Comparative Study of the Presence of Heavy Metals in Edible Vegetable Oils
Abstract
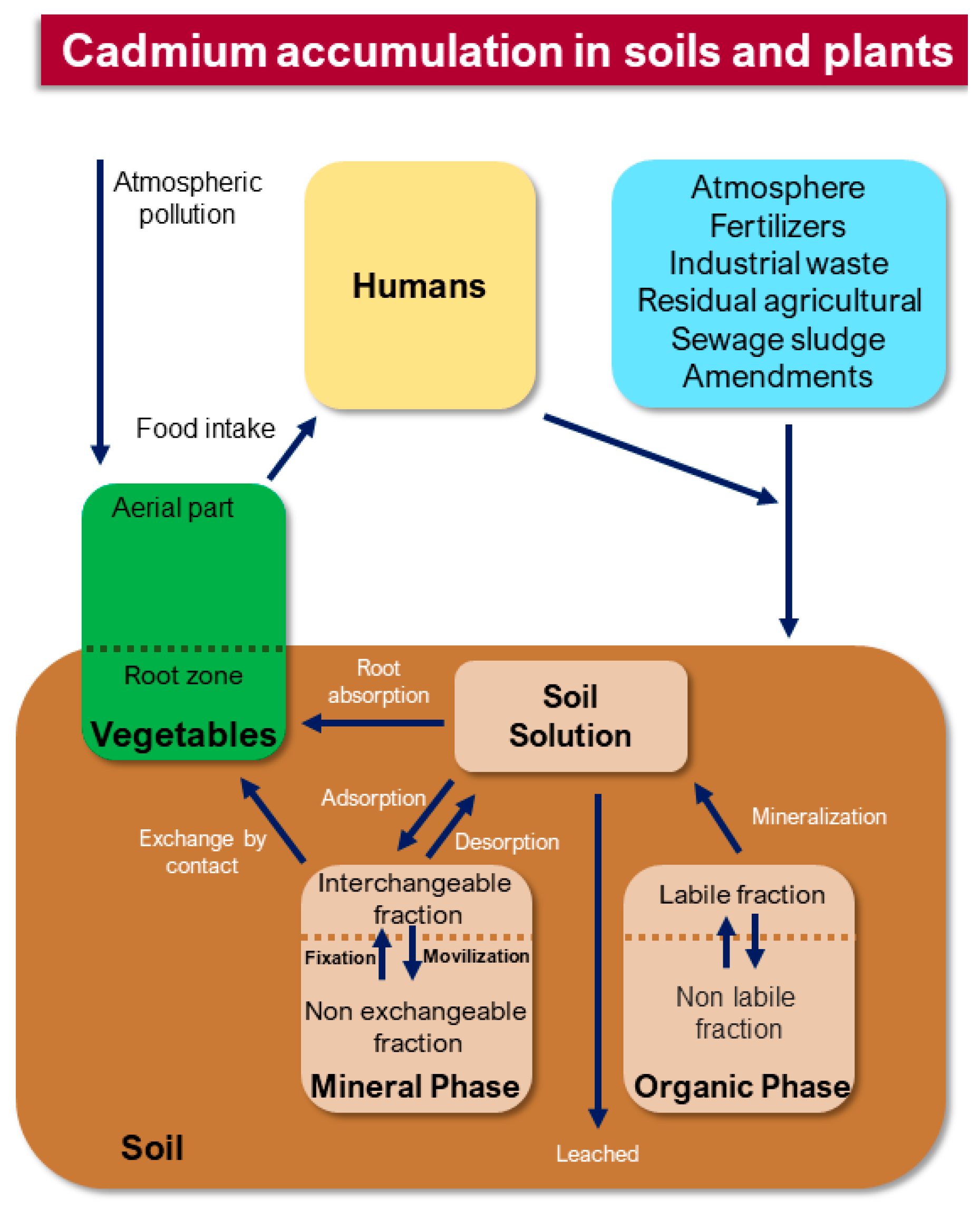
1. Introduction
2. Analysis of Publications and Trend of Heavy Metals in Edible Vegetable Oils
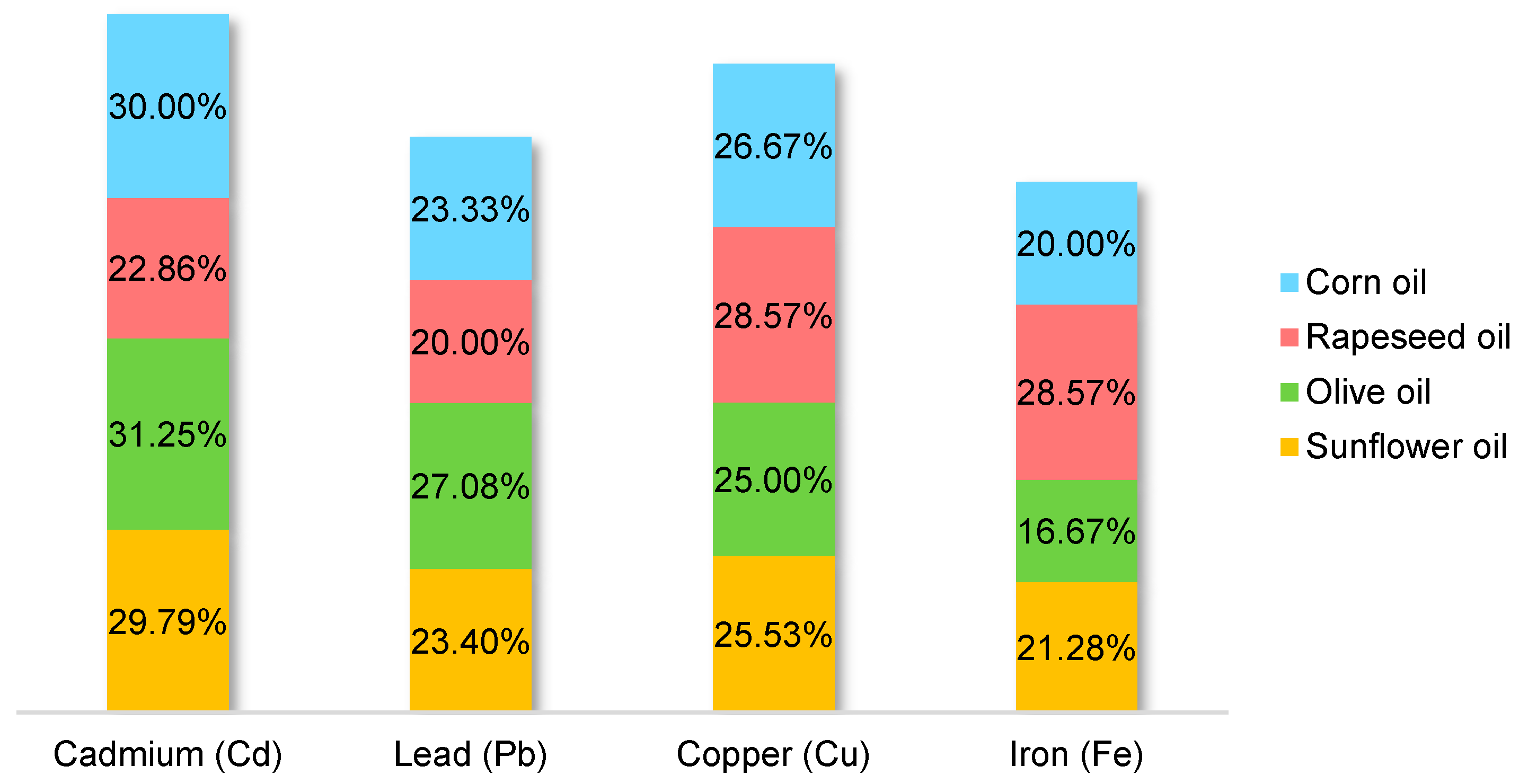
2.1. Comparison between the Most Studied Oils and Metals
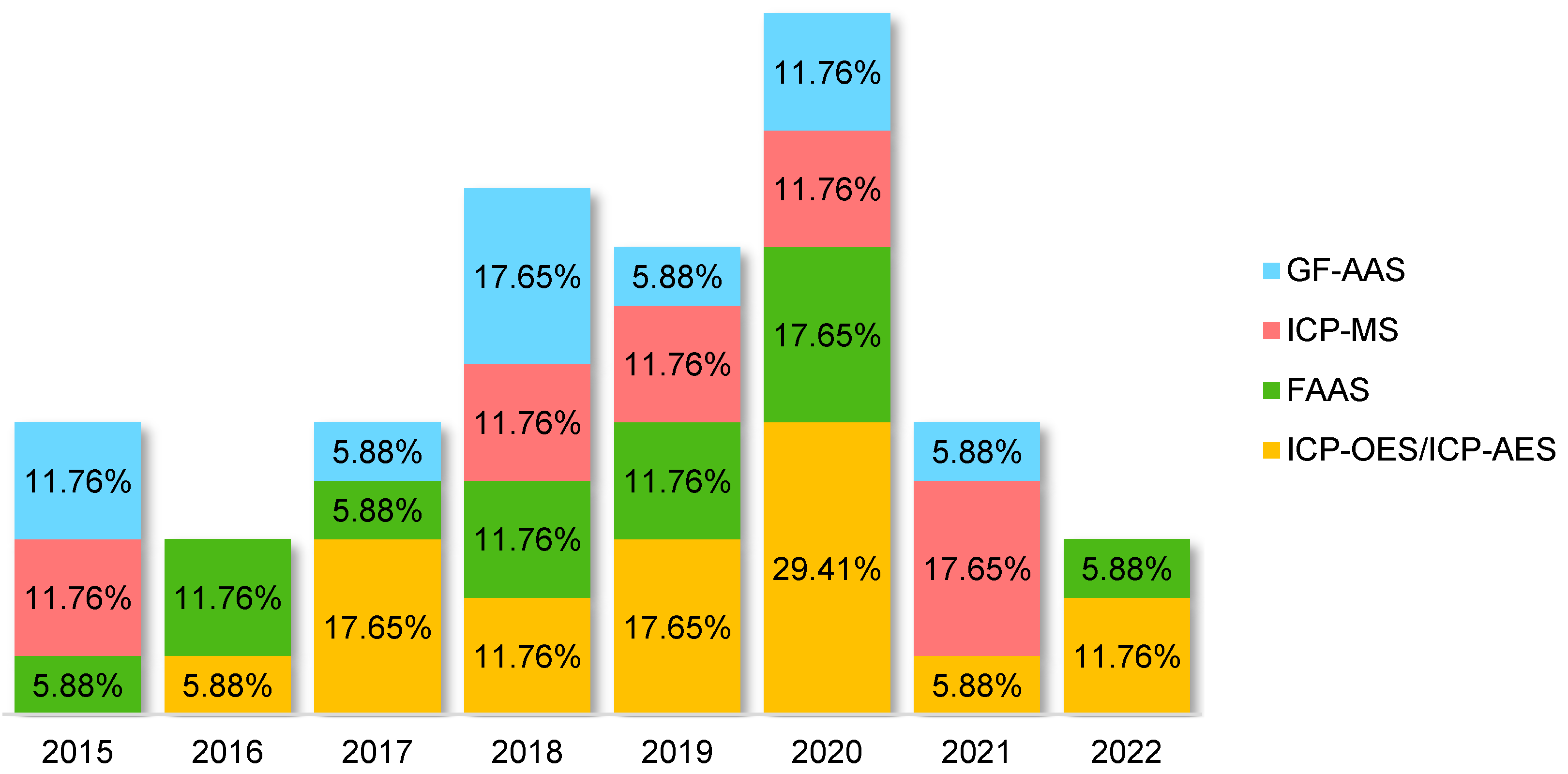
2.2. Comparison between the Oils and the Most Used Techniques
| Types of Oils | Heavy Metal | Analytical Techniques | Limit of Detection (LD) | Reference |
|---|---|---|---|---|
| Rapeseed oil | Cd | ICP-OES | 6.00 × 10−5 | [53] |
| Olive oil | Cd | GF-AAS | 2.00 × 10−6 | [52] |
| Sunflower oil | Cd | GF-AAS | 2.00 × 10−6 | [52] |
| Corn oil | Cd | GF-AAS | 2.00 × 10−6 | [52] |
| Rapeseed oil | Pb | FAAS | 3.06 × 10−4 | [25] |
| Olive oil | Pb | GF-AAS | 2.00 × 10−5 | [53] |
| Sunflower oil | Pb | GF-AAS | 2.00 × 10−5 | [25] |
| Corn oil | Pb | GF-AAS | 2.00 × 10−5 | [52] |
| Rapeseed oil | Cu | ICP-OES | 1.20 × 10−4 | [53] |
| Sunflower oil | Cu | FAAS | 3.50 × 10−4 | [55] |
| Corn oil | Cu | GF-AAS | 4.34 × 10−4 | [56] |
| Olive oil | Cu | GF-AAS | 5.00 × 10−4 | [54] |
| Olive oil | Fe | ICP-OES | 1.00 × 10−3 | [52] |
| Sunflower oil | Fe | ICP-OES | 1.00 × 10−3 | [52] |
| Corn oil | Fe | ICP-OES | 1.00 × 10−3 | [52] |
| Rapeseed oil | Fe | ICP-OES | 9.00 × 10−4 | [25] |
2.3. Results of the Analysis of Heavy Metals in Edible Vegetable Oils
| Heavy Metals | CX-A 1 | WHO 2 | EFSA 3 | Commission Regulation (EU) 2021/1317 4 | Commission Regulation (EU) 2021/1323 5 | GB/T23347-2021 (China) 6 | RD 308/1983 (Spain) 7 |
|---|---|---|---|---|---|---|---|
| Cd | NC 8 | NC/R 9,10 | NC/R 11 | NA 12 | NC 13 | NC | NC |
| Pb | 0.1 14 | NC/R 15 | NC/R 16 | 0.10 | NA | NC | 0.1 |
| Cu | NC 17 | NC | NC/R 18 | NA | NA | ≤0.1 | 0.4 |
| Fe | NC 19 | NC | NC | NA | NA | ≤3.0 | 10 |
2.3.1. Cadmium (Cd)
2.3.2. Lead (Pb)
2.3.3. Copper (Cu)
2.3.4. Iron (Fe)
2.4. Presence of Antimony (Sb) Studies
| Oils | Analytical Techniques | Limit of Detection (LD) 1 | Limit of Quantification (LQ) 1 | Results (Range or Average Value) 1 | Region | References |
|---|---|---|---|---|---|---|
| Walnut oil | ICP-OES | 6.01 × 10−3 | 0.025 | 1.26–1.66 | Turkey | [95] |
| Sweet almond oil | ICP-OES | 6.01 × 10−3 | 0.025 | 1.06–1.60 | Turkey | [95] |
| Soybean oil | ICP-OES | 6.01 × 10−3 | 0.025 | 1.24–1.47 | Turkey | [95] |
| Bitter almond oil | ICP-OES | 6.01 × 10−3 | 0.025 | 1.02–1.47 | Turkey | [95] |
| Coconut oil | ICP-OES | 6.01 × 10−3 | 0.025 | 1.16–1.42 | Turkey | [95] |
| Extra virgin olive oil | ICP-MS | 2.50 × 10−3 | 0.500 | 7.00 × 10−5–4.50 × 10−4 | Italy | [96] |
3. Challenges and Future Prospects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Sustainable Development Goals. Available online: https://www.fao.org/sustainable-development-goals/goals/goal-2/en/ (accessed on 14 January 2023).
- FAO. New FAO Report Reveals Food Insecurity, Nutrition Problems in Europe and Central Asia. Available online: https://www.fao.org/news/story/en/item/1070746/icode/ (accessed on 14 January 2023).
- Ministerio de Salud; República de Chile. Reglamento Sanitario de Los Alimentos (DTO. 977/96). Available online: https://www.minsal.cl/sites/default/files/files/DECRETO_977_96%20actualizado%20a%20Enero%202015(1).pdf (accessed on 17 January 2023).
- Rosas, M.R. Contaminaciones Alimentarias. Offarm 2007, 26, 95–100. [Google Scholar]
- Reyes, Y.C.; Vergara, I.; Torres, O.E.; Díaz, M.; González, E.E. Heavy Metals Contamination: Implications for Health and Food Safety. Ing. Investig. Desarro. 2016, 16, 66–77. [Google Scholar]
- Du, C.; Li, Z. Contamination and Health Risks of Heavy Metals in the Soil of a Historical Landfill in Northern China. Chemosphere 2022, 313, 137349. [Google Scholar] [CrossRef] [PubMed]
- Navia, D.P.; Ayala, A.A.; Villada, H.S. Interacciones Empaque-Alimento: Migración. Rev. Ing. Univ. Medellín 2014, 13, 99–113. [Google Scholar] [CrossRef]
- Garcinuño, R. Contaminación de Los Alimentos Durante Los Procesos de Origen y Almacenamiento. Aldaba 2017, 36, 51–54. [Google Scholar] [CrossRef]
- González Castro, M.I. Determinación de La Migración de Monómeros y Aditivos Plásticos de Envases Alimentarios. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2006. [Google Scholar]
- Cooper, I. Plastics and Chemical Migration into Food. In Chemical Migration and Food Contact Materials; Woodhead Publishing Ltd.: Cambridge, UK, 2007; pp. 228–250. ISBN 9781845690298. [Google Scholar]
- Payán, L.; Poyatos, M.; Muñoz, L.; la Rubia García, M.D.; Pacheco, R.; Ramos, N. Study of the Influence of Storage Conditions on the Quality and Migration Levels of Antimony in Polyethylene Terephthalate-Bottled Water. Food Sci. Technol. Int. 2017, 23, 108201321769030. [Google Scholar] [CrossRef]
- Cobos, R. El Polietilén Tereftalato (PET) Como Envase de Aguas Minerales. Boletín Soc. Española Hidrol. Médica 2016, 31, 179–190. [Google Scholar] [CrossRef]
- Barnes, K.; Sinclair, R.; Watson, D. Chemical Migration and Food Contact Materials; Woodhead Publishing Ltd: Cambridge, UK, 2007. [Google Scholar]
- Maluenda García, M.J. Perspectivas Favorables Para El Sector de Aceites Vegetales. Available online: https://www.agrodigital.com/wp-content/uploads/2019/07/Aceitesvegetalesjl19c.pdf (accessed on 14 January 2023).
- Wang, C.; Scott Buchanan, J.; Kliewer, W.R.; Qian, K. Water-Washing to Reduce Metals in Oils Extracted from Nannochloropsis Algae for Potential FCC Feedstock. Fuel 2015, 155, 63–67. [Google Scholar] [CrossRef]
- Ehishan, N.; Sapawe, N. Performance Studies Removal of Chromium (Cr6+) and Lead (Pb2+) by Oil Palm Frond (OPF) Adsorbent in Aqueous Solution. Mater. Today Proc. 2018, 5, 21897–21904. [Google Scholar] [CrossRef]
- Wuyke, H.; Oropeza, T.; Feo, L. Extraction Induced by Emulsion Breaking for the Determination of As, Co, Cr, Mn, Mo and Pb in Heavy and Extra-Heavy Crude Oil Samples by ICP-MS. Anal. Methods 2017, 9, 1152–1160. [Google Scholar] [CrossRef]
- Galuch, M.B.; Piccioli, A.; Neto, E.; Fier, N.C.; Saldan, N.E.; Garcia, E. Microemulsion as Sample Preparation for Direct Flame Atomic Absorption Spectrometry (FAAS) Determination of Total Iron in Crude and Refined Vegetable Oils. J. Braz. Chem. Soc. 2018, 29, 748–756. [Google Scholar] [CrossRef]
- Kamboh, M.A.; Memon, S.; Zardari, L.; Rashidi Nodeh, H.; Sherazi, S.T.; Yilmaz, M. Removal of Toxic Metals from Canola Oil by Newly Synthesized Calixarene-Based Resin. Turk. J. Chem. 2018, 42, 918–928. [Google Scholar] [CrossRef]
- Valasques, G.; dos Santos, A.M.P.; de Souza, V.; Teixeira, L.; Alves, J.; de Jesus Santos, M.; dos Santos, W.; Bezerra, M. Multivariate Optimization for the Determination of Cadmium and Lead in Crude Palm Oil by Graphite Furnace Atomic Absorption Spectrometry after Extraction Induced by Emulsion Breaking. Microchem. J. 2020, 153, 104401. [Google Scholar] [CrossRef]
- Narukawa, T.; Iwai, T.; Chiba, K. Simultaneous Speciation Analysis of Inorganic Arsenic and Methylmercury in Edible Oil by High-Performance Liquid Chromatography-Inductively Coupled Plasma Mass Spectrometry. Talanta 2020, 210, 120646. [Google Scholar] [CrossRef] [PubMed]
- Seidi, S.; Alavi, L. Novel and Rapid Deep Eutectic Solvent (DES) Homogeneous Liquid–Liquid Microextraction (HLLME) with Flame Atomic Absorption Spectrometry (FAAS) Detection for the Determination of Copper in Vegetables. Anal. Lett. 2019, 52, 2092–2106. [Google Scholar] [CrossRef]
- Cerqueira, U.M.; Valasques, G.; Souza, C.; Araújo, S.; Bezerra, M.; Novaes, C. Extraction Induced by Emulsion Breaking for Ca, Fe, Mg, and Zn Determination in Edible Oils Using High-Resolution Continuous Source Flame Atomic Absorption Spectrometry. Food Anal. Methods 2022, 15, 1–9. [Google Scholar] [CrossRef]
- Soylak, M.; Koksal, M. Deep Eutectic Solvent Microextraction of Lead(II), Cobalt(II), Nickel(II) and Manganese(II) Ions for the Separation and Preconcentration in Some Oil Samples from Turkey Prior to Their Microsampling Flame Atomic Absorption Spectrometric Determination. Microchem. J. 2019, 147, 832–837. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, J.; Ma, T.; Qiu, H.; Ma, S. Determination of Heavy Metal Elements in Different Vegetable Edible Oils in China. Asian J. Chem. 2017, 29, 937–939. [Google Scholar] [CrossRef]
- Sorouraddin, S.; Farajzadeh, M.; Okhravi, T. Development of a New Method for Extraction and Preconcentration of Cadmium and Zinc Ions in Edible Oils Based on Heat-Induced Homogeneous Liquid–Liquid Microextraction. J. Iran. Chem. Soc. 2019, 16, 1537–1543. [Google Scholar] [CrossRef]
- Shariff, R.; Aachary, A.; Pacquette, L.; Mittal, A.; Girdhar, R. Analytical Method Validation and Determination of Iron and Phosphorus in Vegetable Oil by Inductively Coupled Plasma–Mass Spectrometry with Microwave Assisted Digestion. Anal. Lett. 2018, 51, 1–15. [Google Scholar] [CrossRef]
- Bakircioglu, D.; Topraksever, N.; Bakircioglu, Y. Separation/Preconcentration System Based on Emulsion-Induced Breaking Procedure for Determination of Cadmium in Edible Oil Samples by Flow Injection-Flame Atomic Absorption Spectrometry. Food Anal. Methods 2015, 8, 2178–2184. [Google Scholar] [CrossRef]
- Tokay, F.; Bağdat, S. Spectrometric Determination of Iron and Copper in Vegetable Oils after Separation with Schiff Base Impregnated Silica Gel Column: A Simple Approach for Eliminating the High Organic Matrix. Int. J. Food Sci. Technol. 2015, 50, 2694–2699. [Google Scholar] [CrossRef]
- Hassan, M.; Erbas, Z.; Alshana, U.; Soylak, M. Ligandless Reversed-Phase Switchable-Hydrophilicity Solvent Liquid-Liquid Microextraction Combined with Flame-Atomic Absorption Spectrometry for the Determination of Copper in Oil Samples. Microchem. J. 2020, 156, 104868. [Google Scholar] [CrossRef]
- Bakota, E.L.; Winkler-Moser, J.K.; Dunn, R.O.; Liu, S.X. Heavy Metals Screening of Rice Bran Oils and Its Relation to Composition. Eur. J. Lipid Sci. Technol. 2015, 117, 1452–1462. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA) Oilseeds: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/oilseeds.pdf (accessed on 14 January 2023).
- Albergamo, A.; Salvo, A.; Bua, G.; Bartolomeo, G.; Mangano, V.; Rotondo, A.; di Stefano, V.; di Bella, G.; Dugo, G. Chemical Characterization of a Variety of Cold-Pressed Gourmet Oils Available on the Brazilian Market. Food Res. Int. 2018, 109, 517–525. [Google Scholar] [CrossRef]
- Almeida, J.; Anunciação, T.; Brandão, G.; Dantas, A.; Lemos, V.; Teixeira, L. Ultrasound-Assisted Single Drop Microextraction for the Determination of Cadmium in Vegetable Oils Using High-Resolution Continuum Source Electrothermal Atomic Absorption Spectrometry. Spectrochim. Acta Part B Spectrosc. 2015, 107, 159–163. [Google Scholar] [CrossRef]
- Tokay, F.; Bağdat, S. Extraction of Nickel from Edible Oils with a Complexing Agent Prior to Determination by FAAS. Food Chem. 2016, 197, 445–449. [Google Scholar] [CrossRef]
- Karimi, M.; Dadfarnia, S.; Haji Shabani, A.M.; Tamaddon, F.; Azadi, D. Deep Eutectic Liquid Organic Salt as a New Solvent for Liquid-Phase Microextraction and Its Application in Ligandless Extraction and Preconcentraion of Lead and Cadmium in Edible Oils. Talanta 2015, 144, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, A.; Carneiro, C.; Pires, L.; Teixeira, L.; Azcarate, S.; Dias, F. D-Optimal Mixture Design for the Optimization of Extraction Induced by Emulsion Breaking for Multielemental Determination in Edible Vegetable Oils by Microwave-Induced Plasma Optical Emission Spectrometry. Talanta 2020, 219, 121218. [Google Scholar] [CrossRef]
- Ennoukh, F.; Bchitou, R.; Mohammed, F.; Guillaume, D.; Hicham, H.; Bouhaouss, A. Study of the Effects of Extraction Methods on Argan Oil Quality through Its Metal Content. Ind. Crops Prod. 2017, 109, 182–184. [Google Scholar] [CrossRef]
- Gravel’, I.; Galenko, M.; Shapovalova, D.; Kornilova, E.; Shapovalov, S. Sample Preparation for Determining Heavy-Metal Contents in Fatty Oils. Pharm. Chem. J. 2021, 54, 1–4. [Google Scholar] [CrossRef]
- Lin, N.; Tang, F.; Liu, Y.; Shen, D.; Mo, R. Multielemental Analysis of Camellia Oil by Microwave Dry Ashing and Inductively Coupled Plasma Mass Spectrometry. Anal. Lett. 2015, 48, 150112114037002. [Google Scholar] [CrossRef]
- González, N.; Marquès, M.; Nadal, M.; Domingo, J. Temporal Trend of the Dietary Exposure to Metals/Metalloids: A Case Study in Tarragona County, Spain. Food Res. Int. 2021, 147, 110469. [Google Scholar] [CrossRef] [PubMed]
- Ampem, G.; le Gresley, A.; Grootveld, M.; de Mars, S.; Naughton, D.P. The Impact of Partial Oil Substitution and Trace Metal Ions on the Evolution of Peroxidation Products in Thermally Stressed Culinary Oils. Food Chem. 2022, 375, 131823. [Google Scholar] [CrossRef]
- Leal, G.; Adolfo, F.; Maziero, M.; Nascimento, P.; Carvalho, L.; VIANA, C. Emulsion Breaking-Induced Extraction of Cd and Pb from Oily Dietary Supplements Followed by Graphite Furnace Atomic Absorption Spectrometry Detection. J. Food Compos. Anal. 2022, 112, 104651. [Google Scholar] [CrossRef]
- Almeida, J.; Santos, G.; Brandão, G.; Korn, M.D.G.; Teixeira, L. Multivariate Optimization of Ultrasound-Assisted Extraction Using Doehlert Matrix for Simultaneous Determination of Fe and Ni in Vegetable Oils by High-Resolution Continuum Source Graphite Furnace Atomic Absorption Spectrometry. Food Chem. 2018, 273, 130–135. [Google Scholar] [CrossRef]
- Torres, S.; Martínez, L.; Pacheco, P. Determination of Arsenic Species Distribution in Extra Virgin Olive Oils from Arsenic-Endemic Areas by Dimensional Chromatography and Atomic Spectroscopy. J. Food Compos. Anal. 2017, 66, 121–126. [Google Scholar] [CrossRef]
- Baldo, M.; Stortini, A.; Oliveri, P.; Leardi, R.; Moretto, L.M.; Ugo, P. Electrochemical Preconcentration Coupled with Spectroscopic Techniques for Trace Lead Analysis in Olive Oils. Talanta 2019, 210, 120667. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Chen, Z.; Cheng, J.; Tang, F. Simultaneous Determination of Arsenic and Lead in Vegetable Oil by Atomic Fluorescence Spectrometry after Vortex-Assisted Extraction. Anal. Lett. 2017, 50, 2129–2138. [Google Scholar] [CrossRef]
- Damak, F.; Asano, M.; Baba, K.; Suda, A.; Araoka, D.; Wali, A.; Isoda, H.; Nakajima, M.; Ksibi, M.; Tamura, K. Interregional Traceability of Tunisian Olive Oils to the Provenance Soil by Multielemental Fingerprinting and Chemometrics. Food Chem. 2019, 283, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Mirón, C.; Sánchez, R.; Prats, S.; Todolí, J. Total Polyphenol Content and Metals Determination in Spanish Virgin Olive Oils by Means of a Dispersive Liquid-Liquid Aerosol Phase Extraction Method and ICP-MS. Anal Chim. Acta 2019, 1094, 34–46. [Google Scholar] [CrossRef]
- Öner, M.; Bodur, S.; Demir, C.; Yazıcı, E.; Erarpat, S.; Bakirdere, S. An Effective and Rapid Magnetic Nanoparticle Based Dispersive Solid Phase Extraction Method for the Extraction and Preconcentration of Cadmium from Edible Oil Samples before ICP OES Measurement. J. Food Compos. Anal. 2021, 101, 103978. [Google Scholar] [CrossRef]
- Haq, H.; Bibi, R.; Arain, M.; Safi, F.; Ullah, S.; Castro-Muñoz, R.; Boczkaj, G. Deep Eutectic Solvent (DES) with Silver Nanoparticles (Ag-NPs) Based Assay for Analysis of Lead (II) in Edible Oils. Food Chem. 2022, 379, 132085. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Liu, H.; Wang, X.; Xu, W.; Zhu, Y.; Wang, H.; Pang, L.; Lin, C. Ultrasound-Assisted Surfactant-Enhanced Emulsification Microextraction Using a Magnetic Ionic Liquid Coupled with Micro-Solid Phase Extraction for the Determination of Cadmium and Lead in Edible Vegetable Oils. Food Chem. 2018, 256, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, S.F.; Rezaee, R.; Boskabady, M.; Mashayekhi Sardoo, H.; Karimi, G. Exploring the Carcinogenic and Non-Carcinogenic Risk of Chemicals Present in Vegetable Oils. Int. J. Environ. Anal. Chem. 2020, 102, 5756–5784. [Google Scholar] [CrossRef]
- Zhuravlev, A.; Zacharia, A.; Gucer, S.; Chebotarev, A.; Arabadji, M.; Dobrynin, A. Direct Atomic Absorption Spectrometry Determination of Arsenic, Cadmium, Copper, Manganese, Lead and Zinc in Vegetable Oil and Fat Samples with Graphite Filter Furnace Atomizer. J. Food Compos. Anal. 2015, 38, 62–68. [Google Scholar] [CrossRef]
- Manjusha, R.; Shekhar, R.; Kumar, S.J. Ultrasound-Assisted Extraction of Pb, Cd, Cr, Mn, Fe, Cu, Zn from Edible Oils with Tetramethylammonium Hydroxide and EDTA Followed by Determination Using Graphite Furnace Atomic Absorption Spectrometer. Food Chem. 2019, 294, 384–389. [Google Scholar] [CrossRef]
- Adhami, K.; Asadollahzadeh, H.; Ghazizadeh, M. Preconcentration and Determination of Nickel (II) and Copper (II) Ions, in Vegetable Oils by [TBP] [PO4] IL-Based Dispersive Liquid–Liquid Microextraction Technique, and Flame Atomic Absorption Spectrophotometry. J. Food Compos. Anal. 2020, 89, 103457. [Google Scholar] [CrossRef]
- da Silva, A.F.; Papai, R.; Luz, M.S.; Gaubeur, I. Analytical Extraction Procedure Combined with Atomic and Mass Spectrometry for the Determination of Tin in Edible Oil Samples, and the Potential Application to Other Chemical Elements. J. Food Compos. Anal. 2021, 96, 103759. [Google Scholar] [CrossRef]
- Özzeybek, G.; Şahin, İ.; Erarpat, S.; Bakirdere, S. Reverse Phase Dispersive Liquid–Liquid Microextraction Coupled to Slotted Quartz Tube Flame Atomic Absorption Spectrometry as a New Analytical Strategy for Trace Determination of Cadmium in Fish and Olive Oil Samples. J. Food Compos. Anal. 2020, 90, 103486. [Google Scholar] [CrossRef]
- Olafisoye, O.B.; Fatoki, O.S.; Oguntibeju, O.O.; Osibote, O.A. Accumulation and Risk Assessment of Metals in Palm Oil Cultivated on Contaminated Oil Palm Plantation Soils. Toxicol. Rep. 2020, 7, 324–334. [Google Scholar] [CrossRef]
- Luka, M.F.; Akun, E. Investigation of Trace Metals in Different Varieties of Olive Oils from Northern Cyprus and Their Variation in Accumulation Using ICP-MS and Multivariate Techniques. Environ. Earth Sci. 2019, 78, 578. [Google Scholar] [CrossRef]
- Mohajer, A.; Norouzian Baghani, A.; Sadighara, P.; Ghanati, K.; Nazmara, S. Determination and Health Risk Assessment of Heavy Metals in Imported Rice Bran Oil in Iran. J. Food Compos. Anal. 2019, 86, 103384. [Google Scholar] [CrossRef]
- Mitić, M.; Pavlović, A.; Tošić, S.; Maskovic, P.; Kostic, D.; Mitić, S.; Kocić, G.; Mašković, J. Optimization of Simultaneous Determination of Metals in Commercial Pumpkin Seed Oils Using Inductively Coupled Atomic Emission Spectrometry. Microchem. J. 2018, 141, 197–203. [Google Scholar] [CrossRef]
- Mohebbi, M.; Heydari, R.; Ramezani, M. Determination of Cu, Cd, Ni, Pb and Zn in Edible Oils Using Reversed-Phase Ultrasonic Assisted Liquid–Liquid Microextraction and Flame Atomic Absorption Spectrometry. J. Anal. Chem. 2018, 73, 30–35. [Google Scholar] [CrossRef]
- Gunduz, S.; Akman, S. Investigation of Trace Element Contents in Edible Oils Sold in Turkey Using Microemulsion and Emulsion Procedures by Graphite Furnace Atomic Absorption Spectrophotometry. LWT Food Sci. Technol. 2015, 64, 1329–1333. [Google Scholar] [CrossRef]
- European Union (EU). Commission Regulation (EU) 2021/1317 of 9 August 2021 Regulation (EC) No 1881/2006 as Regards Maximum Levels of Lead in Certain Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R1317&from=EN (accessed on 18 January 2023).
- European Union (EU). Commission Regulation (EU) 2021/1323 of 10 August 2021 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Cadmium in Certain Foodstuffs. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R1323&from=EN (accessed on 18 January 2023).
- Spain Export and Investment (ICEX). GB/T23347-2021. Recommended National Standard for Olive and Olive Pomace Oils Exported to the Chinese Market. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/sanidadExterior/docs/China_GB23347-2021_NormaAceitesOlivayOrujo.pdf (accessed on 15 January 2023).
- Spanish Government. Real Decreto 308/1983, de 25 de Enero, Por El Que Se Aprueba La Reglamentación Técnico-Sanitaria de Aceites Vegetales Comestibles. Available online: https://www.boe.es/boe/dias/1983/02/21/pdfs/A04853-04858.pdf (accessed on 18 January 2023).
- FAO/WHO. 73rd Joint FAO/WHO Expert Committee on Food Additives (JECFA) Meeting—Food Additives and Contaminants. Available online: https://www.fao.org/documents/card/en/c/fbf01686-b940-44c2-a077-1052740ad1c6/ (accessed on 15 January 2023).
- EFSA. Cadmium in Food—Scientific Opinion of the Panel on Contaminants in the Food Chain. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2009.980 (accessed on 18 January 2023).
- Codex Alimentarius. General Standard for Contaminants and Toxins in Food and Feed. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf (accessed on 15 January 2023).
- OMS WHO. Guideline for Clinical Management of Exposure to Lead. Available online: https://www.who.int/publications/i/item/9789240037045 (accessed on 15 January 2023).
- EFSA. Scientific Opinion on Lead in Food. EFSA Panel on Contaminants in the Food Chain (CONTAM). Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/j.efsa.2010.1570 (accessed on 15 January 2023).
- FAO. Standard for Named Vegetable Oils CXS 210-1999. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B210-1999%252FCXS_210e.pdf (accessed on 15 January 2023).
- EFSA. Outcome of a Public Consultation on the Draft Scientific Opinion Ofthe EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) on Dietary Reference Values for Copper. Available online: https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2015.EN-874 (accessed on 15 January 2023).
- Yang, Q.; Li, Z.; Lu, X.; Duan, Q.; Huang, L.; Bi, J. A Review of Soil Heavy Metal Pollution from Industrial and Agricultural Regions in China: Pollution and Risk Assessment. Sci. Total. Environ. 2018, 642, 690–700. [Google Scholar] [CrossRef]
- Cruz, A.; Vique, C.; Tovar, M.; Martínez, M. Contenido de Plomo y Cadmio En Aceites de Girasol. Grasas Aceites 2001, 52, 229–234. [Google Scholar]
- Ostrovska, S.; Myasoid, Y.; Kovtunenko, R.; Myakushko, V.; Chernenko, G.; Pismenetska, I.; Baklunov, V. Effect of Cadmium on Children’s Health in Prenatal and Postnatal Periods of Development. Ukraïnsʹkij Žurnal Med. Bìologìï Sport. 2021, 6, 414–422. [Google Scholar] [CrossRef]
- McLaughlin, M.; Singh, B. Cadmium in Soils and Plants; Springer: Dordrecht, The Netherlands, 1999; ISBN 978-94-010-5916-9. [Google Scholar]
- Yang, Y.; Li, H.; Peng, L.; Chen, Z.; Zeng, Q. Assessment of Pb and Cd in Seed Oils and Meals and Methodology of Their Extraction. Food Chem. 2016, 197, 482–488. [Google Scholar] [CrossRef]
- Ziarati, P.; Mirmohammad Makki, F.; Vambol, S.; Vambol, V. Determination of Toxic Metals Content Iranian and Italian Flavoured Olive Oil. Acta Technol. Agric. 2019, 22, 64–69. [Google Scholar] [CrossRef]
- Karthik, D.; Vijayarekha, K. Chemometric Identification of a Few Heavy Metals, Pesticides and Plasticides in Edible Sunflower Oil for Health Risk Assessment. Int. J. Food Prop. 2018, 21, 1442–1448. [Google Scholar] [CrossRef]
- Mohammed, F.; Guillaume, D.; Abdulwali, N.; Zabara, B.; Bchitou, R. Tin Content Is a Possible Marker to Discriminate Argan Oil Against Olive, Sesame, Mustard, Corn, Peanut, and Sunflower Oils. Eur. J. Lipid Sci. Technol. 2019, 121, 1800180. [Google Scholar] [CrossRef]
- Mabood, F.; Hadi, F.; Jan, A.; Ditta, A.; Islam, Z.; Siddiqui, M.; Ali, H.; el Sabagh, A. Assessment of Pb and Ni and Potential Health Risks Associated with the Consumption of Vegetables Grown on the Roadside Soils in District Swat, Khyber Pakhtunkhwa, Pakistan. Environ. Monit. Assess 2022, 194, 906. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Chakraborty, D.; Das, S.; Bhattacharjee, S.C.; Das, P.K. Physicochemical Parameters and Heavy Metal Content in Soybean Oil from Bangladesh. Pak. J. Nut. 2016, 15, 565–571. [Google Scholar] [CrossRef]
- Wroniak, M.; Rękas, A. A Preliminary Study of PCBs, PAHs, Pesticides and Trace Metals Contamination in Cold-Pressed Rapeseed Oils from Conventional and Ecological Cultivations. J. Food Sci. Technol. 2017, 54, 1350–1356. [Google Scholar] [CrossRef]
- Vallieres, C.; Holland, S.; Avery, S. Mitochondrial Ferredoxin Determines Vulnerability of Cells to Copper Excess. Cell Chem. Biol. 2017, 24, 1228–1237.e3. [Google Scholar] [CrossRef]
- Trindade, A.S.N.; Dantas, A.F.; Lima, D.C.; Ferreira, S.L.C.; Teixeira, L.S.G. Multivariate Optimization of Ultrasound-Assisted Extraction for Determination of Cu, Fe, Ni and Zn in Vegetable Oils by High-Resolution Continuum Source Atomic Absorption Spectrometry. Food Chem. 2015, 185, 145–150. [Google Scholar] [CrossRef]
- Szyczewski, P.; Frankowski, M.; Zioła-Frankowska, A.; Siepak, J.; Szyczewski, T.; Piotrowski, P. A Comparative Study of the Content of Heavy Metals in Oils: Linseed Oil, Rapeseed Oil and Soybean Oil in Technological Production Processes. Arch. Environ. Prot. 2016, 42, 37–40. [Google Scholar] [CrossRef]
- Drosaki, E.; Anthemidis, A.N. A Novel Automatic Flow-Batch Extraction Induced by Emulsion Breaking Platform for on-Line Copper Determination in Edible Oil Samples by Atomic Absorption Spectrometry. Talanta 2022, 244, 123423. [Google Scholar] [CrossRef]
- Alrajhi, I.M.; Idriss, H. Concentration of Trace Metals in Some Major Edible Oils of Riyadh. Rev. Int. Contam. Ambient 2020, 36, 977–984. [Google Scholar] [CrossRef]
- Ni, Z.; Chen, Z.; Yu, Q.; Sun, X.; Tang, F. Microwave-Assisted Digestion for Trace Elements Analysis of Tree Nut Oil: Evaluation of Residual Carbon Content. Spectrosc. Lett. 2018, 51, 518–523. [Google Scholar] [CrossRef]
- Ghosh, G.C.; Khan, M.J.H.; Chakraborty, T.K.; Zaman, S.; Kabir, A.H.M.E.; Tanaka, H. Human Health Risk Assessment of Elevated and Variable Iron and Manganese Intake with Arsenic-Safe Groundwater in Jashore, Bangladesh. Sci. Rep. 2020, 10, 5206. [Google Scholar] [CrossRef] [PubMed]
- European Union (EU). Commission Regulation (EU) 2020/1245 of 2 September 2020 Amending and Correcting Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020R1245&from=en (accessed on 19 January 2023).
- Karasakal, A. Determination of Trace and Major Elements in Vegan Milk and Oils by ICP-OES After Microwave Digestion. Biol. Trace. Elem. Res. 2020, 197, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, M.L.; Marconi, E.; Vitiello, G.; Massimi, L. An Optimized Method for Sample Preparation and Elemental Analysis of Extra-Virgin Olive Oil by Inductively Coupled Plasma Mass Spectrometry. Food Chem. 2021, 360, 130027. [Google Scholar] [CrossRef] [PubMed]
| Types of Oils | Analytical Techniques | Limit of Detection (LD) 1 | Limit of Quantification (LQ) 1 | Results (Range or Average Value) 1 | Region | Reference |
|---|---|---|---|---|---|---|
| Rice oil | ICP-MS | 0.50 | - | <LD–0.891 | USA (Peoria, Illinois) | [31] |
| Rapeseed oil | ICP-OES | - | - | 0.88 | China (Shizhuyuan, Chenzhou) | [80] |
| Sunflower oil | GC-MS-AAS | - | - | <LD–0.54 | India (Tamil Nadu) | [82] |
| Corn oil | ICP-OES | 1.00 × 10−4 | 2.00 × 10−4 | 0.02–0.14 | Morocco, Algeria, Jordan | [83] |
| Olive oil | ICP-OES | 1.00 × 10−4 | 2.00 × 10−4 | <LQ–0.10 | Morocco, Algeria, Jordan | [83] |
| Olive oil | ICP-MS | - | - | 0.02–0.09 | Cyprus | [60] |
| Olive oil flavoured 2 | ICP-OES | - | - | <LD–1.004 | Iran, Italy | [81] |
| Olive oil | ICP-OES | - | - | 0.396–4.181 | Iran, Italy | [81] |
| Olive oil | FAAS | 7.40 × 10−5 | 2.48 × 10−3 | 0.026–0.097 | Turkey (Istanbul) | [58] |
| Rapeseed oil | ICP-OES | 1.00 × 10−3 | 3.00 × 10−3 | 0.098–0.099 | Iran | [53] |
| Corn oil | ICP-OES | 1.00 × 10−3 | 3.00 × 10−3 | 0.098–0.100 | Iran | [53] |
| Olive oil | ICP-OES | 1.00 × 10−3 | 3.00 × 10−3 | 0.094–0.097 | Iran | [53] |
| Sesame oil | ICP-OES | 1.00 × 10−3 | 3.00 × 10−3 | 0.090–0.097 | Iran | [53] |
| Sunflower oil | ICP-OES | 1.00 × 10−3 | 3.00 × 10−3 | 0.091–0.100 | Iran | [53] |
| Olive oil | ICP-OES | 3.80 × 10−4 | 1.28 × 10−3 | 2.24 × 10−3–0.1052 | Turkey (Istanbul) | [50] |
| Types of Oils | Analytical Techniques | Limit of Detection (LD) 1 | Limit of Quantification (LQ) 1 | Results (Range or Average Value) 1 | Region | Reference |
|---|---|---|---|---|---|---|
| Rice oil | ICP-MS | 0.10 | - | <LD–0.127 | USA (Peoria, Illinois) | [31] |
| Soybean oil | AAS | 0.12 | - | 0.31–2.35 | Bangladesh | [85] |
| Rapeseed oil | ICP-OES | - | - | 1.96 | China (Shizhuyuan, Chenzhou) | [80] |
| Rapeseed oil | GF-AAS | - | - | 0.012–0.100 | Poland (Lubelskie, Mazowieckie, S’laskie, Opolskie, Wielkopolskie) | [86] |
| Olive oil | ICP-MS | - | - | 0.15–1.48 | Cyprus | [60] |
| Olive oil flavoured 2 | ICP-OES | - | - | 0.984–12.33 | Iran, Italy | [81] |
| Olive oil | ICP-OES | - | - | 8.546–18.783 | Iran, Italy | [81] |
| Rapeseed oil | ICP-OES | 1.00 × 10−3 | 3.00 × 10−3 | 0.099–0.100 | Iran | [53] |
| Corn oil | ICP-OES | 1.00 × 10−3 | 3.00 × 10−3 | 0.099–0.100 | Iran | [53] |
| Olive oil | ICP-OES | 1.00 × 10−3 | 3.00 × 10−3 | 0.099–0.100 | Iran | [53] |
| Sesame oil | ICP-OES | 1.00 × 10−3 | 3.00 × 10−3 | 0.092–0.099 | Iran | [53] |
| Sunflower oil | ICP-OES | 1.00 × 10−3 | 3.00 × 10−3 | 0.098–0.100 | Iran | [53] |
| Coconut oil | ICP-OES | - | - | 0.158 | United Kingdom (London) | [42] |
| Olive oil | ICP-OES | - | - | 0.143 | United Kingdom (London) | [42] |
| Rapeseed oil | ICP-OES | - | - | 0.181 | United Kingdom (London) | [42] |
| Sunflower oil | ICP-OES | - | - | 0.274 | United Kingdom (London) | [42] |
| Sesame oil | FAAS | 3.04 × 10−4 | 1.02 × 10−3 | 1.370–6.641 | Pakistan (Mardan) | [51] |
| Olive oil | FAAS | 3.06 × 10−4 | 1.03 × 10−3 | 1.321–7.249 | Pakistan (Mardan) | [51] |
| Rapeseed oil | FAAS | 3.06 × 10−4 | 1.03 × 10−3 | 1.301–6.765 | Pakistan (Mardan) | [51] |
| Types of Oils | Analytical Techniques | Limit of Detection (LD) 1 | Limit of Quantification (LQ) 1 | Results (Range or Average Value) 1 | Region | Reference |
|---|---|---|---|---|---|---|
| Olive oil | GF-AAS | 5.00 × 10−4 | 2.00 × 10−3 | 0.355 | Ukraine | [54] |
| Soybean oil | HR-CS ET-AAS | 0.041 | 0.14 | 0.83 | Brazil (Salvador) | [88] |
| Sunflower oil | HR-CS ET-AAS | 0.041 | 0.14 | 0.81 | Brazil (Salvador) | [88] |
| Rapeseed oil | HR-CS ET-AAS | 0.041 | 0.14 | 0.81 | Brazil (Salvador) | [88] |
| Linseed oil | FAAS | - | - | 0.10 | Poland | [89] |
| Soybean oil | AAS | 0.11 | - | 9.75–30.50 | Bangladesh | [85] |
| Mustard oil | GF-AAS | 0.04 | - | 0.571–0.582 | India (Hyderabad) | [55] |
| Sunflower oil | GF-AAS | 0.04 | - | 0.436–0.455 | India (Hyderabad) | [55] |
| Sesame oil | GF-AAS | 0.04 | - | 0.150–0.175 | India (Hyderabad) | [55] |
| Coconut oil | GF-AAS | 0.04 | - | 0.300–0.363 | India (Hyderabad) | [55] |
| Oliva | ICP-MS | - | - | 1.02–3.81 | Chipre | [60] |
| Olive oil | ICP-OES | 1.00 × 10−3 | 3.00 × 10−3 | 0.091–0.098 | Iran | [53] |
| Castor oil | AAS | 0.003 | - | 0.158-0.417 | Russia | [39] |
| Sunflower oil | FB-EIEBH | 0.0063 | 0.0209 | 0.025–0.127 | Greece (Thessaloniki) | [90] |
| Olive oil | FB-EIEBH | 0.0063 | 0.0211 | 0.033–0.139 | Greece (Thessaloniki) | [90] |
| Types of Oils | Analytical Techniques | Limit of Detection (LD)1 | Limit of Quantification (LQ) 1 | Results (Range or Average Value) 1 | Region | Reference |
|---|---|---|---|---|---|---|
| Pequi oil 2 | ICP-OES | 0.02 | 0.07 | 1.05–9.64 | China (Hangzhou, Zhejiang) | [92] |
| Walnut oil 2 | ICP-OES | 0.02 | 0.07 | <LD–11.2 | China (Hangzhou, Zhejiang) | [92] |
| Olive oil | ICP-OES | 0.002 | - | 0.025–7.861 | Arabia Saudi (Riad) | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Torres, P.; Puentes, J.G.; Moya, A.J.; La Rubia, M.D. Comparative Study of the Presence of Heavy Metals in Edible Vegetable Oils. Appl. Sci. 2023, 13, 3020. https://doi.org/10.3390/app13053020
González-Torres P, Puentes JG, Moya AJ, La Rubia MD. Comparative Study of the Presence of Heavy Metals in Edible Vegetable Oils. Applied Sciences. 2023; 13(5):3020. https://doi.org/10.3390/app13053020
Chicago/Turabian StyleGonzález-Torres, Pablo, Juan G. Puentes, Alberto J. Moya, and M. Dolores La Rubia. 2023. "Comparative Study of the Presence of Heavy Metals in Edible Vegetable Oils" Applied Sciences 13, no. 5: 3020. https://doi.org/10.3390/app13053020
APA StyleGonzález-Torres, P., Puentes, J. G., Moya, A. J., & La Rubia, M. D. (2023). Comparative Study of the Presence of Heavy Metals in Edible Vegetable Oils. Applied Sciences, 13(5), 3020. https://doi.org/10.3390/app13053020









