervous system pathology [23]. In this study, while the change in MDA and CAT activities was not significant, the differences between the groups due to the increase in SOD activity in the electric field group were found to be statistically significant.
SOD, CAT and GSH activities in the brain tissue of rats exposed acutely to a low-frequency electromagnetic field (60 Hz) for 2 h were found to be significantly lower than the control group. However, when the NO level was compared with the control group, no significant difference was found [24]. In our study, the lowest CAT activity was determined in the EF group, while the lowest SOD activity was detected in the Juglone + EF group.
The cell cycle molecular mechanism of juglone and protein expression levels by apoptosis were measured by Cyclin D1 blot analysis, bcl-2, Bax, cytochrome C, caspase-9 and caspase-3. SKOV3 cells treated with juglone to investigate their motility abilities were demonstrated to inhibit SKOV3 cell proliferation. It has been determined that juglone has an antitumor effect on SKOV3 cells, causing cell cycle arrest and preventing SKOV3 cell proliferation [25]. In our study, the number of apoptotic cells was determined by caspase-3 and tnf-α, and it was concluded that juglone reduced apoptosis because the number of apoptotic cells in the EF group was higher than in the EF + Juglone group.
5. Conclusions
To investigate the proapoptotic mechanism of juglone, the role of reactive oxygen species (ROS) in juglone-induced apoptosis in HL-60 cells was investigated. Glutathione reduction was consistent with ROS production after treatment with juglone. It has been reported that juglone inhibits growth and causes human leukemia cell HL-60 apoptosis through a reactive oxygen species-dependent mechanism [26,27]. In our study, while no significant difference was observed in MDA and catalase levels, a significant difference was observed in SOD activity. In addition, the decrease in the negative effect of EF in the group given juglone was supported by our immunohistochemical and comet results. In studies investigating the apoptotic effect of SGC-7901 cells with different doses of 5, 10, 15 and 20 Mmol/L of juglone in gastric cancer, it was determined that the effect was higher at 20 Mmol/L doses compared to other doses. Juglone may have either pro- or antioxidant characteristics depending on the concentrations [28].
In conclusion, 50 Hz EF created damage to the brain tissue. It was observed that the damage seen in the EF group was less in the EF juglone group. Degeneration in brain tissue was decreased with the antioxidant effects of juglone.
Author Contributions
N.Ş. and E.K. conceived and planned the study. N.Ş., E.K. and R.A. drafted and revised the manuscript. N.Ş., E.K., R.A., Ö.C. and S.Ç. collected the data. N.Ş., E.K. and R.A. analyzed the data. All authors approved the final version of the manuscript for submission. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from Süleyman Demirel University Scientific Research Project Unit of Turkey, contract no. 4550-M2-16.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Süleyman Demirel University Animal Experiments Local Ethics Committee Chairman Turkey (protocol code 21438139-267 and 05.11.2015).
Informed Consent Statement
Not applicable.
Data Availability Statement
All the research data related to this manuscript will be available upon reasonable request from the corresponding authors.
Conflicts of Interest
The authors declared no potential conflict of interest with respect to the research, authorship and/or publication of this article.
References
- Harakawa, S.; Nedachi, T.; Suzuki, H. Extremely low-frequency electric field suppresses not only induced stress response but also stress-related tissue damage in mice. Sci. Rep. 2020, 10, 20930. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Zhang, Y.; Cai, Q.; Chen, G.; Wang, L.; Yang, X.; Zhong, W. Study of electrical stimulation with different electric-field intensities in the regulation of the differentiation of PC12 cells. ACS Chem. Neurosci. 2018, 10, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Bahodirovich, N.B.; Karimovich, H.D. Assessment of behavior and biochemical parameters of blood in experimental animals under conditions of a technogenic rotating electric field. Bull. Sci. Educ. 2020, 23–2, 6–10. [Google Scholar]
- Peña, A.F.; Devine, J.; Doronin, A.; Meglinski, I. Imaging of the interaction of low-frequency electric fields with biological tissues by optical coherence tomography. Opt. Lett. 2013, 38, 2629–2631. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyn, K.; Demidov, V.; Vuong, B.; Harduar, M.K.; Sun, C.; Yang VX, D.; Doganay, O.; Toronov, V.; Xu, Y. Imaging the electro-kinetic response of biological tissues with optical coherence tomography. Opt. Lett. 2013, 38, 2572–2574. [Google Scholar] [CrossRef]
- Peña, A.F.; Doronin, A.; Tuchin, V.V.; Meglinski, I. Monitoring of interaction of low-frequency electric field with biological tissues upon optical clearing with optical coherence tomography. J. Biomed. Opt. 2014, 19, 086002. [Google Scholar] [CrossRef]
- Inbaraj, J.J.; Chignell, C.F. Cytotoxic action of juglone and plumbagin: A mechanistic study using HaCaT keratinocytes. Chem. Res. Toxicol. 2004, 17, 55–62. [Google Scholar] [CrossRef]
- Peng, X.; Nie, Y.; Wu, J.; Huang, Q.; Cheng, Y. Juglone prevents metabolic endotoxemia-induced hepatitis and neuroinflammation via suppressing TLR4/NF-κB signaling pathway in high-fat diet rats. Biochem. Biophys. Res. Commun. 2015, 462, 245–250. [Google Scholar] [CrossRef]
- Anderson, L.E.; Morris, J.E. Large Granular lymphocytic leukemia in rats exposed to intermittent 60 Hz magnetic fields. Bioelectromagnetics 2001, 22, 185–193. [Google Scholar] [CrossRef]
- Miller, C.J. The measurements of electric fields in live line Working. IEEE Trans. Power Appar. Syst. 1967, PAS-86, 493–498. [Google Scholar] [CrossRef]
- Takuma, T.; Kawamoto, T.; Sunaga, Y. Analysis of calibration arrangements for AC field strength meters. IEEE Trans. Power Appar. Syst. 1985, PAS-104, 489–496. [Google Scholar] [CrossRef]
- Bancroft, J.D.İ.; Steven, A.; Turner, D.R. Theory and Practice of Histological Techniques; Churchill Livingstone: New York, NY, USA; Edinburg, UK, 1996; pp. 126–129. [Google Scholar]
- Sainouchi, M.; Tada, M.; Fitrah, Y.A.; Hara, N.; Tanaka, K.; Idezuka, J.; Aida, I.; Nakajima, T.; Miyashita, A.; Akazawa, K.; et al. Brain TDP-43 pathology in corticobasal degeneration: Topographical correlation with neuronal loss. Neuropathol. Appl. Neurobiol. 2022, 48, e12786. [Google Scholar] [CrossRef]
- Higami, Y.; Shimokawa, I.; Okimoto, T.; Ikeda, T. Vulnerability to Oxygen Radicals is More İmportant than İmpaired Repair in Hepatocytic Deoxyribonucleic Acid Damage in Aging. Lab. Investig. J. Tech. Methods Pathol. 1994, 71, 650–656. [Google Scholar]
- Ostling, O.; Johanson, K.J. Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem. Biophys. Res. Commun. 1984, 123, 291–298. [Google Scholar] [CrossRef]
- Alkis, M.E.; Akdag, M.Z.; Dasdag, S. Effects of low-intensity microwave radiation on oxidant-antioxidant parameters and DNA damage in the liver of rats. Bioelectromagnetics 2021, 42, 76–85. [Google Scholar] [CrossRef]
- Lai, H.; Singh, N.P. Acute exposure to a 60 Hz magnetic field increases DNA strand breaks in rat brain cells. Bioelectromagnetics 1997, 18, 156–165. [Google Scholar] [CrossRef]
- Güler, G.; Seyhan, N.; Arıcıoğlu, A. Effects of static and 50 Hz alternating electric fields on superoxide dismutase activity and TBARS levels in guinea pigs. Gen Physiol. Biophys. 2006, 25, 177–193. [Google Scholar]
- Rezaei-Tavirani, M.; Hasanzadeh, H.; Seyyedi, S.; Ghoujeghi, F.; Semnani, V.; Zali, H. Proteomic analysis of extremely low-frequency electromagnetic Field (ELF-EMF) with different intensities in rats hippocampus. Arch. Neurosci. 2018, 5, e62954. [Google Scholar] [CrossRef]
- Türközer, Z.; Güler, G.; Seyhan, N. Effects of expoure to 50 Hz electric field at different strengths on oxidative stress and antioxidant enzyme activities in the brain tissue of guinea pigs. Int. J. Radiat. Biol. 2008, 84, 581–590. [Google Scholar] [CrossRef]
- Salford, L.G.; Brun, A.E.; Elberhardt, J.L.; Malmgren, L.; Persson BR, R. Nerve cell damage in mammalian brain after exposure to microwaves from GSM mobile phones. Environ. Health Perspect. 2003, 111, 881–883. [Google Scholar] [CrossRef]
- Uzar, E.; Yılmaz, H.R.; Yılmaz, M.; Uz, E.; Yürekli, V.A.; Dündar, B.; Koyunuoğlu, H.R.; Çömlekçi, S. Effects of 50 Hz electric field on malondyaldehide and nitric oxide levels in spinal cord of rats at prenatal plus postnatal period. Turk. J. Med. Sci. 2011, 41, 65–72. [Google Scholar] [CrossRef]
- Falone, S.; Mirabilio, A.; Carbone, M.C.; Zimmitti, V.; Loreto, S.D.; Mariggio, M.A.; Mancinelli, R. Chronic exposure to 50 Hz magnetic fields causes a significant weakening of antioxidant defence systems in aged rat brain. Int. J. Biochem. Cell Biol. 2008, 40, 2762–2770. [Google Scholar] [CrossRef] [PubMed]
- Samano, J.M.; Duran PV, T.; Oropeza MA, J.; Diaz, L.V. Effect of acute extreely low frequency electromagnetic field exposure on the antioxidant status and lipid levels in rat brain. Arch. Med. Res. 2012, 43, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Qin, Y.; Qi, L.; Fang, Q.; Zhao, L.; Chen, S.; Li, Q.; Zhang, D.; Wang, L. Juglone exerts antitumor effect in ovarian cancer cells. Iran. J. Basic Med. Sci. 2015, 18, 544–548. [Google Scholar]
- Xu, H.L.; Yu, X.F.; Qu, S.C.; Qu, X.R.; Jiang, F.Y.; Sui, D.Y. Juglone, from Juglans mandshruica maxim, inhibits growth and induces apoptosis in human leukemia cell HL-60 trought a reactive oxygen species-dependent mechanism. Food Chem. Toxicol. 2012, 50, 590–596. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Luo, J.; Zhao, X.; Xu, J.; Zhu, W.; Jiang, Y.; Fang, F. Proliferation inhibition and apoptosis induction of juglone on human cervical cancer caski cells. J. Hyg. Res. 2014, 43, 959–971. [Google Scholar] [CrossRef]
- Ahmad, T.; Suzuki, Y.J. Juglone in oxidative stress and cell signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
