Abstract
This study aimed to examine the sensitivity of the isometric knee extension (IKE) test to detect changes in the lower-limb strength of institutionalized older adults after exercise and inactivity periods. Thirty-four and fifteen institutionalized older adults completed the training and inactive periods, respectively. At each time point, the participants completed two testing sessions. In the first session, they performed the IKE test. As a complement to this evaluation, the second testing session was used to assess their functional capacity and handgrip strength. The sensitivity of the IKE test was examined by comparing the changes generated in this test against the repeatability of the protocol. A 4-week multicomponent Vivifrail program was implemented. After that, a subsample of the participants was re-evaluated after a 14-week inactivity period. Significant changes (p < 0.01; ES ≥ 0.27) in the IKE strength for both the dominant (+0.27 N/kg) and non-dominant legs (+0.25 N/kg) were produced after the training intervention. Likewise, significant decrements (p < 0.01; ES ≥ 0.31) were detected after the inactive period for the dominant (−0.29 N/kg) and non-dominant legs (−0.32 N/kg). All mean changes were found to be superior to the variability threshold of the IKE test for both legs, with superior sensitivity for the non-dominant leg (≥73%). Thus, the IKE test is a sensitive and practical tool for detecting changes in the lower-limb strength of institutionalized older adults after exercise and inactivity periods. Because of its applicability, it seems pertinent to implement the IKE test in a geriatric context.
1. Introduction
Sarcopenia is characterized by a progressive decline in functional capacity, muscle mass, and strength [1] and is the most common aging-related syndrome. In particular, lower-limb strength constitutes a relevant clinical outcome among older adults, specifically for those with sarcopenia, who have a higher risk of disability, frailty, institutionalization, and death [2,3]. A recent estimation from the Eurostat online database (28 European countries) suggests an increase of 60–70% of individuals with sarcopenia by 2045, affecting 12.9 to 22.3% of older people [4]. Age-related deconditioning of lower-limb strength occurs mainly due to the loss of muscle mass, together with the deterioration of neural patterns and tendon proprieties [5,6]. Declines of 24–30% in lower-limb strength were reported in older adults after 12 years of follow-up [7]. Moreover, inactivity periods due to illness, injuries, or hospitalizations—frequent in older adults [8]—accentuate loss in muscular function, reaching decrements of 11–16% after 7–10 days [9,10]. Therefore, assessing lower-limb strength is crucial to develop effective approaches for preventing and even reversing the aforementioned aging-related alterations [11].
Although several tests are available, they present limitations for older adults. For example, the one-repetition maximum and the number of repetitions to failure tests have been used to evaluate the dynamic lower-limb strength in older adults [12,13,14,15]. The one-repetition maximum test refers to an incremental loading evaluation up to the heaviest load that the participant can properly lift without any external help, completing the full range of motion [16]. The number of repetitions to failure test requires the participant to perform as many repetitions as possible with a given load, and then the 1RM is calculated using estimation formulas [17]. Nevertheless, both tests usually produce excessive fatigue and muscle soreness in the older population [18]. Advances in technology-based approaches have made it possible to accurately estimate lower-extremity strength without fatigue using the velocity-based method (i.e., monitoring the barbell velocity) [19,20]. However, this approach requires specific equipment, such as a leg extension or Smith machine, which are not normally present in a geriatric context.
Another practical and widely used tool to screen strength and neuromuscular capacity [21] is the isometric knee extension (IKE) test. This evaluation consists of a 3-s trial of maximal isometric contractions involving the extensor muscles of the knee [22]. The IKE test presents some advantages compared to dynamic methods, namely the technical simplicity, low injury risk, high reliability, and ease of use within clinical settings, as it can be implemented using portable devices, such as strain gauges [23]. In addition to being a specific tool for screening lower-limb strength, the resulting value of the IKE test is highly associated with two relevant health-related outcomes in aging—physical function (e.g., agility, gait speed, and standing ability) and muscle mass [22].
However, to date, there is no information on the sensitivity of this practical test to detect clinical changes in lower-limb strength after training or an exercise cessation period. This interpretation of sensitivity is essential to be confident that changes detected by the IKE test when implemented as an evaluation method are produced by actual improvements or decrements in lower-limb strength and not by the protocol instability (i.e., biological and measurement device variations) [24,25,26].
Against this background, this study aimed to examine the sensitivity of the IKE test within clinical settings to detect changes in the lower-limb strength of institutionalized older adults after exercise training and an inactivity period.
2. Materials and Methods
2.1. Experimental Design
This research was part of the HEAL study, an ongoing multicenter randomized controlled trial [27]. Older adults living in nursing homes completed 4 weeks of the tailored multicomponent exercise program Vivifrail [28]. Their lower- and upper-limb strength and functional capacity were evaluated at baseline (T0) and after the exercise intervention (T1). Then, a subsample was re-evaluated after a 14-week inactivity period due to COVID-19 confinement (T2). Physical evaluations were based on two testing sessions at each time point (i.e., T0, T1, and T2). In the first session, all participants performed the IKE test (main outcome). As a complement and 48 h after the IKE assessment, the second testing session was used to assess the functional capacity of the participants using the Short Physical Performance Battery (SPPB) and Timed Up and Go (TUG) tests, in addition to evaluating the isometric handgrip strength (IHS).
2.2. Participants and Eligibility Criteria
All participants underwent a medical examination to identify any controlled cardiovascular or metabolic disease that would exclude them from the exercise training.
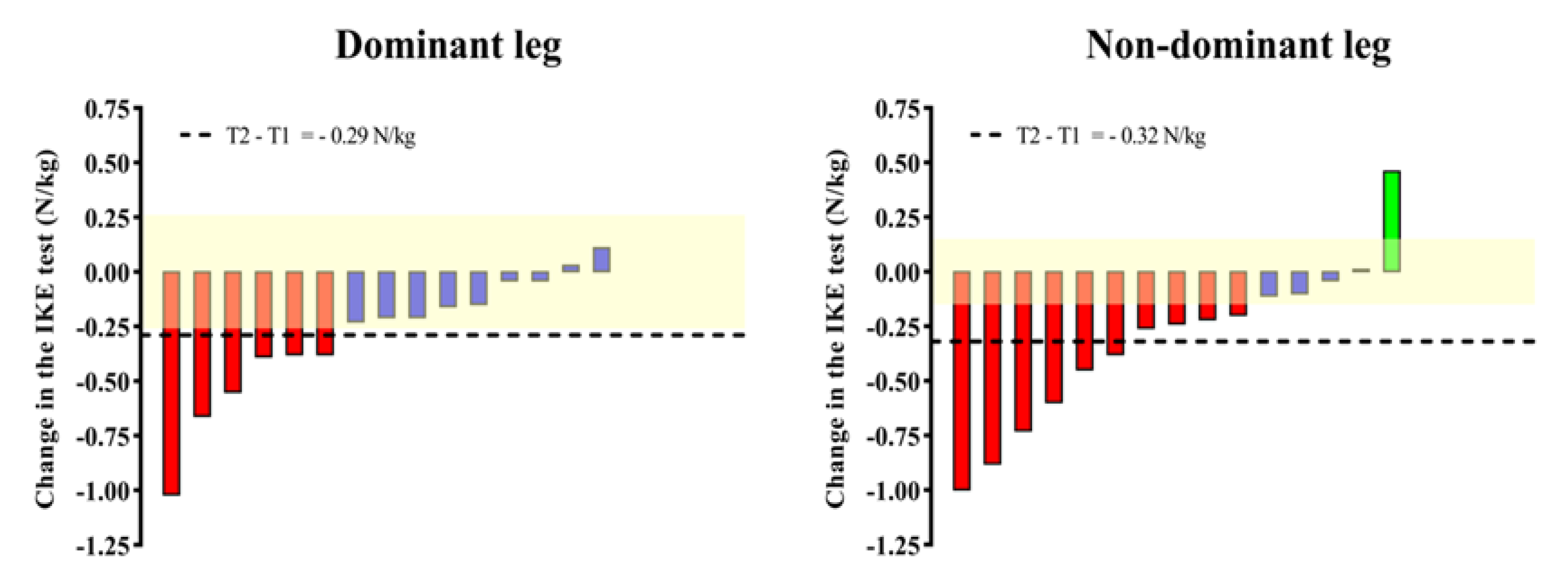
The results from a previous study with older adults revealed changes in the IKE strength (main outcome) with an effect size (ES) of 0.36 [29]. Assuming an alpha value of 0.05 and a power of 95%, a clinically relevant change between the pre- and post-intervention values of IKE strength was identified with 28 subjects using G*Power Software (version 3.1.9.7). Assuming a maximal loss of follow-up of 20%, 36 nursing home residents over 70 years old were recruited. However, two participants dropped out of the exercise intervention due to illness. Therefore, the total number of older adults who completed the 4-week training intervention was 34. Then, a subsample of 15 was re-evaluated after a 14-week inactive period after the training program. A flowchart diagram of the study with patient follow-up is shown in Figure 1.
Figure 1.
Flowchart of the study with participants.
The characteristics of the sample analyzed in the exercise and inactivity periods are detailed in Table 1. The inclusion and exclusion criteria were set according to the Vivifrail exercise program and HEAL study [27]. The study, which was conducted according to the Declaration of Helsinki, was approved by the Ethics Commission of the Local University (ID: 2131/2018). All participants signed a written consent form after being informed of the purpose and experimental procedures.

Table 1.
Characteristics of the study sample analyzed at baseline.
2.3. Outcome Measures
An initial screening using questionnaires was conducted to evaluate the participants’ cognitive state (using the Mini-Mental State Examination, MMSE) [30], disability in daily activities (using the Barthel [31] and Lawton [32] indexes), fear of falling (using the Fall Efficacy Scale—International, FES-I) [33] and sarcopenia (using the SARC-F scale [34]). Thereafter, the IKE, SPPB, IHS, and TUG evaluations were conducted. Two trials of each physical test were recorded 2 minutes apart, and the highest value was used to analyze the pre-post changes after the training or inactive period. The physical assessments are detailed below:
For the isometric knee extension (IKE) test (main outcome), the participants assumed a sitting position on a custom-built bench and conducted a 3-s trial of maximal voluntary contraction for both the dominant and non-dominant legs, which were measured separately at a comfortable knee angle of 110–120° knee (180° = full extension) [22]. A portable strain gauge sampling at 80 Hz (Chronojump, Barcelona, Spain) was secured to the bench and attached at the end to a chain connected to a padded resistance anklet. This padded anklet was specifically designed for maximal isometric testing to guarantee mechanical rigidity and minimize movement [35]. The chain length was adapted depending on the anthropometric characteristics of the participants to obtain the above-mentioned target angle [36]. Before the trial, the knee angle was measured using a human-held goniometer (Nexgen Ergonomics, Point Claire, Quebec, Canada). The strain gauge was calibrated before the session using a 5.0 kg Eleiko disc (Eleiko, Halmstad, Sweden) according to the manufacturer’s instructions. The initial pre-tension was standardized and confirmed using real-time visual feedback to avoid countermovement [35,36]. The maximal voluntary contraction force (instantaneous highest value of force) was obtained from the manufacturer software in relative terms (highest force value/subject’s body mass).
The Short Physical Performance Battery (SPPB) test (secondary outcome) [37] was assessed to evaluate the participants’ functional capacity using the following tests: static balance, 4-m gait speed, and 5 sit-to-stand (STS) repetitions. The static balance test included tandem, semi-tandem, and side-by-side positions. For each balance stand, the timing was evaluated based on when the participants moved their feet, or when 10 s (highest possible score) was achieved. The 4-m gait speed test was conducted from a static position at the usual walking pace. The participants performed the 5-repetitions STS test with their arms across their chest and were timed from the initial sitting position to the final standing position at the end of the fifth repetition. Moreover, the relative mean power (watts/subject’s body mass) resulting from the 5-repetitions STS test was estimated using a mathematical formula [36]. Based on the results achieved in each test, this battery was scored from 0 (worst) to 12 (best). The resulting scores are part of the Vivifrail program to determine each individual’s physical exercise program (described later in detail) [28].
The participants’ isometric handgrip strength (HIS, secondary outcome) was evaluated separately for each hand (i.e., dominant and non-dominant hand) in a seated position, with their shoulders neutrally rotated and adducted, elbows flexed at 90°, forearms in neutral, and wrists between 0 and 30° of dorsiflexion [38]. A calibrated digital dynamometer adjustable (Takei 5401-C, Shinagawa-Ku, Tokyo, Japan) was used. The results derived from this test (in kg) were expressed in relative terms (kg achieved in the test/subject’s body mass).
The Timed Up and Go (TUG) test (secondary outcome) measured the time it took the participants to (i) stand up without using their arms, (ii) walk 3 m, and (iii) turn around and sit back down [39]. A person with a time greater than 20 s is considered to have low agility according to the Vivifrail guidelines. This test has been proposed for measuring agility (i.e., dynamic balance) and as a good predictor of fall risk among older adults [40,41].
All tests started with the cue “3, 2, 1, go” and were directed by experienced strength and conditioning trainers in order to prevent falls. The time-based evaluations (e.g., the TUG test) were measured using the stopwatch option of the Garmin Forerunner 235 (Garmin Ltd., Olathe, KS, USA). All assessments were encouraged and performed under similar climatological conditions (20–24 °C and 45–55% relative humidity), at the same time of day (16:00–18:00 h), and after an identical warm-up (2-min walk and 5 STS repetitions).
2.4. Multicomponent Exercise Intervention and Inactive Period
The nursing home residents participated in 4 weeks of the Vivifrail exercise program, specifically designed to prevent weakness and falls [42,43]. After the initial measurements (T0), the exercise program was individualized depending on the person’s functional capacity level (i.e., their SPPB score). Four functional levels are proposed for 4 tailored exercise regimes: serious limitation or disability (SPPB score of 0–3, Level A), moderate limitation or frail (SPPB score of 4–6, Level B), slight limitation or prefrail (SPPB score of 7–9, Level C), and robust (SPPB score of 10–12, Level D). All regimes included cardiovascular (walking), resistance (handgrip, biceps curl, squat, and knee extension,), balance (walking on toes and heels, in line, around small obstacles, and stepping), and flexibility (arm and hamstring stretching) exercises. Participants at Level A or with low agility (i.e., TUG test > 20 s) performed a 5-day-a-week routine of multicomponent exercises, whereas the remaining groups (Levels B, C, and D) combined strength, balance, and stretching sessions (3 days per week) with walking sessions (2 days per week). The duration of the cardiovascular exercises ranged from 3 min (Level A) to 20 min (Level D), walking at a pace that allowed the participants to keep a conversation continuously. Resistance exercises included 3 sets of 12 repetitions, regulating the load of dumbbells and ankle weights with which the participant was able to perform 30 repetitions. This level of effort (i.e., 12(30); 12 repetitions using an absolute load (kg) that would allow the participants to complete a total of 30 repetitions) [44] was established for all regimes (i.e., Levels A, B, C, and D) according to the Vivifrail guidelines. Dynamic balance exercises were conducted in all regimes depending on the functional capacity, and participants with low agility were assisted by the trainer in order to prevent falls. Three sets of 10 s of one static stretching exercise were performed for both upper- and lower-limb exercises in all regimes. A two-minute rest was imposed between all exercises and sets. Adherence to the program was documented in a daily register during the intervention. The training sessions were directed by qualified strength and conditioning trainers (Degree in Sports Science) and supervised by a medical doctor, nurse, or physiotherapist. All material concerning the tailored Vivifrail exercise program is available online (https://vivifrail.com/resources/, accessed on 2 May 2022).
In the 4th week of training, the exercise program was interrupted by confinement due to COVID-19. This 14-week confinement period was used for evaluating changes in a subsample of participants during an inactive time. Importantly, none of the participants included in this subsample was infected by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) during the confinement period.
2.5. Statistical Analyses
Standard statistical methods were used for the calculation of the means and standard deviations (SD). The normality of the data was verified using the Shapiro–Wilk test. Student’s t-test was used to determine differences between the time points (i.e., T1 vs. T0 and T2 vs. T1). The significance level was set at p < 0.05. The effect size was calculated to estimate the magnitude of the differences between the time points using Hedge’s g and interpreted as large (g > 0.80), moderate (g > 0.50), or small (g > 0.20) [45]. The sensitivity of the IKE test was examined by comparing the changes generated in this test against the repeatability of the protocol itself (using the standard error of measurement, SEM). This variation was recently described for both the dominant (SEM = 0.26 N/kg) and non-dominant legs (SEM = 15 N/kg) in older adults with characteristics similar to those included in the current study (age 87.0 years, BMI 28.5, SPPB 5.4 points) [22]. The statistical calculations were performed using a custom Microsoft Excel spreadsheet and statistical software SPSS version 22 (IBM Corp., Armonk, NY, USA). The figures were designed using GraphPad Prism software version 6.0 (GraphPad Software Inc., La Jolla, CA, USA).
3. Results
3.1. Training Period
Attendance to the training sessions was over 80% (93.7 ± 7.3%). The results of each test include the changes of those participants who were able to perform it at both time points (i.e., T0 and T1) (Table 2). All participants conducted the IHS and IKE tests, although not all could complete the STS (62%), TUG (92%), or 4m gait speed (94%) evaluations due to supramaximal effort for them (i.e., physical limitations to perform the assessment). The training intervention produced significant improvements (p < 0.05 to p < 0.001) for all functional capacity evaluations (ES > 0.48), whereas changes in the IHS did not reach significance (ES < 0.21).

Table 2.
Changes in physical assessment after the 4-week training program.
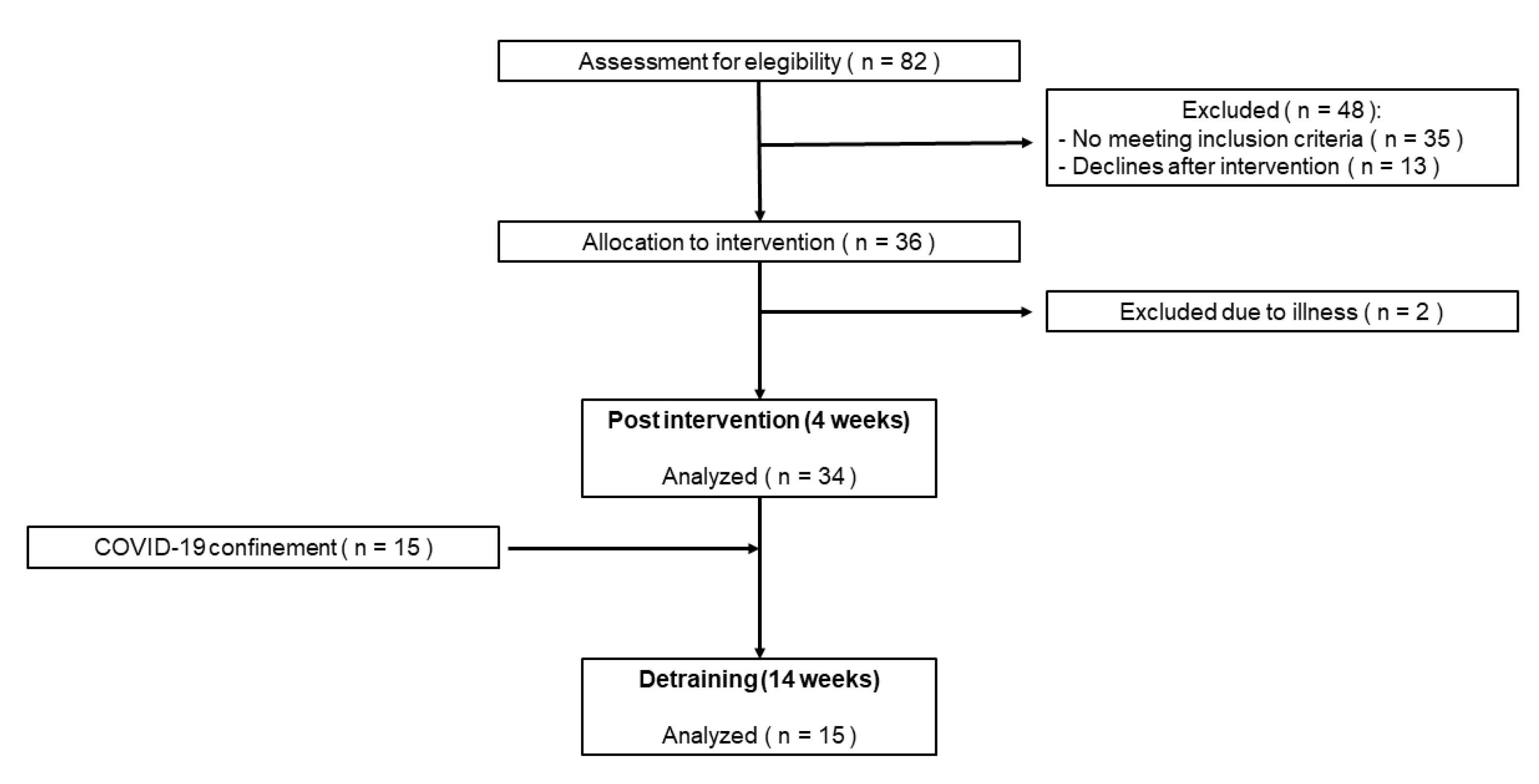
Figure 2 shows the changes in the IKE test. Significant (p < 0.01) changes in the IKE strength for both the dominant (ES = 0.28) and non-dominant legs (ES = 0.27) were detected after the multicomponent program. Specifically, changes in the group means for both legs (dominant = +0.27 N/kg; non-dominant = +0.25 N/kg) were found to be superior to the IKE test variation (i.e., repeatability). Considering individual changes, 53% (for the dominant leg) and 77% (for the non-dominant leg) of the participants showed an increase/decrease in the IKE strength above the variability threshold of the test.
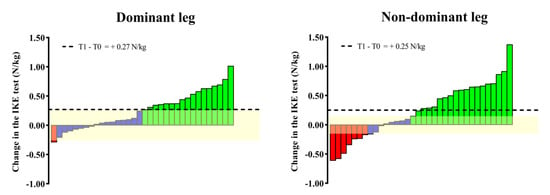
Figure 2.
Individual (bars) and group (horizontal black line) changes in the IKE test after the Vivifrail program. The yellow-shaded range represents the SEM of the IKE test recently reported for older adults. The individual changes that the IKE test was able to detect are represented by green (increase) and red (decrease) bars, while blue bars mean cases whose increase/decrease in performance did not exceed the SEM of the test.
3.2. Detraining Period
Similar to T0, the STS, TUG, and 4m gait speed could not be performed by all the participants included in this inactive period due to supramaximal effort (Table 3). Significant decrements were found for the SPPB score and TUG tests (p < 0.05, ES > 0.26), but not for the STS, gait speed, and IHS (ES > 0.02).

Table 3.
Changes in physical assessment after the 14-week inactive period.
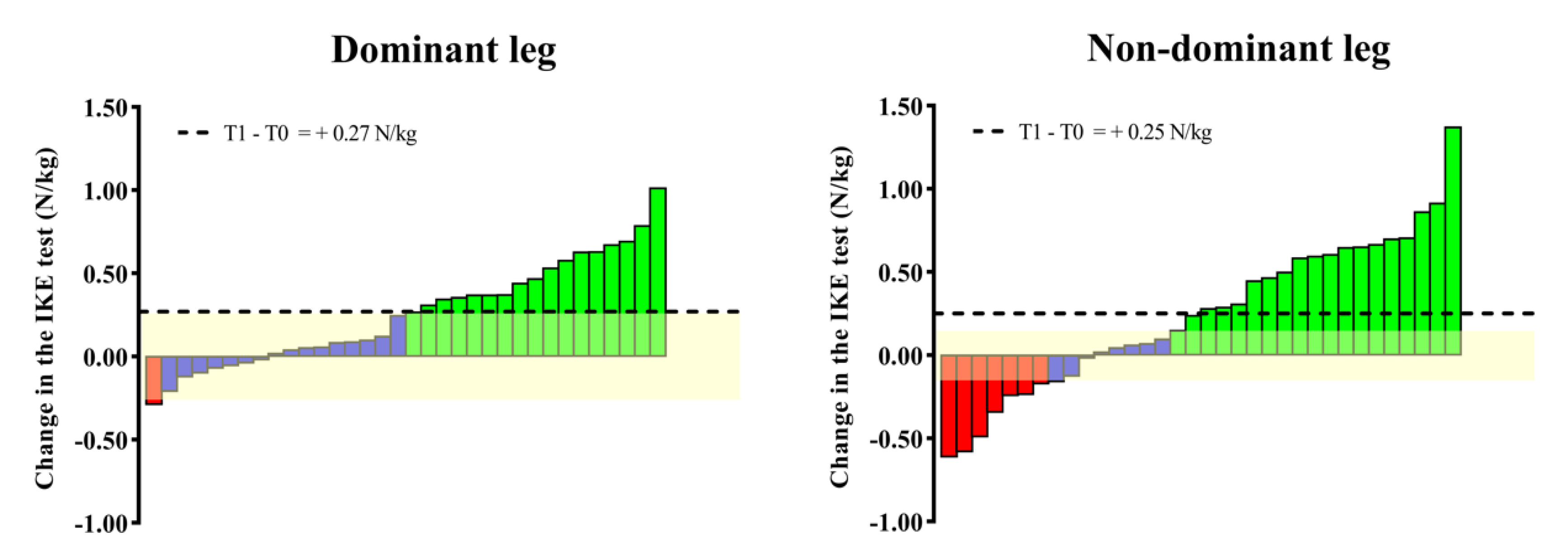
Significant (p < 0.01) declines in the IKE strength for both the dominant (ES = 0.31) and non-dominant legs (ES = 0.37) are shown in Figure 3. Changes in the group means for both legs (dominant = −0.29 N/kg; non-dominant = −0.32 N/kg) were found to be superior to the IKE test variation. Regarding the individual changes, 40% and 73% of the participants achieved changes superior to the variability threshold for the dominant and non-dominant legs, respectively.
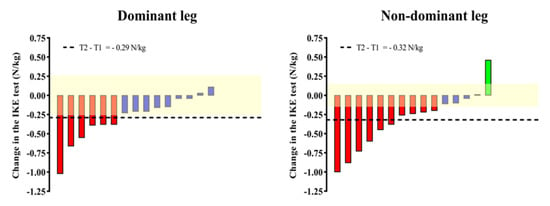
Figure 3.
Individual (bars) and group (horizontal black line) changes in the IKE test after the Vivifrail program. The yellow-shaded range represents the SEM of the IKE test recently reported for older adults. The individual changes that the IKE test was able to detect are represented by green (increase) and red (decrease) bars, while blue bars mean cases whose increase/decrease in performance did not exceed the SEM of the test.
4. Discussion
The results of the current study suggest that the IKE test is a sensitive evaluation to detect changes in the lower-limb strength of institutionalized older adults produced by training and inactivity periods. This finding supports the inclusion of the IKE test as a valid and practical evaluation in nursing homes and clinical settings.
Before including a test in an evaluation battery, practitioners should first analyze its sensitivity. Certainly, a change in any physical capacity should only be considered a real change if it is larger than the variability of the protocol implemented [46], which in turn includes the biological variability of the participant and the error of the device used [47].
The current study found that the IKE test was sensitive enough to detect the group changes generated in institutionalized older adults over 70 years old after both the 4-week training and the 14-week inactive period. However, when individual changes were interpreted, we detected a superior sensitivity of the IKE test when conducted for the non-dominant leg (≥73% individual changes detected) compared to the dominant one (≥40% individual changes detected) (Figure 2 and Figure 3). Since the changes in the lower-limb strength generated after the training and inactive periods were similar for both legs (difference of ≤0.03 N/kg), the higher sensitivity of this IKE evaluation for the non-dominant leg could be explained by its lower variability when implemented for this leg (±0.15 N/kg) compared to the dominant one (±0.26 N/kg) [22]. Considering these findings, future investigations implementing the IKE test for determining changes in lower-limb strength should use the specific variability threshold for each leg instead of a common one to adequately interpret the effects of the intervention conducted. Importantly, a strength of the current study was the use of a previously determined variability threshold [22] in older adults with an age (<2.6 years difference), BMI (<0.8 difference), and physical level (<1.7 points difference in the SPPB) similar to those included in both periods of this study (Table 1). Nevertheless, future studies implementing the IKE test for evaluating older adults with characteristics far from those described above (e.g., healthy community-dwelling older adults or people with Alzheimer’s disease) should use the variability threshold specific to that population and then apply it in practice [48].
On the other hand, this study found that the significance of IKE changes was in line with the significance of the results of the complementary tests included for evaluating functional capacity changes, especially after the training program (Table 2). Enhancements in the knee extensor strength produced by the exercise intervention were probably directly transferred to actions that were proven to be highly dependent on these lower-limb muscles, such as walking or chair raising [49]. Indeed, significant correlations between the IKE test and these functional actions were previously reported for institutionalized older adults [22].
Finally, it is worth highlighting that, together with the handgrip evaluation, the IKE test was the only assessment capable of being performed by 100% of the participants included. When carrying out physical capacity assessments among institutionalized older adults, practitioners should be aware that the very low strength levels commonly present in this population [50] could limit the range of tests they can complete. Considering this aspect, practitioners are encouraged to include the IKE test not only as a sensitive and affordable assessment but also as a practical and low-risk method for evaluating the lower-limb strength of institutionalized older adults.
The main limitation of this study is that the results obtained from the present sample (i.e., institutionalized older adults) cannot be extrapolated to other training or detraining periods or to other older populations (e.g., healthy community-dwelling older adults). Therefore, future researchers must (i) analyze the sensitivity of the IKE test after longer training (i.e., >4 weeks) or shorter detraining periods (i.e., <14 weeks) and (ii) extend the repeatability and sensitivity analyses of the IKE test using a strain gauge in older people with different characteristics beyond those included in the current study.
5. Conclusions
These findings suggest the inclusion of the IKE test as a sensitive and practical evaluation to detect changes in the lower-limb strength of institutionalized older adults after exercise and inactivity periods. Because of its easy-to-use format and applicability (i.e., usable for people with disabilities or reduced mobility), it seems pertinent to implement the IKE test as a complement to other physical assessments proposed in nursing homes and clinical settings, especially among institutionalized older adults who are unable to walk or rise from a chair.
Author Contributions
Conceptualization, Á.B.-R., J.C.-I. and J.G.P.; methodology, Á.B.-R., J.C.-I. and J.G.P.; formal analysis, Á.B.-R. and A.H.-B.; investigation, Á.B.-R., F.F.-L., A.M.C. and E.R.-B.; data curation, Á.B.-R. and A.H.-B.; writing—original draft preparation, Á.B.-R. and A.H.-B.; writing—review and editing, J.C.-I. and J.G.P.; project administration, J.C.-I. and J.G.P.; funding acquisition, J.C.-I. and J.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Science and Innovation (PID2019-108202RA-I00) and the Autonomous Community of the Region of Murcia, Regional Programme for the Promotion of Scientific and Technical Research (Action Plan 2018), Seneca Foundation—Agency of Science and Technology, Region of Murcia (ID: 20872/PI/18).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of University of Murcia (ID: 2131/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to privacy reasons.
Acknowledgments
The authors are grateful to the health professionals and participants for their involvement in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European Consensus on Definition and Diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European Consensus on Definition and Diagnosis. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Losa-Reyna, J.; Alcazar, J.; Carnicero, J.; Alfaro-Acha, A.; Castillo-Gallego, C.; Rosado-Artalejo, C.; Rodríguez-Mañas, L.; Ara, I.; García-García, F.J. Impact of Relative Muscle Power on Hospitalization and All-Cause Mortality in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2022, 77, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Ethgen, O.; Beaudart, C.; Buckinx, F.; Bruyère, O.; Reginster, J.Y. The Future Prevalence of Sarcopenia in Europe: A Claim for Public Health Action. Calcif. Tissue Int. 2017, 100, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Walker, S. Evidence of Resistance Training-Induced Neural Adaptation in Older Adults. Exp. Gerontol. 2021, 151, 111408. [Google Scholar] [CrossRef]
- Thompson, B.J.; Ryan, E.D.; Herda, T.J.; Costa, P.B.; Herda, A.A.; Cramer, J.T. Age-Related Changes in the Rate of Muscle Activation and Rapid Force Characteristics. Age 2014, 36, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Hughes, V.A.; Fielding, R.A.; Fiatarone, M.A.; Evans, W.J.; Roubenoff, R. Aging of Skeletal Muscle: A 12-Yr Longitudinal Study. J. Appl. Physiol. 2000, 88, 1321–1326. [Google Scholar] [CrossRef]
- Gill, T.M.; Gahbauer, E.A.; Han, L.; Allore, H.G. The Relationship between Intervening Hospitalizations and Transitions between Frailty States. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66A, 1238–1243. [Google Scholar] [CrossRef]
- Kortebein, P.; Ferrando, A.; Lombeida, J.; Wolfe, R.; Evans, W.J. Effect of 10 Days of Bed Rest on Skeletal Muscle in Healthy Older Adults. JAMA 2007, 297, 1772–1774. [Google Scholar] [CrossRef] [PubMed]
- Hartley, P.; Romero-Ortuno, R.; Wellwood, I.; Deaton, C. Changes in Muscle Strength and Physical Function in Older Patients during and after Hospitalisation: A Prospective Repeated-Measures Cohort Study. Age Ageing 2021, 50, 153–160. [Google Scholar] [CrossRef]
- Goudarzian, M.; Ghavi, S.; Shariat, A.; Shirvani, H.; Rahimi, M. Effects of Whole Body Vibration Training and Mental Training on Mobility, Neuromuscular Performance, and Muscle Strength in Older Men. J. Exerc. Rehabil. 2017, 13, 573–580. [Google Scholar] [CrossRef]
- Pereira, A.; Izquierdo, M.; Silva, A.J.; Costa, A.M.; González-Badillo, J.J.; Marques, M.C. Muscle Performance and Functional Capacity Retention in Older Women after High-Speed Power Training Cessation. Exp. Gerontol. 2012, 47, 620–624. [Google Scholar] [CrossRef]
- Mazini-Filho, M.; Venturini, G.R.D.O.; Moreira, O.C.; Leitão, L.; Mira, P.A.C.; De Castro, J.B.P.; Aidar, F.J.; Novaes, J.D.S.; Vianna, J.M.; Caputo Ferreira, M.E. Effects of Different Types of Resistance Training and Detraining on Functional Capacity, Muscle Strength, and Power in Older Women: A Randomized Controlled Study. J. Strength Cond. Res. 2022, 36, 984–990. [Google Scholar] [CrossRef]
- Coetsee, C.; Terblanche, E. The Time Course of Changes Induced by Resistance Training and Detraining on Muscular and Physical Function in Older Adults. Eur. Rev. Aging Phys. Act. 2015, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Fukumura, K.; Sato, Y.; Yamasoba, T.; Nakajima, T. Effects of Detraining after Blood Flow-Restricted Low-Intensity Training on Muscle Size and Strength in Older Adults. Aging Clin. Exp. Res. 2014, 26, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.; Haff, G.; Melero, F.; Castilla, A.; Rojas, F.; Fernández, C.; Jaric, S. Feasibility of the Two-Point Method for Determining the One-Repetition Maximum in the Bench Press Exercise. Int. J. Sports Physiol. Perform. 2018, 13, 474–481. [Google Scholar] [CrossRef]
- Kravitz, L.; Akalan, C.; Nowicki, K.; Kinzey, S.J. Prediction of 1 Repetition Maximum in High-School Power Lifters. J. Strength Cond. Res. 2003, 17, 167–172. [Google Scholar] [CrossRef]
- Niewiadomski, W.; Gąsiorowska, A.; Cybulski, G.; Laskowska, D.; Langfort, J. Determination and Prediction of One Repetition Maximum (1RM): Safety Considerations. J. Hum. Kinet. 2008, 19, 109–120. [Google Scholar] [CrossRef]
- Marques, D.L.; Neiva, H.P.; Marinho, D.A.; Nunes, C.; Marques, M.C. Load-Velocity Relationship in the Horizontal Leg-Press Exercise in Older Women and Men. Exp. Gerontol. 2021, 151, 111391. [Google Scholar] [CrossRef]
- Marcos-Pardo, P.J.; González-Hernández, J.M.; García-Ramos, A.; López-Vivancos, A.; Jiménez-Reyes, P. Movement Velocity Can Be Used to Estimate the Relative Load during the Bench Press and Leg Press Exercises in Older Women. PeerJ 2019, 7, e7533. [Google Scholar] [CrossRef]
- Rodríguez-Rosell, D.; Pareja-Blanco, F.; Aagaard, P.; González-Badillo, J.J. Physiological and Methodological Aspects of Rate of Force Development Assessment in Human Skeletal Muscle. Clin. Physiol. Funct. Imaging 2018, 38, 743–762. [Google Scholar] [CrossRef]
- Buendía-Romero, Á.; Hernández-Belmonte, A.; Martínez-Cava, A.; García-Conesa, S.; Franco-López, F.; Conesa-Ros, E.; Courel-Ibáñez, J. Isometric Knee Extension Test: A Practical, Repeatable, and Suitable Tool for Lower-Limb Screening among Institutionalized Older Adults. Exp. Gerontol. 2021, 155, 111575. [Google Scholar] [CrossRef] [PubMed]
- Berg, O.K.; Kwon, O.S.; Hureau, T.J.; Clifton, H.L.; Thurston, T.S.; Le Fur, Y.; Jeong, E.-K.; Trinity, J.D.; Richardson, R.S.; Wang, E.; et al. Skeletal Muscle Mitochondrial Adaptations to Maximal Strength Training in Older Adults. J. Gerontol. Ser. A 2020, 75, 2269–2277. [Google Scholar] [CrossRef]
- Courel-Ibáñez, J.; Martínez-Cava, A.; Morán-Navarro, R.; Escribano-Peñas, P.; Chavarren-Cabrero, J.; González-Badillo, J.J.; Pallarés, J.G. Reproducibility and Repeatability of Five Different Technologies for Bar Velocity Measurement in Resistance Training. Ann. Biomed. Eng. 2019, 47, 1523–1538. [Google Scholar] [CrossRef]
- Hernández-Belmonte, A.; Sánchez-Pay, A. Concurrent Validity, Inter-Unit Reliability and Biological Variability of a Low-Cost Pocket Radar for Ball Velocity Measurement in Soccer and Tennis. J. Sports Sci. 2021, 39, 1312–1319. [Google Scholar] [CrossRef]
- Townshend, A.; Weakley, J.; Chalkley, D.; Johnston, R.; Garc, A.; Dorrell, H.; Pearson, M.; Morrison, M.; Cole, M. Criterion Validity, and Interunit and Between-Day Reliability of the FLEX for Measuring Barbell Velocity During Commonly Used Resistance Training Exercises. J. Strength Cond. Res. 2020, 34, 1519–1524. [Google Scholar]
- Courel-Ibáñez, J.; Pallarés, J.G. Effects of β-Hydroxy-β-Methylbutyrate (HMB) Supplementation in Addition to Multicomponent Exercise in Adults Older than 70 Years Living in Nursing Homes, a Cluster Randomized Placebo-Controlled Trial: The HEAL Study Protocol. BMC Geriatr. 2019, 19, 188. [Google Scholar] [CrossRef]
- Izquierdo, M.; Rodriguez-Mañas, L.; Sinclair, A.P. What Is New in Exercise Regimes for Frail Older People—How Does the Erasmus Vivifrail Project Take Us Forward? J. Nutr. Health Aging 2016, 20, 736–737. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.; Locks, R.R.; Lopes, P.B.; Bento, P.C.B.; Rodacki, A.L.F.; Carraro, A.N.; Pereira, G. Multicomponent Exercise Training Improves Gait Ability of Older Women Rather than Strength Training: A Randomized Controlled Trial. J. Aging Res. 2020, 2020, 6345753. [Google Scholar] [CrossRef]
- Folstein, M.; Folstein, S.; Mchugh, P. Mini-Mental State" A Practical Method for Grading the Cognitive State of Patients for the Clinician Related Papers “MINI-MENTAL STATE” a Practival Method for Grading the Cognitive State of Patients for the Clinician. J. Gsychiaf. Res 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar]
- Lawton, M.P.; Brody, E.M. Assessment of Older People: Self-Maintaining and Instrumental Activities of Daily Living. Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and Initial Validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, T.K.; Morley, J.E. SARC-F: A Simple Questionnaire to Rapidly Diagnose Sarcopenia. J. Am. Med. Dir. Assoc. 2013, 14, 531–532. [Google Scholar] [CrossRef]
- Courel-Ibáñez, J.; Hernández-Belmonte, A.; Cava-Martínez, A.; Pallarés, J.G. Familiarization and Reliability of the Isometric Knee Extension Test for Rapid Force Production Assessment. Appl. Sci. 2020, 10, 4499. [Google Scholar] [CrossRef]
- Tillin, N.A.; Jimenez-Reyes, P.; Pain, M.T.G.; Folland, J.P. Neuromuscular Performance of Explosive Power Athletes versus Untrained Individuals. Med. Sci. Sports Exerc. 2010, 42, 781–790. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A Short Physical Performance Battery Assessing Lower Extremity Function: Association with Self-Reported. J. Gerontol. 1994, 49, 85–94. [Google Scholar] [CrossRef]
- Laukkanen, P.; Heikkinen, E.; Kauppinen, M. Muscle Strength and Mobility as Predictors of Survival in 75–84—Year—Old People. Age Ageing 1995, 24, 468–473. [Google Scholar] [CrossRef]
- Herman, T.; Giladi, N.; Hausdorff, J.M. Properties of the “Timed Up and Go” Test: More than Meets the Eye. Gerontology 2011, 57, 203–210. [Google Scholar] [CrossRef]
- Lusardi, M.M.; Fritz, S.; Middleton, A.; Allison, L.; Wingood, M.; Phillips, E.; Criss, M.; Verma, S.; Osborne, J.; Chui, K.K. Determining Risk of Falls in Community Dwelling Older Adults: A Systematic Review and Meta-Analysis Using Posttest Probability. J. Geriatr. Phys. Ther. 2017, 40, 1–36. [Google Scholar] [CrossRef] [PubMed]
- Buendía-Romero, Á.; Vetrovsky, T.; Estévez-López, F.; Courel-Ibañez, J. Effect of Physical Exercise Cessation on Strength, Functional, Metabolic and Structural Outcomes in Older Adults: A Protocol for Systematic Review and Meta-Analysis. BMJ Open 2021, 11, e052913. [Google Scholar] [CrossRef]
- Courel-Ibáñez, J.; Buendía-Romero, Á.; Pallarés, J.G.; García-Conesa, S.; Martínez-Cava, A.; Izquierdo, M. Impact of Tailored Multicomponent Exercise for Prevent Weakness and Falls on Nursing Home Residents’ Functional Capacity. J. Am. Med. Dir. Assoc. 2021, 23, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Courel-Ibáñez, J.; Pallarés, J.G.; García-Conesa, S.; Buendía-Romero, Á.; Martínez-Cava, A.; Izquierdo, M. Supervised Exercise (Vivifrail) Protects Institutionalized Older Adults Against Severe Functional Decline After 14 Weeks of COVID Confinement. J. Am. Med. Dir. Assoc. 2020, 22, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Belmonte, A.; Courel-Ibáñez, J.; Conesa-Ros, E.; Martínez-Cava, A.; Pallarés, J. Level of Effort: A Reliable and Practical Alternative to the Velocity-Based Approach for Monitoring Resistance Training. J. Strength Cond. Res. 2022, 36, 2992–2999. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioural Sciences. Hillside; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Hopkins, W.G. Measures of Reliability in Sports Medicine and Science. Sports Med. 2000, 30, 375–381. [Google Scholar] [CrossRef]
- Rodríguez-Rielves, V.; Martínez-Cava, A.; Buendía-Romero, Á.; Lillo-Beviá, J.R.; Courel-Ibáñez, J.; Hernández-Belmonte, A.; Pallarés, J.G. Reproducibility of the Rotor 2INpower Crankset for Monitoring Cycling Power Output: A Comprehensive Analysis in Different Real-Context Situations. Int. J. Sports Physiol. Perform. 2021, 1, 120–125. [Google Scholar] [CrossRef]
- Paulo, M.; Oliveira, B.D.; Calixtre, L.B.; Regina, P.; Serrão, S. Reproducibility of Isokinetic Measures of the Knee and Ankle Muscle Strength in Community - Dwelling Older Adults without and with Alzheimer ’ s Disease. BMC Geriatr. 2022, 22, 940. [Google Scholar] [CrossRef]
- Besier, T.F.; Fredericson, M.; Gold, G.E.; Beaupré, G.S.; Delp, S.L. Knee Muscle Forces during Walking and Running in Patellofemoral Pain Patients and Pain-Free Controls. J. Biomech. 2009, 42, 898–905. [Google Scholar] [CrossRef]
- Roberts, H.C.; Syddall, H.E.; Sparkes, J.; Ritchie, J.; Butchart, J.; Kerr, A.; Cooper, C.; Sayer, A.A. Grip Strength and Its Determinants among Older People in Different Healthcare Settings. Age Ageing 2014, 43, 241–246. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).