Abstract
The phenolic profile of wines is often used to evaluate their quality and authenticity. The phenolic composition of twenty-five commercial wines produced in different Croatian regions from eight red and nine white grape varieties was studied. A total of twenty-four polyphenols were analyzed using HPLC-DAD and classified into five groups based on their structure: phenolic acids, flavan-3-ols, anthocyanins, flavonoids, and stilbenes. The red wines contained higher concentrations of phenolic constituents than the white wines, of which gallic acid (11.8–90.3 mg/L), procyanidin B1 (13.7–63.8 mg/L), and catechin (10.5–34.5 mg/L) were the most abundant. In contrast to the white wines, great variability was observed in the red wines, with the autochthonous Plavac Mali and Babić showing the most specific phenolic profiles. The most representative phenolic components in the studied Croatian wines showed strong antioxidant activity. Gallic acid proved to be the most effective DPPH (IC50 = 0.33 µg/mL) and NO scavenger (IC50 = 12.36 µg/mL), while myricetin was the most potent inhibitor of lipid peroxidation (IC50 = 1.68 µg/mL). Our research has contributed to the characterization and varietal differentiation of Croatian wines, highlighting those rich in certain polyphenols as potential nutraceuticals.
1. Introduction
Wine is the most traditional and popular alcoholic beverage consumed worldwide. A highly competitive market and consumer expectations force wineries to produce quality wines. Wine quality is determined by various factors, such as the type of grape varieties, geographical, climatic and pedological factors, viticultural practices, winemaking techniques, and aging conditions. Wine quality evaluation is based on both chemical and sensory analyses. The sensory characteristics of wines are significantly influenced by the phenolic profile, which is often used to evaluate the quality and authenticity of wines [1,2]. Phenolic compounds are a diverse group of highly bioactive substances found in grapes and wine that can be formed and altered during the winemaking process. They are essential for color, flavor, and taste attributes, such as mouthfeel and astringency of wines, especially red wines. The most important phenolic compounds in red wines include anthocyanins and their derivatives, which give the wine its color, flavonols, which are involved in the copigmentation process, and tannins, which are responsible for mouthfeel and astringency. Polymeric pigments, a heterogeneous group of anthocyanin–tannin reaction products, are usually formed during vinification [3]. The technological process to which the grapes are subjected has a significant impact on the phenolic content and composition of the wines. Since red wines are in contact with all parts of the grapes during maceration, they have a higher polyphenol concentration than white wines, whose polyphenol content comes mainly from the pulp [4]. During grape treatment and ripening, numerous chemical changes can take place, creating new compounds and/or degrading others. These changes can affect the polyphenolic profiles of wines. The phenolic composition of wines, which determines their organoleptic properties and provides information about their primary characteristics, can be used as a fingerprint to distinguish them according to their origin in terms of region, grape variety, and vintage [2]. The phenolic compounds most commonly used to evaluate wine quality and authenticity include phenolic acids, flavonoids, tannins, and stilbenes [1]. Polyphenols are not only closely related to wine quality but have also been shown to have health-promoting properties. Numerous studies have shown that moderate consumption of wine, especially red wine, is healthy as it protects against various chronic diseases such as cardiovascular and neurological disorders, metabolic syndrome, cognitive disorders, depression, and some cancers [4,5,6,7]. The positive role of red wine in oxidative stress [8] and in promoting desirable gut bacteria leading to a healthier human body system has also been highlighted [9,10].
Viticulture has a long tradition in Croatia, and there are an estimated 140 autochthonous grape varieties. Although the area under cultivation of foreign varieties has steadily increased over the last 50 years, 14 of the 30 most important varieties in Croatia are still indigenous [11]. Due to the geographical position of Croatia, the continental climate in the eastern and central parts of the country meets the Mediterranean climate in the southern, coastal areas [12]. In this context, Croatia is divided into four wine-growing regions [13]. The importance of determining the authenticity and commercial value of a wine, often related to its geographical origin, is recognized in Croatia, where viticulture and wine production play a significant economic role [12]. In the last two decades, several studies have been conducted to investigate the polyphenolic composition of Croatian wines. Rastija et al. [14] analyzed fifteen individual polyphenols in twelve Croatian wines from the 2002 vintage, and the wines from the Dalmatia wine region with a Mediterranean climate were the richest in polyphenols and contained the highest levels of gallic acid, quercetin, and myricetin. This was also confirmed in a study by Šeruga et al. [15]. Compared to red wines from continental Croatia, the Dalmatian red wines of the 2005–2006 vintage contained the most polyphenols, including the highest levels of gallic acid and catechin. Lukić et al. [16] conducted a comprehensive study of polyphenols in numerous samples of four representative red and six white Croatian wines from 2015. Many of the 58 identified phenolic compounds proved to be relevant differentiators between the wines. The influence of some viticultural and winemaking conditions on the content and composition of various polyphenols in Croatian wines has also been studied. Thus, the influence of commercial yeasts [17] and prolonged grape ripening [18] on the phenolic profile of Plavac Mali red wine was determined. Budić-Leto et al. [19,20] demonstrated a strong correlation between the sensory characteristics of Babić red wine and total polyphenol content and anthocyanin composition under the influence of maceration treatment. In addition, a positive correlation was found between the content of anthocyanins, phenolic acids, and flavan-3-ols of Teran red wine and the skin maceration time. Bubola et al. [21] showed that the concentration of hydroxycinnamic acid in Istrian Malvasia white wine is improved by the removal of leaves. Moreover, Osrečak et al. [22] demonstrated that leaf removal increases the content of many polyphenols in Croatian non-native white wines such as Italian Riesling, Traminer, and Manzoni Bianco. In addition, the antioxidant properties of some Croatian wines were studied. A very high correlation was found between antioxidant activity and total polyphenol content [6,15,23], and consequently better antioxidant properties of the studied red wines than white wines [6,24,25]. It is worth mentioning that the health benefits of phenolic compounds from wine are often attributed precisely to their antioxidant activity [6,26]. Compared to other European and world wines, Croatian wines, especially autochthonous ones, have not been sufficiently researched in terms of the composition of phenolic constituents and their biological properties. Thus, the main objective of this study was to investigate the phenolic profile of a wide range of wines characteristic of certain Croatian wine regions and to evaluate possible differences based on the detected polyphenols and multivariate analyses. Twenty-five commercial wines from eight red and nine white grape varieties were studied, with special attention to Croatian autochthonous wines. In addition, the antioxidant properties of the most representative phenolic constituents were evaluated.
2. Materials and Methods
2.1. Chemicals
HPLC ultra-gradient grade methanol, ethanol, and acetonitrile were supplied by Mallinkrodt-Baker (Deventer, Holland). Other chemicals and references were of HPLC or analytical grade. Orthophosphoric acid and potassium dihydrogen phosphate were obtained from Merck (Darmstadt, Germany). Procyanidins B1 and B2, trans-resveratrol, delphinidin-3-O-glucoside, malvidin-3-glucoside, and sodium nitroprusside were obtained from Extrasynthese (Genay, France). The trans-resveratrol was transformed into its respective cis-isomer by UV irradiation using a 254 nm wavelength for 4 h in a quartz cell [27]. Apigenin, rutin, myricetin, luteolin, and kaempferol were purchased from Carl Roth (Karlsruhe, Germany). Gallic acid, p-hydroxybenzoic acid, syringic acid, o-coumaric acid, p-coumaric acid, chlorogenic acid, caffeic acid, ferulic acid, catechin, epicatechin, naringenin, quercetin, quercitrin, extract from bovine brain, phosphate buffer saline, sulphanilamide, thiobarbituric acid, trichloroacetic acid, DPPH (2,2-diphenyl-1-picrylhydrazyl) were provided by Sigma-Aldrich (Steinheim, Germany). Trolox, butylated hydroxytoluene (BHT), and N-(1-naphthyl)ethylenediamine dihydrochloride were provided by Fluka (Buchs, Switzerland). Ascorbic acid was provided by Acros Organics (Geel, Belgium), butanol by Lach-Ner (Neratovice, Czech Republic), ethanol by Gram-Mol (Zagreb, Croatia), phosphoric acid by T.T.T. (Zagreb, Croatia), and iron(III) chloride by Riedel-de-Haën (Seelze, Germany). Pure water was obtained from the Milli-Q Advantage A10 system (Millipore, MA, USA).
2.2. Wine Samples
In the present study, a total of 25 commercial wine samples were analyzed. The 2007 vintage wines, bottled in glass, were provided by the producers or distributors in 2009. HPLC-DAD analyses were carried out when the samples reached three years of age. Table 1 and Table 2 show the Croatian monovarietal red and white wines studied, respectively, with their corresponding abbreviations, wine types, grape varieties, wine regions, subregions, and vineyard localities.

Table 1.
Data on the studied varietal red wines from different Croatian wine regions.

Table 2.
Data on the studied varietal white wines from different Croatian wine regions.
Croatia is divided into four wine regions [13]. Dalmatia is one of the two coastal wine regions of Croatia covering the area from the city of Zadar and its hinterland to the Konavle region in the extreme south, and from which the wine samples marked with blue circles in Figure 1 originate. Istria and Kvarner is the wine region in the northwestern part of the Croatian Adriatic coast. The samples of Istrian wines are marked in purple in Figure 1. The continental part of Croatia is also represented by two regions. The Croatian Highlands is the wine region in the northwestern part of Croatia, while the Slavonia and Croatian Danube region is located on the predominantly flat eastern mainland of Croatia. In Figure 1, the wine samples from these regions are marked in orange and green, respectively.
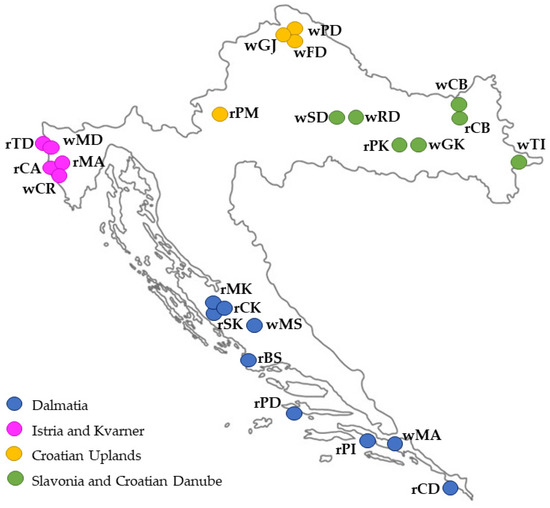
In terms of climate, Croatia is divided into two major climatic regions: the Mediterranean region, which includes Dalmatia, Istria, and Kvarner, and the Continental region, which includes the Croatian Uplands, Slavonia, and the Croatian Danube. The climatic conditions in these two regions of origin of the studied wines are very different. According to the available climatological data for the vineyards in 2007, the average monthly total precipitation during the ripening period was 82.0 mm with a monthly average temperature of 19.7 °C for the vineyards in the Continental region, and 50.0 mm precipitation at 22.9 °C for the vineyards in the Mediterranean region. The Supplementary Materials (Table S1) contain meteorological data from June to September 2007, measured at the meteorological stations near the vineyards where the grapes for the production of the studied wines were grown (data from the Croatian Meteorological and Hydrological Service).
2.3. HPLC Analyses
Samples used for HPLC analysis were taken from two different bottles of each wine. The wine samples and standard solutions were filtered through a syringe filter with a pore size of 0.45 µm. All experiments were performed using an Agilent 1100 series liquid chromatograph equipped with a DAD detector and an Agilent Zorbax SB -C18 (4.6 × 250 mm, 5 μm) column preceded by an Agilent Zorbax SB -C18 (10 × 4.6 mm, 5 μm) guard column.
The HPLC analysis of anthocyanins was performed according to Berente et al. (2000) [28]. Acetonitrile–buffer (5:95) was the mobile phase A, while acetonitrile–buffer (50:50) was the mobile phase B, where 10 mM KH2PO4 + H3PO4 to pH 1.6 was used as a buffer. The oven temperature was 50 °C. An amount of 20 μL of 0.45 μm membrane-filtered samples were injected with a 1 mL/min flow rate. Gradient elution was applied according to the following program: 0–30 min (10–45% B), 30–31 min (45–100% B), 31–34 min (100% B), 34–35 min (100–10%), and 35–45 min (10% B). The wavelength for detection was 518 nm.
Orthophosphoric acid pH 3.00 was the mobile phase A, while methanol was the mobile phase B for the HPLC analysis of flavonoids, phenolic acids, flavan-3-ols, and stilbenes. The eluents were used in the following gradient: 0–7 min (10–30% B), 7–25 min (30% B), 25–50 min (30–70% B), 50–60 min (70–86% B), 60–60.01 min (86–10% B), and 60.01–65 min (10% B). The flow rate was set at 1.0 mL/min. The separation was performed at 35 °C. The aliquot of the injected sample was 25 μL. The wavelengths for detection were 280, 325, or 360 nm, depending on the absorption maximum of the phenolic component analyzed. Peak assignment and identification were performed by comparing the retention times and UV/VIS spectra of the peaks in the sample chromatograms with those of the standards. Quantification of individual phenolic peaks was performed using the external standard method. Response factors (RF) were calculated daily by using freshly prepared reference solutions. Results were expressed in milligrams of the phenolic compound per liter of wine sample. The validation of this method was performed according to the recommendations of the guideline ICH Q2 [29]. The linearity of the method was evaluated using calibration curves with six concentrations (0.1, 0.5, 1, 2, 4, and 50 mg/L), while for gallic acid and procyanidin B1, an additional concentration of 100 mg/L was used. These concentration ranges were set according to the expected concentration of phenolic compounds in the wine samples. The recoveries were determined, and a linear regression analysis was performed, calculating the determinant coefficient, y-axis intercept, and slope to determine the linearity of the method. The analytical curves showed excellent linear behavior over the entire concentration range studied. The mean recoveries were calculated by comparing the calculated amounts with the amount present in the standard solution. The high mean recoveries for all studied compounds indicate the high accuracy of the method. The limit of detection (LOD) and limit of quantification (LOQ) were determined by analyzing the calibration curves. The following equation was used to calculate the LOD and LOQ values: C = (K × SD)/S, where SD is the standard deviation of the y-axis intercept values and S is the mean slope value from the three calibration curves generated. The K values were 3 for LOD and 10 for LOQ [30]. The Supplementary Materials (Table S2) show the corresponding validation parameters.
2.4. DPPH Radical Scavenging Activity
The ability of the selected phenolic compounds in wine to scavenge DPPH radicals was determined according to the method described by Vladimir-Knežević et al. [31]. Solutions of selected compounds at various concentrations (0.20–100 µg/mL) were mixed with a 0.1 mM ethanolic solution of DPPH. The samples were shaken vigorously and kept in the dark for 30 min. Then, the absorbance was measured at 517 nm compared to a suitable blank sample. The ability to scavenge DPPH radicals was calculated using the following equation: (%) = [(A0 − A1)/A0] × 100, where A0 is the absorbance of the control reaction and A1 is the absorbance in the presence of the tested sample.
2.5. NO Radical Scavenging Activity
The selected phenolic compounds in wine were evaluated for their ability to scavenge NO radicals using a slightly modified method described by Patel et al. [32]. Solutions (80 µL) of the selected compounds (6.25–400 µg/mL) were mixed with 80 µL of a 10 mM sodium nitroprusside solution in phosphate-buffered saline (pH = 7.4), the well plates were shaken, and incubated for 120 min at room temperature. Then, 80 µL of Griess reagent (1% sulphanilamide, 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride, and 5% phosphoric acid) was added to the samples under study. The absorbance was measured at 545 nm and NO radical scavenging activity was calculated according to the following equation: (%) = [(A0 − A1)/A0] × 100, where A0 is the absorbance of the control solution and A1 is the absorbance of the test solution.
2.6. Lipid Peroxidation Inhibition Assay
The lipid peroxidation inhibition assay was performed according to the method described by Houghton et al. [33]. The selected compounds were dissolved in dimethyl sulfoxide (0.78–200 µg/mL), and 10 µL of each solution was mixed with 0.5 mL of a suspension of bovine brain extract in phosphate-buffered saline (5 mg/mL, pH = 7.4). After the addition of 1 mM iron(III) chloride solution (0.1 mL), 10 mM phosphate-buffered saline (0.29 mL), and 1 mM ascorbic acid solution (0.1 mL), the test solutions were incubated at 37 °C for 60 min. Then, 1 mL of thiobarbituric acid (1% solution in 0.05 M sodium hydroxide), 1 mL of trichloroacetic acid (2.8%), and 0.1 mL of BHT (2%) were added and the mixture was heated in a boiling water bath for 20 min. After the addition of butanol (2 mL) to the mixture, the absorbance of the supernatant was measured at 532 nm compared to a suitable blank sample. The percentage of lipid peroxidation inhibition was calculated using the following equation: (%) = [(A0 − A1)/A0] × 100, where A0 is the absorbance of the control solution and A1 is the absorbance of the test solution.
2.7. Statistical Analysis
All experiments were performed in triplicate, and the results are expressed as means standard deviation. Linear regression extrapolation was used to calculate IC50 values. The ordinary one-way ANOVA and post hoc Tukey’s multiple comparisons test were used to determine differences between the obtained results. All statistical analyses were accomplished using GraphPad Prism software version 8.4.3 (GraphPad Software, Boston, MA, USA) as well as Microsoft Excel. All values with p < 0.05 were considered statistically significant. Principal component analysis (PCA) was performed for each type of wine to easily correlate and understand the relationship between chemical properties and wine samples. PCA was conducted using XLSTAT (Addinsoft, New York, NY, USA), an add-in for Microsoft Excel.
3. Results and Discussion
3.1. Contents of Phenolic Compounds in Croatian Wines
The content of twenty-four different phenolic compounds in selected Croatian wines was determined by the HPLC-DAD methods and divided into five classes based on their structure: phenolic acids (Table 3), flavan-3-ols, anthocyanins, stilbenes, (Table 4), and flavonoids (Table 5). The red wines contained the highest concentrations of phenolics, while the white wines had lower values. It is known that the content and composition of phenolic constituents are influenced to a considerable extent by the grape variety used, the technological processes to which the grapes are subjected, the type of yeast used in alcoholic fermentation, and the interaction with solid parts of the grapes during maceration [1,26]. Thus, our results are in line with expectations, considering that the pulp, skin, and seeds of grapes contain different classes and amounts of phenolic components, and that red wines are exposed to all parts of the grape during winemaking, while polyphenols in white wines mostly originate from the pulp [2,4].

Table 3.
Contents of hydroxybenzoic and hydroxycinnamic acids in selected Croatian wines (mg/L).

Table 4.
Contents of flavan-3-ols, anthocyanins, and stilbenes in selected Croatian wines (mg/L).

Table 5.
Contents of flavonoids in selected Croatian red wines (mg/L).
3.1.1. Phenolic Acids in Selected Red and White Wines
Three hydroxybenzoic acids and five hydroxycinnamic acids were identified in all the red wines studied, while most white wines did not contain o-coumaric acid, syringic acid, or chlorogenic acid. As shown in Table 3, gallic acid was the most abundant phenolic acid in the red wines; its content ranged from 11.8 to 90.3 mg/L. The red wines also contained significant amounts of syringic acid and hydroxycinnamic acids, such as p-coumaric acid and caffeic acid, whose concentrations ranged from 3.3 to 21.8 mg/L. In the wines studied, the proportion of the above-mentioned acids varies, which indicates their importance for the varietal differentiation of Croatian wines.
The Plavac Mali (rPD and rPI) and Babić (rBS) wines, produced from the autochthonous Croatian grape varieties of the same name, contained the highest levels of phenolic acids (113.0–139.7 mg/L), with gallic acid being the most representative (72.5–90.3 mg/L). In a recent study, a similarly high concentration of gallic acid was found in Plavac Mali of 2019 vintage from the Central and Southern Dalmatia subregion [6]. However, the analysis of 27 different samples of Plavac Mali wine produced in the Croatian coastal regions in 2013–2015 conducted by Žurga et al. [11] showed significantly lower gallic acid content (average 45.8 mg/L) compared to our samples. Similar results were obtained by Lukić et al. [16] when analyzing 20 samples of Plavac Mali wine from the 2015 vintage. Moreover, an even smaller amount of gallic acid (26.9 mg/L) was found in the 2002 vintage Plavac Mali wine from northern Dalmatia than in our samples from the island of Hvar and the Pelješac Peninsula in southern Dalmatia. In the context of these differences, Jagatić Korenika et al. [17] determined the influence of commercial yeasts on the phenolic profile of Plavac Mali wines from the Croatian coastal areas and also confirmed the role of geographical and pedological heterogeneity of vineyard sites. As far as we are aware, this study is the first to provide a detailed polyphenol composition of Babić wine. However, previous analyses of polyphenols in the seeds and skins of Babić grapes confirm such a high content of phenolic acids (130.8 mg/L) in the studied wine sample as in the case of wine from Plavac Mali [34]. Teran is an autochthonous variety widely grown on the Istrian Peninsula. The tested sample from Croatian Istria (rTD) contained 77.9 mg/L of phenolic acids, of which 53.0 mg/L was gallic acid. The percentage of gallic acid was higher than in the previously tested Teran wine from the 2013–2015 vintages [11,16], but almost three times lower than in the wine from 2019 [6].
Of the red wines examined in this study, Cabernet Sauvignon from the southern part of Dalmatia (rCD) had the lowest gallic acid content (11.8 mg/L). By contrast, Cabernet Sauvignon wine samples from the Dalmatian Hinterland (rCK), Croatian Istria (rCA), and Croatian Danube (rCB) contained 30.9–43.3 mg/L, which is consistent with Montenegrin samples [35], higher than other Croatian samples [11,14], and lower than Chinese [36,37] and Macedonian Cabernet Sauvignon wine [38]. In addition to Cabernet Sauvignon, Merlot is also widely grown as a non-native grape variety in the Croatian coastal regions. The percentage of total phenolic acids in Merlot wine from the Dalmatian Hinterland (rMK) was 82.8 mg/L, while in the sample from Croatian Istria (rMA) the content of identified phenolic acids was 60.3 mg/L. The Dalmatian sample of this wine contained the same amount of gallic acid but significantly more p-hydroxybenzoic acid, syringic acid, and p-coumaric acid. The proportions of gallic acid (37.0–38.9 mg/L) in Merlot wines studied were in agreement with the majority of previously studied samples from Croatia [11,16], as well as some samples from Serbia, France, and Spain [39], while the gallic acid concentrations were higher compared to the values determined in some Italian samples [40,41]. Table 3 shows a great similarity between Syrah wine (rSK) and Merlot wine (rMK) from the same geographical area in terms of phenolic acid composition. In this work, the polyphenols of Syrah wine of Croatian origin were identified and quantified for the first time. In comparison with the results of the analysis of polyphenols in Syrah wines from China and Brazil, a common dominance of the proportion of hydroxybenzoic acids is observed, but with mutual differences in their individual concentrations [37,42]. The grape variety Blauer Portugieser is grown mainly in the small region of Plešivica on the Croatian mainland and is used to produce young wine, which is traditionally consumed immediately after fermentation [43]. The wine sample from the Plešivica Hills (rPM), along with Cabernet Sauvignon from the extreme south of the Croatian coast (rCD), is another of the red wines studied with somewhat lower phenolic acid levels. Gallic acid was the most abundant phenolic acid (25.1 mg/L), followed by syringic acid, caffeic acid, and p-coumaric acid with values between 4.7 and 7.4 mg/L. This is the first information on the composition of phenolic acids in this wine variety of Croatian origin. A recent study of several samples from the same subregion showed that the total content of polyphenols and stilbenes decreases with aging and that consumption of young Blauer Portugieser is preferable [43]. An analysis of wine samples from Northern Serbia also showed that the content of phenolic acids in Blauer Portugieser, especially gallic acid, decreases with wine aging [44]. In addition, the gallic acid content in samples of this wine variety from neighboring Hungary averaged 43.0 mg/L, with a high standard deviation indicating high variability of the content [45]. Pinot Noir from the Slavonia region (rPK) was characterized by a high level of hydroxybenzoic acids, primarily gallic acid (57.9 mg/L). Van Leeuw et al. [46], who studied samples of different origins on the Belgian market, also found high levels of gallic acid in this type of wine. Samples from France, Italy, Argentina, and Chile contained 122.1–223.1 mg/L of quantified phenolic acids, of which 23.4–105.3 mg/L was gallic acid.
Istrian Malvasia is an autochthonous Croatian white grape variety grown mainly on the Istrian Peninsula. It is known as Malvazija Istarska in Croatia and Malvasia Istriana in Italy [47]. The percentage of quantified phenolic acids in the sample of Istrian Malvasia from western Istria (wMD) was 6.3 mg/L, dominated by p-hydroxybenzoic acid, p-coumaric acid, and caffeic acid. In contrast to the results of a recent study by Radeka et al. [6], our sample contained significantly less phenolic acids. Table 3 shows that the wMD sample of Istrian Malvasia is more similar in its phenolic acid composition to the sample of the Malvasia Bianca Lunga variety from southern Dalmatia (wMA) than to the other sample from northern Dalmatia (wMS), which is characterized by a low content of hydroxycinnamic acids and a higher content of hydroxybenzoic acids, especially gallic acid (2.3 mg/L), just like the Italian white wine of the Malvazija family [48]. In Croatia, the grape variety Malvasia Bianca Lunga (Malvasia del Chianti) is known as Maraština. It is an old white grape variety grown mainly in the coastal regions of Croatia (Dalmatia). The aroma components of Maraština wine have already been researched [49], but as far as we know, this work provides the first information about its polyphenolic components.
Other white wines contained significant amounts of caffeic acid (1.5–8.7 mg/L) and p-coumaric acid (1.5–4.4 mg/L). In addition, white wine samples of Sauvignon Blanc (wSD), Traminer (wTI), and Rhine Riesling (wRD) contained gallic acid at concentrations of 2.4–4.0 mg/L. The content of p-hydroxybenzoic acid was also significant in most of the tested wines, except for Sauvignon Blanc (wSD), Traminer (wTI), and Welschriesling (wGK) from the Slavonia and Croatian Danube regions. Regarding the total amount of phenolic acids quantified in wSD, our results are in agreement with a previous study on Spanish Sauvignon Blanc wine [50]. Comparing the composition of the predominant phenolic acids in the wSD and Chardonnay (wCR and wCB) wines with those from South Africa, the proportions of gallic acid, caffeic acid, and p-coumaric acid were higher, except for the proportion of gallic acid in Chardonnay wines, which was lower than in the sample from South Africa [51]. The gallic acid content of wTI was consistent with a previous analysis of Traminer wine of the same origin. However, unlike wTI, caffeic acid and p-coumaric acid were not detected in this wine sample [14]. By contrast, Traminer wine from Serbia (Banat wine region) contained significantly higher concentrations of the above hydroxycinnamic acids than wTI [52]. A previously analyzed Croatian Rhine Riesling wine from 2011 contained lower amounts of gallic acid than the sample wRD and no p-coumaric acid [53]. Accordingly, the content of phenolic acids and total phenols in Rhine Riesling wines was found to be significantly different depending on the ripeness of the grapes [54]. Welschriesling (Italian Riesling) is the most widely cultivated grape variety in Croatia, where it is also known as Graševina. The wGJ and wGK wine samples studied in this work were from western and eastern mainland Croatia, respectively. They were characterized by a higher proportion of hydroxycinnamic acids compared to hydroxybenzoic acids. In addition, gallic acid was present in traces (below the calculated limit of quantification), which is not consistent with previous analyzes of Croatian samples from experimental breeding in 2008 and 2009 [22].
3.1.2. Flavan-3-ols, Anthocyanins, and Stilbenes in Selected Red and White Wines
According to the results presented in Table 4, catechin, epicatechin, and their dimers (type B proanthocyanidins) were highly represented polyphenols in red wines. As for phenolic acids, Croatian wines of the Babić (rBS) and Plavac Mali (rPI and rPD) grape varieties were the richest in monomeric and dimeric flavan-3-ols (97.9–140.0 mg/L), while Cabernet Sauvignon wine from the Croatian Danube subregion (rCB) contained the lowest amount of flavan-3-ols (27.2 mg/L). In addition to the autochthonous wines from the Croatian coastal regions, another red wine of the Pinot Noir variety (rPK) from the continental part of Croatia, more precisely from the subregion of Slavonia, stood out for its content of flavan-3-ols (101.2 mg/L). Considering the individual proportions, the highest contents of catechin, epicatechin, and procyanidin B1 were found in rBS, while procyanidin B2 was the most abundant in rPK. In addition to gallic acid, p-coumaric acid, and caffeic acid, individual flavan-3-ols also appear to be of great importance for the differentiation of Croatian wines, as their proportions vary greatly in the wines studied. The content of catechin in Plavac Mali wines (rPI and rPD) was lower, while the content of procyanidin B1 was significantly higher compared to previously studied wine samples [6,11].
As mentioned above, the Croatian Pinot Noir wine (rPK) contained large amounts of flavan-3-ols, although to a much lesser extent than in several Italian, French, Argentine, and Chilean samples. The combined content of catechin, epicatechin, and procyanidins B1 and B2 ranged from 140.9 to a very high value of 767.2 mg/L [46]. In the same article, the results of the determination of flavan-3-ols in Cabernet Sauvignon, Syrah (Italy and France), and Merlot (USA, Australia, Chile, Italy, and Bulgaria) wines from the 2007–2010 vintages are presented. As with Pinot Noir, these commercial wines contained significantly more flavan-3-ols than our corresponding samples. Although the studied Cabernet Sauvignon wines lagged behind those from Spain [55], China [56], and Croatia [16] in terms of flavan-3-ol content, somewhat smaller differences were observed. By contrast, our samples contained similar proportions of monomeric and dimeric flavan-3-ols as the Montenegrin wine [34]. Cabernet Sauvignon wines from Dalmatia (rCD and rCK), Istria (rCA), and Croatian Danube regions (rCB) were characterized by large amounts of procyanidin B1 (13.7–44.6 mg/L) and catechin (10.5–19.3 mg/L), while epicatechin and procyanidin B2 contents were much lower (<LOQ—11.0 mg/L). A very similar composition of flavan-3-ols was also found in the Merlot samples from the Croatian coastal areas (rMK and rMA), which was lower on average compared to previously studied wines of the same variety and the same wine-growing region [16]. In addition to Cabernet and Merlot, the results presented in Table 4 show that the studied Syrah (rSK), Teran (rTD), and Blauer Portugieser (rPM) wines are also very similar in terms of flavan-3-ols composition. The content of flavan-3-ols in rTD was lower compared to the results of previous analyses of the Croatian Teran wine [6,16].
Table 4 shows that, as expected, the white wines had significantly lower amounts of flavan-3-ols. In the majority of the white wines tested, their individual contents were below the limit of quantification. Epicatechin was detected in several samples but could not be quantified in any of them. Pinot Blanc from the Croatian Uplands (wPD) had the highest levels of catechin and procyanidin B1 (6.2 and 6.8 mg/L, respectively). Catechin was also determined only in samples of Chardonnay wine from Istria (wCR) and Traminer from the Croatian Danube (wTI), and procyanidin B1 in the sample of Welschriesling from Međimurje (wGJ), in the amounts of 5.1–5.5 mg/L. Looking at the composition of flavan-3-ol in the Chardonnay samples studied, only one difference can be observed, namely that the sample from Istria (wCR) contained more catechin than the sample from the Croatian Danube region (wCB). The first sample was similar to the sample from northern Italy from the 2014 vintage [57]. Traminer wine (wTI) contained twice as much catechin as the Croatian sample from the 2008 and 2009 experimental vintages [22]. Rhine Riesling from Slavonia (wRD) contained 8.1 mg/L procyanidin B2, which was significantly higher than that of the previously studied sample from northwestern Croatia [53]. There was no statistically significant difference in the other six white wine samples in which 3.0–3.8 mg/L procyanidin B2 was determined (Table 4).
No tested anthocyanins were detected in the white wines, while their content varied widely in the red wines (Table 4). The content of delphindin-3-glucoside was low, reaching a maximum of 0.5 mg/L in the Cabernet Sauvignon from southern Dalmatia (rCD). The same wine also contained a high concentration of 20.2 mg/L malvidin-3-glucoside, which was not the case in other Cabernet Sauvignon wines from Istria, Dalmatia, and the Croatian Danube. A similar malvidin-3-glucoside content to rCD was also found in the Blauer Portugieser from the Croatian Uplands (rPM) at 21.4 mg/L. The other red wines contained 1.4–6.0 mg/L malvidin-3-glucoside. As mentioned above, Blauer Portugieser from the Croatian Uplands (rPM) and Cabernet Sauvignon from southern Dalmatia (rCD) contained the most anthocyanins, but these are still lower values compared to the results of Kumšta et al. [58] and de Andrade et al. [59], who analyzed Czech and Brazilian wines, respectively. As the most abundant anthocyanin in grapes, malvidin-3-glucoside was predominant in all the red wine samples, but its content in the wines studied was significantly lower compared to most previously published results. Thus, its content in Merlot from various European countries was 4.49–41.9 mg/L [38,60], while our samples from the Dalmatian Hinterland (rMK) and Croatian Istria (rMA) contained only 1.6–2.1 mg/L of malvidin-3-O-glucoside, which is also much lower than the samples from Croatia studied by Lukić et al. [16]. This was very similar in the other red wines studied, such as Syrah and Pinot Noir. Our samples contained significantly less malvidin-3-glucoside than the Brazilian [42,46,59] or Hungarian [60] wines. It is interesting to note that not only the examined non-autochthonous but also the Croatian autochthonous red wines had unexpectedly low anthocyanin contents. In comparison with our results, many times higher levels of malvidin-3-glucoside were previously found in Plavac Mali [16,17] and Teran [16] wines.
As shown in Table 4, unlike white wines, red wines contained stilbenes and the proportion of trans-resveratrol was generally much higher than that of cis-resveratrol in all the wines studied, which is consistent with numerous previous relevant studies [2]. In addition to anthocyanins, the Blauer Portugieser from Croatian Uplands (rPM) contained the highest levels of trans-resveratrol (6.8 mg/L) and cis-resveratrol (1.6 mg/L). By contrast, previously studied Portugieser young wines from the same Croatian wine subregion and after 12 months of bottle aging contained significantly less trans-resveratrol (0.6–3.4 mg/L and 0.8–2.0 mg/L, respectively), the concentration of which decreased with aging [43]. Portugieser wine originating from south Hungary [60] and the Czech region of Moravia [61] also contained significantly less resveratrol than the sample studied. In Plavac Mali (rPD and rPI), Babić (rBS), Merlot (rMK and rMA), and Pinot Noir (rPK) a common stilbene content between 1.8 and 4.6 mg/L was found, while the content in the other studied red wines was <LOQ—1.9 mg/L. Katalinić et al. [62] determined the free resveratrol monomers in wine varieties from the Dalmatian region, including Plavac Mali, Babić, and Merlot. They demonstrated that resveratrol contents differ between wines of the same grape variety from different locations. For example, they found that Plavac Mali wine from some locations on the Pelješac Peninsula is particularly rich in resveratrol compared to the central Dalmatian islands of Hvar and Brač. Thus, the resveratrol content is not only dependent on the grape variety, but could also be due to lower temperatures, higher humidity, and precipitation leading to the synthesis of stilbenes in grapes in response to fungal attacks triggered by these environmental conditions. It has already been reported that higher humidity is usually associated with stilbene synthesis, while temperature has a negative effect on the concentration of resveratrol in grapes and wines [35]. Since resveratrol and other stilbenes act as phytoalexins, they play a crucial role in the defense against phytopathogens and are also involved in the adaptation of plants to abiotic environmental factors [63]. Our results also support the fact that, in the production of red wine, maceration with skins and seeds during fermentation results in higher resveratrol concentrations in red wines than in white wines [44].
3.1.3. Flavonoids in the Selected Red and White Wines
Eight flavonoids were analyzed in the selected commercial Croatian wines. The white wines did not contain flavonoids, except for two samples of Welschriesling from Međimurje (wGJ) and Pinot Blanc from the Croatian Uplands (wPD) with traces of quercitrin. In contrast to the white wines, the red wines contained various types of flavonoids in amounts up to 37.6 mg/L (Table 5). The highest amount of flavonoids tested was present in three wine varieties from the same wine region. Merlot (rMK), Cabernet Sauvignon (rCK), and Syrah (rSK) originating from the Dalmatian Hinterland contained 32.4–37.6 mg/L flavonoids. They were followed by the Croatian autochthonous wine varieties Plavac Mali (rPD) and Babić (rBS) from Dalmatia with flavonoid contents of 29.6 and 26.1 mg/L, respectively. The Plavac Mali (rPI) wine sample contained 16.9 mg/L flavonoids, which is significantly less than the previously described rPD sample of the same variety. These Plavac Mali wines are from the same subregion but from different Croatian vineyard locations. Cabernet Sauvignon (rCB) is a red wine originating from a continental wine region and is characterized by high flavonoid content (19.3 mg/L), which, however, is significantly higher than that of the sample of the same variety from Croatian Istria (rCA, 6.6 mg/L) and from the extreme south of Croatia (rCD, 7.1 mg/L). Likewise, the Merlot from Croatian Istria (rMA) contained a much lower amount of flavonoids (7.9 mg/L) than the sample from the Dalmatian Hinterland (rMK). Among the tested flavonoids, the flavonols quercetin (<LOQ—21.7 mg/L) and myricetin (2.9–12.1 mg/L) stood out in terms of quantity in all the studied red wines. As far as the content of quercetin is concerned, the order of the wines from the richest in quercetin to the poorest in quercetin is completely identical to that which we described when considering the contents of total flavonoids tested.
Our results are consistent with some previous studies of Croatian red wines, which showed that quercetin and myricetin are very abundant flavonoids in wines made in the coastal areas [6,14], but with large differences in their proportions. On the contrary, Lukić et al. [16] determined significantly lower amounts of myricetin (<2 mg/L) and quercetin (<0.5 mg/L) in autochthonous wines Plavac Mali and Teran and in non-native Merlot and Cabernet Sauvignon. Syrah wine from the Dalmatian Hinterland (rSK), which was rich in myricetin and quercetin, corresponded to samples of this variety from the 2010 and 2011 vintages in some Brazilian regions, where significant differences in polyphenol composition were found due to climatic conditions [42]. In response to solar radiation, especially UV-B, flavonols accumulate mainly in the epidermal cells of plant tissue and filter the most dangerous wavelength of the solar spectrum for DNA [64]. Therefore, Croatian wines from the coastal regions, where solar radiation is significantly higher compared to the mainland, were richer in flavonols. Among the studied red wines, Merlot (rMK), Cabernet Sauvignon (rCK), and Syrah (rSK) from the Dalmatian Hinterland stood out for their content. Although these wines were obtained from different grape varieties, they had very similar flavonol compositions. The reasons for this are most likely the same location of the vineyard, exposed to high and uniform solar radiation.
3.1.4. Differentiation of the Selected Monovarietal Red and White Wines
Because of the obvious difference between red and white wines, a separate principal component analysis (PCA) was performed using the data in Table 3, Table 4 and Table 5. Analysis of the PCA score plot for the monitored values showed that 61.02 and 53.97% of the total variance in the data could be described by F1 and F2 for red and white wines, respectively. Figure 2 shows three well-separated clusters for the red wines studied and a biplot derived from the polyphenolic compounds. The Croatian autochthonous wines Plavac Mali (rPD and rPI) and Babić (rBS) form a separate group in the first quadrant. The mentioned differences are mostly due to the different contents of gallic acid, p-coumaric acid, ferulic acid, catechin, and procyanidin B1 compared to other red wines. Our study supports the results of Lukić et al. [16] and Žurga et al. [11] about the most specific composition of Plavac Mali compared to other Croatian red wines. In this context, we found for the first time the same varietal differences in Babić wine, which is also produced from Croatian autochthonous grape varieties. A group of non-native wines, including Merlot (rMK), Cabernet Sauvignon (rCK), and Syrah (rSK), was also separated. According to F1, this group shows similarity with Croatian autochthonous red wines from the same wine-growing region, and the differences present can be attributed mostly to higher levels of quercetin and rutin compared to Plavac Mali and Babić wines. One of the possible reasons for this difference is the better adaptation of autochthonous species to local conditions and the resulting lower stress, which leads to a lower accumulation of quercetin [11]. Seven other wines formed a third distinctive group, also comprising other Cabernet Sauvignon samples from different wine-growing regions. PCA confirmed the previously described results that rCD from the extreme south of the Croatian coast is more similar to rCB from the easternmost continental part of Croatia than rCA from Croatian Istria, which contains higher levels of caffeic acid, p-coumaric acid, epicatechin, and procyanidin B1 and lower levels of myricetin compared to rCD and rCB. According to F2, Pinot Noir from the continental region of Slavonia (rPK) in the second quadrant is the least different from the autochthonous red wines Plavac Mali, which is mainly due to the high content of gallic acid, epicatechin, and procyanidins B1 and B2.
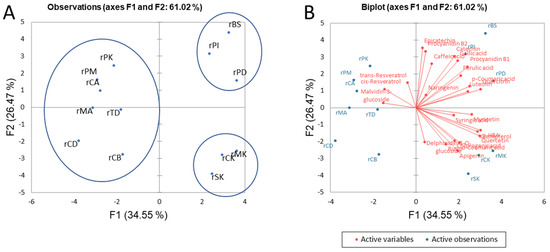
Figure 2.
Principal component analysis of the clustering of Croatian red wines based on phenolic composition (A) and biplot with active variables (B). The abbreviations corresponding to the wine samples are shown in Table 1.
Although the white wines did not differ as much as the red wines, the multivariate analysis revealed more detailed relationships among them. Figure 3 shows the PCA of the clustering of Croatian white wines and a biplot derived from the polyphenolic compounds. In the third quadrant, there is a separate sample of Malvasia Bianca Lunga wine from Northern Dalmatia (wMS), which is significantly different from the other white wines, including wine from the same grape variety (wMA). Compared to other white wines, o-coumaric acid was detected only in the wMS sample. In addition, wMS also contained significantly lower amounts of caffeic acid and p-coumaric acid than other white wines. Compared to the wine of the same variety from Southern Dalmatia (wMA), it also had significantly higher levels of gallic acid and procyanidin B2. Possible reasons for this lie not only in the different locations but also in the winemaking process. Namely, wMS was produced using a special technique based on aging on the lees (sur lie), which has been shown to correlate positively with gallic acid levels [65]. Proteolytic enzymes released during yeast autolysis could be involved in the hydrolysis of tannins and, consequently, in changes in the composition of polyphenols and the associated sensory characteristics of the wine [66]. Two other wines were also distinguished based on their phenolic profile. Pinot Blanc originating from the Croatian Uplands (wPD) and Chardonnay from Istria (wCR) were distinguished from the other white wines by their high content of catechin. In addition, significantly higher levels of caffeic acid and p-coumaric acid in wCR (the second quadrant) than in the other white wines contributed to the separation of this sample from the others. Figure 3 shows the similarity between Traminer (wTI), Sauvignon Blanc (wSD), and Rhine Riesling (wRD) from Slavonia and the Croatian Danube according to F2, which is mainly due to the very comparable compositions of phenolic acids, while the distinction from the other white wines is due to the content of gallic acid. The high content of procyanidin B2 in wRD is the reason why it differs from wTi and wSD.
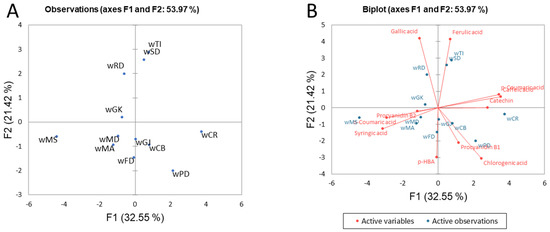
Figure 3.
Principal component analysis of the clustering of Croatian white wines based on phenolic composition (A) and biplot with active variables (B). The abbreviations corresponding to the wine samples are shown in Table 2.
3.2. Antioxidant Activity of Major Phenolic Components in Commercial Croatian Wines
The polyphenols present in wines determine many of their sensory characteristics, such as appearance, color, astringency, bitterness, and taste, as well as their stability to subsequent oxidative processes that lead to browning in white wines and oxidation in red wines. Apart from sensory properties and wine quality, polyphenols have also been shown to be highly beneficial to health, including antioxidant and cardioprotective effects [4,67]. The antioxidant capacity of a wine depends largely on its phenolic profile, since different compounds have different levels of activity that are closely related to their chemical structure [68]. Therefore, our aim was to compare for the first time the antioxidant activity of the main polyphenolic components of Croatian wines and to predict their possible importance in the antioxidant properties of wine. In our study, most of the selected polyphenols also proved to be important distinguishing features between Croatian wines. The obtained results are presented in Table 6 and Suppl. Tables S3–S5. The most representative phenolic components in the studied Croatian wines showed a strong antioxidant effect, mostly exceeding the effect of Trolox as a reference antioxidant. Gallic acid proved to be the most potent DPPH and NO radical scavenger, reaching IC50 values of 0.33 µg/mL and 12.36 µg/mL, respectively. Of the seven polyphenols tested, resveratrol had the weakest potential to inhibit the production of free DPPH (IC50 = 7.56 µg/mL) and NO radicals (IC50 = 35.08 µg/mL) but showed a much better ability to inhibit lipid peroxidation (IC50 = 5.02 µg/mL). The strongest inhibitor of lipid peroxidation was myricetin (IC50 = 1.68 µg/mL), followed by quercetin (IC50 = 2.06 µg/mL), while caffeic acid showed the weakest effect, achieving 50% inhibition of lipid peroxidation at a concentration of 141.76 µg/mL. Our study showed that the selected polyphenols from Croatian wines inhibit lipid peroxidation at very low concentrations (except caffeic acid), which confirms their strong ability to protect against this type of cellular damage as an indicator of oxidative stress in cells and tissues. The high lipid content of cell membranes makes them one of the most important targets for reactive oxygen and nitrogen species. NO is a biologically relevant free radical that, when formed in excessive amounts, reacts with other free radicals, such as superoxide anion, to form a highly reactive nitrogen species [69]. The selected polyphenols were found to be potent NO radical scavengers, indicating their excellent antioxidant properties and related significant contribution to the beneficial health effects of wine.

Table 6.
Comparative overview of antioxidant effects (IC50 values, μg/mL) of the main phenolic compounds from Croatian wine.
4. Conclusions
In this study, twenty-five commercial Croatian wines from eight red and nine white grape varieties were characterized in terms of their phenolic composition. To our knowledge, this is one of the few comparative studies of wines from different wine-growing regions of Croatia. Moreover, for the first time, some data on the phenolic composition of specific wines were reported in detail. The twenty-four polyphenols analyzed were classified as phenolic acids, flavan-3-ols, anthocyanins, flavonoids, and stilbenes. In contrast to the white wines, the red wines contained higher concentrations of polyphenols with wide variability. Gallic acid, p- and o-coumaric acid, caffeic acid, ferulic acid, catechin, epicatechin, procyanidins B1 and B2, quercetin, rutin, and myricetin proved to be important differentiators among the Croatian monovarietal wines. Red wines formed three well-distinguished groups, of which the group of Croatian autochthonous wines Plavac Mali and Babić differed the most in terms of polyphenolic composition. The main representative phenolic components in the studied Croatian wines showed strong antioxidant activity, including gallic acid as the most effective DPPH and NO radical scavenger, while myricetin proved to be the strongest inhibitor of lipid peroxidation. Plavac Mali and Babić wines were the richest sources of gallic acid and catechin. Babić wine also contained the highest levels of epicatechin and myricetin. Merlot, Cabernet Sauvignon, and Shiraz wines from the Dalmatian Hinterland contained high levels of quercetin. Our study provided new insights into the phenolic profiles of the selected red and white wines from different Croatian regions and contributed to their characterization and varietal differentiation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13053031/s1, Table S1: Meteorological data from June to September 2007, collected from the meteorological stations near the vineyards where the grapes were grown; Table S2: Validation parameters of the HPLC method for the determination of non-anthocyanin phenolic compounds in wine; Table S3: DPPH radical scavenging activity (%) of selected phenolic compounds from wine in comparison with a reference antioxidant; Table S4: NO scavenging activity (%) of selected phenolic compounds from wine in comparison with a reference antioxidant; Table S5: Lipid peroxidation inhibitory activity (%) of selected phenolic compounds from wine.
Author Contributions
Conceptualization, S.V.-K.; methodology, M.K. and S.V.-K.; validation, M.K.; formal analysis, M.C. and T.B.; investigation, M.K., M.B.Š.; K.R. and T.P.; writing—original draft preparation, M.K., S.V.-K. and M.B.Š.; writing—review and editing, S.V.-K.; visualization, M.C., K.R. and T.P.; supervision, S.V.-K. and T.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project FarmInova (KK.01.1.1.02.0021) funded by the European Regional Development Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Merkytė, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic compounds as markers of wine quality and authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Casassa, L.F.; Gannet, P.A.; Steele, N.B.; Huff, R. Multi-year study of the chemical and sensory effects of microwave-assisted extraction of musts and stems in Cabernet Sauvignon, Merlot and Syrah wines from the central coast of California. Molecules 2022, 27, 1270. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Weaver, S.R.; Rendeiro, C.; McGettrick, H.M.; Philp, A.; Lucas, S.J.E. Fine wine or sour grapes? A systematic review and meta-analysis of the impact of red wine polyphenols on vascular health. Eur. J. Nutr. 2021, 60, 1–28. [Google Scholar] [CrossRef]
- Radeka, S.; Rossi, S.; Bestulić, E.; Budić-Leto, I.; Kovačević Ganić, K.; Horvat, I.; Orbanić, F.; Zaninović Jurjević, T.; Dvornik, Š. Bioactive compounds and antioxidant activity of red and white wines produced from autochthonous Croatian varieties: Effect of moderate consumption on human health. Foods 2022, 11, 1804. [Google Scholar] [CrossRef]
- Lucarini, C.M.; Durazzo, A.; Lombardi-Bocci, G.; Souto, E.B.; Cecchini, F.; Santini, A. Wine polyphenols and health: Quantitative research literature analysis. Appl. Sci. 2021, 11, 4762. [Google Scholar] [CrossRef]
- Pavlidou, E.; Mantzorou, M.; Fasoulas, A.; Tryfonos, C.; Petridis, D.; Giaginis, C. Wine: An aspiring agent in promoting longevity and preventing chronic diseases. Diseases 2018, 6, 73. [Google Scholar] [CrossRef]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellor, D.D.; Panagiotakos, D.B.; McKune, A.J.; Kellett, J.; Naumovski, N. The effects of grape and red wine polyphenols on gut microbiota—A systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef]
- Nemzer, B.; Kalita, D.; Yashin, A.Y.; Yashin, Y.I. Chemical composition and polyphenolic compounds of red wines: Their antioxidant activities and effects on human health—A review. Beverages 2022, 8, 1. [Google Scholar] [CrossRef]
- Žurga, P.; Vahčić, N.; Pasković, I.; Banovi, M.; Malenica Staver, M. Croatian wines from native grape varieties have higher distinct phenolic (nutraceutic) profiles than wines from non-native varieties with the same geographic origin. Chem. Biodivers. 2019, 16, e1900218. [Google Scholar] [CrossRef] [PubMed]
- Leder, R.; Petric, I.V.; Jusup, J.; Banović, M. Geographical discrimination of Croatian wines by stable isotope ratios and multielemental composition analysis. Front. Nutr. 2021, 8, 625613. [Google Scholar] [CrossRef] [PubMed]
- Zakon o Vinu (Law on Wine). Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_03_32_641.html (accessed on 13 August 2022).
- Rastija, V.; Srečnik, G.; Medić-Šarić, M. Polyphenolic composition of Croatian wines with different geographical origins. Food Chem. 2009, 115, 54–60. [Google Scholar] [CrossRef]
- Šeruga, M.; Novak, L.; Jakobek, L. Determination of polyphenols content and antioxidant activity of some red wines by differential pulse voltammetry, HPLC and spectrophotometric methods. Food Chem. 2011, 124, 1208–1216. [Google Scholar] [CrossRef]
- Lukić, I.; Radeka, S.; Budić-Leto, I.; Bubola, M.; Vrhovsek, U. Targeted UPLC-QqQ-MS/MS profiling of phenolic compounds for differentiation of monovarietal wines and corroboration of particular varietal typicity concepts. Food Chem. 2019, 300, 125251. [Google Scholar] [CrossRef]
- Jagatić Korenika, A.-M.; Tomaz, I.; Preiner, D.; Plichta, V.; Jeromel, A. Impact of commercial yeasts on phenolic profile of Plavac Mali wines from Croatia. Fermentation 2021, 7, 92. [Google Scholar] [CrossRef]
- Mucalo, A.; Zdunić, G.; Maletić, E. Prolonged ripening on the vine affects the polyphenolic profile of grapes and wine of ’Plavac Mali’ (Vitis vinifera L.). Acta Hortic. 2019, 1248, 417–424. [Google Scholar] [CrossRef]
- Budić-Leto, I.; Zdunić, G.; Gajdoš Kljusurić, J.; Pezo, I.; Alpeza, I.; Lovrić, T. Effects of polyphenolic composition on sensory perception of Croatian red wine Babić. J. Food Agric. Environ. 2008, 6, 138–142. [Google Scholar]
- Budić-Leto, I.; Lovrić, T.; Gajdoš-Kljusurić, J.; Pezo, I.; Vrhovšek, U. Anthocyanin composition of the red wine Babić affected by maceration treatment. Eur. Food Res. Technol. 2006, 222, 397–402. [Google Scholar] [CrossRef]
- Bubola, M.; Rusjan, D.; Lukić, I. Crop level vs. leaf removal: Effects on Istrian Malvasia wine aroma and phenolic acids composition. Food Chem. 2020, 312, 126046. [Google Scholar] [CrossRef]
- Osrečak, M.; Karoglan, M.; Kozina, B.; Preiner, D. Influence of leaf removal and reflective mulch on phenolic composition of white wines. J. Int. Sci. Vigne Vin 2015, 49, 183–193. [Google Scholar] [CrossRef]
- Maletić, E.; Kontić, K.; Preiner, D.; Jeromel, A.; Patz, C.-D.; Dietrich, H. Anthocyanin profile and antioxidative capacity of some autochthonous Croatian red wines. J. Food Agric. Environ. 2009, 7, 48–51. [Google Scholar] [CrossRef]
- Katalinić, V.; Milos, M.; Modun, D.; Musić, I.; Boban, M. Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem. 2006, 86, 593–600. [Google Scholar] [CrossRef]
- Vinković Vrček, I.; Bojić, M.; Žuntar, I.; Mendaš, G.; Medić-Šarić, M. Phenol content, antioxidant activity and metal composition of Croatian wines deriving from organically and conventionally grown grapes. Food Chem. 2011, 124, 354–361. [Google Scholar] [CrossRef]
- Visioli, F.; Panaite, S.-A.; Tomé-Carneiro, J. Wine’s phenolic compounds and health: A Pythagorean view. Molecules 2020, 25, 4105. [Google Scholar] [CrossRef]
- Nour, V.; Trandafir, I.; Muntean, C. Ultraviolet irradiation of trans-resveratrol and HPLC determination of trans-resveratrol and cis-resveratrol in Romanian red wines. J. Chromatogr. Sci. 2012, 50, 920–927. [Google Scholar] [CrossRef]
- Berente, B.; De la Calle García, D.; Reichenbächer, M.; Danzer, K. Method development for the determination of anthocyanins in red wines by high-performance liquid chromatography and classification of German red wines by means of multivariate statistical methods. J. Chromatogr. A 2000, 871, 95–103. [Google Scholar] [CrossRef]
- ICH Harmonised Tripartite Guideline. Validation of Analytical Procedures: Text and Methodology Q2 (R1). Available online: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf (accessed on 13 August 2022).
- de Souza Dias, F.; Lovillo, M.P.; Barroso, C.G.; David, J.M. Optimization and validation of a method for the direct determination of catechin and epicatechin in red wines by HPLC/fluorescence. Microchem. J. 2010, 96, 17–20. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Štefan, M.B.; Alegro, A.; Köszegi, T.; Petrik, J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules 2011, 16, 1454–1470. [Google Scholar] [CrossRef]
- Patel, A.; Patel, A.; Patel, A.; Patel, N.M. Determination of polyphenols and free radical scavenging activity of Tephrosia purpurea Linn leaves (Leguminosae). Pharmacogn. Res. 2010, 2, 152–158. [Google Scholar] [CrossRef]
- Houghton, P.J.; Zarka, R.; de las Heras, B.; Hoult, J.R. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995, 61, 33–36. [Google Scholar] [CrossRef]
- Ćurko, N.; Kovačević Ganić, K.; Gracin, L.; Đapić, M.; Jourdes, M.; Teissedre, P.L. Characterization of seed and skin polyphenolic extracts of two red grape cultivars grown in Croatia and their sensory perception in a wine model medium. Food Chem. 2014, 145, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Šćepanović, R.P.; Wendelin, S.; Raičević, D.; Eder, R. Characterization of the phenolic profile of commercial Montenegrin red and white wines. Eur. Food Res. Technol. 2019, 245, 2233–2245. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Shi, P.; Li, F. Phenolic profile and antioxidant capacity of ten dry red wines from two major wine-producing regions in China. Adv. J. Food Sci. Technol. 2014, 6, 344–349. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, B.; Lu, J.; Xu, S. Analysis of metabolites in Cabernet Sauvignon and Shiraz dry red wines from Shanxi by 1H NMR spectroscopy combined with pattern recognition analysis. Open Chem. 2018, 16, 446–452. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Ricci, A.; Nedelkovski, D.; Dimovska, V.; Parpinello, G.P.; Versari, A. Targeted analysis of bioactive phenolic compounds and antioxidant activity of Macedonian red wines. Food Chem. 2015, 171, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Majkić, T.M.; Torović, L.D.; Lesjak, M.M.; Četojević-Simin, D.D.; Beara, I.N. Activity profiling of Serbian and some other European Merlot wines in inflammation and oxidation processes. Food Res. Int. 2019, 121, 151–160. [Google Scholar] [CrossRef]
- Girelli, A.; Mele, C.; Salvagni, L.; Tarola, A.M. Polyphenol content and antioxidant activity of Merlot and Shiraz wine. Anal. Lett. 2015, 48, 1865–1880. [Google Scholar] [CrossRef]
- Fermo, P.; Comite, V.; Sredojević, M.; Ćirić, I.; Gašić, U.; Mutić, J.; Baošić, R. Elemental analysis and phenolic profiles of selected Italian wines. Foods 2021, 10, 158. [Google Scholar] [CrossRef]
- Sartor, S.; Malinovski, L.I.; Caliari, V.; da Silva, A.L.; Bordignon-Luiz, M.T. Particularities of Syrah wines from different growing regions of Southern Brazil: Grapevine phenology and bioactive compounds. J. Food Sci. Technol. 2017, 54, 1414–1424. [Google Scholar] [CrossRef]
- Alpeza, I.; Lukić, K.; Vanzo, A.; Kovačević Ganić, K. Bioactive complexity of the red wine “Portugizac”; Is younger more beneficial? Agric. Conspec. Sci. 2021, 86, 329–335. [Google Scholar]
- Atanacković Krstonošić, M.; Cvejić Hogervorst, J.; Torović, L.; Puškaš, V.; Miljić, U.; Mikulić, M.; Gojković Bukarica, L. Influence of 4 years of ageing on some phenolic compounds in red wines. Acta Aliment. 2019, 48, 449–456. [Google Scholar] [CrossRef]
- Nikfardjam, M.S.P.; Márk, L.; Avar, P.; Figler, M.; Ohmacht, R. Polyphenols, anthocyanins, and trans-resveratrol in red wines from the Hungarian Villány region. Food Chem. 2006, 98, 453–462. [Google Scholar] [CrossRef]
- Van Leeuw, R.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Antioxidant capacity and phenolic composition of red wines from various grape varieties: Specificity of Pinot Noir. J. Food Compos. Anal. 2014, 36, 40–50. [Google Scholar] [CrossRef]
- Meneghetti, S.; Poljuha, D.; Frare, E.; Costacurta, A.; Morreale, G.; Bavaresco, L.; Calo, A. Inter- and intra-varietal genetic variability in Malvasia. Mol. Biotechnol. 2012, 50, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Fanizzi, F.P.; Maffia, M. A comparative study of phenols in Apulian Italian wines. Foods 2017, 6, 24. [Google Scholar] [CrossRef]
- Mucalo, A.; Lukšić, K.; Budić-Leto, I.; Zdunić, G. Cluster thinning improves aroma complexity of white Maraština (Vitis vinifera L.) wines compared to defoliation under Mediterranean climate. Appl. Sci. 2022, 12, 7327. [Google Scholar] [CrossRef]
- del Barrio-Galán, R.; del Valle-Herrero, H.; Bueno-Herrera, M.; López-de-la-Cuesta, P.; Pérez-Magariño, S. Volatile and non-volatile characterization of white and rosé wines from different Spanish Protected Designations of Origin. Beverages 2021, 7, 49. [Google Scholar] [CrossRef]
- de Villiers, A.; Majek, P.; Lynen, F.; Crouch, A.; Lauer, H.; Sandra, P. Classification of South African red and white wines according to grape variety based on the non-coloured phenolic content. Eur. Food Res. Technol. 2005, 221, 520–528. [Google Scholar] [CrossRef]
- Mitić, M.N.; Obradović, M.V.; Grahovac, Z.B.; Pavlović, A.N. Antioxidant capacities and phenolic levels of different varieties of Serbian white wines. Molecules 2010, 15, 2016–2027. [Google Scholar] [CrossRef]
- Jagatić Korenika, A.-M.; Žulj, M.M.; Puhelek, I.; Plavša, T.; Jeromel, A. Study of phenolic composition and antioxidant capacity of Croatian macerated white wines. Mitt. Klostern. 2014, 64, 171–180. [Google Scholar]
- Jakobović, S.; Jero, A.; Maslov Bandić, L.; Jakobović, M. Influence of grape ripeness of Rhine Riesling on the composition of polyphenolic compounds in must and wine. J. Food Agric. Environ. 2015, 13, 29–35. [Google Scholar]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B.; Laureano, O.; da Silva, J.M.R. Monomeric, oligomeric, and polymeric flavan-3-ol composition of wines and grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, Z.-W. Comparison on phenolic compounds and antioxidant properties of Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Molecules 2012, 17, 8804–8821. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Gatti, M.; Bavaresco, L.; Lucini, L. Untargeted metabolomics to investigate the phenolic composition of Chardonnay wines from different origins. J. Food Compos. Anal. 2018, 71, 87–93. [Google Scholar] [CrossRef]
- Kumšta, M.; Pavloušek, P.; Kárník, P. Use of anthocyanin profiles when differentiating individual varietal wines and terroirs. Food Technol. Biotechnol. 2014, 52, 383–390. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, R.H.S.; do Nascimento, L.S.; Pereira, G.E.; Hallwass, F.; Paim, A.P.S. Anthocyanic composition of Brazilian red wines and use of HPLC-UV–Vis associated to chemometrics to distinguish wines from different regions. Microchem. J. 2013, 110, 256–262. [Google Scholar] [CrossRef]
- Mark, L.; Nikfardjam, M.S.P.; Avar, P.; Ohmacht, R. A validated HPLC method for the quantitative analysis of trans-resveratrol and trans-piceid in Hungarian wines. J. Chromatogr. Sci. 2005, 43, 445–449. [Google Scholar] [CrossRef]
- Baron, M.; Sochor, J.; Tomaskova, L.; Prusova, B.; Kumsta, M. Study on antioxidant components in rosé wine originating from the wine growing region of Moravia, Czech Republic. Erwerbs-Obstbau 2017, 59, 253–262. [Google Scholar] [CrossRef]
- Katalinić, V.; Ljubenkov, I.; Pezo, I.; Generalić, I.; Stričević, O.; Miloš, M.; Modun, D.; Boban, M. Free resveratrol monomers in varietal red and white wines from Dalmatia (Croatia). Period. Biolog. 2008, 110, 77–83. [Google Scholar]
- Valletta, A.; Iozia, L.M.; Leonelli, F. Impact of environmental factors on stilbene biosynthesis. Plants 2021, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lüscher, J.; Brillante, L.; Kurtural, S.K. Flavonol profile is a reliable indicator to assess canopy architecture and the exposure of red wine grapes to solar radiation. Front. Plant Sci. 2019, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Stefenon, C.A.; Bonesi, C.D.M.; Marzarotto, V.; Barnabé, D.; Spinelli, F.R.; Webber, V.; Vanderlinde, R. Phenolic composition and antioxidant activity in sparkling wines: Modulation by the ageing on lees. Food Chem. 2014, 145, 292–2099. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Bayón, M.A.; Andujar, O.; Moreno-Arribas, M.V. Scientific evidence beyond the application of inactive dry yeast preparations in winemaking. Food Res. Int. 2009, 42, 754–761. [Google Scholar] [CrossRef]
- Haseeb, S.; Alexander, B.; Lopez Santi, R.; Sosa Liprandi, A.; Baranchuk, A. What’s in wine? A clinician’s perspective. Trends Cardiovasc. Med. 2019, 29, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical scavenging mechanisms of phenolic compounds: A quantitative structure-property relationship (QSPR) study. Front. Nutr. 2022, 9, 882458. [Google Scholar] [CrossRef] [PubMed]
- Mervić, M.; Bival Štefan, M.; Kindl, M.; Blažeković, B.; Marijan, M.; Vladimir-Knežević, S. Comparative antioxidant, anti-acetylcholinesterase and anti-α-glucosidase activities of Mediterranean Salvia species. Plants 2022, 11, 625. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).