Abstract
In recent decades, the worldwide production of microalgae has been carried out on an industrial scale. In recent years, the market for natural products has grown because of changes in consumer preferences for more natural products. The objective of this study was to demonstrate the hepatoprotective capacity of fucoxanthin extract obtained from an industrial culture of the microalgae Phaeodactylum tricornutum (Culture Collection of Alga and Protists in Scotland). The microalga was grown in an artificial and natural seawater mixture (1:9), using Walne’s culture medium in columns and raceway photobioreactors (RWP) inside a greenhouse. The carotenoid content in the tested systems continued to increase from day 5 of the culture, when the stationary phase was reached. The final biomass powder contained 4.9 mg (2.59%) of pure fucoxanthin. The possible hepatoprotective activity of fucoxanthin was then studied in the HepG2 cell line for 24 h in culture, and compared with the cytotoxicity of methotrexate (MTX). In conclusion, the active ingredient showed hepatoprotective activity against MTX in the human hepatocyte cell line HEPG-2 at a concentration of 0.25 mg/mL. The current results also suggest that it has beneficial properties for liver health and is a suitable ingredient for all types of nutraceutical products.
1. Introduction
The natural supplement industry has grown rapidly in recent years. According to Transparency Market Research, the value of the global market for nutraceutical products reached a remarkable figure of USD 493.06 billion in 2022 and is expected to reach 991.09 by 2030, growing at a compound annual rate of 6.3%.
As a result of COVID-19, natural supplement consumption has increased, and physical and mental health have become more important [1]. Moreover, the Public Health Ministry of Spain has reported that one of the most prevalent health problems is related to the liver system [2]. This report clearly indicates that liver cirrhosis and chronic liver diseases were responsible for 1% of deaths in 2017 in Spain and Europe. According to the American College of Gastroenterology, liver cirrhosis can be defined as scarring of the liver resulting in abnormal liver function due to chronic (long-term) liver injury [3].
A mortality rate of 9 per 100,000 inhabitants has been reported for this condition. It should be noted that this rate is higher in males, with 1.5% of deaths being caused by this disease (a mortality rate of 14 per 100,000 inhabitants), while the rate was almost three times lower in women at 0.5%, (a mortality rate of 5 per 100,000 inhabitants) [2].
Algae and microalgae have always been among the most frequently used natural resources in the production of new nutraceutical compounds [4]. For this purpose, food supplement compounds have been developed, which are present in a non-food matrix (pills, capsules, powder, etc.) of a concentrated natural bioactive substance, usually present in food. This, taken at a dose higher than that existing in these foods, has a more favorable effect on health than normal food. In addition, the market has always positively accepted all products related to marine resources.
Food supplements are legally defined in Europe as food products whose purpose is to complement the normal diet and that consist of concentrated sources of nutrients or other substances that have a nutritional or physiological effect. They can be in either simple or combined form and are marketed in dosage form, that is to say, in capsules, pills, tablets and other similar forms, powder sachets, liquid ampoules, dropper bottles, and other similar forms of liquids and powders to be taken in small unit quantities [5].
The main objective of this study was to investigate the hepatoprotective activity of a spirulina extract in combination with an oil rich in fucoxanthin. Fucoxanthin (FX) is a marine carotenoid found in multiple micro- and macroalgae, such as Cylindrotheca closterium, Phaeodactylum tricornutum, Undaria pinnatifida and Laminaria japonica [6]. Both microalgae are cultivated continuously throughout the year in bioreactors. The final product is a mix of Arthospira platensis (spirulina) cultivated in raceways (outside of the EU, in China), whereas Phaeodactylum tricornutum is cultivated in column-type photobioreactors and raceways inside a greenhouse in the Neoalgae facilities (Gijón, Spain).
The cyanobacterium spirulina has been cultivated in different parts of the world as far away as Lake Texcoco in Mexico, and Lake Chad in Africa, where it grows naturally and has formed the basis of feeding diverse cultures for centuries [7]. According to [8], spirulina is an excellent matrix for food supplements. This is not only due to its nutritional characteristics and high amino acid concentrations, but also because it allows the creation of mixtures and new food formulas. Using these characteristics, the microencapsulation of spirulina and fucoxanthin extracts will be studied in this paper.
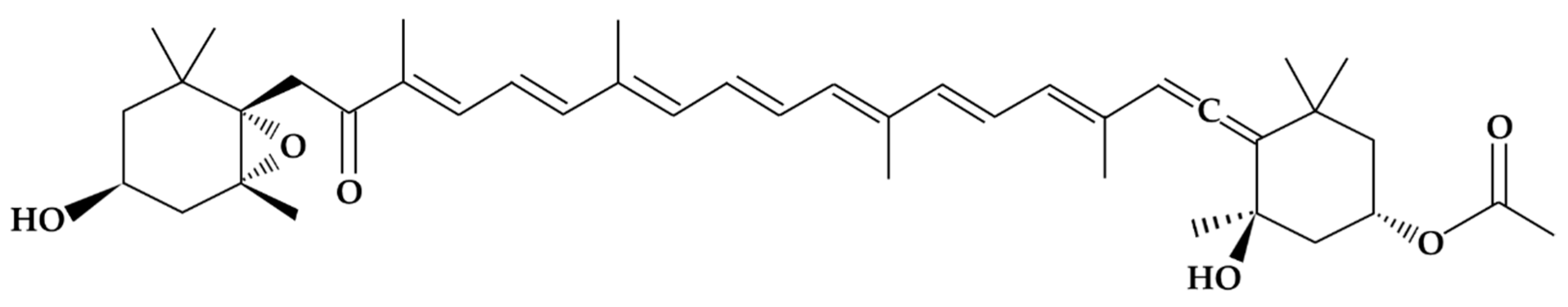
Fucoxanthin is a marine carotenoid that belongs to the xanthophyll family (carotenoids possessing at least one oxygenated functional group). This carotenoid is mainly present in brown edible seaweeds, such as wakame (Undaria pinnatifida, Kombu Laminaria digitata and Hijiki Sargassum phusiphorme) and diatoms (such as P. tricornutum), and is widely consumed in Asian countries. The FX content in microalgae is much higher than that in brown seaweeds; therefore, microalgae have been regarded as potential commercial producers, as indicated by [9]. FX, whose molecular structure was fully described in 1990 [10], has an allenic bond, a conjugated carbonyl, a 5,6-monoepoxide, and an acetyl group that contribute to the unique structure of the molecule (Figure 1).
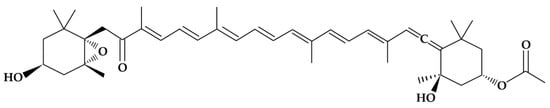
Figure 1.
Chemical structure of the fucoxanthin molecule [11].
It is also considered one of the most abundant carotenoids in nature, accounting for more than 10% of the total carotenoids produced in nature [12]. There are also other plant sources of fucoxanthin, such as Undaria pinnatifida (wakame) and other macroalgae. Consumption of these species has been very common for many years, particularly in Japan and other East Asian countries [13]. Other sources of fucoxanthin, such as P. tricornutum, do not have a history of use as human food, but rather as food for fish and mollusks intended for human consumption [14]. P. tricornutum is also a source of polyunsaturated fatty acids such as omega-6 [15]. Fucoxanthin has been evaluated in vitro for its biological activity [16].
In a biorefinery environment, P. tricornutum can be stimulated to elicit lipid biosynthesis through nutrient depletion and increased CO2 supply, whereas eicosapentaenoic acid (EPA) synthesis can be induced by supplying CO2 and urea as well as strong light [17]. Similarly, in a study in which P. tricornutum was supplemented in the diet of rats, P. tricornutum was estimated to be equivalent to a daily EPA intake of 33 mg/rat [18]. This study found the EPA content of P. tricornutum to be high (2.04% of dry weight). Fucoxanthin has been found in different dietary supplements marketed in the United States. A search for fucoxanthin in the Dietary Supplement Label Database (DSLD) returned 121 dietary supplements. The daily serving sizes of fucoxanthin in these products vary, containing 15–30 mg fucoxanthin per day. Fucoxanthin and its metabolites have shown anti-obesity effects, primarily by promoting energy expenditure in the adipose tissue and modulating blood glucose and insulin levels [19]. Fucoxanthin is an ingredient that is relevant to the supplement market in the United States, and it targets a population of people 4 years of age and above (Table 1).

Table 1.
Sample list of dietary supplements marketed in the U.S. that contain fucoxanthin.
In this study, we evaluated the combination of spirulina and fucoxanthin. spirulina and plant sources of fucoxanthin are valued for their health benefits, as well as their nutritional value. These health benefits have been reported as one of the reasons for the long life expectancy of the Japanese population, which is the highest in the world [20]. This has been attributed to their chemical composition, which produces antitumor, antiallergic, and antithrombotic activities [21,22,23].
There are different types of encapsulation in which different compounds are used, such as maltodextrin and guar gum. One of the reasons for using encapsulation was to provide protection to the active ingredients (especially fucoxanthin) against environmental conditions (oxygen, light, temperature, and water), thus avoiding their oxidation and increasing their useful life. The encapsulation of extracts containing different microalgae products and marine active principles has been mentioned by [24], who used an emulsion phase separation technique involving an ionotropic gelation method to prepare chitosan microcapsules to stabilize fish oil, such as krill oil, and [25], who used a solvent evaporation method to encapsulate astaxanthin in a chitosan matrix cross-linked with glutaraldehyde. In our case, we used spirulina as a microencapsulation agent using a European patent [26], and obtained a green powder containing between 1.65% and 2.25% fucoxanthin (including analytics that are now ready to be incorporated into any food or nutraceutical matrix). The objective of this study was to generate a complete development process to produce fucoxanthin as a nutritional supplement. This new procedure goes from cultivation to the final production of the supplement. Subsequently, the nutritional supplement was evaluated to demonstrate its capacity for liver protection.
2. Materials and Methods
2.1. P. tricornutum Cultivation and Lipid Production
2.1.1. Organism and Culture Conditions
The diatom Phaeodactylum Tricornutum was obtained from the Scottish Association of Science (SAMS) Culture Collection of Algae and Protists (CCAP, 56.45155615297254, −5.440805919920754) as CCAP1052/1A. Microalgae were grown in an artificial and natural seawater mixture (1:9). Natural seawater was filtered and sterilized using UV, and artificial seawater was prepared using tap water and NaCl (35 g/L NaCl). The mixture was then sterilized in an autoclave (121 °C, 20 min) when the final culture volume was less than 2 L, followed by bleaching (40 g/L NaClO) and sodium thiosulfate sterilization protocol for larger volumes (v:v). We used Walne’s medium [27] with several modifications and a vitamin solution in this experiment (Table 2).

Table 2.
Composition of the culture medium designed by [28] for the massive cultivation of marine phytoplankton commonly used as food for several species of shellfish.
2.1.2. Scale-Up Procedure
Microalgae were stored in 200 mL flasks stored at 15 °C under a light intensity of 6500 lx with a photoperiod of 16:8 h−1 (light/dark), allowing them to stay in a dormant state. In the first step, 100 mL of the dormant microalgae was diluted in a 400 mL graduated beaker of previously sterilized culture medium. An aeration source was added, and the temperature was raised to 25 °C. The next step was performed under the same culture conditions using a final volume of 2 L. CO2 was not used during the experiments. All volumes were established in triplicate. The following steps of the scale-up process were developed inside a glasshouse, where the control of the culture parameters was limited. Aeration was performed in the same way. Methacrylate bottles (Nalgene) were used for 10 L and 20 L of culture. For higher volumes in the column photobioreactor (2 m height, 0.2 m internal diameter), two sequential culture steps were performed (50 and 100 L). The last step took place in open raceways of two different dimensions (6 m length, 1.1 m width, 1.1 or 1.4 m height), with a final volume between 1200 and 1500 L.
2.1.3. Harvesting
In this experiment, 100 L of culture was harvested using a disk stack centrifuge from the GEA Westfalia Separator GMBH model OSE 5-91-037. This equipment separates the liquids from the solids owing to the high speed (8000–9000 rpm) of the drum. The fresh microalgae paste is concentrated in the discs with a solid concentration between 15 and 17% depending on the concentration of the culture.
- Growth parameters
Optical density (OD) values were obtained using 2 mL of culture volume with a spectrophotometer (Biochrom Libra S11 Visible Spectrophotometer) at three different wavelengths (680, 668, and 750 nm). DW was measured using a 10 mL sample of culture filtered through previously dried glass fiber filters (47 mm PRAT DUMAS, France) and washed with 10 mL of distilled water for productivity analysis. Light (Light Meter HS1010A), pH, and temperature (ADWA AD11) measurements were also performed in this experiment.
- Pigment analytical measurements
Chlorophyll extraction and quantification were performed using ethanol as the organic solvent, as described by [29]. Five milliliters of the culture was centrifuged at 3000 rpm for 10 min. After discarding the supernatants, 5 mL of distilled water was added and centrifuged at 4500 rpm for 10 min. This rinse was repeated twice, and after discarding the supernatant, 5 mL 98% ethanol was added to the pellet. The tubes were stored in the absence of light at 6 °C for 24 h and then centrifuged at 4500 rpm for 10 min. Chlorophyll quantification was then performed spectrophotometrically using the supernatants and the following equation:
where Axxx is the absorbance at xxx nm after removing the sample absorbance at 750 nm, against a blank of the solvent used; ν means the volume of solvent used (mL); l is the spectrophotometric cell length (cm); and V is the sample volume (mL).
µgclorofila/mLmedium = (11.64A663 − 2.16A645 − 0.10A630)ν/(lV),
Carotenoid extraction and quantification were performed using dimethyl sulfoxide (DMSO) as an organic solvent following the method described by [30], assuming fucoxanthin as the main carotenoid produced by P. tricornutum [31]. Fifty milliliters of the culture was centrifuged at 3600 rpm for 15 min. After discarding the supernatants, the pellets were stored in a freezer and dried via lyophilization. Five milliliters of DMSO was added to each 3.5 mg of biomass, homogenized and incubated at 70 °C for 5 min. The tubes were centrifuged at 4600 rpm for 10 min. Quantification of total carotenoids was performed spectrophotometrically using the supernatants and the following equation:
CChl a (mg · L−1) = 13.34A666 − 4.85A650
CChl b (mg · L−1) = 24.58A650 − 6.65A666
Total car (mg · L−1) = (1000 × D480 − 1.29 × CChl a − 53.76 × CChl b)/220
- Lipid extraction
Total lipid extractions were performed following the method described by [32], where 13.5 mL of chloroform, 6.5 mL of methanol, and 5.25 mL of distilled water (68% NaCl) were mixed with 1 g of dried biomass. The mixture was magnetically stirred for 10 min and then centrifuged at 3000 rpm for 10 min. The resultant organic lower phase was transferred to a clean glass vial and evaporated on a rotary evaporator to remove chloroform. The difference between the weight of the vial after evaporation and the weight of the empty vial was taken to be the weight of the lipids. The lipid content (% of dry weight) was the weight of lipids divided by the weight of the starting dry algal biomass (1 g).
- Stress
Experiments to study the effect of culture age were performed using three open raceways photobioreactors (RWP) inoculated with inoculum from a back-up system (RWP4). Two of them, RWP1 and RWP2 (R1 and R2) were inoculated again, while R3 was renewed. The final volume was 1000 L in all systems, and they were maintained for 15 days without handling. Four harvests were performed throughout this experiment: T0 (after culture establishment), T1 (when the steady state was reached), T2 (on the 10th day), and T3 (on the 15th day). To avoid excessive water evaporation from the culture systems, 150 L of artificial seawater was added on the 7th day. Experiments to study the effect of nitrogen deprivation were performed using three open raceways, two of which were established as experimental systems (N1 and N2), while the last one was maintained as a control system (Control). In this experiment, algae were harvested on the 5th day.
2.2. Hematoprotection Assay
The experimental system used in this study was HepG2 cells (human hepatocyte cell line HB-8065, ATCC, Manassas, VA, USA). These cells were stored in the GAIKER culture bank and checked for the absence of contaminating mycoplasmas when thawed. HepG2 cells were grown at 37 °C in a humidified atmosphere containing 5% CO2. Cells were maintained in the culture medium for a minimum of two weeks before starting the assays to allow optimal cell growth. The cells were subcultured before reaching 70% confluence (cell concentration/volume).
A cell suspension was prepared 24 h before the hepatoprotective activity assay was performed. The cell suspension (200 µL/well; 20,000 cells/well) was dispensed into 96-well plates using a multichannel pipette. The plates were then incubated at 37 °C and 5% CO2 for 24 h.
Cells cultured in 96-well plates were treated with four concentrations of the product under study for 24 h (37 °C, 5% CO2) together with 5 mg/mL methotrexate (MTX, M8407, Sigma, St. Louis, MO, USA), a known hepatotoxic agent [33,34,35], at 5 mg/mL. The product was assayed at a maximum concentration of 0.25 mg/mL. Serial dilutions (1:2) of the product were prepared in the culture medium and assayed at concentrations of 0.25, 0.125, 0.0625, and 0.03125 mg/mL.
After incubation with the test products or positive control, the cells were washed with PBS and stained with Thiazolyl Blue Tetrazolium Bromide (MTT, M2128, SIGMA) solution. The plates were then incubated at 37 °C for 2 h. At the end of this period, the medium was removed and 100 µL of dimethyl sulfoxide (DMSO, D2650, SIGMA Aldrich, Burlington, MA, USA) was added to solubilize the colored precipitate. The plates were left in the dark and stirred for 15 min. Absorbance was measured at 540 nm using a spectrophotometer plate reader. Finally, the cell viability percentage was calculated from these results using a spectrophotometer and plotted on a graph against the positive test/control product concentration. Three independent experiments with at least four replicates were performed for each experimental point.
2.3. Statistical Analysis
Growth measures and chlorophyll extraction were conducted in triplicate, total carotenoid extraction was carried out in duplicate, and lipid extraction was performed as a single measure. All values are given as mean ± standard deviation with error bars denoting the standard deviation. Microsoft® Excel® (Microsoft 365 MSO, 16.0.14228.20200, 64 bits) was used for data processing and graphical representation. Parametric one-way analysis of variance (ANOVA) and Kruskal–Wallis non-parametric tests were performed using RStudio (R-4.0.3). Means were compared using Tukey’s and Dunn’s tests, respectively, at a significance level of p < 0.05. Similarly, for hepatoprotection assays, values are given as the mean of the percentage of viability ± standard error mean (SEM). A one-factor analysis of variance (ANOVA) with Bonferroni–Dunn’s correction was performed to assess differences in the percentage of cell viability with respect to the MTX Group (p < 0.05).
3. Results
3.1. Scaling-Up and Fucoxanthin Production by P. tricornutum
3.1.1. Environmental Parameters
There was no significant difference in any of the environmental parameters measured between the culture systems during the culture-age experiment (pH, temperature, and irradiance).
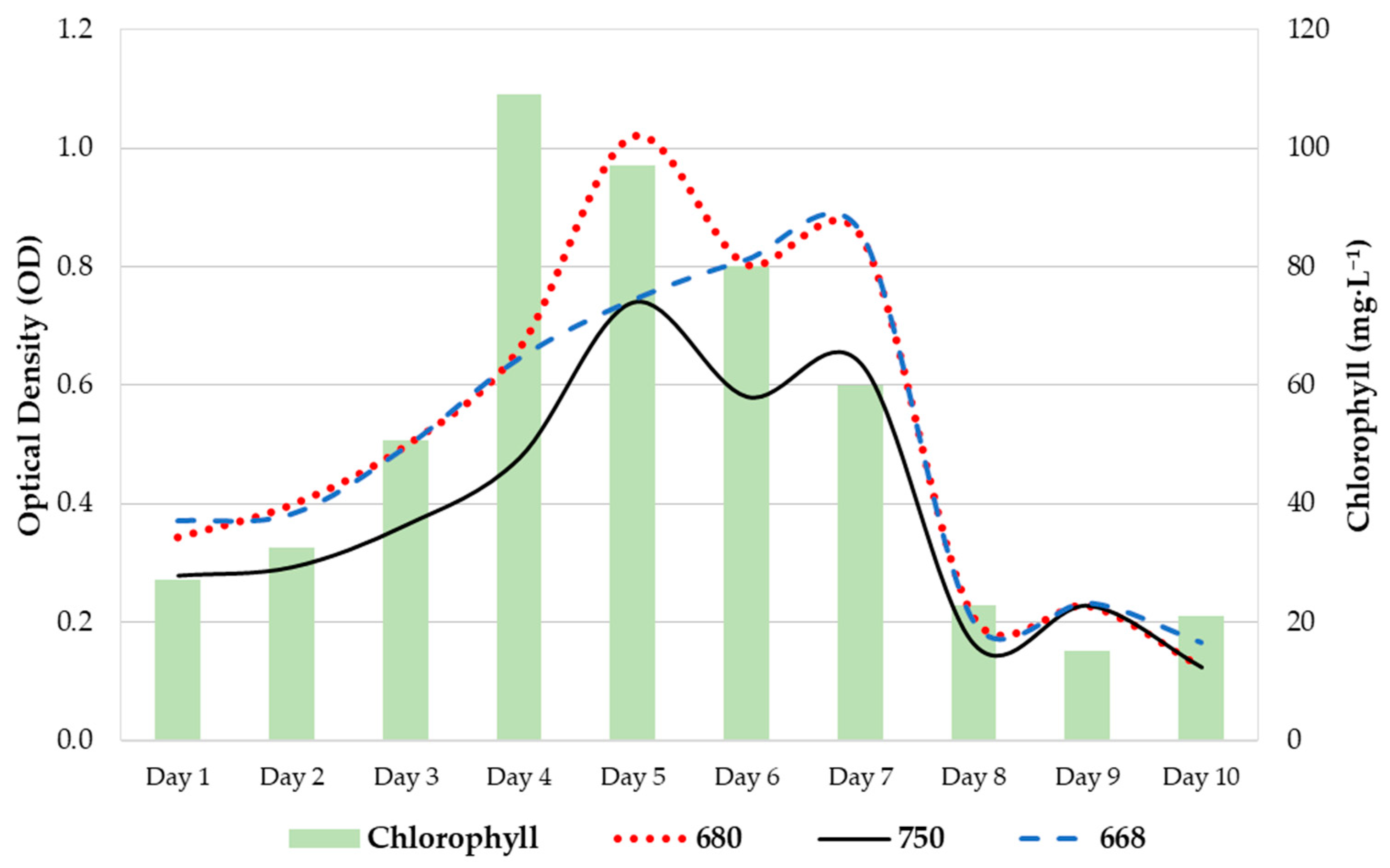
In Figure 2, the bars represent the chlorophyll extraction values (mg/L), and the lines represent the optical density values at 680 nm (red line), 750 nm (black line), and 668 nm (blue line).
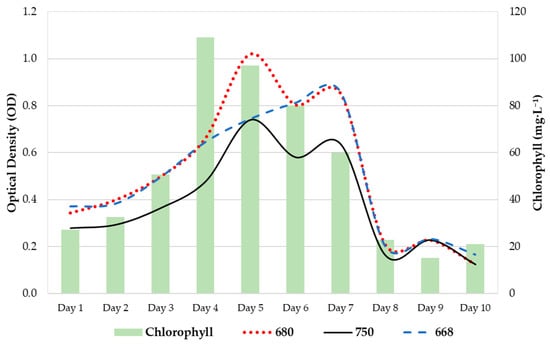
Figure 2.
Growth diagram of P. tricornutum in column-type photobioreactors for 10 days.
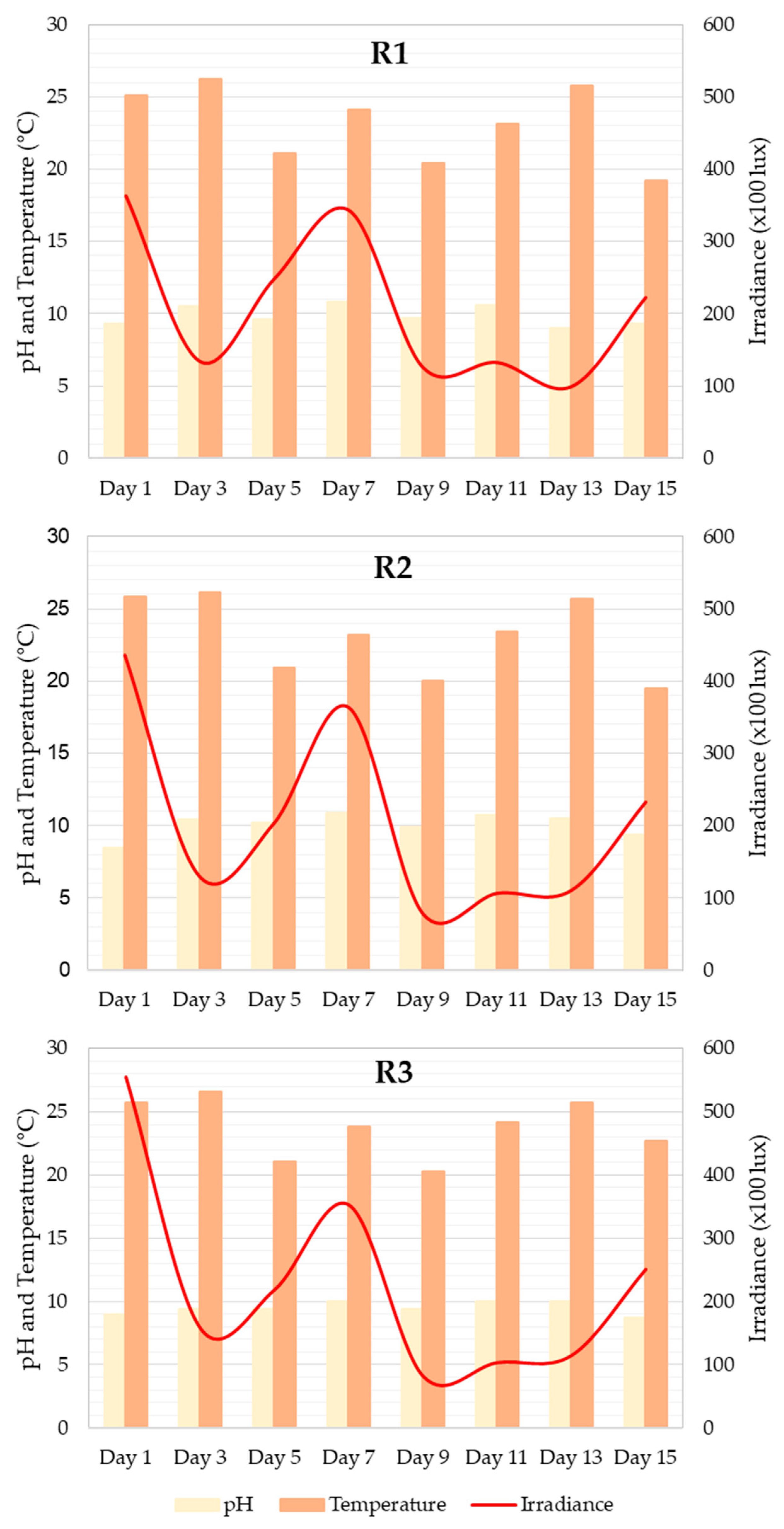
Flocculation was recorded in system R1 at the beginning and end of the experiment. This phenomenon was not observed during the nitrogen deprivation experiments. However, significant differences were observed between the levels of irradiance registered by the systems (p value = 0.049), whereas higher differences were noticed between the control and N2 systems (Figure 3). There were no significant differences in pH or temperature between the systems, although the highest values were observed between the control and N2 systems.
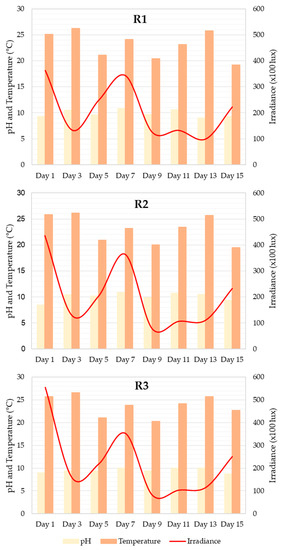
Figure 3.
Graph of measurements of pH, temperature (°C), and irradiance (×100 lux) recorded throughout the 15 days of the experiment in the RWP1 (R1), RWP3 (R2), and RWP4 (R3) experimental systems.
3.1.2. Culture Growth
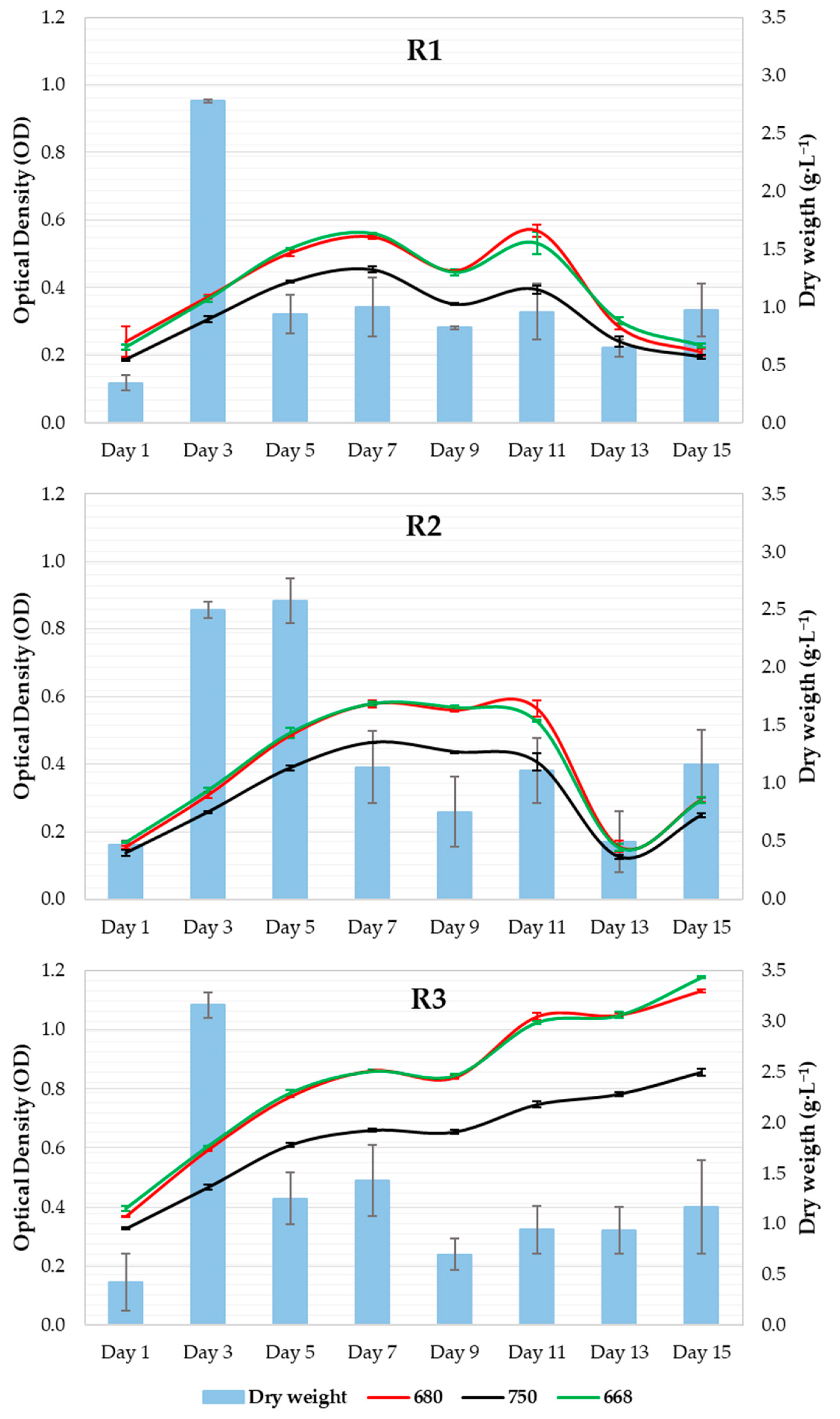
Similar behaviors were observed in the R1 and R2 systems during all culture-age experiments (Figure 4). R3 registered significantly higher OD values throughout the experiment (p value < 0.05) and continued to increase, whereas R1 and R2 showed a strong decrease between the 11th and 13th days. The R2 systems showed a slight increase in OD on the last day of culture, whereas no variation was observed in R1. The productivity values (DW) varied for all systems throughout the experiment.
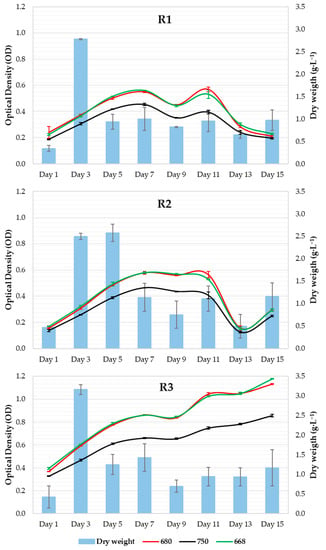
Figure 4.
Microalgal growth curve as a function of dry weight.
3.2. Pigment and Carotenoid Content
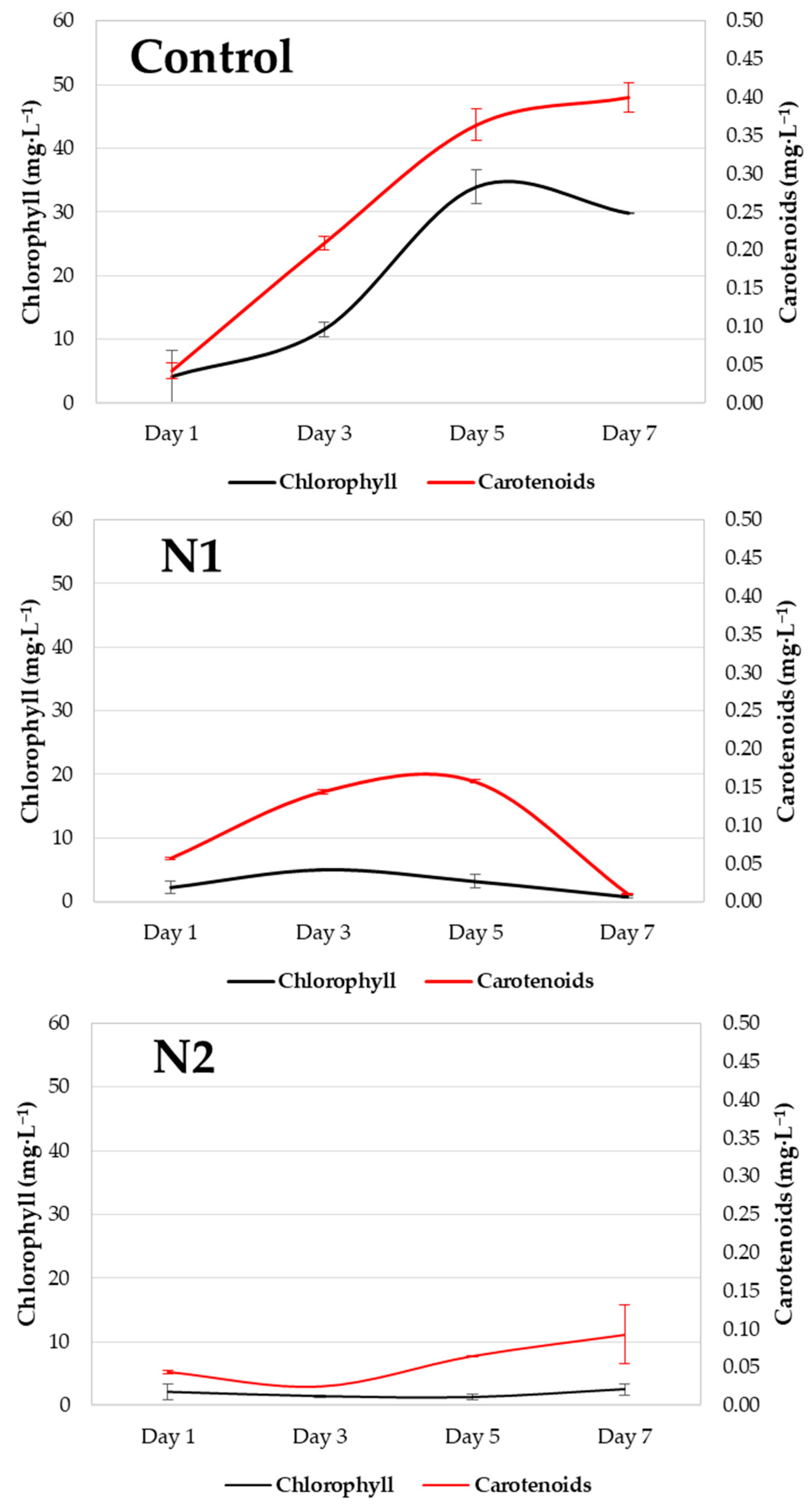
In Figure 5, the black lines represent the chlorophyll content, whereas the red lines represent the carotenoid content. Error bars indicate the standard deviation. During the nitrogen deprivation experiment, the chlorophyll content (mg/L) increased in the control system, but no changes were observed in the N1 and N2 systems. Between the 5th and 7th days, the control system experienced a marked decline. There were significant differences in the chlorophyll content (p value = 0.030), which was greater between the system and N2 (p value = 0.032). There was no significant difference in carotenoid content (p value = 0.218), although greater differences were recorded between the control and N2 treatments (p value = 0.286).
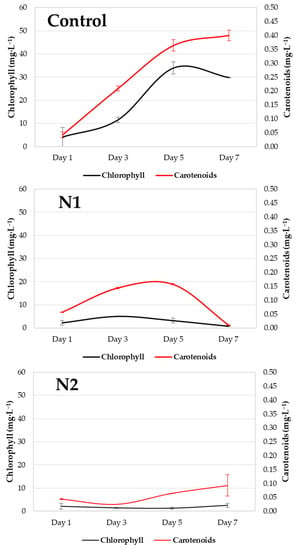
Figure 5.
Graph of chlorophyll (mg/L) and carotenoid (mg/L) content recorded throughout the 7 days of the experiment in the control (Control) and experimental (N1 and N2) systems.
3.3. Characteristics of the Biomass Obtained
This biomass contained 99% powder and 1% humidity, and the 350 mg of mix contained 172.9 mg from Arthrospira platensis, 172.9 mg of microalgae oil from P. tricornutum, and 4.2 mg of tocopherol.
The powder contained 4.9 mg (2.59%) of pure fucoxanthin. These contents were analyzed by Ofice SL Laboratori d’investigació cerealista in Castellgali, who used HPLC-UV (DAD) with the PNT-M-841 method.
3.4. Extraction and Encapsulation
Fucoxanthin was extracted from P. tricornutum via solid–liquid extraction using an organic solvent (hexane) (Table 3). We decided to use hexane because it does not leave any solvent residue and its use is permitted in the food industry.

Table 3.
Analytics of residues after carrying out the extraction and encapsulation process.
The first step of the extraction process after harvesting the P. tricornutum culture involves a breakdown phase. Microalgae were diluted in distilled water and subsequently disrupted. Once the biomass was broken, it was lyophilized.
Subsequently, the product was extracted with ethanol. The extraction occurred at a temperature between 25 and 50 °C and while stirring at 30–50 rpm. The supernatant was then decanted and centrifuged. Once all the liquid phase was collected, it was placed in a rotary evaporator for 1–4 h at 25–50 °C between 0.02 and 0.08 MPa (vacuum), resulting in the removal of hexane. The hexane is evaporated and passes through a spiral where it is cooled, condenses, and becomes liquid. The liquid hexane was recovered and reused. This process yielded an oily black fucoxanthin-rich extract.
In the final formulation, the oily fucoxanthin-rich extract was included within the matrix of the broken spirulina, as the support for obtaining the powdered product. To prepare the powder ingredients (spirulina and P. tricornutum extract), a second phase of mixing was performed. Spirulina and oil extracts were prepared in a liquid medium (distilled water). The water/spirulina/oily extract mixture was produced in proportions of 80:10:10 (water 80%, spirulina 9.9%, Phaeodactylum 9.9%, tocopherol 0.25%, with the initial fucoxanthin content in the plant material being 0.1–0.3%). These percentages can vary depending on the desired amount of fucoxanthin.
Once the mixture was combined, it was lyophilized. After the product was dehydrated, a powder was obtained containing 49.4% spirulina, 49.4% Phaeodactylum extract and 1.2% tocopherol. The mixed powder was then vacuum packed, and the parameters of the packaging machine were controlled in each process (% vacuum:98 and sealing time:4 s).
After vacuum packaging, the product was frozen to preserve its properties. At that time, the final encapsulated mixture was ready for hepatoprotective capacity tests.
3.5. Hepatoprotective Assay
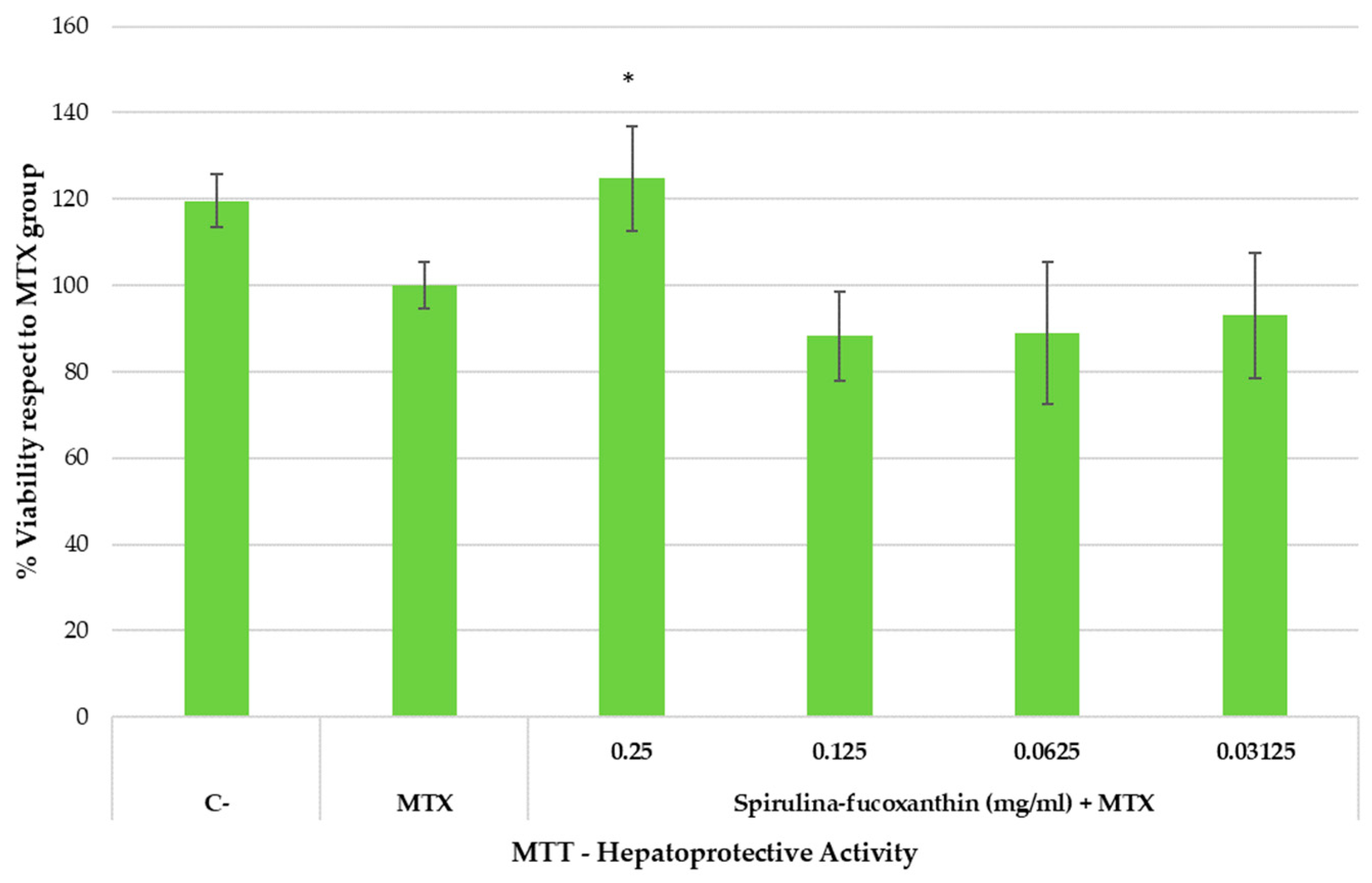
Cytotoxicity assays are basic and essential evaluations performed to determine the toxicity of compounds intended for use in the biomedical field. The cytotoxicity of MTX and possible hepatoprotective activity of fucoxanthin were studied in HepG2 cells for 24 h in culture. The effects of fucoxanthin and free MTX on cell metabolic activity were assessed using the MTT assay as an indicator of cellular cytotoxicity. The results of the MTT assay for nanocarriers are presented in Figure 6.
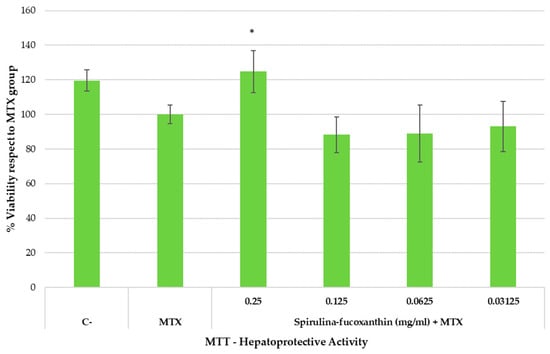
Figure 6.
Hepatoprotective effect (MTT reduction assay) in HepG2 cell line exposed to methotrexate (MTX) for 24 h.
The cells were exposed to the indicated concentrations of the active substance and the hepatotoxic compound MTX for 24 h. C and MTX conditions represent untreated and MTX-treated cells, respectively. The mean (top of the bars) and SEM (whiskers) of four replicates and three independent trials (n = 3) are shown. Asterisks represent statistically significant differences compared to MTX at the same dose (p value < 0.05).
4. Discussion
4.1. Environmental Parameters and Culture
Figure 3 shows that there were no significant differences between the values for light (p value = 0.949), pH (p value = 0.241), and temperature (p value = 0.896) taken in the different systems with a 95% confidence interval (significance level = 0.05). The greatest differences in pH were detected between systems R2 and R3 (p value = 0.218). The light measurements recorded between systems R1 and R2 differed the most (p value = 0.750). The temperature was very similar in the R1 and R2 systems (p value = 0.869), and was slightly different from that of the R3 system (p value = 0.6).
The pH values are represented by the black bars. A maximum value of pH = 10.8 was recorded on day 7 for R1, and a minimum value of 9 was recorded on day 13, which represents a variation in pH of 1.8 throughout the experiment. In the case of R2, the extreme pH values were 10.9 and 8.5, on days 7 and 1 of the culture, respectively, which represents an oscillation of 2.4. Finally, for R3, the maximum pH value was 10 on days 7, 6, and 13, and the minimum was 8.7 on day 15, a variation of 1.3. The microalgae in this study are a species that can tolerate these small changes in pH in the culture; however, these changes can modify the growth of the microalgae, as indicated by [36].
pH is one of the most important factors in microalgae cultures because microalgae membranes are not completely impermeable to hydrogen ions and hydroxy groups. Although P. tricornutum is a tolerant species, these changes can be leveraged in the culture. A pH increase occurs as a result of the absence of CO2 input because the ions are pulled out as bicarbonate by the microalgae. Calcium carbonate precipitation occurs when pH reaches 10, and microalgae may be affected [36]. There are studies in the literature that indicate the onset of flocculation (microalgae aggregation) when pH values are higher than 10.5 [37]. However, this may not be the only explanation for the phenomenon. Flocculation was observed only at the beginning and end of the culture experiment, regardless of pH value, according to [38]. In the absence of nitrogen, this was not observed, although the pH values remained lower than the previously mentioned umbral pH values.
Irradiance values can only be used to establish whether the culture conditions remain constant in all systems. To use this variation to infer its influence on the culture, it would be necessary to constantly record this parameter, because slight changes in light intensity can lead to changes in culture growth and productivity rates [39]. In various studies, light has been shown to influence not only the growth and cell division of P. tricornutum, but also the pigment content (i.e., fucoxanthin). High irradiance levels are harmful to orbital aerated cultures in fucoxanthin production and culture aeration is essential for growth under high light intensity [40]. However, high light intensity can induce photoinhibition and photodamage, leading to cellular death [41]. In Haematococcus sp., Dunaliella sp. and Muriellopsis sp., increased primary carotenoid production was detected under high-irradiance conditions. However, light seems to affect fucoxanthin production in P. tricornutum in a different manner. Some studies have reported increased carotenoid production under medium light conditions [40], while others have suggested that the highest accumulation rates occur at low light intensities [42].
The culture temperature remained constant throughout all experiments, and its influence on P. tricornutum has already been described. The highest division rate was registered at 23 °C, carbon fixation increased in direct relation to temperature (14–25 °C), and cells tended to have larger chloroplasts above 21 °C and lower light intensities [43]. Recent studies have noticed higher chlorophyll content in cultures growing at 20–23 °C, due to higher productivity levels [44]. It has also been documented that microalgae morphology is affected by temperature [44], although we did not observe this in our experiments. Here, culture temperature ranged between 19.5 °C and 26.6 °C in both experiments, and the temperature was very close to the best-established culture conditions for P. tricornutum; however, slight fluctuations were registered, so its influence cannot be dismissed.
No significant differences in DW were observed between the systems (Figure 4). In the nitrogen deprivation experiment, DW showed higher levels on the 1st day of culture, while it remained constant during the rest of the samplings. In this case, OD showed different behaviors in all systems: a marked increase in OD in the control system, a slight increase in N1, and no increase in N2. The control and N1 systems showed a drastic decline between the 5th and 7th days. No significant differences in OD were found among the systems or in the DW values.
No significant differences were found in carotenoid content. During the P. tricornutum culture, the carotenoid content in the tested systems continued to increase from day 5 of the culture, when the stationary phase was reached. This increase continued throughout the experiment until day 11 in all cases, at which point a stabilizing trend was detected in two of the systems (R2 and R3). The results indicate that the best time to carry out carotenoid extraction occurred on day 11 of culture, under the growth conditions established in the experiment.
Sudden changes in light resulted in a rapid decrease in carotenoid content, so continuous monitoring of the state of the crop is crucial to avoid the loss of fucoxanthin content, an aspect that must be taken into account when carrying out commercial fucoxanthin exploitation.
4.2. Hepatoprotective Assay
As shown in Figure 6, spirulina–fucoxanthin significantly increased the percentage of cell viability (reduced the hepatotoxic impact) at the highest assayed concentration compared to the MTX group (cells treated only with MTX), suggesting hepatoprotective activity. On the one hand, the healthy group (C-) presented 119.6 ± 6.26% cell viability. On the other hand, when hepatocytes were incubated with MTX, cell viability was 100.00 ± 5.27%. However, when stressed hepatocytes (incubated with MTX) were incubated with test product at 0.25 mg/mL, the cell viability was 124.74 ± 12.21% (similar to the healthy control). A significant increase in cell viability was observed at 0.25 mg/mL of spirulina–fucoxanthin.
5. Conclusions
We draw the following conclusions from this study:
MTX (5 mg/mL) produced adequate hepatotoxic stress, and a decrease in cell viability of the human hepatocyte cell line was observed.
The active ingredient (spirulina–fucoxanthin) showed hepatoprotective activity against the hepatotoxic compound MTX in the human hepatocyte cell line HEPG-2 at a concentration of 0.25 mg/mL.
The current results also suggest that spirulina–fucoxanthin has beneficial properties for liver health and is a suitable ingredient for all types of natural functional foods.
Author Contributions
Conceptualization, J.F.D.-R. and M.Á.-G.; methodology, J.F.D.-R. and L.C.-C.; software, F.G.-d.-C.; validation, J.F.D.-R., V.C.-B. and E.R.; formal analysis, J.F.D.-R. and D.S.-M.; investigation, J.F.D.-R. and L.C.-C.; resources, D.S.-M., F.G.-d.-C. and L.C.-C.; data curation, F.G.-d.-C. and L.C.-C.; writing—original draft preparation, J.F.D.-R.; writing—review and editing, V.C.-B. and E.R.; visualization, J.F.D.-R. and M.Á.-G.; supervision, V.C.-B. and E.R.; project administration, J.F.D.-R. and E.R.; funding acquisition, J.F.D.-R. and M.Á.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by REA in the Horizon Europe Framework Programme within the funding scheme for fair, healthy and environmentally friendly food systems from primary production to consumption under the scope of the IMPRESS Project 101084437 (HORIZON-CL6-2022-FARM2FORK-02-two-stage).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher on reasonable request.
Acknowledgments
The authors gratefully acknowledge the support of the Neoalgae Micro-Seaweed Products (Spain) for this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- How COVID-19 Is Changing Consumer Behavior—Now and Forever. Available online: https://www.mckinsey.com/~/media/mckinsey/industries/retail/our%20insights/how%20covid%2019%20is%20changing%20consumer%20behavior%20now%20and%20forever/how-covid-19-is-changing-consumer-behaviornow-and-forever.pdf (accessed on 16 February 2023).
- Indicadores de Salud 2020. Evolución de los Indicadores del Estado de Salud en España y su Magnitud en el Contexto de la Unión Europea. Madrid: Ministerio de Sanidad. Available online: https://www.sanidad.gob.es/estadEstudios/estadisticas/inforRecopilaciones/docs/Indicadores_de_Salud_2020.pdf (accessed on 16 February 2023).
- Liver Cirrhosis. Available online: https://gi.org/topics/liver-cirrhosis/ (accessed on 3 May 2023).
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An Overview to the Health Benefits of Seaweeds Consumption. Mar. Drugs 2021, 19, 341. [Google Scholar] [CrossRef] [PubMed]
- European Council. Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the Approximation of the Laws of the Member States Relating to Food Supplements (Consolidated Text); European Council: Strasbourg, France, 2002; Volume 183, p. 51. [Google Scholar]
- Kim, S.M.; Jung, Y.-J.; Kwon, O.-N.; Cha, K.H.; Um, B.-H.; Chung, D.; Pan, C.-H. A Potential Commercial Source of Fucoxanthin Extracted from the Microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Abdulqader, G.; Barsanti, L.; Tredici, M.R. Harvest of Arthrospira platensis from Lake Kossorom (Chad) and its household usage among the Kanembu. J. Appl. Phycol. 2000, 12, 493–498. [Google Scholar] [CrossRef]
- Lupatini Menegotto, A.L.; Souza, L.E.; Colla, L.M.; Costa, J.A.; Sehn, E.; Bittencourt, P.R.; Moraes Flores, É.L.; Canan, C.; Colla, E. Investigation of techno-functional and physicochemical properties of Spirulina platensis protein concentrate for food enrichment. LWT 2019, 114, 108267. [Google Scholar] [CrossRef]
- Wan-Loy, C.; Siew-Moi, P. Marine Algae as a Potential Source for Anti-Obesity Agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef]
- Englert, G.; Bjørnland, T.; Liaaen-Jensen, S. 1D and 2D NMR study of some allenic carotenoids of the fucoxanthin series. Org. Magn. Reson. 1990, 28, 519–528. [Google Scholar] [CrossRef]
- Pajot, A.; Hao Huynh, G.; Picot, L.; Marchal, L.; Nicolau, E. Fucoxanthin from Algae to Human, an Extraordinary Bioresource: Insights and Advances in up and Downstream Processes. Mar. Drugs 2022, 20, 222. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef]
- Yamanaka, R.; Akiyama, K. Cultivation and utilization of Undaria pinnatifida (wakame) as food. J. Appl. Phycol. 1993, 5, 249–253. [Google Scholar] [CrossRef]
- Bohlin, K. Zur Morphologie und Biologie einzelliger Algen. Öfversigt Kongliga Vetenskadademiens Förhanligar 1897, 54, 507–529. [Google Scholar]
- Pudney, A.; Gandini, C.; Economou, C.K.; Smith, R.; Goddard, P.; Napier, J.A.; Spicer, A.; Sayanova, O. Multifunctionalizing the marine diatom Phaeodactylum tricornutum for sustainable co-production of omega-3 long chain polyunsaturated fatty acids and recombinant phytase. Sci. Rep. 2019, 9, 11444. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, R.; Nurkolis, F.; Taslim, N.A.; Subali, D.; Surya, R.; Gunawan, W.B.; Alisaputra, D.; Mayulu, N.; Salindeho, N.; Kim, B. Carotenoids Composition of Green Algae Caulerpa racemosa and Their Antidiabetic, Anti-Obesity, Antioxidant, and Anti-Inflammatory Properties. Molecules 2023, 28, 3267. [Google Scholar] [CrossRef]
- Cui, Y.; Thomas-Hall, S.R.; Schenk, P.M. Phaeodactylum tricornutum microalgal as a rich source of omega-3 oil: Progress in lipid induction techniques towards industry adoption. Food Chem. 2019, 297, 124937. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Côme, M.; Ulmann, L.; Chini Zittelli, G.; Faraloni, C.; Nazih, H.; Ouguerram, K.; Chénais, B.; Mimouni, V. Preventive Effects of the Marine Microalga Phaeodactylum tricornutum, Used as a Food Supplement, on Risk Factors Associated with Metabolic Syndrome in Wistar Rats. Nutrients 2019, 11, 1069. [Google Scholar] [CrossRef] [PubMed]
- Gammone, M.A.; Riccioni, G.; D’Orazio, N. Marine Carotenoids against Oxidative Stress: Effects on Human Health. Mar. Drugs 2015, 13, 6226–6246. [Google Scholar] [CrossRef] [PubMed]
- Marmot, M.; Bell, R. Social determinants and non-communicable diseases: Time for integrated action. BMJ 2019, 364, l251. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef]
- Oomizu, S.; Yanase, Y.; Suzuki, H.; Kameyoshi, Y.; Hide, M. Fucoidan prevents C epsilon germline transcription and NFkappaB p52 translocation for IgE production in B cells. Biochem. Biophys. Res. Commun. 2006, 350, 501–507. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Q.; Chen, L.; Ren, S.; Xu, P.; Tang, Y.; Luo, D. Higher specificity of the activity of low molecular weight fucoidan for thrombin-induced platelet aggregation. Thromb. Res. 2010, 125, 419–426. [Google Scholar] [CrossRef]
- Bustos, R.; Romo, L.; Yáñez, K.; Díaz, G.; Romo, C. Oxidative stability of carotenoid pigments and polyunsaturated fatty acids in microparticulate diets containing krill oil for nutrition of marine fish larvae. J. Food Eng. 2003, 56, 289–293. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M.; Argüelles-Monal, W. Microencapsulation of astaxanthin in a chitosan matrix. Carbohydr. Polym. 2004, 56, 41–45. [Google Scholar] [CrossRef]
- Casado-Bañares, V. Encapsulated Oil. US20220007698A1, 12 January 2022. [Google Scholar]
- Andersen, R.A. Algal Culturing Techniques. J. Phycol. 2005, 41, 906–908. [Google Scholar] [CrossRef]
- Walne, P.R. Culture of Bivalve Molluscs: 50 Years of Experience at Conwy; Fishing News Books Ltd.: Farnham, UK, 1974; 189p. [Google Scholar]
- Henriques, M.; Silva, A.; Rocha, J. Extraction and quantification of pigments from a marine microalga: A simple and reproducible method. In Communicating Current Research and Educational Topics and Trends in Applied Microbiology; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2007; Volume 1, pp. 586–593. [Google Scholar]
- Solovchenko, A.; Merzlyak, M.N.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. Coordinated carotenoid and lipid syntheses induced in parietochloris incisa (Chlorophyta, Trebouxiophyceae) mutant deficient in Δ5 desaturase by nitrogen starvation and high light. J. Phycol. 2010, 46, 763–772. [Google Scholar] [CrossRef]
- Rebolloso, M.M.; Navarro, P.A.; Ramos, J.J.; Guil, J.L. Biomass Nutrient Profiles of the Microalgae Phaeodactylum tricornutum. J. Food Biochem. 2001, 25, 57–76. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Bath, R.K.; Brar, N.K.; Forouhar, F.A.; Wu, G.Y. A review of methotrexate-associated hepatotoxicity. J. Dig. Dis. 2014, 15, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Shergy, W.J.; Polisson, R.P.; Caldwell, D.S.; Rice, J.R.; Pisetsky, D.S.; Allen, N.B. Methotrexate-Associated Hepatotoxicity: Retrospective Analysis Of 210 Patients with Rheumatoid Arthritis. Am. J. Med. 1988, 85, 771–774. [Google Scholar] [CrossRef]
- West, S.G. Methotrexate hepatotoxicity. Rheum. Dis. Clin. N. Am. 1997, 23, 883–915. [Google Scholar] [CrossRef]
- Abalde, J.; Cid, Á.; Fidalgo Paredes, P.; Torres, E.; Herrero, C. Microalgas: Cultivo y Aplicaciones. Available online: https://ruc.udc.es/dspace/bitstream/handle/2183/25013/Abalde_Julio_1995_Microalgas_cultivo_aplicaciones.pdf?sequence=2&isAllowed=y (accessed on 11 May 2023).
- Spilling, K.; Seppälä, J.; Tamminen, T. Inducing autoflocculation in the diatom Phaeodactylum tricornutum through CO2 regulation. J. Appl. Phycol. 2011, 23, 959–966. [Google Scholar] [CrossRef]
- Formosa-Dague, C.; Castelain, M.; Martin-Yken, H.; Dunker, K.; Dague, E.; Sletmoen, M. The Role of Glycans in Bacterial Adhesion to Mucosal Surfaces: How Can Single-Molecule Techniques Advance Our Understanding? Microorganisms 2018, 6, 39. [Google Scholar] [CrossRef]
- Parisa, H.; Wafâa, B.; Morteza, Z.; Bing, H.; Brigitte, M.; Ewa, L.; Aurélie, C.-M.; Gaëtane, W.-C.; Véronique, M.-J.; Gaël, B. Marchand Justine and Schoefs Benoît, Response of CO2-starved diatom Phaeodactylum tricornutum to light intensity transition, Phil. Trans. R. Soc. B 2017, 372, 2016039620160396. [Google Scholar] [CrossRef]
- Gómez-Loredo, A.; Benavides, J.; Rito-Palomares, M. Growth kinetics and fucoxanthin production of Phaeodactylum tricornutum and Isochrysis galbana cultures at different light and agitation conditions. J. Appl. Phycol. 2016, 28, 849–860. [Google Scholar] [CrossRef]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015, 125, 423–436. [Google Scholar] [CrossRef] [PubMed]
- McClure, D.D.; Luiz, A.; Gerber, B.; Barton, G.W.; Kavanagh, J.M. An Investigation into the Effect of Culture Conditions on Fucoxanthin Production Using the Marine Microalgae Phaeodactylum tricornutum. Algal Res. 2018, 29, 41–48. [Google Scholar] [CrossRef]
- Fawley, M.W. Effects of Light Intensity and Temperature Interactions on Growth Characteristics of Phaeodactylum tricornutum (Bacillariophyceae). J. Phycol. 1984, 20, 67–72. [Google Scholar] [CrossRef]
- Bojko, M.; Brzostowska, K.; Kuczyńska, P.; Latowski, D.; Olchawa-Pajor, M.; Krzeszowiec, W.; Waloszek, A.; Strzałka, K. Temperature Effect on Growth, and Selected Parameters of Phaeodactylum tricornutum in Batch Cultures. Acta Biochim. Pol. 2013, 60, 861–864. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).