Abstract
One of the most discussed topics concerns the sample preparation before the analysis and, therefore, all the operations necessary to eliminate the interferents, clean up the specimens, and extract the analytes of interest, reducing the matrix effect. This review highlights the fundamental steps in the treatment of postmortem samples used in forensic analysis. Through critical literature research, it was possible to choose among the countless works that could provide a general overview of the state-of-the-art in this field. Different biological matrices have been considered; blood and urine (the traditional biological fluids) are used to investigate the presence of substances that may have caused death, whilst other body fluids, such as bile and oral fluids, are still under discussion for their usability (and suitability). In the second part of the review, all the solid matrices obtained after autopsy were further divided into conventional and unconventional matrices to facilitate proper understanding. The choice of literature was also made according to the most widely used pretreatment techniques and the most representative innovative techniques.
1. Introduction
In the medicolegal context and in autopsy cases, the first target is to evaluate and quantify the possible presence of substances, such as legal drugs, illicit drugs, pesticides, and other exogenous compounds or the relevant metabolites, that can be ascribed as the cause of death. In this field, it is essential to ensure accuracy (simultaneous presence of precision and trueness), reliability, and validity of the method used, and to be able to extract and quantify different substances from various complex matrices [1]. Amphetamines, benzodiazepines, cocaine, cannabis, and opiates are the most common drugs, but no less critical and frequent are other illicit compounds like new psychoactive substances (NPS) or pesticides [2].
The accuracy of the procedure depends on the sample selection and its storage. These are the critical steps during the analysis: the sample pretreatment, the extraction and the clean-up of the analyte(s), and the instrumental analysis [1,3]. The most popular techniques used in forensic analytical chemistry are gas chromatography mass spectrometry (GC-MS), gas chromatography tandem mass spectrometry (GC-MS/MS), high-performance liquid chromatography mass spectrometry (HPLC-MS), high-performance liquid chromatography tandem mass spectrometry (HPLC-MS/MS), ultra-high-performance liquid chromatography mass spectrometry (UHPLC-MS) and tandem mass spectrometry (UHPLC-MS/MS) [4,5]. However, to increase the sensitivity of determination, an appropriate sample pretreatment is necessary to remove the interference(s) of the matrix, extract, and concentrate the analytes to reduce the matrix effects [6]. In forensic toxicology, drug tests have traditionally been performed in whole blood, plasma, and urine specimens considered as “conventional” biological fluids [7].
Sometimes it is not convenient to use blood samples in case of suspected postmortem redistribution, a phenomenon much studied in recent years, which can cause false results [8,9]. For this reason, it is increasingly common to use other biological matrices like vitreous humor, muscle, bile, liver, brain, stomach, and oral fluid. Occasionally, hair, nails, and bone are also used primarily in scenarios of severe decomposition or cruel death where the integrity of the cadaver is severely compromised [10].
Over the years, different techniques have been developed to maximize the efficiency of extraction and the accuracy of the analysis. Some of these are protein precipitation (PP), liquid-liquid extraction (LLE), and solid-phase extraction (SPE), which are relatively laborious, solvents- and time-consuming [11,12,13,14,15]. To obtain a fast and precise extraction, the QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) approach is preferred and has been applied to several forensic samples [16,17,18,19]. Novel strategies for sample preparation, such as microextraction by packed sorbent, solid phase microextraction, polymer monolith microextraction, and more, have already been discussed in the literature [6]. This review aims to overview the information to bring out the use of the most effective extraction techniques in samples consisting of a wide range of complex biological matrices obtained after the autopsy in the field of forensic toxicology.
2. Biological Fluids for Forensic Analyses
In the following sections, some of the most widely used sample preparation techniques will be highlighted, focusing on the historically most used biological matrices in the forensic field. In addition to blood and urine, other fluid matrices have also been considered, such as vitreous humor, bile, and oral cavity fluids that can provide additional information to the analysis or be used as a screening tool when blood and urine are not available.
2.1. Blood
Traditionally, blood was considered the main biological matrix to extract and determine drugs or other substances in the clinical and forensic fields. Usually, the literature calls this “whole blood” to denote that it refers to blood and its cellular components and to differentiate it from serum or plasma in which solid components have been eliminated.
In forensic toxicology, the determination of substances in the blood is strongly affected by their half-life, time, and mode of administration, condition of the corpse, and redistribution phenomena. Analyses are often carried out on blood obtained from peripheral sites, such as the femoral vein, but in some critical situations, as exsanguination caused by severe injury, specimens are collected from the chest or the abdominal cavity considering the substantial differences between the “two types of blood”. Numerous studies have been conducted to assess the differences between peripheral blood and cardiac blood, which is more abundant and allows the identification of those substances that are extensively metabolized [3,20]. After sampling, the critical stage is the treatment of the sample to extract the analytes of interest, reducing the matrix effect and improving the accuracy of the analysis. The techniques most used in this subject area are protein precipitation, solid or liquid extractant phase extraction, and microextraction. However, the main goal in our day is to simplify these procedures as much as possible to reduce time and solvent consumption [21]. These classic techniques have been revised, modified, and automated to reduce the number of operations performed and optimize the extraction process [22].
Usui K. et al. have modified QuEChERS extraction to detect some critical drugs and poisons in the forensic field. Their method consists of two main steps: firstly, a fraction of whole blood collected was diluted with distilled water and put into a plastic tube (pre-packed Agilent SimplyQ) containing solid sorbent phase shacked and centrifugated to promote separation of two phases (aqueous layer and acetonitrile layer), with the supernatant directly injected to analyze acidic drugs. The second step, required for basic drugs, consisted in dispersive solid-phase extraction (d-SPE) using a pre-packed tube containing primary–secondary amine, end-capped octadecylsilane, and magnesium sulfate. An aliquot obtained of supernatant by first extraction was added into the tube, mixed, and successfully centrifugated to clean up the blood. The supernatant was utilized for quantification in LC-MS/MS. This method foresees very short times compared to the execution of a SPE cation exchange (necessary for the separation of acidic and basic analytes). It is also important to underline the versatility of the method, which can be applied in the same way even by inexperienced operators without major variations in the results, as also discussed by the authors [16]. Junior E.F. et al. have also tried applying d-SPE to extract drugs and pesticides from blood in postmortem cases. The authors evaluated two extraction solvents (acetonitrile and ethyl acetate) and compared the method with solid-liquid extraction low-temperature partitioning (SLE-LTP) regarding selectivity, matrix effect, cleaning, stability of the sample, and operator precision. The two methods were carried out using the same sample and first acetonitrile and then ethyl acetate, and it has been shown that the use of acetonitrile caused less interference from lipids and fatty acids and that the recovery of analytes was maximum for d-SPE compared to SLE-LTP [23].
Lau T. et al. used solid phase extraction to clean up blood specimens obtained from the femoral vein and heart during their study aimed at identifying synthetic cathinones using LC-MS/MS. For the extraction that was used, mixed-mode cation exchanges cartridges were to retain basic ionized substances. All samples were firstly vortexed, sonicated after adding a phosphate buffer, and then centrifugated. After these preliminary steps, the sample was inserted in a column, and after washing with acetic acid and methanol, the analytes were eluted with dichloromethane:isopropanol:ammonium hydroxide (93:5:2, v:v:v). The eluate was dried and, after reconstitution, was injected for analysis. Based on our knowledge, this validation method appeared to be the most sensitive among those found in the literature. Thanks to the careful choice of mixed-mode cation exchange cartridges, based on the chemical properties of the analytes sought, the authors obtained a limit of quantification (LOQ) around 1 ng/mL using only 0.25 mL of blood [24].
Pouliopoulos A et al. have validated a modified mini-QuEChERS to extract 15 psychotropic drugs from blood samples obtained after autopsy. The extraction method was optimized using different pH conditions. First, potassium carbonate (for basic condition), sodium chloride (for acid condition), and magnesium sulfate were mixed with 100 µL of sample and 600 µL of acetonitrile. Then, the mixture was vortexed and centrifugated, and the supernatant was dried. The use of basic conditions showed the best results, using acetonitrile and excluding other solvents such as cyclohexane, butyl acetate, and dichloromethane. This single-step method was also compared with two steps of extraction that consisted of the pretreatment of the sample as described above and a second clean-up step with magnesium sulfate and primary secondary ammine, which, however, caused a lower recovery of the analytes [25].
Odoardi S. et al. have applicated the dried blood spot method to detect a wide range of drugs of abuse. This method is already widely used in clinical analysis due to its minimal invasiveness. The authors have promoted its use in forensic analysis, allowing a further reduction of solvents and time. This study used Bond Elut Dried Matrix Spotting cards from Agilent, where three drops of the mixture of blood and internal standard were deposed and allowed to dry at room temperature. Three spots formed were cut and put into a tube with methanol (0.1% of formic acid), and after the appropriate time, were centrifugated, and the supernatant was dried [22].
Takitane J. et al. have used liquid-liquid extraction (LLE) to treat postmortem whole blood to detect cocaine and its metabolites and cocaine crack biomarkers, exceeding the limits of other techniques such as SPE and protein precipitation that led, firstly, to too high costs, the secondly, to a sample cleaning process which was sometimes not sufficient. For the extraction, 100 µL of blood, 25 µL of methanol:water (50:50, v:v), and 25 µL of internal standard was mixed, after which was added 100 µL of carbonate buffer (pH 9.3) and 1 mL of a mix methyl tert-butyl ether:2-propanol (70:30, v:v). After this, the mixture was vortexed, centrifugated, and the upper phase was dried. There are numerous applications of this technique for the extraction of cocaine and metabolites from biological matrices, but they often involve the use of very toxic chlorinated solvents. It was interesting to note how in this case the use of these solvents was bypassed [26].
Vejar-Vivar C. developed a method to evaluate the presence of tricyclic antidepressants and benzodiazepines in postmortem blood using a microextraction by packed sorbent coupled to electrospray-time of flight mass spectrometry ((MEPS)-ESI-TOF-MS). Before putting the sample in MEPS, the blood was deproteinized by adding acetonitrile and the mixture was vortexed and centrifugated to obtain two phases. The supernatant (acetonitrile layer) was dried to eliminate the acetonitrile, which is incompatible with MEPS. The dried residue was reconstituted with acid water (formic acid, pH 3), loaded in MEPS for clean-up, and directly analyzed [27].
Merone GM et al. have proposed a new fast method for qualitatively detecting illicit drugs and drugs in postmortem blood samples and other matrices for rapid and reliable screening. Their study aimed to develop a green method, minimizing handling, costs, and use of toxic solvents without damaging the accuracy of the analysis. The single expected step was to precipitate proteins using a low concentration of methanol that was added to the sample. The mixture was vortexed and centrifuged to obtain a clear supernatant that was diluted with an aqueous solution (0.1% formic acid) and injected into liquid chromatography tandem mass spectrometry (LC-MS/MS) instrumentation [28]. Merone GM et al. discussed in their review that ionic liquids are now considered the main alternative to extraction solvents, which are usually toxic for operators. These ionic liquids can be used in a wide range of applications, including the treatment and extraction process of biological samples before forensic analysis [29].
De Boeck M. et al. have used ionic liquid-based liquid–liquid microextraction to extract benzodiazepines from the blood for LC-MS/MS analysis. They used BMIm PF6 as an ionic liquid added at 1 mL of blood and dispersed with agitation to promote the extraction by the ionic liquid phase. After the sample was centrifugated, two phases were obtained (upper blood phase and ionic liquid lower phase), and the lower phase was collected and analyzed [30]. Savini F. et al. used whole blood to determine the concentration of alcohol in 31 forensic cases using GC-FID. Before analysis, blood samples collected from the femoral vein and lyophilized were stored with the addition of sodium fluoride. After reconstitution using water, they were put into hermetic vials where different ethanol concentrations were added. All samples were vortexed and then injected into GC. The investigation and quantification of post-mortem ethanol levels hides various pitfalls, due to the characteristics of the molecule, which is absorbed and rapidly distributed throughout the body, and above all, by some factors such as the production of alcohol by bacteria which can distort the quantified levels. We consider this work important because it highlights the problems of this type of investigation and illustrates the solution [31].
Ogawa T. et al. demonstrated the superiority of novel extraction methods using ISOLUTE PDL+ compared with another extraction method, such as protein precipitation or QuEChERS, in the pretreatment of whole blood for forensic applications. The authors have used this phospholipids and protein removal column to clean up the blood before determining psychoactive drugs in several forensic cases. The sample was diluted with sodium dihydrogen phosphate/disodium hydrogen phosphate, after which internal standard, acetonitrile, and methanol were added. The mixture was loaded in the column and inserted into a centrifuge. After centrifugation, the purified analytes were collected on the bottom of the tube, where 100 µL of 0.1% trifluoracetic acid in acetonitrile was added. After evaporating to dryness, the sample was reconstituted with methanol for analysis [32]. Locatelli M. et al. used a new type of extraction based on the use of substrate coated with sol-gel sorbent called fabric-phase sorptive extraction (FPSE) in the analysis of seven antidepressants in whole blood. This innovative extraction technique patented by Kabir and Furton has been used over the years in different fields thanks to application versatility and high-performance efficiency, which overcomes the disadvantages of classical techniques [33,34,35,36,37,38]. In this work, Locatelli M. et al. applied FPSE in the forensic field directly on the collected blood avoiding further pretreatment (e.g., protein precipitation, filtration, etc.). They tested eight different coated membranes and other parameters such as sorbent chemistry, time of extraction, and sample volume extraction solvent. The authors demonstrated that the membrane coated with Carbowax 20 M for 20 min. of extraction time represents the most efficient solution, so they chose this membrane for the real sample application. Briefly, the membrane was cut and washed first with acetonitrile:methanol and then with Milli-Q water and put into the sample for 20 min After the extraction, the membrane was inserted into 150 µL of methanol for 5 min to back-extract the analytes and finally analyzed them. This extraction technique, although it involves the accurate preparation phases of the sorbent, allows for good resolution even using an analysis tool other than mass spectrometry, which is the most used tool in the forensic field, but which is not always present in all laboratories [39].
2.2. Urine
Urine, together with blood, is commonly used in forensic toxicology, as urine also represents a deposit of drugs, illicit drugs, and other substances and metabolites that may provide information on the victim’s exposure before death. In forensic cases, urine can be obtained with a withdrawal from the bladder and successively analyzed, considering that the urine contains less protein and lipids than blood, resulting in it being more accessible and faster to treat. Ferrari J.E. et al. investigated the presence of new psychoactive substances in both blood and urine collected postmortem, and to compare the two matrices, they applied the same treatment. Using the QuEChERS method, they tested four extraction protocols with different amounts of acetonitrile and water added to an aliquot of urine. All were vortexed, and then magnesium sulfate was added, as sodium acetate, re-vortexed and centrifuged. The supernatant was separated and put into a vial containing primary secondary ammine and magnesium sulfate and once again vortexed and centrifugated to clean up the sample. Finally, the supernatant obtained was dried and resuspended prior to analysis [40]. Mouskeftara T. et al. compared three different extractions and clean-up methods to determine the concentration of insecticides and fungicides in postmortem urine samples. Simple protein precipitation was carried out by adding 150 µL of methanol: water (50:50, v:v) into the sample, and then the mixture was centrifugated, and the supernatant was separated from the precipitate. For solid phase extraction, 2 mL of urine was loaded on a C18 cartridge, and after washing with water, the analytes were eluted using 1.5 mL of methanol. For dispersive solid phase extraction (d-SPE) were tested four different protocols were used. However, a large number of operations to be conducted led the authors to discard this method of extraction, which seemed to induce errors. SPE method did not ensure sufficient recovery of analytes, so protein precipitation was considered the best technique [41]. Merone GM et al. in their work already discussed in the “blood section” have also used urine to develop screening methods for illicit substances. In this case, urine was processed with enzymatic hydrolysis of any metabolites present. To 500 µL of the sample was added 100 µL of enzymatic solution (glucuronidase), which was left in incubation at 60 °C for 3 h. After dilution with a solution of internal standard, an amount of sample was directly injected into LC-MS/MS. The use of a minimum volume of enzymatic hydrolysis and non-toxic solvents is the real novelty represented by this work, which differs from the others present in the literature [28].
Song A. et al. developed a new type of headspace solid-phase microextraction using carbon nanotubes as a sorbent to increase the effectiveness of extraction of amphetamine-type stimulants from postmortem urine. The work involved the preparation of this sorbent and covering appropriate fiber as supporting material and then the headspace solid phase microextraction. Briefly, 5 mL of diluted urine was placed into a vial under stirring with an aluminum cap and then into a thermostatic bath (80 °C) after the syringe needle of SPME containing the coated fiber was inserted through the aluminum cover for the time of extraction (10–60 min). Then the coated fiber was subjected to thermal desorption at 250 °C prior to GC analysis. It is interesting to note how the study and development of such an efficient sorbent of this type can increase the performance of an already very high-performing extraction technique like SPME [42]. In recent work, Yamaguchi K. et al. validated a method to discover novel zolpidem metabolites in urine samples collected from victims of fire who consumed Myslee® 5 mg (5 mg of zolpidem per tablet). The urine was centrifugated after adding 0.1% acid formic in acetonitrile, and the collected supernatant was dried and resuspended with methanol/water. After another centrifugation, the supernatant obtained was used for liquid chromatography coupled to quadrupole-time-of-flight mass spectrometer (LC-QqTOFMS) analysis [43].
Wang H. et al. tested a novel effective method for quantification of ethyl sulfate and ethyl glucuronide in postmortem urine. These are the most used biomarkers, which allow the victim to assess alcohol consumption. In their study, the authors used a simple treatment of the sample based on fast-dried urine spots, significantly reducing the interest compounds’ matrix effect. A small amount of urine (20 µL) was loaded on Watman 903 filter paper and the spot was circled with a pencil. After this, the loaded filter was put into a microwave for one minute and was then cut into 4 pieces. All pieces were added into a vial containing 500 µL of methanol and subjected to extraction in an ultrasonic bath. The upper phase was collected, dried, and restored with formic acid in water. Finally, the mixture was centrifugated, and the supernatant was used for analysis [44]. Very frequent in the forensic field is the use of on-site drug screening kit work based on urine immunoassay. Sometimes false positives can occur, especially for some substances (e.g., amines produced during decomposition time can cause an amphetamine false positive) or, in some cases, false negatives can also occur, when the substances of interest interact with antibodies of other endogenous compounds. Tanaka T. et al. compared five analytical methods on postmortem urine, three on-site kits, GC-MS, and LC-MS/MS. The latter two were used for confirmation analyses. For analyses with a fast immunoassay kit, samples need minimal treatment before being applied. Usually, the sample was centrifuged, and the supernatant was taken. However, mass analysis samples required more accurate treatment to minimize contaminants and matrix effect. In this work, urine was treated with glucuronidase and cleaned up with the QuEChERS method, as described in other previous studies [45].
2.3. Vitreous Humor
Vitreous humor is one of the most widely used matrices under conditions where blood or urine do not provide the necessary information or are unavailable. This fluid is composed primarily of water and other components such as lipids, proteins, and electrolytes. It is taken with a thin needle between the crystalline lens and retina during the autopsy. Compared with other matrices (blood or solid matrices), vitreous humor is a clear matrix; thus, it contains fewer interferences and is sheltered from bacterial contaminations [46]. This is a significant advantage, especially in some situations, as discussed by Savini F. et al. in their work about the determination of ethanol in postmortem specimens, where they have highlighted that in some cases, quantification of alcohol can be altered by microbial activity during putrefaction time capable of producing ethanol with the fermentation of glucose. In this context, the authors have shown the correlation between ethanol concentration in blood and vitreous humor, confirming the possibility of using vitreous humor as an alternative to other matrices [31].
Many classes of drugs can be detected in this matrix, such as benzodiazepines, opioids, cocaine, and their metabolites. However, the determination of compounds that are highly protein-bound is more difficult [20]. Techniques used to treat and clean up vitreous humor samples are the same as other fluid matrices, but the operations and results can be more accurate by being a cleaner matrix. Birk L. et al. used a simple protein precipitation protocol to treat postmortem samples while developing their method for simultaneous quantifying benzodiazepines. 150 µL of the sample was mixed with 200 µL of acetonitrile:acetone:methanol (1:1:1, v:v:v) and internal standard. The mixture was vortexed and centrifugated to obtain the extract for analysis [47]. Akhgari M. et al. treated vitreous humor with dispersive liquid–liquid microextraction before the analysis. They compared the results with the same protocols using a urine sample to quantify methadone and tramadol in forensic cases. Briefly, the sample was added into a vial containing chloroform as extraction solvent and methanol as dispersion solvent. The mixture was firmly vortexed to facilitate the dispersion of the two phases. The precipitate phase (extraction solvent) obtained after centrifugation was taken and evaporated, and the dry product was reconstituted with methanol for analysis in UHPLC and GC-MS [48].
Interesting research was conducted by Legg K.M. et al., who have validated an automated process based on immunoaffinity to detect insulin analogous to postmortem vitreous humor. Finding insulin analogs is a very ambitious task due to the size and complexity of these molecules and their close similarity with endogenous insulin, which often requires lengthy and laborious work. The authors proposed a method using robotic immunoaffinity-micro chromatography followed by the separation of alpha and beta chains, followed by only beta chains mass analysis. The insulin extraction from the sample was conducted using Agilent AssayMap, and the micro chromatography was obtained using two monoclonal mouse anti-insulin antibodies immobilized on cartridges. The insulin was purified in the column and eluted with acid conditions [49]. Ntoupa P.S.A. et al. validated a method to study the distribution of antidepressants in postmortem humor vitreous, using for the extraction of analytes a solid-phase extraction containing a mixed mode sorbent (C8 nonpolar sorbent and strong cation exchange sorbent). 1 mL of the unknown sample was added to the internal standard, and 5 mL of phosphate buffer (pH 6). All were vortexed and centrifugated to obtain the supernatant loaded onto the column. For the wash, deionized water was used, and then 1 mL of acetic acid was added and rewashed with methanol before being dried under a vacuum. The elution was carried out with isopropanol:ethyl acetate:ammonium hydroxide (15:82:3, v:v:v), and the eluate was dried, followed by derivatization of the residue by adding heptafluorobutyric anhydride (HFBA) and ethyl acetate at 50 °C for 30 min. The last step was to dry the reconstituted mixture with 50 µL of ethyl acetate before analysis. We have noticed that there are few works that concern the determination of drugs within the vitreous humor, and even fewer that have the objective of simultaneously determination of more than one drug in this matrix. In this work, the authors have developed a method to do so by also comparing with the results obtained from peripheral blood [50]. To further reduce time and costs for sample preparation, Kovacs L. et al. have tested a new extraction approach using disposable pipette extraction (DPX) to determine the concentration of codeine, morphine, and heroin in postmortem vitreous humor. The sorbent in the pipette is loosely packed so that the elution can be done without a vacuum; moreover, this technique requires low conditioning steps, and the procedure can be automated. For the extraction the following steps were performed:
- The sorbent was conditioned with water and methanol for 20 s. to equilibrate the system
- the sample was drawn up and put in contact with the sorbent for 30 s.
- the sample was dispensed.
- the sorbent was washed with deionized water and acetonitrile.
- the analytes of interest were eluted with a mixture of dichloromethane:isopropanol:ammonium hydroxide (78:20:2, v:v:v).
- the eluate was dried and then N-Methyl-N-(trimethylsilyl)tri-fluoroacetamide (MSTFA) was added for the silylation of molecules prior to GC analysis [51].
2.4. Alternative Biological Fluids: Bile and Oral Fluids
In addition to the primary biological fluids of forensic interest, researchers have tried to understand what information could be obtained from other types of samples, such as bile and oral fluids, and whether this information correlated with conventional matrices. The subject of many studies was bile that can be considered an excellent matrix for identifying all substances with a biliary excretion. The main problem is that bile is a very complex fluid subjected to several changes after death. Some works in the literature testify to the use and evaluation of bile as a matrix for forensic use, and Bèvalot F. et al. proposed an interesting review that compared the concentration of several substances in bile and blood, emphasizing that in some cases, the concentration in bile was higher than in blood [8].
Mercurio I. et al. quantified the concentration of morphine in bile compared with blood and liver using GC-MS. However, the complexity of this matrix requires careful sample preparation that needs more steps. Briefly, the sample was first hydrolyzed with glucuronidase overnight at 50 °C, for basic analytes were carried out an extraction using a column-type ISOLUTE HCX conditioned with methanol, deionized water, and phosphate buffer (pH 6). After adding phosphate buffer (pH 6), the sample was loaded into the column, and the interferences were washed first with water and hydrochloric acid and then with methanol. The analytes were eluted with dichloromethane:isopropanol (80:20, v:v). Before the analysis, the eluate was dried, reconstituted in methylene chloride, and derivatized with 20 μL of N,O-bis(trimethylsilyl)trifluoroacetamide with 1% of trimethylsilyl (TMS). The authors conclude that the results obtained do not show a precise correlation between the bile and the other matrices, probably caused by the complex mechanisms that are the basis of biliary excretion, and for this reason suggest the use of this matrix only as a screening test in cases where the blood are not available [52]. In addition, oral cavity fluids can be collected from corpses to obtain critical information about the presence of illicit substances. In most cases, heroin metabolites that have a short window of detection could be detected more readily in oral fluids than in other conventional matrices. Reisinger A.J. et al. investigated a new method for the evaluation of substances in postmortem oral cavity fluids using an innovative device for simple and fast sampling. This device consists of a cellulose pad that is placed in the mouth of the corpse for 15 min and allows it to absorb fluids, then is placed into a buffer protected by a filter, and the obtained mixture (cavity fluid released/buffer) was transferred into a vial with methanol and internal standard before the LC-MS/MS analysis [53].
3. Main Solid Matrices for Forensic Purposes
In this section, attention is paid to solid matrices that can be used for analysis in the forensic field, examining in detail both the pretreatment step of the samples and the techniques used to extract analytes of interest from them. It was necessary to divide the matrices of this group into conventional and unconventional based on our knowledge and on data collected from literature to emphasize which of these is most frequently used, but with the main objective that remains being to offer a general vision of extraction techniques that guarantee the best performance.
3.1. Muscle Tissue
In some cases of brutal death or when the cadaver is burned or decomposed, conventional fluids are not available. A good alternative is represented by muscle tissue. Rees K.A. et al. have validated a method for simultaneously quantifying cocaine and its metabolites after extracting the analytes from rectus femoris. After preliminary tests on muscle samples obtained from Sika Deer, subsequent confirmation was made on samples obtained from the autopsy. The muscle was sectioned and chopped into fine pieces, then homogenized at 18,000 rpm. An accurately weighed quantity was diluted with 8 mL phosphate buffer (0.1 M; pH 6), centrifugated, and extracted by SPE with mixed-mode sorbent. After derivatization, analytes were quantified by GC-ion trap-MS/MS [54,55].
Frost J. et al. have used muscle samples to simultaneously determine codeine, morphine, and their metabolite. After homogenization, the tissue was mixed with ammonium carbonate and extracted using an Agilent Bond Elut-C18 column. The column was conditioned using 1 mL of methanol, 1 mL of water, and 2 mL of ammonium carbonate pH 9. After washing and vacuum drying, the column was eluted with 0.5 mL methanol:0.5 M acetic acid (9:1, v:v). The eluent was evaporated, and the residue was reconstituted with 50 mL 50 mM ammonium acetate pH 7.0 and injected into the LC-MS [56]. Staeheli S.N. et al., after validating the method, used muscle tissue and other solid samples to investigate postmortem redistribution of fentanyl and its metabolite. Extraction of tissue was performed after homogenization using two LLE; for the first extraction was added 250 mL of 0.2 M phosphate buffer and 0.8 mL of butyl acetate:ethyl acetate (1:1, v:v). After agitation and centrifugation, the supernatant obtained was transferred into a vial, and the remaining sample was treated with the second LLE. The supernatant obtained was added to the previous extract. After the addition of 25 mL of formic acid, the sample was evaporated to dryness under a stream of nitrogen and successively reconstituted with the mobile phase [57,58].
The use of complex and effective extraction techniques like those listed above (SPE and LLE) is important when dealing with solid matrices because a simple purification by protein precipitation often leads to an alteration of the results due to the high matrix effect.
3.2. Liver Tissue
Sometimes in the liver, many toxic substances are present in higher concentrations than in other matrices. This type of specimen is favored when blood is unavailable due to exsanguination.
Kalh J.H. et al. used a liver sample for quantitative analysis of fentanyl and its analogs, assessing the correlation between the liver, brain, and blood. Homogenized liver tissue was buffered with sodium phosphate, vortexed, and then centrifuged. The extraction step was performed using positive pressure solid-phase United Chemical Technologies Clean Screen mixed mode columns. After conditioning, the supernatant obtained from the sample was added to the columns, and then the analytes were eluted with dichloromethane:isopropanol:ammonium hydroxide (78:20:2, v:v:v) and evaporated. Before the analysis in UHPLC-MS/MS, the dried sample was reconstituted with 50 mL of 0.1% formic acid in water [59]. Takayasu T. et al. investigated the distribution of zolpidem in body fluid and organ tissue. In this study, a portion of the liver was homogenized and applied to the Extrelut NT column. After waiting for 1 h, elution was carried out with 20 mL of ethyl acetate:chlorobutane (4:1, v:v). The extract was dried and reconstituted with the same mixture prior to analysis [60]. Magalhaes E.J. et al. proposed an interesting method for the determination of cocaine in the human liver obtained from overdose victims. They have validated an efficient method for extraction of analytes from the liver using solid-liquid extraction with low-temperature partitioning (SLE-LTP) by evaluating the influence of different variables such as pH of extract, volume, and composition of extraction solvent and sorbent material. This technique allows researchers to obtain relatively pure extracts that can be directly analyzed and are based on low steps (Figure 1). After evaluating the extraction parameters, they decided to continue using physiological pH (7.4) and 8 mL of pure acetonitrile without the addition of sorbent material (primary secondary amines, PSA) and NaCl [61].
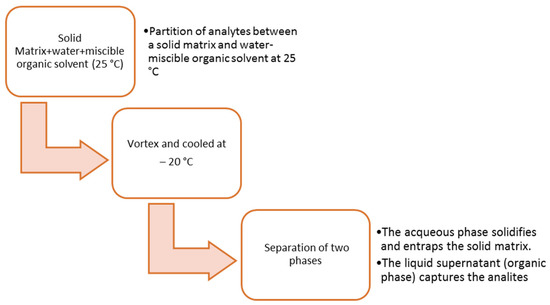
Figure 1.
Classical operations in solid–liquid extraction low temperature partitioning.
To reduce the pretreatment procedures and consumption of organic solvent, Menck R.A. et al. have developed an analytical method for quantifying barbiturates in postmortem liver samples using three-phase hallow fiber–liquid phase microextraction (HF-LPME) and GC-MS. This technique is very efficient for cleaning up the sample and eliminating the potential interference problems and allows researchers to overcome the difficulties of classic solid-phase extraction with homogenates tissue because some particles in the sample can block the pores of the cartridge. Briefly, after homogenization of the sample, 0.8 mL of hydrochloric acid (0.1 M) was added. 9 cm of hollow fiber was then used for the extraction, and the fiber lumen was filled with the acceptor phase (25 mL of a NaOH aqueous solution pH 13) and immersed in the sample solution with a U-configuration. The extraction was conducted for 5 min in an ultrasonic bath. Successively, the extraction phase was withdrawn and dried before the analysis [62].
3.3. Brain Tissue
The brain is the primary site of action for many drugs, particularly lipophilic substances that accumulate in central nervous tissue. For this reason, high concentrations of these types of drugs can be observed in brain specimens from acute fatalities. Nielsen M.K.K. et al. have validated a method for quantification of 30 common pharmaceuticals and metabolites in brain tissue from a forensic case. They use a fully automated robotic system to perform a solid-phase extraction. In this way, manual labor and human errors are minimized [63]. Cheers R. et al. have used brain tissue in their study about the distribution of synthetic opioids in postmortem specimens. The sample was first diluted in distilled water and homogenized, then pH 6 phosphate buffer was added, vortexed, and centrifugated. The supernatant was added on to a UTC Xcel I cartridge and driven through the column at 1–2 psi after washing and conditioning the column. The samples were eluted with ethyl acetate:ammonium hydroxide (98:2, v:v), and the eluate was dried under nitrogen [64].
Knuth M. et al. used a classic solid-phase extraction to extract analytes of interest from brain tissue. The aim of their work was to analyze cocaine adulterants in the human brain in cases of drug-related death [65]. Two extraction techniques used, one after the other, have been necessary for Pastor-Belda M. et al. to develop and validate a new method for the quantification of polycyclic aromatic hydrocarbons for forensic assessment. The brain was also used as a matrix for the evaluation of these. The first step was to add 3 mL of water and 3.5 mL of acetonitrile in 300 mg of tissue, and after manual agitation, the organic and aqueous phases were separated by addition of 0.5 g NaCl (Salting-out Liquid-Liquid Extraction, SALLE). The supernatant (2 mL of acetonitrile) was used for Dispersive Liquid-Liquid Microextraction (DLLME), and this was added 50 mL of carbon tetrachloride (CCl4) was. The mixture was injected into 9 mL water containing 5% NaCl (w:v). After centrifugation, the portion precipitated by CCl4 was recovered and injected in GC [66]. Unceta N. et al. developed an innovative and simple technique in their study about the quantification of serotonin uptake inhibitors in plasma, urine, and brain tissue. Stir bar sorptive extraction has been employed as a new sample pretreatment and consists of a stir bar coated by a thin layer of polydimethylsiloxane (PDMS) and used to stir samples and simultaneously extract analytes. After the extraction step, the bar was removed from the sample, dried, and then the analytes were removed by thermal or liquid desorption. Before starting the extraction, brain tissue was homogenized with 1 mL of water using an ultrasonic cell disruptor, was centrifugated, and supernatant was subjected to extraction [67].
3.4. Skeletal Tissue
In cases of severe decomposition, fragmentation, or skeletonization, the analysis of bones is the only source to determine past exposure to drugs and other substances. However, skeletal tissue is considered a complex matrix, so the extraction of drugs is much more complicated than conventional matrices. Orfanidis A. et al. have developed a method to determine the presence of most drugs of abuse in bone obtained from autopsies. For extraction of the analytes from the matrix was applied a simple method already validated by McGrath K.K. et al.; briefly, the bone was cleaned, and any remains of another kind of tissue were removed, then to 1 g of bone was added 3 mL of methanol, after which the mixture was adjusted to pH 10 with ammonium hydroxide. The mixture was stirred for 5 h and was put into an ultrasonic bath for 1 h. When the extraction was completed, the sample was centrifugated, and the supernatant was dried under a stream of nitrogen and reconstituted before analysis [68,69].
Vendenbosch M. et al. have also used methanol to extract opioids and their metabolites from skeletal tissue. First, they validated the method using animal specimens, evaluating the distribution of methadone and its metabolite in bones and other work evaluating the distribution of clomipramine, citalopram, and midazolam after chronic dosing in rats [70,71,72]. Particularly interesting is the study of Majda A. et al., in which a method for quantifying psychotropic drugs in bone marrow was developed. They used Direct Immersion Solid-Phase Microextraction (DI-SPME) with liquid chromatography coupled with a mass spectrometer (LC-TOF-MS). The work aimed to optimize all the parameters to obtain the maximum extraction with DI-SPME, such as sample volume, absorption time, post-absorption washing, and desorption time [73].
Snamina M. et al. have developed a method for the quantification of antidepressants in human bone marrow aspirated. This method has provided microwave-assisted extraction that includes several steps, illustrated in Figure 2, after which the analysis was conducted in UHPLC-TOF-MS [74].
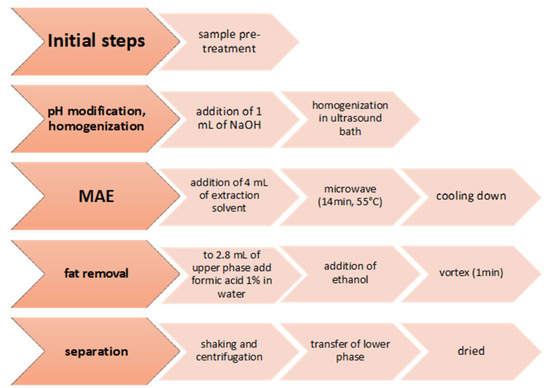
Figure 2.
The sample preparation procedure of human bone marrow aspirate.
Lopez F.L. et al. have used human bone as a matrix to detect carbamazepine in one case of overdose after psychiatric conditions. After cleaning and pulverizing bone, the powder was vortexed with 2 mL of methanol and then incubated for 1 h under ultrasounds. The mixture was centrifugated; the supernatant was dried and resuspended in phosphate-buffered saline pH 6 for solid-phase extraction [75].
Additionally, dry bone samples of people who have died over 23 years were used by Giordano G. et al. to detect some drugs. For this study, they used samples from the cranium, vertebrae, and ribs. After pulverization, all specimens were subjected to accelerated solvent extraction (ASE) using ASE 350 instrument by Thermo-Fischer Scientific. The authors used a mixture of n-hexane and acetone (4:1, v:v) and led extraction at 60 °C and 1500 psi, following the times foreseen by the protocol. The extraction results were dried and restored before the analysis [76]. Szpot P. et al. have examined the presence of Diclofenac and its distribution in different biological matrices obtained from patients who have died for various reasons. In a case reported by them, dry bones of a fetus were used to conduct the forensic analysis. After homogenization, a small amount of water was added, and the sample was inserted in an ultrasonic bath. The operators successfully performed a liquid-liquid extraction using ethyl acetate followed by a classical operation such as centrifugation, evaporation, and reconstitution prior to the analysis [77].
3.5. Hair
Hair analysis is widely used in pharmacotoxicology to monitor the consumption of substances and to extend control to previous periods. The use of this matrix was then widespread in postmortem toxicology. Over the years, many studies have been carried out to detect several typologies of drugs in hair compared with their presence in other conventional matrices, e.g., blood and urine.
To prepare hair samples for LC-MS/MS analysis to quantify some newly designed stimulant drugs, Niebel A. et al. used two extractions in series. Collected samples were washed and decontaminated with water and acetone and then cut. For the first extraction they used a solution of water:acetonitrile:methanol was (50:25:25, v:v:v), conducted at 40 °C for 18 h with agitation (900 rpm). The upper phase obtained was stored, and the lower phase led to the second extraction similarly. After this, both supernatants (by first and second extraction) were mixed and purified using a filter composed of regenerated cellulose [78]. Kintz P. et al., in their paper, proposed a method to extract and determine any presence of steroids in hair samples collected postmortem. In this case, the treatment of the sample involves several steps:
- cleaning of hairs with dichloromethane
- addition of methanol and sonication for 60 min.
- centrifugation and exsiccation of the first supernatant that was successively diluted with buffer (first aliquot)
- in the remained sample on the bottom was added 1mL of NaOH and shacked (second aliquot).
Both aliquots were subjected to liquid–liquid extraction with ethyl acetate, and the obtained extract was dried and restored in dichloromethane to be further subjected to a solid phase extraction using the ISOLUTE C18 column [79]. Numerous studies and works in literature treat the hair as a forensic sample to investigate a wide range of substances (e.g., antidepressants, benzodiazepines, barbituric derivates, cocaine, amphetamine, derivates of opioids, and many drugs of abuse). Among these, we can note the typical attention of all the authors regarding the fundamental operations in the treatment preceding this type of specimen analysis. Is commonly highlighted the importance of washing and decontamination of the sample, cutting the segments of interest based on the clinical case of the victim, homogenization, and choice of the best extraction techniques, frequently using liquid–liquid extraction, in some cases followed by an additional solid phase extraction [80,81,82,83,84]. Recently, the technique of micro-segmentation of hair has been increasingly used and consists of cutting a fragment of 4 mm which corresponds to the average daily hair growth, to identify the use of a particular substance on a specific day [85,86].
3.6. Other Matrices (Lung, Adipose Tissue, Nails, and Larvae)
However, in some cases, it is necessary to use other types of matrices, especially when “conventional matrices” are unavailable. In literature, a higher accumulation in an unconventional site is demonstrated or compared to the drug levels in the various body districts. They are reported as “unconventional matrices” because their use is not very common, but it is already witnessed in some studies.
In cases of suspected death caused by toxic solvents or very volatile substances such as pesticides, the lung may be one of the accumulation sites. It is considered a valid alternative to conventional matrices in forensics analysis. Rallis G.N. et al. proposed using matrix solid-phase dispersion (MSPD) to extract organochlorine pesticides and polychlorinated biphenyls from lung tissue. The optimization of this technique has allowed reducing the number of pre-analysis operations and the consumption of the solvents. The first step was the preparation of a dry homogeneous mixture of 0.5 g lung tissue and 2 g of Florisil, used as dispersion sorbent. The powder obtained was inserted into a syringe barrel column, corked with glass wool, and then the elution was carried out using n-hexane:dichloromethane (11:89, v:v). The collected eluate was dried and resuspended before analysis in gas chromatography with an electron capture detector (GC-ECD) [87].
Another tissue used in certain situations is kidney tissue, especially for identifying drug metabolites excreted in urine and often accumulated in this organ. Al-Asmari A.I. extended the use of a method already validated on biological fluid and solid matrices such as kidneys to determine concentrations of heroin biomarkers and their metabolites in heroin-related fatalities. Obviously, the extraction procedure was modified, with an initial dilution and homogenization into a Stomacher bag. Successively the extraction was carried out by solid phase using a Clean Screen cartridge previously conditioned. The sample loaded into the column was collected in two fractions: the first fraction was obtained after elution with hexane:ethyl acetate (1:1 v:v); the second was eluted using dichloromethane:isopropanol:ammonium hydroxide (78:20:2, v:v:v). The analysis was conducted in LC-MS/MS [88]. Kidney tissue was also used by Bottinelli C. et al. to determine the postmortem concentration of insulins. The authors have validated an extraction method based on five steps that can be reported as follow
- The tissue was cut and homogenized in an ultrasonic bath in water:ethanol (20:80, v:v, pH 3)
- the extraction step provided two successive centrifugations after adding extraction solvents (hexane, acetonitrile, phosphate buffer)
- another centrifugation using Amicon Ultra Filter (3 kDa) to concentrate the sample
- the last step was immunopurification using a cell of an IP plate Iso-Insulin Elisa; after deposition of the sample in this cell, a stirring was applied
- the elution was carried out using a 2% formic acid solution of water:acetonitrile (80:20, v:v).
The extract was analyzed successfully in LC-Hight Resolution MS (LC-HRMS) [89].
Adipose tissue consists mainly of lipids. For this reason, it could be a depot for lipophilic substances. However, apparent difficulties in sample handling and processing make it a matrix little used and even little discussed in the literature. One recent study by Baumer A. et al. used the passive equilibrium sampling with silicone (polydimethylsiloxane, PDMS), a fast technique applicable to a wide range of biological matrices, also including those with high lipid content such as adipose tissue. Using a combination of chemical analysis and in vivo bioassay, the authors determined the presence of chemical mixtures in postmortem tissue, and persistent organic pollutants (POP) or polychlorinated biphenyls (PCB) were highest in adipose tissue. This technique involves three main steps: PMDS foil is rinsed with ethyl acetate using Soxhlet extraction and dried before the passive sampling; one layer of PMDS is put between two layers of adipose tissue, so the extraction takes place statically; finally, the PMDS sheet was treated with ethyl acetate to extract the adsorbed analytes for 2 h. on a roller mix. Then the volume of the extraction solvent was reduced with a nitrogen stream before the analysis [90]. Hasegawa K. et al. quantified 5-fluoro-ADB, a synthetic cannabinoid, in different fluids and solid tissue from a human cadaver. The highest level of this substance has been found in adipose tissue, which required special preparation before the analysis compared to other samples used. The specimen was first chopped, immersed into a plastic tube containing 4 mL of acetonitrile, and after was heated at 80 °C. The mixture was homogenized by adding stainless steel beads and using a bead beater-type homogenizer. After 5 min, the content was transferred into another tube, and more acetonitrile was added to obtain a homogeneous mixture of liquid fat and solvent. An aliquot of this mix was centrifugated, and the supernatant was subjected to solid-phase extraction following the QuEChERS method and finally analyzed in LC-MS/MS [91].
In addition to matrices obtained from tissue or organs, other specimens that may provide detailed information on the consumption or abuse of drugs are recurrent. Examples of the most frequently used ones are dental calculus, nails, or larvae from decomposed corpses. Sørensen L.K. et al. validated a method to investigate the entrapment of several drugs in dental calculus, which is composed of different calcium phosphates that, in time, precipitated on the teeth. After sampling, sample washing was necessary to remove any contaminants, such as saliva and blood. This was carried out by repeated cycles of adding methanol, stirring, centrifugation, and recovery of the supernatant. After the wash was added, citric acid 0.5 M was homogenized and kept under agitation for 1 h. Methanol was added successively and the mixture was centrifugated to obtain a supernatant that was be treated with two different extractions depending on the type of analyte to be extracted. The first extraction was conducted with a cartridge coated with weak polymeric cation exchange (WCX) sorbent. The sample was diluted with water and added to ammonia to obtain pH 6–7, and then passed through the cartridge. The analytes of interest were eluted using methanol with formic acid, a small amount of DMSO was added in the elute before being dried. However, to determine morphine-3-glu, morphine-6-glu, pregabalin, gabapentin, and salicylic acid, it was necessary to perform a second extraction using a cartridge coated with polymeric strong cation exchange (SCX) sorbent. The procedure is like the previous one, but the sample was mixed with 0.1 mL of hydrochloride acid 12 M prior to extraction. The elution was performed with 5% ammonia in methanol [92].
Nail samples are considered an excellent alternative to hair samples, especially for the long-term determination of substances. Cobo-Golpe M. et al. used nail samples to evaluate the incorporation of antipsychotic drugs into keratinized matrices. Nails were washed with dichloromethane for decontamination and then were dried in an oven (80 °C). The sample was pulverized using two cycles of a ball mill. The powder obtained was agitated after the addition of acetonitrile:water (50:50, v:v) and then was centrifugated, and then the supernatant was dried. For the extraction, the sample was restored with 0.2 mL of methanol and 2 mL of formic acid 2% in water. It was subjected to solid phase extraction with Oasis MCX cartridges that were washed and dried after loading the sample. Elution was carried out with dichloromethane:ammonium hydroxide:2-propanol (75:0.5:24.5, v:v:v), and then the eluate was dried using nitrogen [93].
In cases of severe decomposition state of the corpses, the stability of many compounds of interest can be very low, as well as it being very difficult to treat and examine putrefied tissue. In this regard, the possibility of using larvae to quantify drugs in postmortem cases was evaluated. Aguiar F. J. et al. validated a method to extract and determine some prescription drugs (diazepam, carbamazepine, flunitrazepam, etc.), cocaine and its metabolites, and pesticides using larvae from decomposed cadavers. The authors first optimized the extraction process to minimize the matrix effect and increase analytes detection, using solid-liquid extraction with low-temperature partitioning (SLE-LTP). Larvae specimens were homogenized and water and organic solvent (acetonitrile, mixture of acetonitrile and ethyl acetate) was added. The obtained sample was sonicated, centrifugated, and frozen at −20 °C. Freezing causes a separation of the two phases (aqueous and organic), so the organic phase was used to analyze LC-MS/MS. The parameters evaluated during optimization are the composition of the organic mixture, the addition of salt, the time of sonication, or the possibility of manual agitation [94].
4. Conclusions
The information presented in the current review paper confirms that the common goal of the researchers is to develop new methods of sample extraction and cleaning that reduce the number of operations and, thus, the probability of error in the entire analytical process. As already stated, one of the main problems has always been the time needed to treat the samples and the high volume of solvents used, which are often toxic to operators. In this context, the validation of novel techniques using more selective sorbents, mentioned in the review, can make this process easier and less costly in time and money. Another critical perspective is the continuous study of techniques that allows analytes to be effectively extracted from complex matrices that still classify them as “unconventional”. The possibility of having a wider choice is fundamental in forensic analytics, especially when the corpse is in an advanced state of decomposition or exsanguination, and when conventional matrices are unavailable.
Author Contributions
All Authors contributed equally in terms of data curation, writing, and editing. M.L., A.G., F.S., U.d.G., A.K., H.I.U., C.D. and I.A.: conceptualization; M.L., A.G., F.S., U.d.G., A.K., H.I.U., C.D. and I.A.: Supervision and Project Administration; V.G., M.P., L.C., M.L., A.G., F.S., U.d.G., A.K., H.I.U., C.D. and I.A.: Writing—Original Draft Preparation and Writing—Review & Editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable, review paper.
Informed Consent Statement
Not applicable, review paper.
Data Availability Statement
Data and information are available on request to the authors.
Acknowledgments
Authors would like to thank the University “G. d’Annunzio” and the University of Catania for the support in the literature survey.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, Y.; Concheiro-Guisan, M. Microextraction sample preparation techniques in forensic analytical toxicology. Biomed. Chromatogr. 2019, 33, e4444. [Google Scholar] [CrossRef] [PubMed]
- Drummer, O.H. Forensic Toxicology. In Molecular, Clinical and Environmental Toxicology; Luch, A., Ed.; Birkhäuser: Basel, Switzerland, 2010; Volume 2: Clinical Toxicology, pp. 579–603. [Google Scholar]
- Skopp, G. Preanalytic aspects in postmortem toxicology. Forensic Sci. Int. 2004, 142, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Mogollon, N.G.S.; Quiroz-Moreno, C.D.; Prata, P.S.; De Almeida, J.R.; Cevallos, A.S.; Torres-Guiérrez, R.; Augusto, F. New advances in toxicological forensic analysis using mass spectrometry techniques. J. Anal. Methods Chem. 2018, 2018, 4142527. [Google Scholar] [CrossRef] [PubMed]
- Peters, F.T. Recent advances of liquid chromatography-(tandem) mass spectrometry in clinical and forensic toxicology. Clin. Biochem. 2011, 44, 54–65. [Google Scholar] [CrossRef]
- Samanidou, V.; Kovatsi, L.; Fragou, D.; Rentifis, K. Novel strategies for sample preparation in forensic toxicology. Bioanalysis 2011, 3, 2019–2046. [Google Scholar] [CrossRef]
- Frederick, D.L. Toxicology testing in alternative specimen matrices. Clin. Lab. Med. 2012, 32, 467–492. [Google Scholar] [CrossRef]
- Bévalot, F.; Cartiser, N.; Bottinelli, C.; Guitton, J.; Fanton, L. State of the art in bile analysis in forensic toxicology. Forensic Sci. Int. 2016, 259, 133–154. [Google Scholar] [CrossRef]
- Yarema, M.C.; Becker, C.E. Key concepts in postmortem drug redistribution. Clin. Toxicol. 2005, 43, 235–241. [Google Scholar] [CrossRef]
- Rodda, L.N.; Volk, J.A.; Moffat, E.; Williams, C.M.; Lynch, K.L.; Wu, A.H. Evaluation of intraosseous fluid as an alternative biological specimen in postmortem toxicology. J. Anal. Toxicol. 2018, 42, 163–169. [Google Scholar] [CrossRef]
- Sempio, C.; Morini, L.; Vignali, C.; Groppi, A. Simple and sensitive screening and quantitative determination of 88 psychoactive drugs and their metabolites in blood through LC–MS/MS: Application on postmortem samples. J. Chromatogr. B 2014, 970, 1–7. [Google Scholar] [CrossRef]
- Nahar, L.; Smith, A.; Patel, R.; Andrews, R.; Paterson, S. Validated method for the screening and quantification of baclofen, gabapentin and pregabalin in human post-mortem whole blood using protein precipitation and liquid chromatography–tandem mass spectrometry. J. Anal. Toxicol. 2017, 41, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Fagiola, M.; Hahn, T.; Avella, J. Screening of novel psychoactive substances in postmortem matrices by liquid chromatography–tandem mass spectrometry (LC–MS-MS). J. Anal. Toxicol. 2018, 42, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Mata, D.C.; Davis, J.F.; Figueroa, A.K.; Stanford, M.J. Ultra-performance liquid chromatography with tandem mass spectrometry for the quantitation of seventeen sedative hypnotics in six common toxicological matrices. J. Anal. Toxicol. 2016, 40, 58–63. [Google Scholar] [CrossRef]
- Tartaglia, A.; Locatelli, M.; Kabir, A.; Furton, K.G.; Macerola, D.; Sperandio, E.; Piccolantonio, S.; Ulusoy, H.I.; Maroni, F.; Bruni, P.; et al. Comparison between Exhaustive and Equilibrium Extraction Using Different SPE Sorbents and Sol-Gel Carbowax 20M Coated FPSE Media. Molecules 2019, 24, 382. [Google Scholar] [CrossRef] [PubMed]
- Usui, K.; Hayashizaki, Y.; Hashiyada, M.; Funayama, M. Rapid drug extraction from human whole blood using a modified QuEChERS extraction method. Leg. Med. 2012, 14, 286–296. [Google Scholar] [CrossRef]
- Alves, E.A.; Agonia, A.S.; Cravo, S.M.; Afonso, C.M.; Netto, A.D.P.; Bastos, M. GC-MS method for the analysis of thirteen opioids, cocaine and cocaethylene in whole blood based on a modified quechers extraction. Curr. Pharm. Anal. 2017, 1, 2017. [Google Scholar]
- Westland, J.L.; Dorman, F.L. QuEChERS extraction of benzodiazepines in biological matrices. J. Pharm. Anal. 2013, 3, 509–517. [Google Scholar] [CrossRef]
- Usui, K.; Hashiyada, M.; Hayashizaki, Y.; Igari, Y.; Hosoya, T.; Sakai, J. Application of modified QuEChERS method to liver samples for forensic toxicological analysis. Forensic Toxicol. 2013, 32, 139–147. [Google Scholar] [CrossRef]
- Jones, S.; McGowan, C.; Boyle, S.; Ke, Y.; Chan, C.H.M.; Hwang, H. An overview of sample preparation in forensic toxicology. Wiley Interdiscip. Rev. Forensic Sci. 2022, 4, 13. [Google Scholar] [CrossRef]
- Tartaglia, A.; D’Ambrosio, F.; Ramundo, P.; Ferrone, V.; Ricci, D.; Locatelli, M. Innovative approach to increase sensibility and selectivity in analytical chemistry: QuEChERS method. Rev. Sep. Sci. 2020, 2, 19–34. [Google Scholar] [CrossRef]
- Odoardi, S.; Anzillotti, L.; Strano-Rossi, S. Simplifying sample pretreatment: Application of dried blood spot (DBS) method to blood samples, including postmortem, for UHPLC–MS/MS analysis of drugs of abuse. Forensic Sci. Int. 2014, 243, 61–67. [Google Scholar] [CrossRef]
- Júnior, E.F.; Caldas, E.D. Simultaneous determination of drugs and pesticides in postmortem blood using dispersive solid-phase extraction and large volume injection-programmed temperature vaporization-gas chromatography–mass spectrometry. Forensic Sci. Int. 2018, 290, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Lau, T.; Concheiro, M.; Cooper, G. Determination of 30 synthetic cathinones in postmortem blood using LC–MS-MS. J. Anal. Toxicol. 2020, 44, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Pouliopoulos, A.; Tsakelidou, E.; Krokos, A.; Gika, H.G.; Theodoridis, G.; Raikos, N. Quantification of 15 psychotropic drugs in serum and postmortem blood samples after a modified mini-QuEChERS by UHPLC–MS-MS. J. Anal. Toxicol. 2018, 42, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Takitane, J.; Leyton, V.; Andreuccetti, G.; Gjerde, H.; Vindenes, V.; Berg, T. Determination of cocaine, metabolites and a crack cocaine biomarker in whole blood by liquid–liquid extraction and UHPLC–MS/MS. Forensic Sci. Int. 2018, 289, 165–174. [Google Scholar] [CrossRef]
- Vejar-Vivar, C.; Bustamante, L.; Lucena, R.; Ortega, C.; Valenzuela, M.; Mardones, C. Direct coupling of MEPS to ESI-QqTOF-MS for the simultaneous analysis of tricyclic antidepressants and benzodiazepines in postmortem blood. Microchem. J. 2021, 171, 6. [Google Scholar] [CrossRef]
- Merone, G.M.; Tartaglia, A.; Rossi, S.; Santavenere, F.; Bassotti, E.; D’Ovidio, C.; Rosato, E.; De Grazia, U.; Locatelli, M.; Del boccio, P.; et al. Fast LC–MS/MS screening method for the evaluation of drugs, illicit drugs, and other compounds in biological matrices. Talanta Open 2022, 5, 9. [Google Scholar] [CrossRef]
- Merone, G.M.; Tartaglia, A.; Rosato, E.; D’Ovidio, C.; Kabir, A.; Ulusoy, H.I.; Locatelli, M. Ionic liquids in analytical chemistry: Applications and recent trends. Curr. Anal. Chem. 2021, 17, 1340–1355. [Google Scholar] [CrossRef]
- De Boeck, M.; Dehaen, W.; Tytgat, J.; Cuypers, E. Ionic Liquid-Based Liquid–Liquid Microextraction for Benzodiazepine Analysis in Postmortem Blood Samples. J. Forensic Sci. 2018, 63, 1875–1879. [Google Scholar] [CrossRef]
- Savini, F.; Tartaglia, A.; Coccia, L.; Palestini, D.; D’Ovidio, C.; De Grazia, U.; Merone, G.M.; Bassotti, E.; Locatelli, M. Ethanol determination in post-mortem samples: Correlation between blood and vitreous humor concentration. Molecules 2020, 25, 2724. [Google Scholar] [CrossRef]
- Ogawa, T.; Kondo, F.; Iwai, M.; Matsuo, T.; Kubo, K.; Seno, H. Novel extraction method using an ISOLUTE PLD+ protein and phospholipid removal column for liquid chromatography-tandem mass spectrometry analysis of 20 psychoactive drugs in postmortem whole blood samples. Forensic Sci. Int. 2022, 331, 11. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractors. United States Patents 9557252, 31 January 2017. [Google Scholar]
- Manousi, N.; Kabir, A.; A Zachariadis, G. Green bioanalytical sample preparation: Fabric phase sorptive extraction. Bioanalysis 2021, 13, 693–710. [Google Scholar] [CrossRef]
- Kabir, A.; Samanidou, V. Fabric phase sorptive extraction: A paradigm shift approach in analytical and bioanalytical sample preparation. Molecules 2021, 26, 865. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G.; Tinari, N.; Grossi, L.; Innosa, D.; Macerola, D.; Locatelli, M. Fabric phase sorptive extraction-high performance liquid chromatography-photo diode array detection method for simultaneous monitoring of three inflammatory bowel disease treatment drugs in whole blood, plasma and urine. J. Chromatogr. B 2018, 1084, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, M.; Tinari, N.; Grassadonia, A.; Tartaglia, A.; Macerola, D.; Piccolantonio, S.; Kabir, A. FPSE-HPLC-DAD method for the quantification of anticancer drugs in human whole blood, plasma, and urine. J. Chromatogr. B 2018, 1095, 204–213. [Google Scholar] [CrossRef]
- Locatelli, M.; Furton, K.G.; Tartaglia, A.; Sperandio, E.; Ulusoy, H.I.; Kabir, A. An FPSE-HPLC-PDA method for rapid determination of solar UV filters in human whole blood, plasma and urine. J. Chromatogr. B 2019, 1118, 40–50. [Google Scholar] [CrossRef]
- Locatelli, M.; Covone, S.; Rosato, E.; Bonelli, M.; Savini, F.; Furton, K.G.; Gazioglu, I.; D’Ovidio, C.; Kabir, A.; Tartaglia, A. Analysis of seven selected antidepressant drugs in post–mortem samples using fabric phase sorptive extraction followed by high performance liquid chromatography-photodiode array detection. Forensic Chem. 2022, 31, 9. [Google Scholar] [CrossRef]
- Ferrari Júnior, E.; Caldas, E.D. Determination of new psychoactive substances and other drugs in postmortem blood and urine by UHPLC–MS/MS: Method validation and analysis of forensic samples. Forensic Toxicol. 2022, 40, 88–101. [Google Scholar] [CrossRef]
- Mouskeftara, T.; Virgiliou, C.; Iakovakis, A.; Raikos, N.; Gika, H.G. Liquid chromatography tandem mass spectrometry for the determination of nine insecticides and fungicides in human postmortem blood and urine. J. Chromatogr. B 2021, 1179, 13. [Google Scholar] [CrossRef]
- Song, A.; Wang, J.; Lu, G.; Jia, Z.; Yang, J.; Shi, E. Oxidized multiwalled carbon nanotubes coated fibers for headspace solid-phase microextraction of amphetamine-type stimulants in human urine. Forensic Sci. Int. 2018, 290, 49–55. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Miyaguchi, H.; Ohno, Y.; Kanawaku, Y. Qualitative analysis of 7-and 8-hydroxyzolpidem and discovery of novel zolpidem metabolites in postmortem urine using liquid chromatography–tandem mass spectrometry. Forensic Toxicol. 2022, 40, 263–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.; Fan, E.; Wang, F.; Li, B.; Rao, Y. A novel fast-dried urine spot-based method for the analysis of EtS and EtG in urine by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2021, 1171, 122642. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yoshida, K.; Kasai, K.; Yoshizumi, S.; Sato, H. Assessment of Triage DOA®, Status DS10, and DRIVEN-FLOW® M8-Z on-site drug screening kits for postmortem urine. Leg. Med. 2022, 54, 101993. [Google Scholar] [CrossRef] [PubMed]
- de Campos, E.G.; da Costa, B.R.B.; Dos Santos, F.S.; Monedeiro, F.; Alves, M.N.R.; Santos Junior, W.J.R.; De Martinis, B.S. Alternative matrices in forensic toxicology: A critical review. Forensic Toxicol. 2022, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Birk, L.; Ossanes, D.; Petry, A.U.S.; de Menezes, F.P.; Gonzaga, A.P.; Schlickmann, P.F.; de Oliveira, T.F. Bioanalytical method for simultaneous determination of benzodiazepines in vitreous humor using liquid chromatography-tandem mass spectrometry. J. Forensic Leg. Med. 2022, 91, 8. [Google Scholar] [CrossRef]
- Akhgari, M.; Mirahmadi Sani, N.; Mousavi, Z. Determination of Methadone and Tramadol in Vitreous Humor Speci-mens Using Dispersive Liquid Liquid Microextraction and Ultra High Performance Liquid Chromatography. Int. J. Med. Toxicol. Forensic Med. 2021, 11, 31530. [Google Scholar] [CrossRef]
- Legg, K.M.; Labay, L.M.; Aiken, S.S.; Logan, B.K. Validation of a fully automated immunoaffinity workflow for the detection and quantification of insulin analogs by LC–MS-MS in postmortem vitreous humor. J. Anal. Toxicol. 2019, 43, 505–511. [Google Scholar] [CrossRef]
- Ntoupa, P.S.A.; Armaos, K.P.; Athanaselis, S.A.; Spiliopoulou, C.A.; Papoutsis, I.I. Study of the distribution of antidepressant drugs in vitreous humor using a validated GC/MS method. Forensic Sci. Int. 2020, 317, 11. [Google Scholar] [CrossRef]
- Kovatsi, L.; Rentifis, K.; Giannakis, D.; Njau, S.; Samanidou, V. Disposable pipette extraction for gas chromatographic determination of codeine, morphine, and 6-monoacetylmorphine in vitreous humor. Rev. Sep. Sci. 2011, 34, 1716–1721. [Google Scholar] [CrossRef]
- Mercurio, I.; Ceraso, G.; Melai, P.; Gili, A.; Troiano, G.; Agostinelli, F.; Bacci, M. Significance of Morphine Concentration in Bile, Liver, and Blood: Analysis of 52 Cases of Heroin Overdoses. Am. J. Forensic Med. Pathol. 2019, 40, 329–335. [Google Scholar] [CrossRef]
- Reisinger, A.J.; Miller, A.C.; Shaw, L.A.; Champion, J.L.; Neiswonger, M.A. Oral cavity fluid as an investigative approach for qualitative and quantitative evaluations of drugs in postmortem subjects. J. Anal. Toxicol. 2019, 43, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.A.; Seulin, S.; Yonamine, M.; Leyton, V.; Munoz, D.R.; Gianvecchio, V.A.; Osselton, M.D. Analysis of skeletal muscle has potential value in the assessment of cocaine-related deaths. Forensic Sci. Int. 2013, 226, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Rees, K.A.; McLaughlin, P.A.; Osselton, M.D. Validation of a gas chromatography–ion trap-tandem mass spectrometry assay for the simultaneous quantification of cocaine, benzoylecgonine, cocaethylene, morphine, codeine, and 6-acetylmorphine in aqueous solution, blood, and skeletal muscle tissue. J. Anal. Toxicol. 2012, 36, 1–11. [Google Scholar] [CrossRef]
- Frost, J.; Løkken, T.N.; Brede, W.R.; Hegstad, S.; Nordrum, I.S.; Slørdal, L. A validated method for simultaneous determination of codeine, codeine-6-glucuronide, norcodeine, morphine, morphine-3-glucuronide and morphine-6-glucuronide in post-mortem blood, vitreous fluid, muscle, fat, and brain tissue by LC–MS. J. Anal. Toxicol. 2015, 39, 203–212. [Google Scholar] [CrossRef]
- Staeheli, S.N.; Baumgartner, M.R.; Gauthier, S.; Gascho, D.; Jarmer, J.; Kraemer, T.; Steuer, A.E. Time-dependent postmortem redistribution of butyrfentanyl and its metabolites in blood and alternative matrices in a case of butyrfentanyl intoxication. Forensic Sci. Int. 2016, 266, 170–177. [Google Scholar] [CrossRef]
- Staeheli, S.N.; Poetzsch, M.; Kraemer, T.; Steuer, A.E. Development and validation of a dynamic range-extended LC-MS/MS multi-analyte method for 11 different postmortem matrices for redistribution studies applying solvent calibration and additional 13C isotope monitoring. Anal. Bioanal. Chem. 2015, 407, 8681–8712. [Google Scholar] [CrossRef] [PubMed]
- Kahl, J.H.; Gonyea, J.; Humphrey, S.M.; Hime, G.W.; Boland, D.M. Quantitative analysis of fentanyl and six fentanyl analogs in postmortem specimens by UHPLC–MS-MS. J. Anal. Toxicol. 2018, 42, 570–580. [Google Scholar] [CrossRef]
- Takayasu, T.; Ishida, Y.; Kimura, A.; Kawaguchi, M.; Kondo, T. Distribution of zolpidem in body fluids and organ tissues in five autopsy cases. Forensic Toxicol. 2008, 26, 80–84. [Google Scholar] [CrossRef]
- Magalhães, E.J.; de Queiroz, M.E.L.R.; de Oliveira Penido, M.L.; Paiva, M.A.R.; Teodoro, J.A.R.; Augusti, R.; Nascentes, C.C. Determination of cocaine in postmortem human liver exposed to overdose. Application of an innovative and efficient extraction/clean up procedure and gas chromatography–mass spectrometry analysis. J. Chromatogr. A 2013, 1309, 15–21. [Google Scholar] [CrossRef]
- Menck, R.A.; de Oliveira, C.D.R.; de Lima, D.S.; Goes, L.E.; Leyton, V.; Pasqualucci, C.A.; Yonamine, M. Hollow fiber–liquid phase microextraction of barbiturates in liver samples. Forensic Toxicol. 2013, 31, 31–36. [Google Scholar] [CrossRef]
- Nielsen, M.K.K.; Nedahl, M.; Johansen, S.S.; Linnet, K. Validation of a fully automated solid-phase extraction and ultra-high-performance liquid chromatography–tandem mass spectrometry method for quantification of 30 pharmaceuticals and metabolites in post-mortem blood and brain samples. Drug Test. Anal. 2018, 10, 1147–1157. [Google Scholar] [CrossRef]
- Chesser, R.; Pardi, J.; Concheiro, M.; Cooper, G. Distribution of synthetic opioids in postmortem blood, vitreous humor and brain. Forensic Sci. Int. 2019, 305, 10. [Google Scholar] [CrossRef] [PubMed]
- Knuth, M.; Temme, O.; Daldrup, T.; Pawlik, E. Analysis of cocaine adulterants in human brain in cases of drug-related death. Forensic Sci. Int. 2018, 285, 86–92. [Google Scholar] [CrossRef]
- Pastor-Belda, M.; Campillo, N.; Arroyo-Manzanares, N.; Torres, C.; Pérez-Cárceles, M.D.; Hernández-Córdoba, M.; Viñas, P. Bioaccumulation of polycyclic aromatic hydrocarbons for forensic assessment using gas chromatography–mass spectrometry. Chem. Res. Toxicol. 2019, 32, 1680–1688. [Google Scholar] [CrossRef]
- Unceta, N.; Ugarte, A.; Sánchez, A.; Gómez-Caballero, A.; Goicolea, M.A.; Barrio, R.J. Development of a stir bar sorptive extraction based HPLC-FLD method for the quantification of serotonin reuptake inhibitors in plasma, urine and brain tissue samples. J. Pharm. Biomed. Anal. 2010, 51, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Orfanidis, A.; Gika, H.; Mastrogianni, O.; Krokos, A.; Theodoridis, G.; Zaggelidou, E.; Raikos, N. Determination of drugs of abuse and pharmaceuticals in skeletal tissue by UHPLC–MS/MS. Forensic Sci. Int. 2018, 290, 137–145. [Google Scholar] [CrossRef]
- McGrath, K.K.; Jenkins, A.J. Detection of drugs of forensic importance in postmortem bone. Am. J. Forensic Med. Pathol. 2009, 30, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Vandenbosch, M.; Pajk, S.; Van Den Bogaert, W.; Wuestenbergs, J.; Van de Voorde, W.; Cuypers, E. Postmortem Analysis of Opioids and Metabolites in Skeletal Tissue. J. Anal. Toxicol. 2022, 46, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Vandenbosch, M.; Somers, T.; Cuypers, E. Distribution of methadone and metabolites in skeletal tissue. J. Anal. Toxicol. 2018, 42, 400–408. [Google Scholar] [CrossRef]
- Vandenbosch, M.; Somers, T.; Cuypers, E. Distribution of clomipramine, citalopram, midazolam, and metabolites in skeletal tissue after chronic dosing in rats. Drug Test. Anal. 2019, 11, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Majda, A.; Mrochem, K.; Wietecha-Posłuszny, R.; Zapotoczny, S.; Zawadzki, M. Fast and efficient analyses of the post-mortem human blood and bone marrow using DI-SPME/LC-TOFMS method for forensic medicine purposes. Talanta 2020, 209, 120533. [Google Scholar] [CrossRef]
- Snamina, M.; Wietecha-Posłuszny, R.; Zawadzki, M. Postmortem analysis of human bone marrow aspirate-Quantitative determination of SSRI and SNRI drugs. Talanta 2019, 204, 607–612. [Google Scholar] [CrossRef]
- Fernández-López, L.; Mancini, R.; Rotolo, M.C.; Navarro-Zaragoza, J.; Hernández del Rincón, J.P.; Falcón, M. Carbamazepine Overdose after Psychiatric Conditions: A Case Study for Postmortem Analysis in Human Bone. Toxics 2022, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Biehler-Gomez, L.; Seneci, P.; Cattaneo, C.; Di Candia, D. Detecting drugs in dry bone: A pilot study of skeletal remains with a post-mortem interval over 23 years. Int. J. Leg. Med. 2021, 135, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Szpot, P.; Wachełko, O.; Zawadzki, M. Diclofenac Concentrations in Post-Mortem Specimens—Distribution, Case Reports, and Validated Method (UHPLC-QqQ-MS/MS) for Its Determination. Toxics 2022, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Niebel, A.; Westendorf, L.; Krumbiegel, F.; Hartwig, S.; Parr, M.K.; Tsokos, M. Prevalence and concentrations of new designer stimulants, synthetic opioids, benzodiazepines, and hallucinogens in postmortem hair samples: A 13-year retrospective study. Drug Test Anal. 2022, 14, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P.; Gheddar, L.; Blanchot, A.; Ameline, A.; Raul, J.S. In a Case of Death Involving Steroids, Hair Testing is More Informative than Blood or Urine Testing. J. Anal. Toxicol. 2021, 45, 829–834. [Google Scholar] [CrossRef]
- Wen, D.; Shi, Y.; Zhang, X.; Xie, B.; Liu, W.; Yu, F.; Ma, C. Determination of barbiturates in hair samples by using a validated UHPLC-HRMS method: Application in investigation of drug-facilitated sexual assault. Forensic Sci. Res. 2022, 7, 78–87. [Google Scholar] [CrossRef]
- Kintz, P.; Villain, M.; Cheze, M.; Pepin, G. Identification of alprazolam in hair in two cases of drug-facilitated incidents. Forensic Sci. Int. 2005, 153, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Carfora, A.; Campobasso, C.P.; Cassandro, P.; Petrella, R.; Borriello, R. Long-term detection in hair of zolpidem, oxazepam and flunitrazepam in a case of drug-facilitated sexual assault. J. Anal. Toxicol. 2022, 46, 16–20. [Google Scholar] [CrossRef]
- Rygaard, K.; Nielsen, M.K.K.; Linnet, K.; Banner, J.; Johansen, S.S. Concentrations of citalopram and escitalopram in postmortem hair segments. Forensic Sci. Int. 2022, 336, 111349. [Google Scholar] [CrossRef] [PubMed]
- Carelli, C.; Radogna, A.; Bolcato, V.; Vignali, C.; Moretti, M.; Merli, D.; Morini, L. Old and New Synthetic and Semi-synthetic Opioids Analysis in Hair: A Review. Talanta Open 2022, 5, 100108. [Google Scholar] [CrossRef]
- Kuwayama, K.; Miyaguchi, H.; Iwata, Y.T.; Kanamori, T.; Tsujikawa, K.; Yamamuro, T.; Inoue, H. Strong evidence of drug-facilitated crimes by hair analysis using LC–MS/MS after micro-segmentation. Forensic Toxicol. 2019, 37, 480–487. [Google Scholar] [CrossRef]
- Kuwayama, K.; Miyaguchi, H.; Kanamori, T.; Tsujikawa, K.; Yamamuro, T.; Segawa, H.; Iwata, Y.T. Possibility of drug-distribution measurement in the hair of drowned bodies: Evaluation of drug stability in water-soaked hair using micro-segmental analysis. Int. J. Leg. Med. 2022, 137, 89–98. [Google Scholar] [CrossRef]
- Rallis, G.N.; Sakkas, V.A.; Boumba, V.A.; Vougiouklakis, T.; Albanis, T.A. Determination of organochlorine pesticides and polychlorinated biphenyls in post-mortem human lung by matrix solid-phase dispersion with the aid of response surface methodology and desirability function. J. Chromatogr. A 2012, 1227, 1–9. [Google Scholar] [CrossRef]
- Al-Asmari, A.I. Postmortem Liver and Kidney Tissue Concentrations of Heroin Biomarkers and Their Metabolites in Heroin-Related Fatalities. J. Forensic Sci. 2020, 65, 2087–2093. [Google Scholar] [CrossRef]
- Bottinelli, C.; Bévalot, F.; Cartiser, N.; Fanton, L.; Guitton, J. Detection of insulins in postmortem tissues: An optimized workflow based on immunopurification and LC–MS/HRMS detection. Int. J. Leg. Med. 2021, 135, 1813–1822. [Google Scholar] [CrossRef]
- Baumer, A.; Jäsch, S.; Ulrich, N.; Bechmann, I.; Landmann, J.; Stöver, A.; Escher, B.I. Chemical mixtures in human post-mortem tissues assessed by a combination of chemical analysis and in vitro bioassays after extraction with silicone. Environ. Int. 2021, 157, 106867. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, K.; Wurita, A.; Minakata, K.; Gonmori, K.; Yamagishi, I.; Nozawa, H.; Suzuki, O. Identification and quantitation of 5-fluoro-ADB, one of the most dangerous synthetic cannabinoids, in the stomach contents and solid tissues of a human cadaver and in some herbal products. Forensic Toxicol. 2015, 33, 112–121. [Google Scholar] [CrossRef]
- Sørensen, L.K.; Hasselstrøm, J.B.; Larsen, L.S.; Bindslev, D.A. Entrapment of drugs in dental calculus–detection validation based on test results from post-mortem investigations. Forensic Sci. Int. 2021, 319, 110647. [Google Scholar] [CrossRef]
- Cobo-Golpe, M.; de-Castro-Ríos, A.; Cruz, A.; Páramo, M.; López-Rivadulla, M.; Lendoiro, E. Determination of antipsychotic drugs in nails and hair by liquid chromatography tandem mass spectrometry and evaluation of their incorporation into keratinized matrices. J. Pharm. Biomed. Anal. 2020, 189, 113443. [Google Scholar] [CrossRef] [PubMed]
- Aguiar França, J.; Brandao, M.; Sodré, F.F.; Caldas, E.D. Simultaneous determination of prescription drugs, cocaine, aldicarb and metabolites in larvae from decomposed corpses by LC–MS–MS after solid–liquid extraction with low temperature partitioning. Forensic Toxicol. 2015, 33, 93–103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
