Application of the Falling Number Method in the Evaluation of the α-Amylase Activity of Malt Flour
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Malting Procedure
2.2.2. Enzyme Extraction from Malt Flour
2.2.3. Determination of α-Amylase Activity
2.2.4. Modification of the Falling Number (FN) Method
2.2.5. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delwiche, S.R.; Rausch, S.R.; Vinyard, B.T. Evaluation of a Standard Reference Material for Falling Number Measurement. Cereal Chem. 2020, 97, 441–448. [Google Scholar] [CrossRef]
- He, Y.; Lin, Y.L.; Chen, C.; Tsai, M.H.; Lin, A.H.M. Impacts of Starch and the Interactions Between Starch and Other Macromolecules on Wheat Falling Number. Compr. Rev. Food Sci. Food Saf. 2019, 18, 641–654. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). ISO 3093:2009; Wheat, Rye and Their Flours, Durum Wheat and Durum Wheat Semolina—Determination of the Falling Number According to Hagberg-Perten. International Organization for Standardization (ISO): Geneve, Switzerland, 2015. [Google Scholar]
- AACC International. AACC Approved Methods of Analysis—Determination of Falling Number (56-81.03: 1999), 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- International Association for Cereal Science and Technology (ICC). Determination of the Falling Number According to Hagberg—As a Measure of the Degree of Alpha-Amylase Activity in Grain and Flour (107/1: 1995); International Association for Cereal Science and Technology (ICC): Vienna, Austria, 1995. [Google Scholar]
- American Society of Brewing Chemists. ASBC Methods of Analysis—Sprout Damage by the Falling Number Method (Barley 12A), 14th ed.; American Society of Brewing Chemists: St. Paul, MN, USA, 2011. [Google Scholar]
- Perten, H. Application of the Falling Number Method for Evaluating Alpha-Amylase Activity. Cereal Chem. 1964, 41, 127–140. [Google Scholar]
- Kiszonas, A.M.; Engle, D.A.; Pierantoni, L.A.; Morris, C.F. Relationships between Falling Number, α-Amylase Activity, Milling, Cookie, and Sponge Cake Quality of Soft White Wheat. Cereal Chem. 2018, 95, 373–385. [Google Scholar] [CrossRef]
- Mares, D.J.; Mrva, K. Wheat Grain Preharvest Sprouting and Late Maturity Alpha-Amylase. Planta 2014, 240, 1167–1178. [Google Scholar] [CrossRef]
- Ral, J.P.; Whan, A.; Larroque, O.; Leyne, E.; Pritchard, J.; Dielen, A.S.; Howitt, C.A.; Morell, M.K.; Newberry, M. Engineering High α-Amylase Levels in Wheat Grain Lowers Falling Number but Improves Baking Properties. Plant Biotechnol. J. 2016, 14, 364–376. [Google Scholar] [CrossRef]
- Newberry, M.; Zwart, A.B.; Whan, A.; Mieog, J.C.; Sun, M.; Leyne, E.; Pritchard, J.; Daneri-Castro, S.N.; Ibrahim, K.; Diepeveen, D.; et al. Does Late Maturity Alpha-Amylase Impact Wheat Baking Quality? Front. Plant Sci. 2018, 9, 1356. [Google Scholar] [CrossRef]
- Cannon, A.E.; Marston, E.J.; Kiszonas, A.M.; Hauvermale, A.L.; See, D.R. Late-Maturity α-Amylase (LMA): Exploring the Underlying Mechanisms and End-Use Quality Effects in Wheat. Planta 2022, 255, 2. [Google Scholar] [CrossRef]
- Shao, Y.; Tsai, M.H.; He, Y.; Chen, J.; Wilson, C.; Lin, A.H.M. Reduction of Falling Number in Soft White Spring Wheat Caused by an Increased Proportion of Spherical B-Type Starch Granules. Food Chem. 2019, 284, 140–148. [Google Scholar] [CrossRef]
- Raschke, A.M.; Taylor, J.; Taylor, J.R.N. Use of Falling Number and Rapid Visco Analyser Instruments to Estimate Sorghum Malt Diastatic Power. J. Cereal Sci. 1995, 21, 97–102. [Google Scholar] [CrossRef]
- Yu, N.; Laurenz, R.; Siler, L.; Ng, P.K.W.; Souza, E.; Lewis, J.M. Evaluation of α-Amylase Activity and Falling Number around Maturity for Soft White and Soft Red Wheat Varieties in Michigan. Cereal Res. Commun. 2015, 43, 672–681. [Google Scholar] [CrossRef]
- Bhatty, R.S. Production of Food Malt from Hull-Less Barley. Cereal Chem. 1996, 73, 75–80. [Google Scholar]
- Bera, S.; Sabikhi, L.; Singh, A.K. Assessment of Malting Characteristics of Different Indian Barley Cultivars. J. Food Sci. Technol. 2018, 55, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.H.; Henson, C.A. A Comparison of Barley Malt Quality Measurements and Malt Sugar Concentrations. J. Am. Soc. Brew. Chem. 2008, 66, 151–161. [Google Scholar] [CrossRef]
- Arends, A.M.; Fox, G.P.; Henry, R.J.; Marschke, R.J.; Symons, M.H. Genetic and Environmental Variation in the Diastatic Power of Australian Barley. J. Cereal Sci. 1995, 21, 63–70. [Google Scholar] [CrossRef]
- Muralikrishna, G.; Nirmala, M. Cereal α-Amylases—An Overview. Carbohydr. Polym. 2005, 60, 163–173. [Google Scholar] [CrossRef]
- McCleary, B.V.; McNally, M.; Monaghan, D.; Mugford, D.C.; Black, C.; Broadbent, R.; Chin, M.; Cormack, M.; Fox, R.; Gaines, C.; et al. Measurement of α-Amylase Activity in White Wheat Flour, Milled Malt, and Microbial Enzyme Preparations, Using the Ceralpha Assay: Collaborative Study. J. AOAC Int. 2002, 85, 1096–1102. [Google Scholar] [CrossRef]
- AACC International. AACC Approved Methods of Analysis—Measurement of Alpha-Amylase in Plant and Microbial Materials Using the Ceralpha Method (22-02.01: 2001), 11th ed.; AACC International: St. Paul, MN, USA, 2010. [Google Scholar]
- Bland, J.M.; Altman, D.G. Measuring Agreement in Method Comparison Studies. Stat. Methods Med. Res. 1999, 8, 135–160. [Google Scholar] [CrossRef]
- Myles, P.S.; Cui, J.I. Using the Bland-Altman Method to Measure Agreement with Repeated Measures. Br. J. Anaesth. 2007, 99, 309–311. [Google Scholar] [CrossRef]
- Bunce, C. Correlation, Agreement, and Bland-Altman Analysis: Statistical Analysis of Method Comparison Studies. Am. J. Ophthalmol. 2009, 148, 4–6. [Google Scholar] [CrossRef]
- Mangan, D.; Szafranska, A.; McKie, V.; McCleary, B.V. Investigation into the Use of the Amylase SD Assay of Milled Wheat Extracts as a Predictor of Baked Bread Quality. J. Cereal Sci. 2016, 70, 240–246. [Google Scholar] [CrossRef]
- Doğan, N.Ö. Bland-Altman Analysis: A Paradigm to Understand Correlation and Agreement. Turkish J. Emerg. Med. 2018, 18, 139–141. [Google Scholar] [CrossRef] [PubMed]
- Van Stralen, K.J.; Jager, K.J.; Zoccali, C.; Dekker, F.W. Agreement between Methods. Kidney Int. 2008, 74, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Delwiche, S.R.; Wang, N.S. Hydrolysis of Wheat Starch and Its Effect on the Falling Number Procedure: Mathematical Model. Biotechnol. Bioeng. 2002, 79, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.Y.; Delwiche, S.R.; Wang, N.S. Hydrolysis of Wheat Starch and Its Effect on the Falling Number Procedure: Experimental Observations. J. Sci. Food Agric. 1999, 79, 19–24. [Google Scholar] [CrossRef]

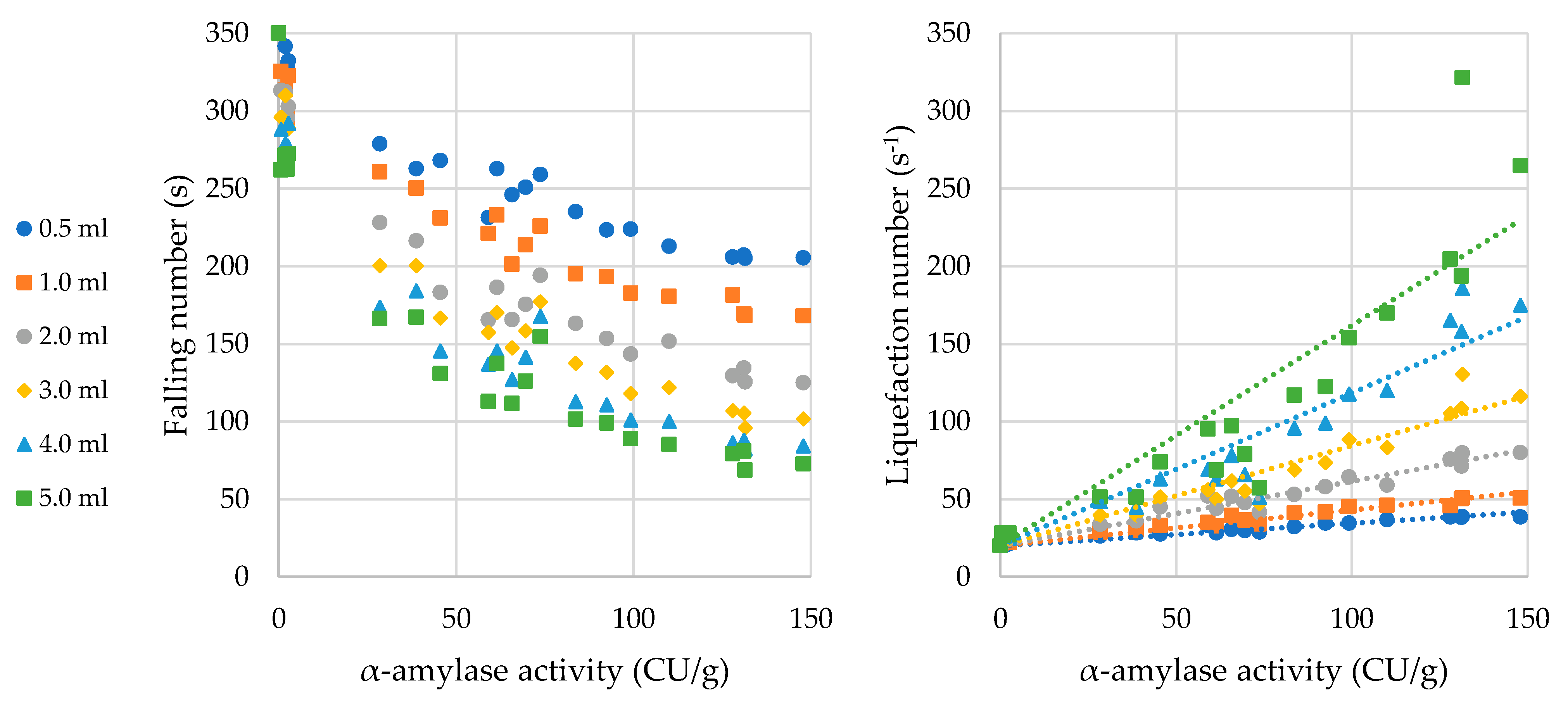
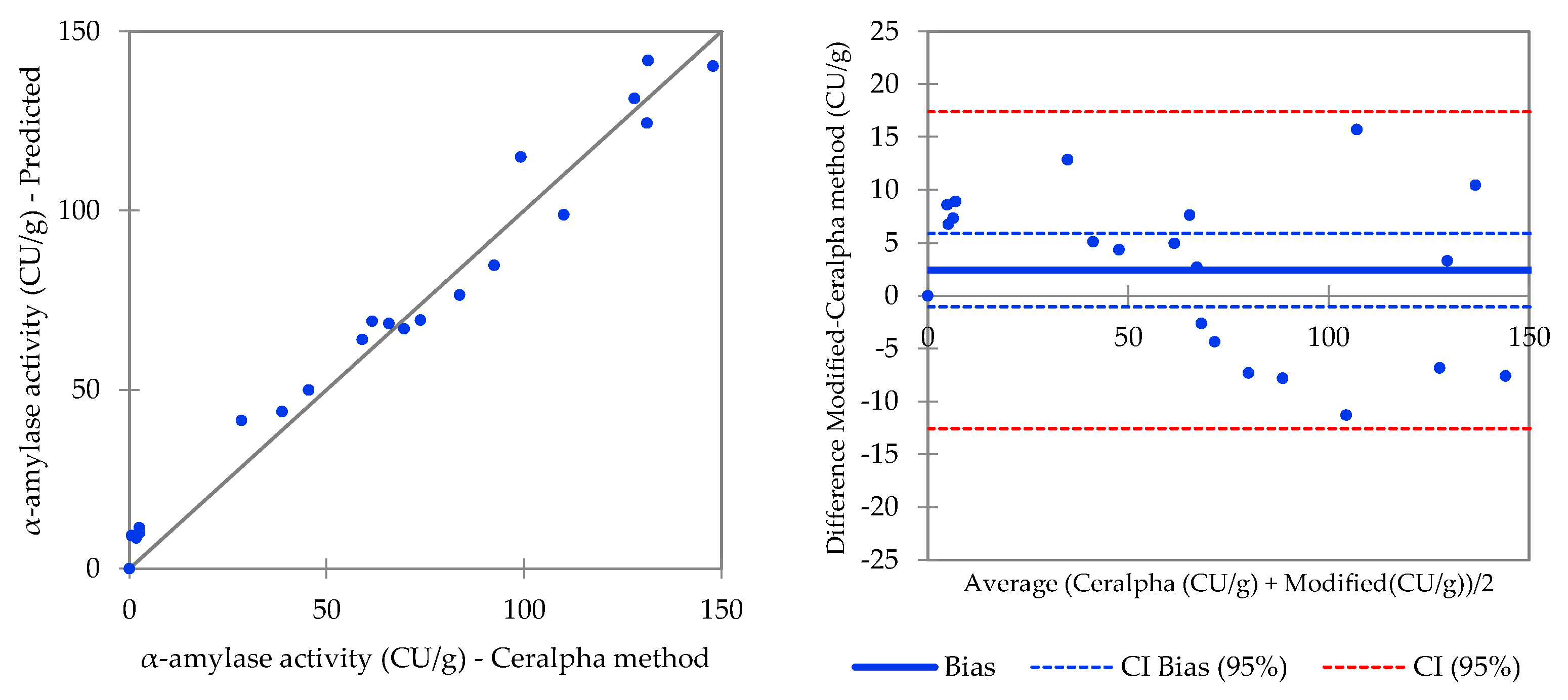
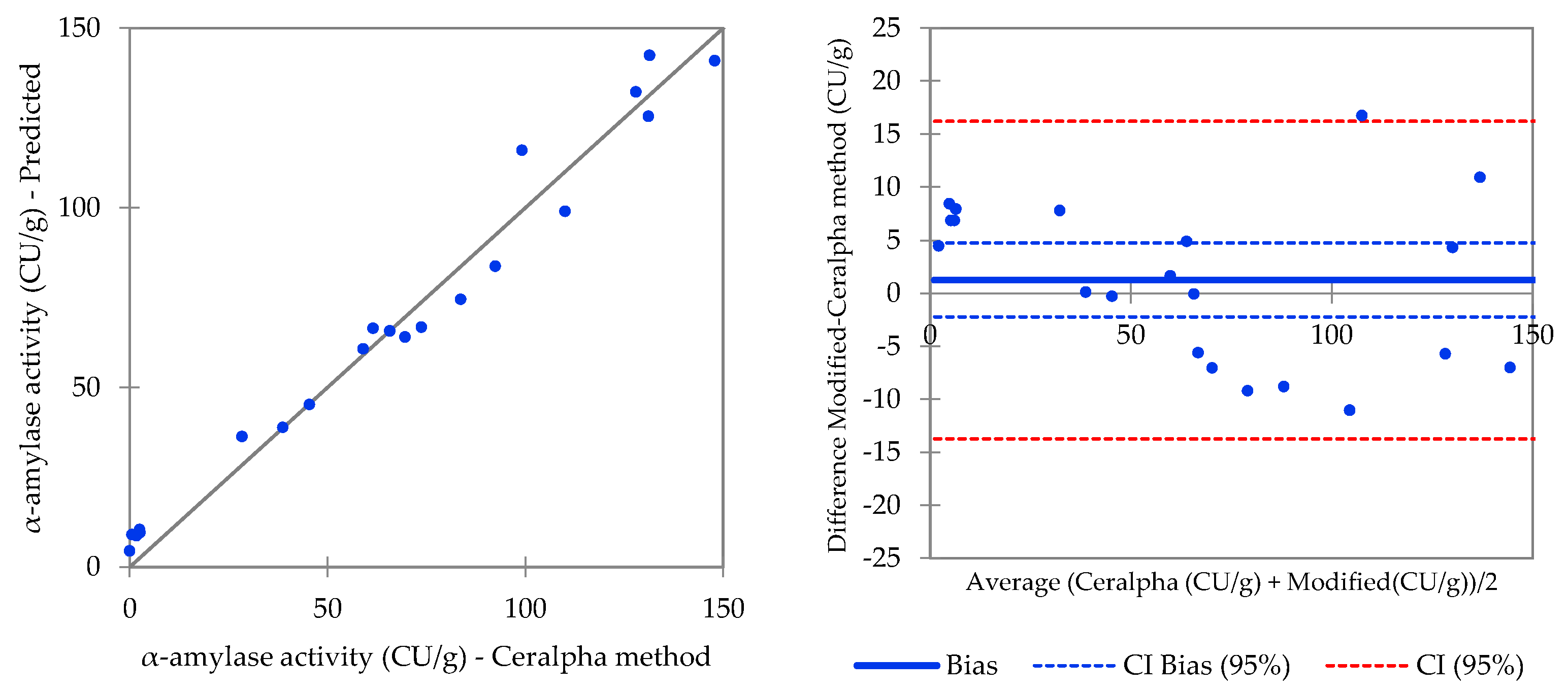
| Procedure 1 (Malt Flour) | Procedure 2 (Malt Extract 5% w/v) | ||||
|---|---|---|---|---|---|
| Wheat Starch 1 (g) | Malt Flour 1 (g) | Water (mL) | Wheat Starch 1 (g) | Malt Extract (mL) | Water (mL) |
| 5.95 | 0.05 | 25 | 6.00 | 0.5 | 24.5 |
| 5.90 | 0.1 | 25 | 6.00 | 1.0 | 24.0 |
| 5.80 | 0.2 | 25 | 6.00 | 2.0 | 23.0 |
| 5.70 | 0.3 | 25 | 6.00 | 3.0 | 22.0 |
| 6.00 | 4.0 | 21.0 | |||
| 6.00 | 5.0 | 20.0 1 | |||
| Sample Produced after | Barley Malt Zlatko (CU/g) | Barley Malt Osvit (CU/g) | Barley Malt Tristan (CU/g) | Wheat Malt Lorena (CU/g) |
|---|---|---|---|---|
| Steeping | 2.5 ± 0.26 | 1.8 ± 0.20 | 2.7 ± 0.23 | 0.6 ± 0.26 |
| 1st day of germination | 73.8 ± 0.38 | 38.7 ± 3.77 | 61.5 ± 3.33 | 28.4 ± 1.61 |
| 2nd day of germination | 99.2 ± 1.32 | 69.6 ± 1.80 | 110.0 ± 0.28 | 45.5 ± 0.93 |
| 3rd day of germination | 127.9 ± 1.48 | 83.7 ± 0.42 | 131.2 ± 5.69 | 59.0 ± 2.10 |
| 4th day of germination | 131.4 ± 0.81 | 92.5 ± 2.19 | 147.9 ± 3.29 | 65.8 ± 1.43 |
| Equation 1 | r | Bias (CU/g) | SEB (CU/g) | CIB (95%) (CU/g) | ULOQ (CU/g) | Repeatability (CU/g) | |
|---|---|---|---|---|---|---|---|
| Malt flour (g) | |||||||
| 0.05 | AA = (LN − 20)/0.215 | 0.956 | 3.99 | 12.18 | −1.55–9.54 | 2445.1 | 8.55 |
| 0.1 | AA = (LN − 20)/0.386 | 0.984 | 2.41 | 7.64 | −1.07–5.89 | 1363.0 | 6.42 |
| 0.2 | AA = (LN − 20)/0.834 | 0.973 | −0.65 | 11.61 | −5.94–4.63 | 629.4 | 4.69 |
| 0.3 | AA = (LN − 20)/1.616 | 0.926 | −4.35 | 21.47 | −14.12 ± 5.42 | 325.2 | 4.69 |
| Malt extract (mL) | |||||||
| 0.5 | AA = (LN − 20)/0.1452 | 0.970 | 2.93 | 10.63 | −1.91–7.77 | 3618.8 | 7.67 |
| 1.0 | AA = (LN − 20)/0.2304 | 0.974 | 3.38 | 9.35 | −0.88–7.63 | 2280.6 | 5.56 |
| 2.0 | AA = (LN − 20)/0.4154 | 0.977 | 1.89 | 9.66 | −2.50–6.29 | 1264.9 | 4.31 |
| 3.0 | AA = (LN − 20)/0.6445 | 0.965 | −0.91 | 13.41 | −7.01–5.20 | 815.3 | 4.91 |
| 4.0 | AA = (LN − 20)/0.9832 | 0.954 | −2.40 | 15.95 | −9.66–4.86 | 534.4 | 3.00 |
| 5.0 | AA = (LN − 20)/1.4179 | 0.907 | −3.87 | 24.50 | −15.03–7.28 | 370.6 | 3.14 |
| Equation 1 | r | Bias (CU/g) | SEB (CU/g) | CIB (95%) (CU/g) | ULOQ (CU/g) | Repeatability (CU/g) | |
|---|---|---|---|---|---|---|---|
| Malt flour (g) | |||||||
| 0.05 | AA = 3645.0 × exp(−0.019 × FN) | 0.973 | 0.86 | 11.07 | −4.18–5.90 | 1167.5 | 11.31 |
| 0.1 | AA = 1114.4 × exp(−0.016 × FN) | 0.987 | 1.24 | 7.64 | −2.24–4.72 | 425.4 | 6.70 |
| 0.2 | AA = 546.5 × exp(−0.015 × FN) | 0.987 | 3.10 | 7.44 | −0.28–6.49 | 222.6 | 3.86 |
| 0.3 | AA = 401.4 × exp(−0.016 × FN) | 0.989 | 0.68 | 7.07 | −2.53–3.90 | 155.5 | 2.46 |
| Malt extract (mL) | |||||||
| 0.5 | AA = 6089.6 × exp(−0.019 × FN) | 0.974 | 1.20 | 10.97 | −3.79–6.20 | 1961.8 | 8.61 |
| 1.0 | AA = 2335.6 × exp(−0.017 × FN) | 0.979 | 1.18 | 9.76 | −3.26–5.62 | 839.5 | 6.16 |
| 2.0 | AA = 962.9 × exp(−0.015 × FN) | 0.978 | 0.93 | 9.99 | −3.61–5.48 | 377.8 | 4.23 |
| 3.0 | AA = 536.3 × exp(−0.014 × FN) | 0.981 | 1.11 | 9.37 | −3.15–5.37 | 235.0 | 3.92 |
| 4.0 | AA = 442.9 × exp(−0.014 × FN) | 0.978 | 0.68 | 9.99 | −3.87–5.22 | 187.1 | 2.19 |
| 5.0 | AA = 391.7 × exp(−0.015 × FN) | 0.973 | 0.59 | 11.17 | −4.50–5.67 | 160.3 | 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jukić, M.; Šumanovac, F.; Nakov, G.; Šimić, G.; Komlenić, D.K.; Ivanova, N.; Lukinac, J. Application of the Falling Number Method in the Evaluation of the α-Amylase Activity of Malt Flour. Appl. Sci. 2023, 13, 3218. https://doi.org/10.3390/app13053218
Jukić M, Šumanovac F, Nakov G, Šimić G, Komlenić DK, Ivanova N, Lukinac J. Application of the Falling Number Method in the Evaluation of the α-Amylase Activity of Malt Flour. Applied Sciences. 2023; 13(5):3218. https://doi.org/10.3390/app13053218
Chicago/Turabian StyleJukić, Marko, Franjo Šumanovac, Gjore Nakov, Gordana Šimić, Daliborka Koceva Komlenić, Nastia Ivanova, and Jasmina Lukinac. 2023. "Application of the Falling Number Method in the Evaluation of the α-Amylase Activity of Malt Flour" Applied Sciences 13, no. 5: 3218. https://doi.org/10.3390/app13053218
APA StyleJukić, M., Šumanovac, F., Nakov, G., Šimić, G., Komlenić, D. K., Ivanova, N., & Lukinac, J. (2023). Application of the Falling Number Method in the Evaluation of the α-Amylase Activity of Malt Flour. Applied Sciences, 13(5), 3218. https://doi.org/10.3390/app13053218










