Abstract
Microalgae, eukaryotic unicellular plants that are distributed worldwide, have been shown to exert anti-proliferative and anticancer activities on various human cancer cell lines. An example of a microalgal bioactive compound is a chlorophyll breakdown product named Pheophorbide a (Ppa), which has been reported to have anti-proliferative properties against various cell lines. This compound has also been tested with light exposure in photodynamic therapy for cancer treatment. In this paper, we screened eleven marine microalgae against a panel of cancer cells, and evaluated the synergistic anti-proliferative effect with Pheophorbide a, with and without photo-activation. The results showed significant anti-proliferative activity against melanoma cells when Ppa was combined with fraction E of the diatom Cylindrotheca closterium plus 1 h photo-activation. Its activity was also analyzed using gene expression and Western blot experiments. Altogether, these data give new insights into the possible application of microalgae for photodynamic therapy.
1. Introduction
Cancer ranks as one of the leading causes of death worldwide (https://www.who.int/health-topics/cancer#tab=tab_1; accessed on 19 January 2023). Photodynamic therapy (PDT) is an evolving strategy for the non-invasive treatment of superficial tumors and is used for both pre-malignant and various cancerous diseases [1]. It consists of the administration of a photosensitiser drug (or pro-drug) successively and locally exposed to visible light with minimal risk of toxicity [1,2]. A chlorophyll derivative product, named Pheophorbide a (Ppa), is a photosensitizer that can have anticancer properties [3]. Interestingly, Ppa showed anticancer properties with or without PDT and with or without conjugation with other chemotherapeutic drugs [3]. In particular, Ppa showed anti-proliferative activities on hepatocellular carcinoma (Hep3B; [4]), uterine sarcoma (MES-SA; [5]), breast adenocarcinoma (MDA-MB-231, MCF-7; [6,7]), oral squamous cell carcinoma (YD10B; [8]) and glioblastoma cells (U87MG; [9]). The mechanism of action is not always reported, and, when studied, it showed a cell-death generally induced by intrinsic or extrinsic apoptosis [3].
Microalgae are characterized by enormous biodiversity in terms of species adapted to living in very different environments, in both marine and freshwater ecosystems, including extreme conditions. Their culturing is faster compared to macroorganisms and they are known to produce a plethora of molecules with possible exploitation in different industrial fields [10,11]. Most common bioactive molecules from microalgae belong to pigments, polyphenols, polysaccharides, lipids, oxylipins, glycolipids, steroids, peptides and macrolides (as reviewed by [12]). These molecules have demonstrated different activities, such as anticancer, anti-diabetes, anti-atherosclerosis, antiviral, antibacterial and immunomodulatory activities [13,14,15,16,17,18,19,20,21], and have also found applications in the aquaculture (such as for fish oil replacement in fish diets, as live prey diets or powder products), biodiesel and biomaterial sectors [22,23,24,25,26]. Considering that some microalgae (biomass or extracts) have received the “GRAS” (generally recognized as safe) status [27], there are now various microalgae-derived powder-based products on the market and recent investigations have also proposed them as ingredients in fresh pasta, cookies and yogurt [28,29,30].
In this study, various microalgal species (i.e., two Chlamydomonas sp., Dunaliella tertiolecta-Chlorophyta, Asterionellopsis glacialis, Cylindrotheca closterium, Ditylum brightwellii, Trieres mobiliensis, Pseudo-nitzschia arenysensis, Pseudo-nitzschia fraudulenta-Bacillariophyta, Scrippsiella acuminata-Miozoa, Rhinomonas reticulata-Cryptista) were tested on human cancer cell lines (i.e., melanoma, hepatocarcinoma and myeloma) and a non-cancerous cell line. The microalgae selected for this study included both diatoms, flagellates and one dinoflagellate, in order to screen different microalgal classes and increase the probability of finding activity. In addition, considering that different strains of the same genus may exert different bioactivities [10], we also selected two Chlamydomonas (Chlorophyta) and two Pseudo-nitzschia species (Bacillariophyta). Some of these microalgae were already tested in other studies for other possible applications. For example, Lauritano et al. [31] showed that the diatom Cylindrotheca closterium had anti-inflammatory activity and identified lysophosphatidylcholines and chlorophyll-derived molecules as active components. Total extracts of the diatom Trieres mobiliensis (formerly Odontella mobiliensis) also showed anti-inflammatory activity on THP-1 (human acute monocytic leukemia cell line) with dose-dependent inhibition of tumor necrosis factor-α production [10]. Pasquet et al. [32] demonstrated a strong anti-proliferative activity of the flagellate Dunaliella tertiolecta (Chlorophyta) on the breast cancer cell line MCF-7 and the prostate cancer cell line LNCaP. The natural xanthophyll pigment violaxanthin was identified as the bioactive component of the active D. tertiolecta dichloromethane extracts [32]. Recently, Martinez and co-workers [16] tested another Dunaliella tertiolecta strain (CCMP 1320) on melanoma, hepatocellular liver carcinoma and two lung adenocarcinoma cell lines (A2058, HepG2, HCC827 and Calu-3, respectively) and isolated a glycerol 1-(9Z,12Z,15Z-octadecatrienoate)-2-(4Z,7Z,10Z,13Z-hexadecatetraenoate)-3-O-b-D-galactopyranoside as the active component with anti-proliferative activity against melanoma. The focus of this research was to investigate the effects of microalgal extracts on various cancer cell lines (including both circulating and solid tumor cell lines) combined with the pure compound Pheophorbide a, with and without light exposure, to evaluate their possible application for PDT in cancer treatment.
2. Materials and Methods
2.1. Microalgae Culture
A series of microalgae (Table 1), including both diatoms, flagellates and one dinoflagellate, were cultivated in triplicate in 10L polycarbonate bottles. Cylindrotheca closterium, Ditylum brightwellii, Trieres mobiliensis, Pseudo-nitzchia arenysensis, Pseudo-nitzchia fraudulenta and Scrippsiella acuminata belong to the Stazione Zoologica Anton Dohrn culture collection. They were previously collected in the Mediterranean Sea and identified using microscopy. The other microalgae were bought at the Roscoff culture collection (RCC), the Provasoli–Guillard National center for marine algae and microbiota (originally named culture collection of marine phytoplankton, from which the abbreviation code CCMP), and culture collection of algae and protozoa (CCAP). Diatoms were grown in Guillard’s f/2 medium [33], flagellates in f/2 without silicates and the dinoflagellate in Keller medium [34]. The triplicates were cultured at 19 °C with a 12:12 h light:dark cycle and at 100 μmol photons m−2 s−1 in a dedicated controlled chamber (as in [10]). An inoculum of 5000 cells·mL−1 was used and algae were centrifuged (for 30 min at 4 °C at 3900× g) at the end of the stationary phase (selected because this is the stage when secondary compounds may be produced in higher quantities; [35]). Culture growth was evaluated from samples fixed with one drop of Lugol (about 2% final concentration) and counted in a Sedgewick Rafter counting chamber under an Axioskop 2 microscope (20×) (Carl Zeiss GmbH, Jena, Germany). Samples were stored at −80 °C until successive chemical analysis.

Table 1.
The table reports the microalgal species cultured for this study, name abbreviation (Abb), their class, growth media, sampling location and cell concentration at the end of the stationary phase when they were pelleted and stored for the successive analyses.
2.2. Chemical Extraction
Extractions were performed according to Martinez et al. [16]. For the microalga, Cylindrotheca closterium, an additional step of fractionation was done according to Cutignano et al. [36].
2.3. Cells
DMEM medium was used for human melanoma cells (A2058; ATCC® CRL-11147TM) and human keratinocytes (HACAT; CLS n. 300493; https://www.ceinge.unina.it/en/cell-cultures, accessed on 17 January 2022; CEINGE, Napoli, Italy), EMEM for hepatocellular liver carcinoma cells (HepG2; ATCC® HB-8065TM). Media were supplemented with 10% fetal bovine serum, 50 U·mL−1 penicillin, and 50 μg·mL−1 streptomycin (Sigma–Aldrich, St. Louis, MO, USA). Human plasma cell myeloma (JJN3; DSMZ n. ACC541; https://www.ceinge.unina.it/en/cell-cultures, accessed on 17 January 2022; CEINGE, Napoli, Italy) were cultured in DMEM HG + ISCOVE (1:1), 20% fetal bovine serum and 2% glutamine.
2.4. Antibody
Antibodies (from Cell Signaling Technology; Danvers, MA, USA) used were: rabbit monoclonal anti-glutathione peroxidase 4 (GPX4; 52455s), rabbit monoclonal anti-tubulin (9F3, 2118s), rabbit monoclonal anti-nuclear receptor coactivator 4 (NCOA4; E8H8Z, 66849s), rabbit monoclonal anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 14C10, 2118s), the rabbit monoclonal anti-B-cell lymphoma (Bcl-2, D17C4, 3498S), rabbit monoclonal anti-Bcl-2-associated X protein (Bax, D2E11, 5023S), anti-rabbit IgG, HRP-linked antibody (7074s) and anti-mouse IgG, and HRP-linked antibody (7074s).
2.5. In Vitro Cell Viability
To study the effects of microalgal extracts and Pheophorbide a (CAS15664-29-6; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) on cell viability, A2058, HepG2 and HACAT cell lines were seeded in 96-well microtiter plates (1 × 104 cells·well−1) and incubated at 37 °C for 24 h. Then, fresh medium was used, including extracts and Ppa: (1) 50 μg·mL−1 microalgal extract + 10 μg·mL−1 Ppa, (2) 25 μg·mL−1 microalgal extract + 10 μg·mL−1 Ppa, (3) 25 μg·mL−1 microalgal extract + 5 μg·mL−1 Ppa, (4) 10 μg·mL−1 Ppa. Extracts were dissolved in dimethyl sulfoxide (DMSO; maximum concentration 1% v/v) and tested at least in triplicate. After 72 h of incubation, cell viability was evaluated using the MTT assay (3-(4,5-dimethyl-2-thizolyl)-2,5-diphenyl-2H-tetrazolium bromide; A2231,0001, Applichem Panreac Tischkalender, Darmstadt, GmbH) as in [37]. For JJN3 cells, grown mostly as single cells in suspension, after 72 h of incubation, cell viability was assessed using OranguTM Cell Counting Solution (OR01-1000) by directly adding it to the medium. After 1 h in the incubator, the absorbance was measured at 450 nm in the same above-mentioned microplate reader. Photo-activation experiments were performed by exposing cells treated with extracts, fractions and/or Ppa for 1 h under 1.000 Lux·m−2, immediately followed by the MTT or Orangu assay.
The viability of the melanoma cell line A2058 exposed to Ppa (i.e., 0.005, 0.01, 0.05, 0.1, 0.5, 1.0, 5.0, 10 and 50 µM) and photo-activation for 1 h (to evaluate the combination of Ppa and light for possible photodynamic therapy) was also evaluated with the MTT protocol. Cylindrotheca closterium (Cc) was the most active species combined with Ppa and further fractionation and fraction/Ppa testing was performed (for 24 h). When photo-activation was tested, 1 h light exposure was performed before the MTT assay (as above). Cell survival was expressed as a percentage of viable cells in treated samples, with respect to untreated control cultures with only DMSO.
2.6. RNA Extraction, Retrotranscription and PCR Array
RNA was extracted from A2058 cells seeded in 6-well plates (at 500,000 cells/well) for 16 h and then incubated with either Ppa 1.91 µM or Cc fraction E 10 µg·mL−1, or with a combination of Ppa 1.91 µM and Cc fraction E 10 µg·mL−1, for 14 h at 37° C followed by 1 h of photo-activation. RNA was extracted following Trisure Reagent (Meridian Bioscience, Memphis, TN, USA) manufacturer’s instructions. RNA quality, quantity and purity check, retrotranscription and PCR array (cat. PAHS-212ZE-4, Qiagen, Hilden, Germany) were performed as in Riccio et al. [37]. The results described the changes in gene expression between cells treated in the presence of Ppa 1.91 µM, Cc fraction E 10 µg·mL−1, or a combination of Ppa 1.91 µM plus Cc fraction E 10 µg·mL−1, each one plus photo-activation, and cells treated in the presence of DMSO alone. Only expression values greater than a two-fold difference with respect to the controls were considered significant.
2.7. Protein Extraction and Western Blotting
A2058 cells seeded for protein extraction were incubated with either Ppa 1.91 µM, Cc fraction E 10 µg·mL−1, or a combination of Ppa 1.91 µM and Cc fraction E 10 µg·mL−1 for 14 h, followed by 1 h photo-activation. Protein extraction and Western blot were performed as in Riccio et al. [37]. GAPDH was used as reference to normalize immunoreactive bands. Arithmetic means ± the standard deviations (SD) were calculated and compared using a two-tailed Student t test. Differences at p < 0.05 were considered significant.
3. Results
3.1. Anti-Proliferative Activity of Microalgal Extracts
Total extracts of Asterionellopsis glacialis, Chlamydomonas sp. (CCMP2536), Chlamydomonas sp. (CCMP225), Cylindrotheca closterium, Dunaliella tertiolecta, Ditylum brightwellii, Trieres mobiliensis, Rhinomonas reticulata, Pseudo-nitzschia arenysensis, Pseudo-nitzschia fraudolenta and Scrippsiella acuminata in combination with Pheophorbide a were screened on various cancer cells. The results showed that the most active combination was Cylindrotheca closterium and Pheophorbide a against A2058 melanoma cell lines (Supplementary Table S1). A2058 cells were incubated in the presence of (1) 50 μg·mL−1 microalgae extract + 10 μg·mL−1 Ppa, (2) 25 μg·mL−1 microalgae extract + 10 μg·mL−1 Ppa, (3) 25 μg·mL−1 microalgae extract + 5 μg·mL−1 Ppa, (4) 10 μg·mL−1 Ppa. As shown in Supplementary Table S1, the first combination showed 32% cell viability while the second and the third showed 46% cell viability after incubation.
3.2. Pheophorbide a Activity against Melanoma Cells
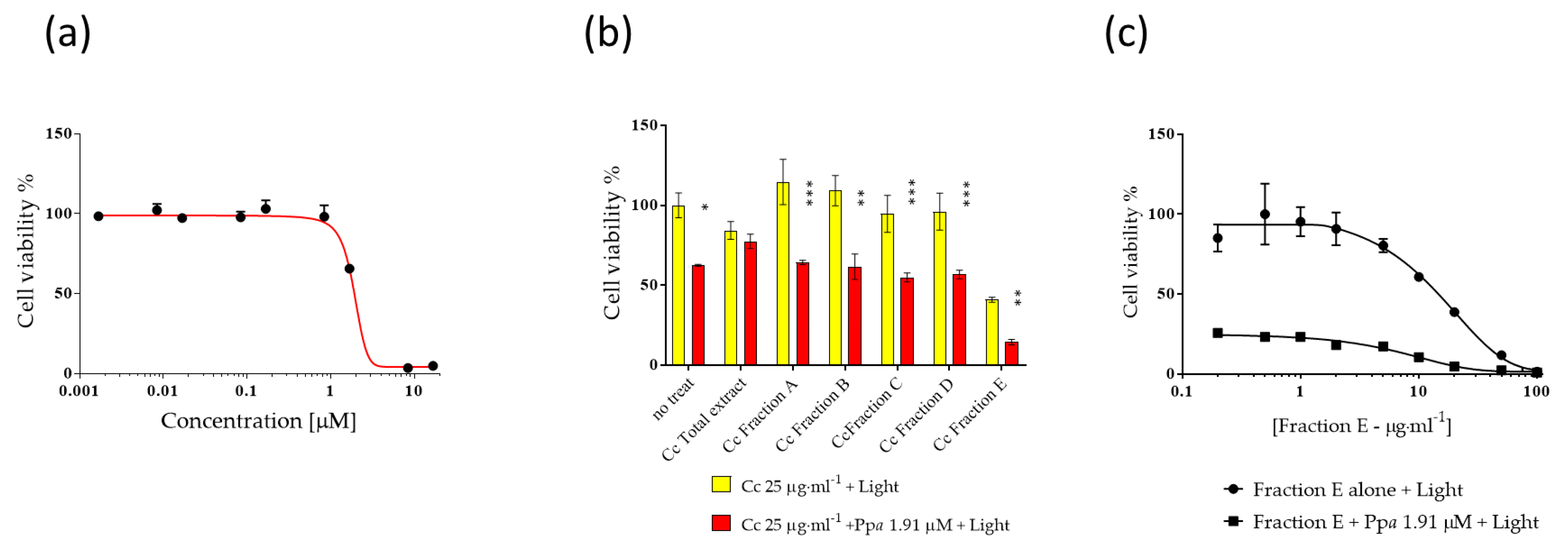
Ppa was evaluated at concentrations from 0.001 to 20 µM in order to establish IC50 values, (Figure 1a) for 24 h followed by 1 h photo-activation. IC50 value of Ppa on A2058 cells was 1.91 μM. Then Ppa (at IC50 concentration) was tested in combination with fractions (from A to E) of Cc at 25 µg·mL−1 (plus 1 h light exposure) in order to evaluate possible combinatorial antiproliferative effects (Figure 1b). Fraction E was the most active in reducing cell viability below 20% (i.e., 14%). At this point, A2058 cells were treated for 24 h in the presence of fraction E alone followed by 1 h photo-activation and fraction E in combination with Ppa followed by 1 h photo-activation at different concentrations (Figure 1c) to evaluate the IC50 of fraction E. IC50 of fraction E in the absence or in the presence of Ppa was very similar (10.17 ± 0.20 and 9.9 ± 0.17 µg·mL−1, respectively). On the contrary, lower activity was observed against normal human keratinocytes (HaCat, Figure S1).
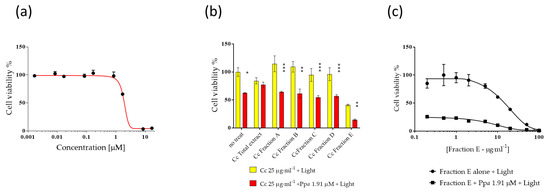
Figure 1.
Activity of Pheophorbide a (Ppa) and Cylindrotheca closterium (Cc) against melanoma cells (A2058). The figure shows (a) the dose-dependent activity of Ppa, (b) Cc fractions (from A to E) activity alone or in the presence of 1.91 μM of Ppa (IC50) plus light, (c) the dose-dependent activity of Fraction E from Cc alone or with 1.91 μM Ppa (IC50 value) plus light. No treat means without Cc. Control cells were incubated with complete cell medium and DMSO. Results are expressed as percent survival after 24 h exposure followed by 1 h photo-activation (* p < 0.05; ** p < 0.01; *** p < 0.001).
3.3. Mechanism of Action at Gene and Protein Level
PCR array for A2058 cells treated in the presence of Ppa 1.91 μM or fraction E 10 μg·mL−1 or a combination of Ppa 1.91 μM and fraction E, each followed by 1 h photo-activation, showed a series of up- or down-regulated genes (Table 2). Some of these genes were common to the three treatments, while others were peculiar to a specific treatment (Table 2). For instance, the three treatments induced the up-regulation of tumor necrosis factor, estrogen receptor 1 and defensin beta 1, while they decreased the expression of amyloid beta (A4) precursor protein, “glucosidase, alpha; acid” and transmembrane protein 57. The combination treatment of fraction E with Ppa and photo-activation was the only treatment able to also induce the decreased expression of tumor necrosis factor receptor superfamily, member 1A, cathepsin S and cathepsin B (Table 2).

Table 2.
Expression levels of cell death-related genes.
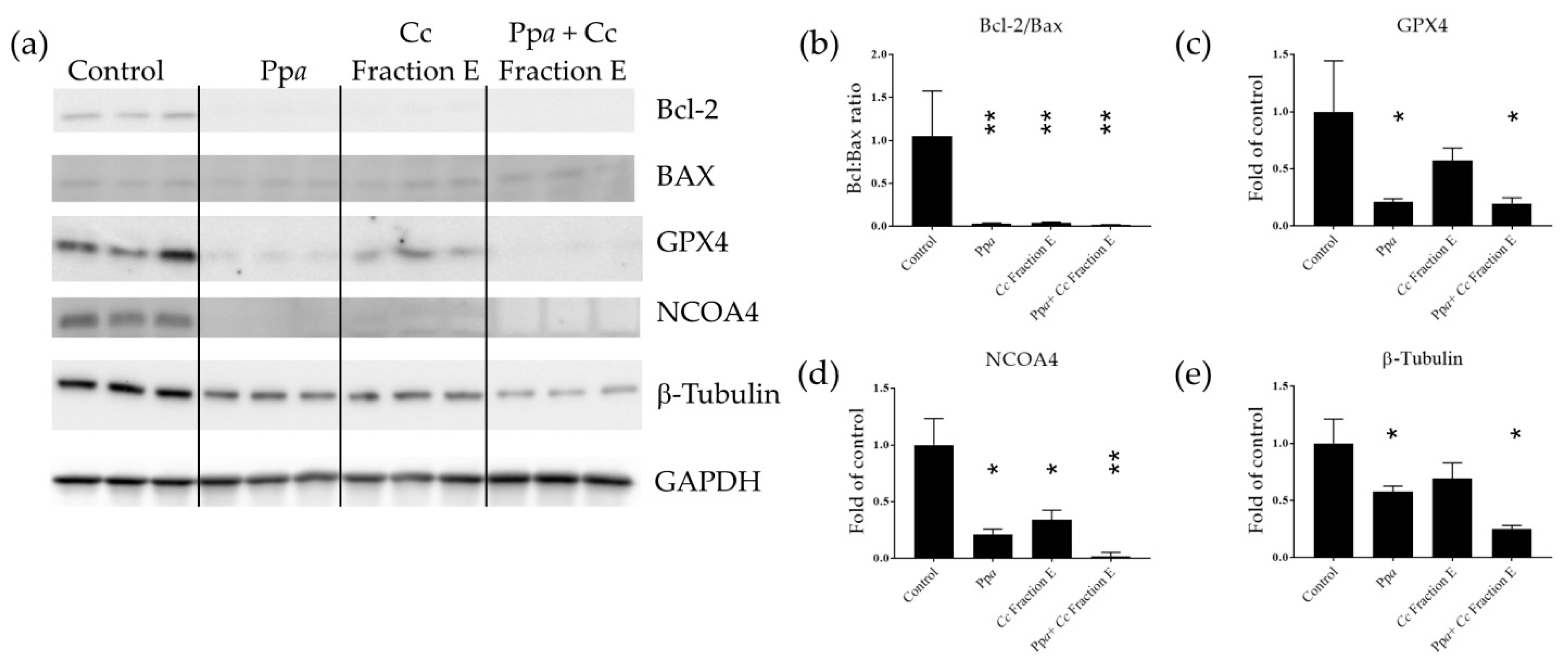
B-cell lymphoma-2 (Bcl-2) and Bcl-2-associated X protein (Bax) expression were also evaluated to better understand the mechanism of action induced. These two proteins are involved in the early stage of apoptotic processes [38,39]. Considering that Ppa treatment was reported to be associated with lipid peroxidation [40], the level of glutathione peroxidase 4 (GPX4) and nuclear receptor coactivator 4 (NCOA4) was also analyzed at the protein level. GPX4 is known to protect membrane lipids against peroxidation; indeed, GPX4 reduction has been associated with lipid peroxide accumulation [41]. NCOA4 is responsible for ferritin transport to lysosomes and degradation [42], impairing iron trafficking [43]. Our results showed that Ppa treatment induced a significant reduction in Bcl-2, GPX4, and NCOA4 protein levels (Figure 2a–d and Figure S2a), and it did not affect Bax protein levels (Figure 2a and Figure S2b). Cc Fraction E treatment induced a reduction in Bcl-2 and NCOA4 (Figure 2a,b,d and Figure S2a) and increased the level of Bax protein (Figure 2a and Figure S2b). Combination treatment by testing Ppa, Cc fraction E plus photo-activation reduced Bcl-2, GPX4, and NCOA4 protein levels (Figure 2a–d and Figure S2a), and increased Bax protein level (Figure 2a,b and Figure S2b). The reduction in the Bcl-2/Bax ratio triggered the mitochondrial-dependent apoptotic pathway [38,44]. Loss of NCOA4 led to ferritin accumulation [42] and tumor growth suppression in melanoma cell models [45]. β-tubulin, initially selected as a possible reference gene for Western blotting, was also found to be differentially expressed (Figure 2e).
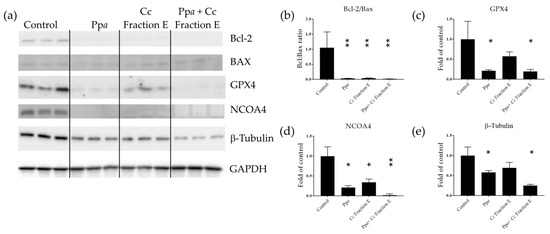
Figure 2.
Western blotting analyses. (a) Immunodetection of Bcl-2, Bax, GPX4, NCOA4 and beta-tubulin in treated cells with Ppa, Cc fraction E or both, followed by photo-activation; (b) Bcl-2/Bax ratio; (c) band intensity for GPX4, (d) NCOA4, (e) β-Tubulin normalized with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (* p < 0.05; ** p < 0.01).
4. Discussion
Looking for new therapeutics and combinations of drugs leading to higher cancer selectivity and fewer side effects is still a big challenge. Ppa is a chlorophyll degradation product which has been shown to exert anticancer properties with or without photo-activation; the so-called photodynamic therapy [3]. Our data show that the most active combination against melanoma cells was induced by fraction E from the diatom Cylindrotheca closterium, plus Ppa followed by 1 h photo-activation. In a previous study, we showed the composition of C. closterium fractions A–D [31] and suggested that the anti-inflammatory activity observed for C. closterium fractions C and D was related to the presence of lysophosphatidylcholines and the chlorophyll-derived molecules Ppa and hydroxypheophorbide a [31]. Compared to fractions A–D, fractions E also contained a larger amount of Pheophytin a, which also is a breakdown product of chlorophyll but it has a long aliphatic tail making it more non-polar (unpublished data). This composition difference in the fractions may explain why fraction E is more active compared to the others. Pheophytin a isolated from the seagrass Syringodium isoetifolium also showed anticancer properties against adenocarcinomic A549 cells [46]. Similarly, Pheophytin a from the plant Leucaena leucocephala showed anticancer, anti-migration and anti-invasion activity in human gastric cancer cell line (AGS) [47].
When we studied the mechanism of action in the current study, Ppa triggered the mitochondrial-related apoptotic pathway in melanoma cells as also previously reported in hepatocellular carcinoma [4], uterine carcinosarcoma [5] and breast tumor [7]. Our results suggested the activation of the intrinsic apoptotic pathway (Bcl-2/Bax disregulation) triggered by Ppa and Cc fraction E treatment followed by 1 h photo-activation. Other transcripts have been found to be differentially expressed as well. Up-regulated genes by Ppa, fraction E or the combination of both plus photo-activation included TNF, estrogen receptor and defensin beta 1. The role of TNF in melanoma is still controversial [48]. TNF has been shown to have a key role in immunity and immunomodulation and is associated with both the inhibition and promotion of tumor proliferation [48,49]. The role of the estrogen receptor in melanoma progression and genesis is still under investigation [50]. Hoi et al. [7] also showed that Ppa does not require the estrogen receptor for transport into cells. Defensin beta 1 has been found to be constitutively expressed in various epithelia [51,52]. Moreover, defensin beta 1 was reduced in squamous cell carcinoma and its down-regulation was directly related to malignancy transformation [53]. Defensin beta 1 was also down-regulated in prostate carcinomas [54] and in renal cell carcinomas (RCC) [55]. The down-regulated transcripts by the three treatments also included the amyloid beta precursor, a glucosidase alpha acid and a transmembrane protein 57. The amyloid beta precursor protein has been always linked to Alzheimer disease, but recently, the increased expression of this protein has been found in multiple types of cancers [56]. Hence, its down-regulation is in line with the reduction in cancer cell proliferation. A transcript coding for “glucosidase, alpha; acid”, also known as acid alpha-glucosidase (GAA), is a lysosomal enzyme, and its suppression has shown to be associated with improved chemosensitivity and apoptosis in pancreatic cancer cells [57]. Hamura et al. [57] found a reduction in tumor growth and prolonged tumor-related survival after GAA silencing in a murine model. In addition, apoptosis activation was also observed. Transmembrane proteins have been found to be differentially expressed in different tumors, but their function is still mostly unknown [58]. Finally, treatment with Ppa plus fraction E plus photo-activation also induced the down-regulation of tumor necrosis factor receptor superfamily (member 1A), cathepsin B and S. Tumor necrosis receptor 1 levels were found to be increased in patients with hepatocellular carcinoma [49], but to our knowledge its dysregulation has never been reported to be associated with melanoma. Cathepsin B inhibition has been found to reduce cell invasiveness of melanoma cells [59]. Cathepsin S has been associated with different cancer types. It is a mediator of tumor progression and has been associated with angiogenesis and metastasis formation, hence, representing a promising target for cancer therapy [60].
The microtubule subunit β-tubulin, initially selected as a possible reference gene for Western blotting, was also found to be differentially expressed (i.e., reduced expression induced by the treatment) in our study. In fact, levels of β-tubulin were affected by Ppa treatment. The intrinsic apoptotic pathway has been found to also be activated by chemotherapeutic agents targeting tubulin proteins, such as paclitaxel (Taxol®) and docetaxel [61]. However, in addition to the intrinsic apoptotic pathway mediators, we also found that GPX4 and NCOA4 protein levels were reduced after Ppa treatment and photo-activation suggesting that the treatment may induce lipid peroxidation.
This study shows that the known compound Ppa, a specific microalgal fraction plus photo-activation, can have an additive anti-proliferative role on melanoma cells. As reported by Prüser et al. [62], the US Food and Drug Administration (FDA) has granted the GRAS status to various microalgae, including Auxenochlorella protothecoides (formerly Chlorella protothecoides), Spirulina, Dunaliella salina, Haematococcus pluvialis, Prototheca zopfii, Ulkenia sp., Crypthecodinium cohnii and Schizochytrium sp. For some of these, the GRAS status refers to the entire biomass, while for others only a part of the extract or a fraction. Spirulina and Chlorella are most commonly used in Europe and, recently, whole alga (Odontella aurita and Tetraselmis chui) or only specific components (Ulkenia sp. oil, Schizochytrium sp. oil, Haematococcus pluvialis oleoresin) have been approved under the Commission Implementing Regulation (EU) 2017/2470 [62]. This “safe” status has been expanded to a greater number of species in recent years and this trend will positively influence the addition of microalgal extracts, fractions and components in nutraceutical and pharmaceutical products. These may have properties that are useful for various human pathologies, such as antioxidant, anti-inflammatory and anticancer properties [12,31]. Future studies on the combinatorial effects of microalgal extracts with known drugs, such as those used for chemotherapy, with or without photo-activation, can shed light on new activities against cancer cells and on possible photodynamic therapies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13042590/s1, Figure S1: Antiproliferative activity of the diatom Cylindrotheca closterium, Pheophorbide a, and their combination on normal human cells HACAT. Results are expressed as percent survival after 24 h exposure followed by 1 h photo-activation (* p < 0.05; ** p < 0.01; *** p < 0.001); Figure S2: The intensity of the bands of immunodetected Bcl-2 (a) and Bax (b) were normalized with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (* p < 0.05; ** p < 0.01); Table S1: Antiproliferative activity of various microalgae on cancer human cells.
Author Contributions
Conceptualization, A.S., A.I. and C.L.; formal analysis, A.S., G.R. and C.L.; writing—original draft preparation, A.S., G.R. and C.L.; writing—review and editing, A.S., G.R., A.I. and C.L.; supervision, A.I. and C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the project “Antitumor Drugs and Vaccines from the Sea (ADViSE)” (CUP B43D18000240007–SURF 17061BP000000011; PG/2018/0494374) funded by POR Campania FESR 2014–2020 “Technology Platform for Therapeutic Strategies against Cancer”-Action 1.1.2 and 1.2.2. Assunta Saide and Gennaro Riccio were supported by research grants within this project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors thank the Servier Medical Art (SMART) website (https://smart.servier.com/; accessed on 1 February 2022) by Servier for elements in the graphical abstract and PubChem (https://pubchem.ncbi.nlm.nih.gov/compound/Pheophorbide-a#section=3D-Conformer; accessed on 18 April 2022) for the 3D structure of Pheophorbide a. Authors thank Massimo Perna and Mariano Amoroso for their technical support. Authors also thanks Espen H. Hansen from The Arctic University of Norway (UiT), Norway, for dereplication support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dobson, J.; de Queiroz, G.F.; Golding, J.P. Photodynamic Therapy and Diagnosis: Principles and Comparative Aspects. Vet. J. 2018, 233, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, M.; Muchowicz, A.; Firczuk, M.; Gabrysiak, M.; Winiarska, M.; Wańczyk, M.; Bojarczuk, K.; Golab, J. Aminolevulinic Acid (ALA) as a Prodrug in Photodynamic Therapy of Cancer. Molecules 2011, 16, 4140–4164. [Google Scholar] [CrossRef]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide a: State of the Art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.-K.; Chan, J.Y.-W.; Au, S.W.-N.; Kong, S.-K.; Tsui, S.K.-W.; Waye, M.M.-Y.; Mak, T.C.-W.; Fong, W.-P.; Fung, K.-P. Pheophorbide a, an Active Compound Isolated from Scutellaria Barbata, Possesses Photodynamic Activities by Inducing Apoptosis in Human Hepatocellular Carcinoma. Cancer Biol. Ther. 2006, 5, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.-K.; Liu, X.-Z.; Zhang, D.-M.; Fong, W.-P.; Fung, K.-P. Pheophorbide a Based Photodynamic Therapy Induces Apoptosis via Mitochondrial-Mediated Pathway in Human Uterine Carcinosarcoma. Cancer Biol. Ther. 2009, 8, 533–539. [Google Scholar] [CrossRef]
- Bui-Xuan, N.-H.; Tang, P.M.-K.; Wong, C.-K.; Fung, K.-P. Photo-Activated Pheophorbide-a, an Active Component of Scutellaria Barbata, Enhances Apoptosis via the Suppression of ERK-Mediated Autophagy in the Estrogen Receptor-Negative Human Breast Adenocarcinoma Cells MDA-MB-231. J. Ethnopharmacol. 2010, 131, 95–103. [Google Scholar] [CrossRef]
- Hoi, S.W.-H.; Wong, H.M.; Chan, J.Y.-W.; Yue, G.G.L.; Tse, G.M.-K.; Law, B.K.-B.; Fong, W.P.; Fung, K.P. Photodynamic Therapy of Pheophorbide a Inhibits the Proliferation of Human Breast Tumour via Both Caspase-Dependent and -Independent Apoptotic Pathways in In Vitro and In Vivo Models. Phytother. Res. 2012, 26, 734–742. [Google Scholar] [CrossRef]
- Ahn, M.Y.; Yoon, H.-E.; Kwon, S.-M.; Lee, J.; Min, S.-K.; Kim, Y.-C.; Ahn, S.-G.; Yoon, J.-H. Synthesized Pheophorbide A-Mediated Photodynamic Therapy Induced Apoptosis and Autophagy in Human Oral Squamous Carcinoma Cells. J. Oral Pathol. Med. 2013, 42, 17–25. [Google Scholar] [CrossRef]
- Cho, M.; Park, G.-M.; Kim, S.-N.; Amna, T.; Lee, S.; Shin, W.-S. Glioblastoma-Specific Anticancer Activity of Pheophorbide a from the Edible Red Seaweed Grateloupia Elliptica. J. Microbiol. Biotechnol. 2014, 24, 346–353. [Google Scholar] [CrossRef]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Romano, G.; Costantini, M.; Sansone, C.; Lauritano, C.; Ruocco, N.; Ianora, A. Marine Microorganisms as a Promising and Sustainable Source of Bioactive Molecules. Mar. Environ. Res. 2017, 128, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Saide, A.; Martínez, K.A.; Ianora, A.; Lauritano, C. Unlocking the Health Potential of Microalgae as Sustainable Sources of Bioactive Compounds. Int. J. Mol. Sci. 2021, 22, 4383. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Ianora, A. Marine Organisms with Anti-Diabetes Properties. Mar. Drugs 2016, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Martínez Andrade, K.; Lauritano, C.; Romano, G.; Ianora, A. Marine Microalgae with Anti-Cancer Properties. Mar. Drugs 2018, 16, 165. [Google Scholar] [CrossRef]
- Martínez, K.A.; Lauritano, C.; Druka, D.; Romano, G.; Grohmann, T.; Jaspars, M.; Martín, J.; Díaz, C.; Cautain, B.; de la Cruz, M.; et al. Amphidinol 22, a New Cytotoxic and Antifungal Amphidinol from the Dinoflagellate Amphidinium Carterae. Mar. Drugs 2019, 17, 385. [Google Scholar] [CrossRef]
- Martínez, K.A.; Saide, A.; Crespo, G.; Martín, J.; Romano, G.; Reyes, F.; Lauritano, C.; Ianora, A. Promising Antiproliferative Compound From the Green Microalga Dunaliella Tertiolecta Against Human Cancer Cells. Front. Mar. Sci. 2022, 9, 778108. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2020, 18, 2. [Google Scholar] [CrossRef]
- Giordano, D.; Costantini, M.; Coppola, D.; Lauritano, C.; Núñez Pons, L.; Ruocco, N.; di Prisco, G.; Ianora, A.; Verde, C. Biotechnological Applications of Bioactive Peptides From Marine Sources. In Advances in Microbial Physiology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 73, pp. 171–220. ISBN 978-0-12-815190-7. [Google Scholar]
- Brillatz, T.; Lauritano, C.; Jacmin, M.; Khamma, S.; Marcourt, L.; Righi, D.; Romano, G.; Esposito, F.; Ianora, A.; Queiroz, E.F.; et al. Zebrafish-Based Identification of the Antiseizure Nucleoside Inosine from the Marine Diatom Skeletonema Marinoi. PLoS ONE 2018, 13, e0196195. [Google Scholar] [CrossRef]
- Lauritano, C.; Martín, J.; de la Cruz, M.; Reyes, F.; Romano, G.; Ianora, A. First Identification of Marine Diatoms with Anti-Tuberculosis Activity. Sci. Rep. 2018, 8, 2284. [Google Scholar] [CrossRef]
- Riccio, G.; Ruocco, N.; Mutalipassi, M.; Costantini, M.; Zupo, V.; Coppola, D.; de Pascale, D.; Lauritano, C. Ten-Year Research Update Review: Antiviral Activities from Marine Organisms. Biomolecules 2020, 10, 1007. [Google Scholar] [CrossRef]
- Muller-Feuga, A. The Role of Microalgae in Aquaculture: Situation and Trends. J. Appl. Phycol. 2000, 12, 527–534. [Google Scholar] [CrossRef]
- Hossain, N.; Mahlia, T.M.I.; Saidur, R. Latest Development in Microalgae-Biofuel Production with Nano-Additives. Biotechnol. Biofuels 2019, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Orejuela-Escobar, L.; Gualle, A.; Ochoa-Herrera, V.; Philippidis, G.P. Prospects of Microalgae for Biomaterial Production and Environmental Applications at Biorefineries. Sustainability 2021, 13, 3063. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable Products from Biotechnology of Microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Eryalçın, K.M. Nutritional Value and Production Performance of the Rotifer Brachionus Plicatilis Müller, 1786 Cultured with Different Feeds at Commercial Scale. Aquac. Int. 2019, 27, 875–890. [Google Scholar] [CrossRef]
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a Future Food Source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Gouveia, L.; Coutinho, C.; Mendonça, E.; Batista, A.P.; Sousa, I.; Bandarra, N.M.; Raymundo, A. Functional Biscuits with PUFA-Ω3 from Isochrysis Galbana. J. Sci. Food Agric. 2008, 88, 891–896. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, C.M.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Isochrysis Galbana and Diacronema Vlkianum Biomass Incorporation in Pasta Products as PUFA’s Source. LWT—Food Sci. Technol. 2013, 50, 312–319. [Google Scholar] [CrossRef]
- Matos, J.; Afonso, C.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M. Yogurt Enriched with Isochrysis Galbana: An Innovative Functional Food. Foods 2021, 10, 1458. [Google Scholar] [CrossRef]
- Lauritano, C.; Helland, K.; Riccio, G.; Andersen, J.H.; Ianora, A.; Hansen, E. Lysophosphatidylcholines and Chlorophyll-Derived Molecules from the Diatom Cylindrotheca Closterium with Anti-Inflammatory Activity. Mar. Drugs 2020, 18, 166. [Google Scholar] [CrossRef]
- Pasquet, V.; Morisset, P.; Ihammouine, S.; Chepied, A.; Aumailley, L.; Berard, J.-B.; Serive, B.; Kaas, R.; Lanneluc, I.; Thiery, V.; et al. Antiproliferative Activity of Violaxanthin Isolated from Bioguided Fractionation of Dunaliella Tertiolecta Extracts. Mar. Drugs 2011, 9, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Guillard, R.R.L. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings—1st Conference on Culture of Marine Invertebrate Animals Greenport; Smith, W.L., Chanley, M.H., Eds.; Springer: Boston, MA, USA, 1975; pp. 29–60. ISBN 978-1-4615-8714-9. [Google Scholar]
- Keller, M.D.; Selvin, R.C.; Claus, W.; Guillard, R.R.L. Media for the Culture of Oceanic Ultraphytoplankton1,2. J. Phycol. 1987, 23, 633–638. [Google Scholar] [CrossRef]
- Ribalet, F.; Wichard, T.; Pohnert, G.; Ianora, A.; Miralto, A.; Casotti, R. Age and Nutrient Limitation Enhance Polyunsaturated Aldehyde Production in Marine Diatoms. Phytochemistry 2007, 9, 2059–2067. [Google Scholar] [CrossRef] [PubMed]
- Cutignano, A.; Nuzzo, G.; Ianora, A.; Luongo, E.; Romano, G.; Gallo, C.; Sansone, C.; Aprea, S.; Mancini, F.; D’Oro, U.; et al. Development and Application of a Novel SPE-Method for Bioassay-Guided Fractionation of Marine Extracts. Mar. Drugs 2015, 13, 5736–5749. [Google Scholar] [CrossRef]
- Riccio, G.; Nuzzo, G.; Zazo, G.; Coppola, D.; Senese, G.; Romano, L.; Costantini, M.; Ruocco, N.; Bertolino, M.; Fontana, A.; et al. Bioactivity Screening of Antarctic Sponges Reveals Anticancer Activity and Potential Cell Death via Ferroptosis by Mycalols. Mar. Drugs 2021, 19, 459. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Korsmeyer, S.J.; Shutter, J.R.; Veis, D.J.; Merry, D.E.; Oltvai, Z.N. Bcl-2/Bax: A Rheostat That Regulates an Anti-Oxidant Pathway and Cell Death. Semin. Cancer Biol. 1993, 4, 327–332. [Google Scholar]
- Rapozzi, V.; Miculan, M.; Xodo, L. Evidence That Photoactivated Pheophorbide a Causes in Human Cancer Cells a Photodynamic Effect Involving Lipid Peroxidation. Cancer Biol. Ther. 2009, 8, 1318–1327. [Google Scholar] [CrossRef]
- Li, J.; Cao, F.; Yin, H.; Huang, Z.; Lin, Z.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Santana-Codina, N.; Mancias, J.D. The Role of NCOA4-Mediated Ferritinophagy in Health and Disease. Pharmaceuticals 2018, 11, 114. [Google Scholar] [CrossRef]
- Ryu, M.-S.; Zhang, D.; Protchenko, O.; Shakoury-Elizeh, M.; Philpott, C.C. PCBP1 and NCOA4 Regulate Erythroid Iron Storage and Heme Biosynthesis. J. Clin. Investig. 2017, 127, 1786–1797. [Google Scholar] [CrossRef] [PubMed]
- Raisova, M.; Hossini, A.M.; Eberle, J.; Riebeling, C.; Wieder, T.; Sturm, I.; Daniel, P.T.; Orfanos, C.E.; Geilen, C.C. The Bax/Bcl-2 Ratio Determines the Susceptibility of Human Melanoma Cells to CD95/Fas-Mediated Apoptosis. J. Investig. Dermatol. 2001, 117, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Shekoohi, S.; Rajasekaran, S.; Patel, D.; Yang, S.; Liu, W.; Huang, S.; Yu, X.; Witt, S.N. Knocking out Alpha-Synuclein in Melanoma Cells Dysregulates Cellular Iron Metabolism and Suppresses Tumor Growth. Sci. Rep. 2021, 11, 5267. [Google Scholar] [CrossRef] [PubMed]
- Shailaja, V.L.; Christina, V.S.; Mohanapriya, C.D.; Sneha, P.; Lakshmi Sundaram, R.; Magesh, R.; George Priya Doss, C.; Gnanambal, K.M.E. A Natural Anticancer Pigment, Pheophytin a, from a Seagrass Acts as a High Affinity Human Mitochondrial Translocator Protein (TSPO) Ligand, in Silico, to Reduce Mitochondrial Membrane Potential (∆ψmit) in Adenocarcinomic A549 Cells. Phytomedicine 2019, 61, 152858. [Google Scholar] [CrossRef]
- Chen, C.-Y. The Anti-Cancer and Anti-Metastasis Effects of Phytochemical Constituents from Leucaena Leucocephala. Biomed. Res. 2017, 28, 2893–2897. [Google Scholar]
- Donia, M.; Kjeldsen, J.W.; Svane, I.M. The Controversial Role of TNF in Melanoma. OncoImmunology 2016, 5, e1107699. [Google Scholar] [CrossRef]
- Puimège, L.; Libert, C.; Van Hauwermeiren, F. Regulation and Dysregulation of Tumor Necrosis Factor Receptor-1. Cytokine Growth Factor Rev. 2014, 25, 285–300. [Google Scholar] [CrossRef]
- Dika, E.; Patrizi, A.; Lambertini, M.; Manuelpillai, N.; Fiorentino, M.; Altimari, A.; Ferracin, M.; Lauriola, M.; Fabbri, E.; Campione, E.; et al. Estrogen Receptors and Melanoma: A Review. Cells 2019, 8, 1463. [Google Scholar] [CrossRef]
- Weinberg, A.; Jin, G.; Sieg, S.; McCormick, T. The Yin and Yang of Human Beta-Defensins in Health and Disease. Front. Immunol. 2012, 3, 294. [Google Scholar] [CrossRef]
- O’Neil, D.A.; Porter, E.M.; Elewaut, D.; Anderson, G.M.; Eckmann, L.; Ganz, T.; Kagnoff, M.F. Expression and Regulation of the Human β-Defensins HBD-1 and HBD-2 in Intestinal Epithelium. J. Immunol. 1999, 163, 6718–6724. [Google Scholar] [CrossRef]
- Scola, N.; Gambichler, T.; Saklaoui, H.; Bechara, F.G.; Georgas, D.; Stücker, M.; Gläser, R.; Kreuter, A. The Expression of Antimicrobial Peptides Is Significantly Altered in Cutaneous Squamous Cell Carcinoma and Precursor Lesions. Br. J. Dermatol. 2012, 167, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Donald, C.D.; Sun, C.Q.; Lim, S.D.; Macoska, J.; Cohen, C.; Amin, M.B.; Young, A.N.; Ganz, T.A.; Marshall, F.F.; Petros, J.A. Cancer-Specific Loss of β-Defensin 1 in Renal and Prostatic Carcinomas. Lab. Investig. 2003, 83, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Young, A.N.; Amin, M.B.; Moreno, C.S.; Lim, S.D.; Cohen, C.; Petros, J.A.; Marshall, F.F.; Neish, A.S. Expression Profiling of Renal Epithelial Neoplasms: A Method for Tumor Classification and Discovery of Diagnostic Molecular Markers. Am. J. Pathol. 2001, 158, 1639–1651. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Yoo, B.K.; Kim, H.-S.; Gilmore, H.L.; Lee, Y.; Lee, H.; Kim, S.-J.; Letterio, J.; Lee, H. Amyloid-β Precursor Protein Promotes Cell Proliferation and Motility of Advanced Breast Cancer. BMC Cancer 2014, 14, 928. [Google Scholar] [CrossRef] [PubMed]
- Hamura, R.; Shirai, Y.; Shimada, Y.; Saito, N.; Taniai, T.; Horiuchi, T.; Takada, N.; Kanegae, Y.; Ikegami, T.; Ohashi, T.; et al. Suppression of Lysosomal Acid Alpha-Glucosidase Impacts the Modulation of Transcription Factor EB Translocation in Pancreatic Cancer. Cancer Sci. 2021, 112, 2335–2348. [Google Scholar] [CrossRef] [PubMed]
- Schmit, K.; Michiels, C. TMEM Proteins in Cancer: A Review. Front. Pharmacol. 2018, 9, 1345. [Google Scholar] [CrossRef]
- Matarrese, P.; Ascione, B.; Ciarlo, L.; Vona, R.; Leonetti, C.; Scarsella, M.; Mileo, A.M.; Catricalà, C.; Paggi, M.G.; Malorni, W. Cathepsin B Inhibition Interferes with Metastatic Potential of Human Melanoma: An in Vitro and in Vivo Study. Mol. Cancer 2010, 9, 207. [Google Scholar] [CrossRef]
- McDowell, S.H.; Gallaher, S.A.; Burden, R.E.; Scott, C.J. Leading the Invasion: The Role of Cathepsin S in the Tumour Microenvironment. Biochim. Biophys. Acta BBA-Mol. Cell Res. 2020, 1867, 118781. [Google Scholar] [CrossRef]
- McGrogan, B.T.; Gilmartin, B.; Carney, D.N.; McCann, A. Taxanes, Microtubules and Chemoresistant Breast Cancer. Biochim. Biophys. Acta BBA-Rev. Cancer 2008, 1785, 96–132. [Google Scholar] [CrossRef]
- Prüser, T.F.; Braun, P.G.; Wiacek, C. Microalgae as a Novel Food. Ernahrungs Umsch. 2021, 68, 78–85. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).