Abstract
The quadriceps femoris muscle (QF) is of clinical importance since it has been correlated with pathologies at knee level, such as anterior cruciate ligament (ACL) injury, pain processes and complex clinical conditions. Among the variables that have been related to these clinical conditions are anthropometric measurements, architecture and muscular behavior of the QF. The aim of this study was to determine the relationship between the rectus femoris (RF) and vastus intermedius (VIM) muscles’ behavior measured by rehabilitative ultrasound imaging (RUSI) M-mode under maximal voluntary isometric contraction (MVIC) and anthropometric measurements. This was a cross-sectional, observational study. Sixty-two asymptomatic volunteers were included (20.42 ± 4.97 years, most women 59.7%). RUSI measurements were muscle contraction/rest thickness and contraction/relaxation velocity. Anthropometric measurements were, lower limb length, RF length, QF tendon length, distance between spines, proximal, middle and distal thigh perimeter. Statistically significant correlations (p < 0.05) were found between VIM thickness at rest and contraction with thigh perimetry, RF length and dominant lower limb length. For the RF, a correlation was found between the thickness at rest and the length of this muscle (p = 0.003). There is a correlation between anthropometric variables and muscular behavior measured by RUSI M-mode.
1. Introduction
The quadriceps femoris muscle (QF) is part of the anterior muscles of the thigh. It is a muscle group made up of five muscle bellies: rectus femoris (RF), vastus lateralis (VL), intermedius (VIM), medialis (VM) and tensor vastus intermedius (TVI). At the distal level they are inserted through the common quadriceps tendon while at the proximal level the insertion varies in each one of them. It is the main knee extensor, essential in human movements such as standing, walking and running [1,2].
There is evidence showing differences in the behavior of the QF’s different muscle bellies depending on the type of contraction (concentric, eccentric or isometric) that is requested to the subject and the angulation of the movement. Some studies highlight the difference in the participation of the muscle bellies movement, evidencing that the VIM has greater participation in knee extension while the VL muscle dominance appears to manifest across the concentric-isometric-eccentric transition [3,4]. However, other studies conclude that the monoarticular muscles (VM, VL and VIM) and the biarticular muscles (RF) act as a functional unit [5].
The QF is of clinical importance, since it has been correlated with pathologies at knee level, such as anterior cruciate ligament (ACL) injury, pain processes and complex clinical conditions [6,7].
In a previous study carried out on 17,397 elite athletes, it was determined that knee injury has a prevalence of 39.8%, among which the most prevalent was the ACL injury, 20.3%, followed by injuries of the meniscus 14.5% [8].
Because of the high prevalence of ACL injuries, research related to the causes, sequelae and treatment related to this type of injury has been increasing. The etiology of ACL injury is complex and there are multiple intrinsic and extrinsic factors that have been described in the literature [9,10].
Several risk factors are associated with an increased risk of ACL injury, such as family history of injury (2.5 times greater odds for sustaining a primary ACL injury), sex, bony geometry (decreased intercondylar femoral notch size, decreased depth of concavity of the medial tibial plateau, increased slope of the tibial plateaus), strength, stiffness, laxity and alterations in muscle behavior, among others [10,11,12,13].
One of the most used treatments in the management of ACL injuries is surgery; however, the rates of re-rupture and rupture of the contralateral ACL are high [14,15]. Due to the high social and personal impact that surrounds ACL injury, it is considered of vital importance to implement protocols for the prevention, treatment and rehabilitation of this type of injury.
On the other hand, anthropometric relationships have been described in other pathologies, in the case of knee osteoarthritis, the triceps-skinfold thickness (peripheral fat) in males and the waist-hip ratio (central fat) in females were strongly associated with the presence of this pathology. Similarly, it has been suggested that high body mass index may be related to patellar tendinopathy [16,17].
Neuromusculoskeletal alterations related to the strength, morphology and muscle activation of the QF are described in subjects with knee injuries and in subjects who have had surgery [18,19]. Previous studies have shown that QF neuromuscular function remains altered in the short and long term after knee arthroscopy for meniscal surgery and/or ACL reconstruction. Compared with patients who had undergone meniscus surgery, patients with ACL reconstruction showed greater asymmetries in maximal voluntary contraction torque (15% vs. 5%, p = 0.049) and VL thickness (6% vs. 0%, p = 0.021). Patients demonstrated persistent quadriceps muscle weakness 6 months after ACL reconstruction [19].
Morphometric correlations have been established between QF architecture and injury, sarcopenia, frailty, and alterations in muscle strength and activation [20,21,22,23]. Moreover, it is known that muscle performance is related to muscle architecture, strength and muscle activation capacity, which are directly related to a better quality of life, functionality and, in the case of injury, with a better prognosis for recovery [24,25,26,27].
For this reason, it is considered essential to have tools that allow a valid and reliable evaluation of the variables that could condition muscular behavior, as well as range of normal values.
Currently, there are several tools that allow the evaluation of activity, activation, functionality and muscle architecture, such as electromyography (EMG), magnetic resonance imaging (MRI) and ultrasonography (US) [28,29,30].
The characteristics of US, such as its low associated cost and speed of use, have led to an increase in its implementation in the clinical and research area in physiotherapy and rehabilitation, where it is called Rehabilitative Ultrasound Imaging (RUSI) [31,32,33].
RUSI has proven to be a valid and reliable tool when compared to gold standards such as MRI in the morphological evaluation of lower limb muscles [28,34,35,36,37,38].
RUSI in B-mode provides information related to muscle morphometry (thickness, cross-sectional area (CSA), pennation angle, fascicle length) while RUSI in M-mode shows temporal parameters related to activation [39,40].
RUSI M-mode shows by means of a time-depth graph the moment of activation and relaxation of the musculature from changes in the movement of the connective tissue within the muscle [41,42]. It also makes it possible to determine the velocity at which these changes are generated, both at the beginning and at the end of muscle activation [43].
Currently, RUSI M-mode is used to assess the muscle activity of the knee extensor complex [44,45,46]. In a study conducted in subjects who underwent ACL reconstruction with a five-year follow-up, a decrease in the thickness of the RF muscle was found when compared to a control group. However, no changes in contraction/relaxation velocity were found. This study suggests the evaluation of the relationship between the muscular behavior of the RF and VL, VM and VIM in future investigations [47].
The close anatomical relationship between the RF and the VIM could condition the muscular behavior of one with respect to the other, both in the changes in thickness that occur during muscle contraction, as well as in the contraction and relaxation velocity.
Based on the current scientific evidence where QF muscle behavior is evaluated by RUSI, the authors considered it of interest to determine if the muscle behavior of RF and VIM are related to each other, as well as to describe the anthropometric variables that could be correlated.
To the best of the author’s knowledge this is the first study aimed at determining the relationship between RF and VIM behavior measured by RUSI M-mode under maximal voluntary isometric contraction (MVIC) and anthropometric measurements.
2. Materials and Methods
2.1. Study Design
This was a cross-sectional, observational study, according to the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) guidelines and checklist.
2.2. Participants
The sample size was calculated using the Wilcoxon Signed Rank test applied to the average velocity contraction between RF and VIM of the first 34 subjects recruited in the study.
Accepting a risk α of 0.05 and a power higher of 80%, a sample of 62 subjects was estimated.
Participants were recruited through flyers and advertising at the Physiotherapy and Pain Research Center, Universidad de Alcalá (Madrid, Spain) over a 4-month period, between June to September of 2022. The research was carried out in the same facilities as the recruitment.
The inclusion criteria were healthy volunteers, between 18 and 55 years old who signed the informed consent. The exclusion criteria were surgical interventions in the hip, thigh or knee area, hip, knee or lower limb fractures, pregnant women, recurrent pain in the dominant lower limb, systemic diseases and subjects with a BMI > 30 kg/m2.
2.3. Ethical Considerations
The present study was developed in accordance with the fundamental guidelines for clinical research in humans of the Declaration of Helsinki. Ethics approval was obtained from the Ethics Committee for Research and Animal Experimentation of the Universidad de Alcalá (Ethics ID Number: CEID/2022/03/044). Volunteers were fully informed of the purpose and procedures of the study and provided written informed consent.
Participants’ personal data were numerically coded and stored in a computer database. Access to the database was limited to the principal investigator.
The General Data Protection Regulation (GDPR, EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data and Law 14/2007, on biomedical research involving human beings, were respected during the present research.
2.4. Clinical Measurements
The demographic and anthropometric characteristics obtained were age, sex, height, weight and dominant lower limb, dominant lower limb length, RF length, QT tendon length, distance between spines (anterior superior iliac spine (ASIS) and anterior inferior iliac spine (AIIS)) and thigh perimetry (proximal third perimeter, middle third perimeter and distal third perimeter).
Height and body weight were measured with a digital stadiometer. The rest of the distances were measured with a measuring tape. All measurements were obtained in kilograms and centimeters (cm).
2.5. RUSI Measures
RUSI measures were collected using a VINNO E35 US device with a linear 5–13 MHz probe and a 48 mm footprint. All ultrasound measurements were carried out by a physiotherapist expert with more than 10 years of experience. For the RUSI measurements, a water-based gel was used as a conductor on the skin of the volunteers.
RUSI measurements were thickness at rest, thickness at contraction, average contraction velocity and average relaxation velocity of both RF and VIM.
To achieve an optimal image, the US parameters (frequency, depth, gain, focus, dynamic range, etc.) were optimized according to the characteristics of each participant. Image optimization was considered correct when it was possible to observe from the skin to the cortical bone of the femur.
During the training of patients for MVIC, the depth parameter on the ultrasound was adjusted to avoid loss of information during muscle contraction, due to the increase in muscle thickness under contraction. The position of the probe was kept perpendicular to the tissue and the measurements of muscle thickness were performed without considering the thickness of the intermuscular fasciae. The probe was placed at the midpoint of the RF and the VIM. Minimum possible pressure was guaranteed during the US evaluation in order not to alter the muscular behavior during contraction.
A set of three consecutive measurements was performed, and the average value was reported. Data were reported in cm and cm/s.
2.6. Measurement Procedure
The participants were evaluated in a single session, with an average duration of 45 min. Only the dominant leg of each participant was evaluated.
Participants were placed in a supine position with a pillow under their head. During the examination, a neutral position of the trunk, pelvis and lower limbs was maintained.
The ASIS, the proximal insertion of the RF in the AIIS, the end of the muscular portion of the RF at the beginning of the quadriceps tendon (QT) and the superior pole of the patella were corroborated by US with the objective of determining the landmarks for the measurements.
The RF was divided into three equal parts to determine the perimetry of the tight (proximal third perimeter, middle third perimeter and distal third perimeter) (Figure 1).
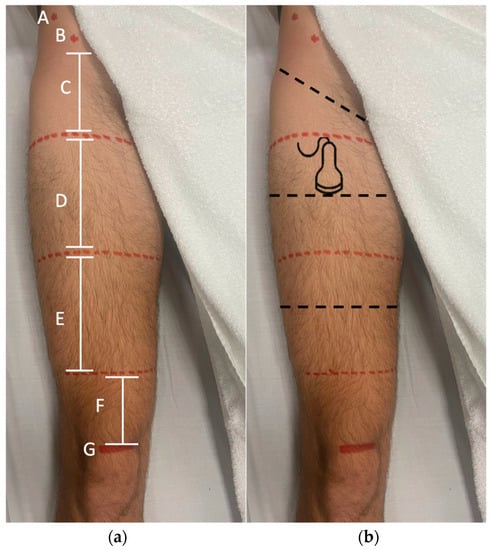
Figure 1.
Landmarks of the measurements. (a) The RF was divided into three equal parts, ASIS (A), AIIS (B), proximal third perimeter (C), medial third perimeter (D), distal third perimeter (E), quadriceps tendon length (F), superior pole of the patella (G). (b) The dotted black line in the middle of the division of the thirds is where the perimetry and RUSI evaluation of the RF was performed.
To determine the lower limb length the inferior aspect of the ASIS and the inferior aspect of the medial malleolus were considered [48].
For RUSI measurements the physiotherapist took position on the same side as the leg to be evaluated.
The midpoint of the RF was located, and the probe was placed in a transverse position. With the M-mode active the participant was asked to perform a MVIC of the QF, with the following verbal command “pull the patella superiorly tightly as fast and hard as possible and hold the leg in the same position” [49].
Participants held the contraction for 3 s after which they were asked to relax as fast as possible. Three consecutive measurements were performed with 2 min rest in between to avoid fatigue (Figure 2).
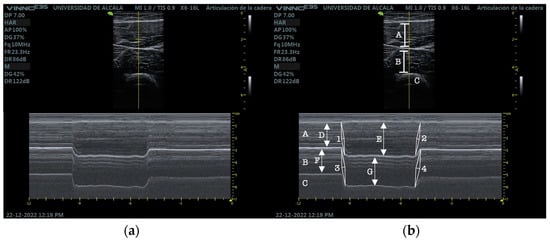
Figure 2.
(a) RUSI M-mode RF and VIM. (b) RUSI M-mode RF and VIM measurements. RF (A), VIM (B), cortical bone of the femur (C), RF at rest (D), RF at isometric contraction (E), RF contraction velocity (1), RF relaxation velocity (2), VIM at rest (F), VIM at isometric contraction (G), VIM contraction velocity (3), VIM relaxation velocity (4).
All participants were trained prior to measurement to avoid offsets.
2.7. Statistical Analysis
Statistical analysis was performed with the program R Ver. 4.1.3. (R Foundation for Statistical Computing, Institute for Statistics and Mathematics, Welthandelsplatz 1, 1020 Vienna, Austria). The significance level was set at p < 0.05.
The distribution of the quantitative variables was tested with the Kolmogorov–Smirnov test with Lillierfors correction.
Quantitative variables are shown with mean ± standard deviation and qualitative variables with absolute and relative values (%).
The presence of significant differences in the average velocity of contraction and relaxation and in the ratio of contraction between the RF and the VIM was tested using the Student’s T test for dependent samples or the Wilcoxon Signed Rank test depending on the distribution of the variables. The effect size was defined in the variables by a normal distribution with Cohen’s D test, defining it as small (<0.5), moderate (0.5–0.8) and large (0.8); in the variables with a non-normal distribution, it was defined by the non-parametric r statistic as small (<0.4), moderate (0.4–0.6), and large (>0.6).
The three variables were modeled according to the muscle using a Generalized Estimating Equations (GEE) model that allows the assumption of dependence of the observations. The selection of the best model was made by evaluating the lowest value in the Quasi-likelihood under Independence Model Criterion (QIC) and with an ANOVA test between models. The best correlation structure was selected evaluating the lowest QIC and the lowest difference between the naive and robust sandwich estimators. The precision of the final model was estimated using the Receiver Operating Characteristic (ROC) curve, taking values greater than 80% in the Area Under Curve (AUC) as the cut-off point.
The correlation between the average of the thickness US variables and the clinical-demographic variables was calculated using the Pearson correlation matrix or by means of a punctual polychoric-polyserial matrix in the case of qualitative variables, defining it as negligible (<0.29), low (0.3–0.49), moderate (0.5–0.69), high (0.70–0.89) and very high (>0.90) [50].
3. Results
The sample consisted of 62 subjects of 20.42 ± 4.97 years, with most of the participants being women (59.7%) with right dominance (88.7%) (Table 1).

Table 1.
Clinical and demographic characteristics of the participants.
Significant differences are shown in the variable average velocity contraction (cm/sg) (Z = 9.661, p < 0.001) with a velocity difference of −2.1 (−2.742, −1.588) cm/s greater in the VIM and a large and significant effect size, and in the variable average velocity relaxation (cm/sg) (Z = 9.548, p < 0.001) with a velocity difference of −1.122 (−1.392, −0.885) cm/s greater in the VIM and with an effect size that is also large and significant (Table 2).

Table 2.
Ratio and velocities of rectus femoris contraction vs. vastus intermedius.
In the GEE model, both the QIC and the ANOVA test indicate that the model with the variables’ contraction ratio and average velocity relaxation is the most appropriate (Supplementary Materials Table S1). It verifies that the exchangeable correlation structure is the one that presents the lowest QIC and the smallest difference between the naive and sandwich estimators (Supplementary Materials Table S2).
The odds ratio of the final model indicates that, significantly, for each point increase in the contraction ratio the probability that the target muscle is the RF decreases 0.9 times (0.827, 0.98) whereas, for each point of increase in the average velocity relaxation the probability that the target muscle is the rectus femoris increases 1.023 times (1.016, 1.03), that is, the RF is associated with increments in the average velocity relaxation while the VIM is associated with increments in the contraction ratio (Table 3).

Table 3.
Final model.
The ROC curve shows a significant AUC of 82.544% 95%CI (75.246–89.843%), which indicates a high discriminatory capacity of the model (Figure 3).
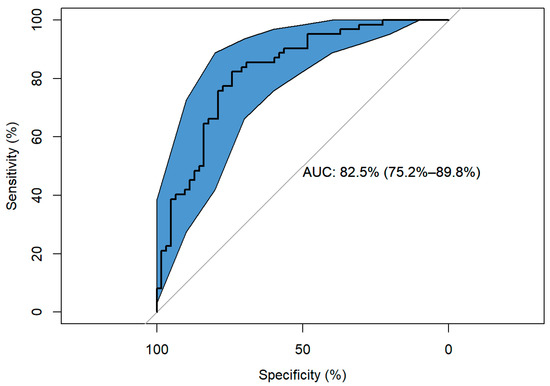
Figure 3.
Response ROC curve.
Significant correlations (p < 0.05) occur in the RF mean thickness at rest vs. RF length, VIM mean contraction thickness vs. distal third perimeter, VIM mean contraction thickness vs. dominant lower limb length, VIM mean contraction thickness vs. middle third perimeter, VIM mean contraction thickness vs. RF length, VIM mean thickness at rest vs. distal third perimeter, VIM mean thickness at rest vs. dominant lower limb length, VIM mean thickness at rest vs. middle third perimeter, VIM mean thickness at rest vs. proximal third perimeter and VIM mean thickness at rest vs. RF length. Comparisons with low positive correlations are shown (Figure 4) (Table 4) (Supplementary Materials Table S3).
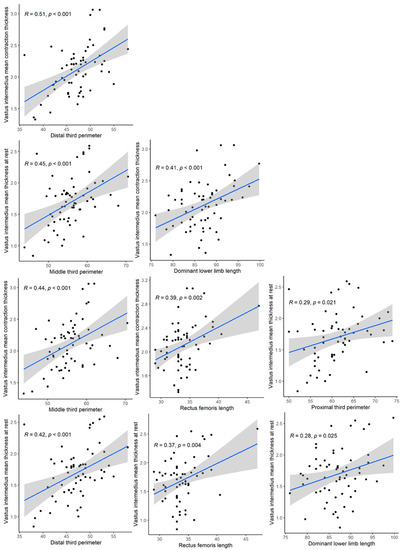
Figure 4.
Significant correlations (p < 0.05).

Table 4.
Baseline outcomes vs. thickness measurements correlations.
4. Discussion
The main purpose of this study was to determine the relationship between RF and VIM behavior measured in RUSI M-mode under MVIC and anthropometric measurements. The hypothesis of this study is that there could be a linear correlation between anthropometric variables and muscle behavior measured by ultrasound. The results obtained in this research showed a significant correlation in the comparison of some variables, especially those related to the VIM. In relation to the variables’ QT length, distance between spines, RF and VIM contraction ratio, no significant correlations were found.
There is a correlation between resting VIM thickness and three other variables: three-thirds thigh perimeters, RF length and lower limb length. VIM thickness contraction correlates with three other variables: proximal, medial and distal thigh perimeters, RF length and lower limb length. The only correlation found in relation to RF was resting thickness with RF length. In the correlations described above, the higher the RUSI measurement, the higher the anthropometric variables were.
Regarding the statistical analysis it must be noted that this test indicates that the best model is the one that assumes an exchangeable correlation structure versus independence, so the model that assumes a fixed correlation between each pair of RF and VIM measurements in each subject fits better than the one that assumes no correlation.
This is a statistical argument that reinforces considering the data related, because the best model is the one that assumes this correlation structure, although biomechanically it is possible to consider two independent muscles.
The “exchangeable” one is the one with the least amount of information not explained by the model and the one where the difference between the standard and robust errors is the smallest (Figure 3).
In previous studies where QF has been evaluated, RUSI has proven to be a valid and reliable tool with excellent ICC in the determination of muscle thickness (ICC = 0.95), contraction and relaxation velocity (ICC 0.99 (CI 0.99–1.00)) [47,51,52].
The results for RF thickness at rest (2.11 ± 0.44) and contraction (2.72 ± 0.52), are comparable to those obtained in previous studies describing resting RF values of (2.1 ± 0.22) [45] and contraction (2.72 ± 0.17) [47].
The data obtained in relation to VIM thickness at rest (1.69 ± 0.40) are like those obtained in a previous study in a similar population (1.71 ± 0.44) [53].
Regarding the values of thickness in contraction of the VIM, the results obtained (2.11 ± 0.38) are not comparable to others, since to the authors’ knowledge there are no reference values described in the literature to date.
A previous study demonstrated a loss of VIM thickness at rest after ACL reconstruction surgery [54]. It is considered that it could be of interest to determine the possible changes in thickness at contraction, since they could provide information regarding the functionality of the muscle. The results obtained in this investigation in relation to this variable could serve as a criterion for comparison in future studies.
These results give more robustness to the existing normality values described for the thickness of RF and VIM in healthy subjects. Considering the clinical significance of the loss of RF and VIM thickness in both subjects with ACL injuries and in subjects who are in a state of frailty or sarcopenia, we consider that for future research these data could be of interest to determine global reference values.
There is evidence for the validity and reliability of contraction and relaxation velocity measurements in different muscle groups, however, there is still not enough evidence to establish normative values [42,55,56].
The results obtained for RF contraction and relaxation velocities (4.42 ± 2.51 and 2.39 ± 0.77) differ considerably from those described in studies with similar methodologies (1.07 ± 0.08 and 0.93 ± 007) [47]. The research team believes that this difference may be due to variables related to the indications given to the subject at the time of the contraction [49], different populations sampled and no data about the dynamometry to be compared. It is also known that the volume intensity of the verbal command can modify the response obtained, since higher verbal commands have greater muscular recruitment [57,58].
With respect to the results obtained for the contraction and relaxation velocity of the VIM (7.44 ± 7.07 and 3.58 ± 1.25), there are no comparison values since this study was conducted.
Although no changes in contraction and relaxation velocity have been found in the RF in subjects who have undergone ACL reconstruction surgery, this has only been evaluated in the long term and has not been studied in the short or medium term [47]. Similarly, these changes have not been evaluated in the VIM in subjects who have received surgical or conservative treatment.
Given the biomechanical relevance of the VIM in the knee joint, carrying out an evaluation with RUSI M-mode could provide information regarding variables that may not be taken into account and that would be of importance when following up the recovery process of the patients.
There is a considerable difference between the results obtained in the RUSI variables between RF and VIM, both in thickness and velocities. The difference in thickness between RF and VIM could be conditioned by the muscle morphology, the type of muscle contraction and the position of the knee during the evaluation [59,60].
Differences in contraction and relaxation velocity between RF and VIM could be due to early activation of VIM relative to RF, which could condition contraction velocity [60].
The results of the morphometric variables are within the mean parameters of normality established for each one of them [61,62,63].
The lack of correlation found in the present study between anthropometric variables and muscle activation (contraction ratio and contraction/relaxation velocity) demonstrates that there are variables intrinsic to the musculature that could justify the changes in muscle behavior.
Based on the observations and results obtained in the present investigation, we consider that in future research the morphometric variables that could condition muscular behavior should be evaluated (pennation angle, fascicle length or cross-sectional area). Likewise, it would be interesting to replicate the present methodology in clinical populations, as in the case of subjects with ACL injuries.
The implementation of the methodology used in this research could be of clinical interest as an evaluation and monitoring tool in processes in which a change in the muscle morphology of the RF and VIM, as well as changes in the velocity of contraction/relaxation, are sought to be evidenced.
The implementation of RUSI as a visual feedback tool has proven to be useful in improving performance and retention in motor control exercise in different regions of the body [64]. Implementing RUSI M-mode in future research lines could be an interesting tool in the management of pathologies that involve changes in the motor control of the QF musculature, allowing to explain to patients in a simple way the changes in the image that are associated with the movement of the contracting muscle in a simple way.
The results described here should be analyzed with caution considering the specific conditions of the study and the sample from which they were obtained.
Limitations
This study had several limitations. One of the major ones being that it was carried out with healthy subjects, and only evaluated the dominant leg.
Furthermore, the thickness of all the tissues that form the perimetry of the thigh (muscles, adipose and connective tissue etc.) has not been evaluated. The significant correlation between the thickness of the VIM and the perimetry raises the question whether this correlation is also present in other tissues in the same area.
Similarly, it would have been interesting to evaluate the behavior of the rest of the muscles of QF; however, this was outside the research objective of the present study.
5. Conclusions
In this study it was determined that the VIM thickness correlated with the perimeter in the three thirds of the thigh at rest and under MVIC, and with the length of the lower limb. In both RF and VIM muscles, there is a correlation with the RF length, being the only statistically significant variable in both.
The anthropometry of the lower limb correlated with muscle morphometry, mainly of the VIM.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13042589/s1. Table S1: Model variables selection; Table S2: Values for each correlation structure; Table S3: Baseline outcomes vs. thickness measurements correlations.
Author Contributions
Conceptualization, F.N.-C., M.G.-E., P.B.-S., D.P.-M. and S.F.-C.; methodology, J.N.C.-Z., D.P.-M., M.G.-E. and A.A.-O.; formal analysis, S.F.-C., J.N.C.-Z. and P.B.-S.; investigation, F.N.-C., D.P.-M., and S.F.-C.; resources, D.P.-M. and S.F.-C.; data curation, A.A.-O., J.Q.-P. and P.B.-S.; writing—original draft preparation, F.N.-C., D.P.-M. and S.F.-C.; writing—review and editing, F.N.-C., J.Q.-P., D.P.-M. and S.F.-C.; supervision, D.P.-M. and S.F.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee for Research and Animal Experimentation of the University of Alcala (Ethics ID Number: CEID/2022/03/044 and date of approval 10 Junio 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data sets used and/or analyzed in the current study or any query regarding the research process are available from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Waligora, A.C.; Johanson, N.A.; Hirsch, B.E. Clinical Anatomy of the Quadriceps Femoris and Extensor Apparatus of the Knee. Clin. Orthop. Relat. Res. 2009, 467, 3297–3306. Available online: https://link.springer.com/article/10.1007/s11999-009-1052-y (accessed on 19 December 2022). [CrossRef] [PubMed]
- Franchi, T. Tensor vastus intermedius: A review of its discovery, morphology and clinical importance. Folia Morphol. 2021, 80, 792–798. Available online: https://pubmed.ncbi.nlm.nih.gov/33084009/ (accessed on 22 December 2022). [CrossRef] [PubMed]
- Zhang, L.Q.; Wang, G.; Number, G.W.; Press, J.M.; Koh, J.L. In vivo load sharing among the quadriceps components. J. Orthop. Res. 2003, 21, 571. Available online: https://www.ncbi.nlm.nih.gov/www.elsevier.com/locatelorthres (accessed on 8 February 2023). [CrossRef] [PubMed]
- Pincivero, D.M.; Gandhi, V.; Timmons, M.K.; Coelho, A.J. Quadriceps femoris electromyogram during concentric, isometric and eccentric phases of fatiguing dynamic knee extensions. J. Biomech. 2006, 39, 246–254. Available online: https://pubmed.ncbi.nlm.nih.gov/16321626/ (accessed on 8 February 2023). [CrossRef]
- Oda, T.; Malis, V.; Finni, T.; Kinugasa, R.; Sinha, S. Dynamics of Quadriceps Muscles during Isometric Contractions: Velocity-Encoded Phase Contrast MRI Study. Diagnostics 2021, 11, 2280. Available online: https://pubmed.ncbi.nlm.nih.gov/34943517/ (accessed on 8 February 2023). [CrossRef]
- Maniar, N.; Cole, M.H.; Bryant, A.L.; Opar, D.A. Muscle Force Contributions to Anterior Cruciate Ligament Loading. Sports Med. 2022, 52, 1737–1750. Available online: https://link.springer.com/article/10.1007/s40279-022-01674-3 (accessed on 19 December 2022). [CrossRef]
- Werner, S. Anterior knee pain: An update of physical therapy. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2286–2294. Available online: https://link.springer.com/article/10.1007/s00167-014-3150-y (accessed on 19 December 2022). [CrossRef]
- Majewski, M.; Susanne, H.; Klaus, S. Epidemiology of athletic knee injuries: A 10-year study. Knee 2006, 13, 184–188. [Google Scholar] [CrossRef]
- Chia, L.; Silva, D.D.O.; Whalan, M.; McKay, M.J.; Sullivan, J.; Fuller, C.W.; Pappas, E. Non-contact Anterior Cruciate Ligament Injury Epidemiology in Team-Ball Sports: A Systematic Review with Meta-analysis by Sex, Age, Sport, Participation Level, and Exposure Type. Sports Med. 2022, 52, 2447–2467. [Google Scholar] [CrossRef]
- Smith, H.C.; Vacek, P.; Johnson, R.J.; Slauterbeck, J.R.; Hashemi, J.; Shultz, S.; Beynnon, B.D. Risk Factors for Anterior Cruciate Ligament Injury: A Review of the Literature—Part 1: Neuromuscular and Anatomic Risk. Sport Health 2012, 4, 69–78. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3435896/ (accessed on 8 December 2022). [CrossRef]
- Anderson, A.F.; Dome, D.C.; Gautam, S.; Awh, M.H.; Rennirt, G.W. Correlation of Anthropometric Measurements, Strength, Anterior Cruciate Ligament Size, and Intercondylar Notch Characteristics to Sex Differences in Anterior Cruciate Ligament Tear Rates. Am. J. Sports Med. 2001, 29, 58–66. Available online: https://journals.sagepub.com/doi/10.1177/03635465010290011501?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed (accessed on 7 February 2023). [CrossRef] [PubMed]
- Hasani, S.; Feller, J.A.; Webster, K.E. Familial Predisposition to Anterior Cruciate Ligament Injury: A Systematic Review with Meta-analysis. Sports Med. 2022, 52, 2657–2668. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.C.; Vacek, P.; Johnson, R.J.; Slauterbeck, J.R.; Hashemi, J.; Shultz, S.; Beynnon, B.D. Risk Factors for Anterior Cruciate Ligament Injury: A Review of the Literature—Part 2: Hormonal, Genetic, Cognitive Function, Previous Injury, and Extrinsic Risk Factors. Sports Health 2012, 4, 155–161. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3435909/ (accessed on 7 February 2023). [CrossRef] [PubMed]
- Gans, I.; Retzky, J.S.; Jones, L.C.; Tanaka, M.J. Epidemiology of Recurrent Anterior Cruciate Ligament Injuries in National Collegiate Athletic Association Sports: The Injury Surveillance Program, 2004-2014. Orthop. J. Sports Med. 2018, 6, 2325967118777823. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6024527/ (accessed on 7 February 2023). [CrossRef] [PubMed]
- Salmon, L.; Russell, V.; Musgrove, T.; Pinczewski, L.; Refshauge, K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy 2005, 21, 948–957. Available online: https://pubmed.ncbi.nlm.nih.gov/16084292/ (accessed on 7 February 2023). [CrossRef] [PubMed]
- Deng, M.; Mansfield, M. Association between Body Weight and Body Mass Index and Patellar Tendinopathy in Elite Basketball and Volleyball Players, a Systematic Review and Meta-Analysis. Healthcare 2022, 10, 1928. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9601617/ (accessed on 8 December 2022). [CrossRef]
- Sanghi, D.; Srivastava, R.N.; Singh, A.; Kumari, R.; Mishra, R.; Mishra, A. The association of anthropometric measures and osteoarthritis knee in non-obese subjects: A cross sectional study. Clinics 2011, 66, 275–279. Available online: https://www.elsevier.es/en-revista-clinics-22-articulo-the-association-anthropometric-measures-osteoarthritis-S1807593222015289 (accessed on 8 December 2022). [CrossRef]
- Hart, J.M.; Pietrosimone, B.; Hertel, J.; Ingersoll, C.D. Quadriceps Activation Following Knee Injuries: A Systematic Review. J. Athl. Train. 2010, 45, 87–97. [Google Scholar] [CrossRef]
- Casartelli, N.C.; Item-Glatthorn, J.F.; Friesenbichler, B.; Bizzini, M.; Salzmann, G.M.; Maffiuletti, N.A. Clinical Medicine Quadriceps Neuromuscular Impairments after Arthroscopic Knee Surgery: Comparison between Procedures. J. Clin. Med. 2019, 8, 1881. Available online: www.mdpi.com/journal/jcm (accessed on 8 December 2022). [CrossRef]
- Tim-Yun Ong, M.; Fu, S.C.; Mok, S.W.; Franco-Obregón, A.; Lok-Sze Yam, S.; Shu-Hang Yung, P. Persistent quadriceps muscle atrophy after anterior cruciate ligament reconstruction is associated with alterations in exercise-induced myokine production. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2022, 29, 35–42. [Google Scholar] [CrossRef]
- Rustani, K.; Kundisova, L.; Capecchi, P.L.; Nante, N.; Bicchi, M. Ultrasound measurement of rectus femoris muscle thickness as a quick screening test for sarcopenia assessment. Arch. Gerontol. Geriatr. 2019, 83, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Dutaillis, B.; Maniar, N.; Opar, D.A.; Hickey, J.T.; Timmins, R.G. Lower Limb Muscle Size after Anterior Cruciate Ligament Injury: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 1209–1226. Available online: https://link.springer.com/article/10.1007/s40279-020-01419-0 (accessed on 8 December 2022). [CrossRef] [PubMed]
- Lee, H.-J.; Lee, K.-W.; Takeshi, K.; Lee, Y.-W.; Kim, H.-J. Correlation analysis between lower limb muscle architectures and cycling power via ultrasonography. Sci. Rep. 2021, 11, 5362. [Google Scholar] [CrossRef] [PubMed]
- Suchomel, T.J.; Nimphius, S.; Stone, M.H. The Importance of Muscular Strength in Athletic Performance. Sports Med. 2016, 46, 1419–1449. Available online: https://link.springer.com/article/10.1007/s40279-016-0486-0 (accessed on 9 December 2022). [CrossRef] [PubMed]
- Schmitt, L.C.; Paterno, M.V.; Hewett, T.E. The Impact of Quadriceps Femoris Strength Asymmetry on Functional Performance at Return to Sport Following Anterior Cruciate Ligament Reconstruction. J. Orthop. Sports Phys. Ther. 2012, 42, 750–759. Available online: https://www.jospt.org/doi/10.2519/jospt.2012.4194 (accessed on 8 December 2022). [CrossRef] [PubMed]
- Laudisio, A.; Giovannini, S.; Finamore, P.; Loreti, C.; Vannetti, F.; Coraci, D.; Incalzi, R.A.; Zuccal, G.; Macchi, C.; Padua, L.; et al. Muscle strength is related to mental and physical quality of life in the oldest old. Arch. Gerontol. Geriatr. 2020, 89, 104109. [Google Scholar] [CrossRef]
- Matta, T.T.; Nascimento, F.X.; Trajano, G.S.; Simão, R.; Willardson, J.M.; Oliveira, L.F. Selective hypertrophy of the quadriceps musculature after 14 weeks of isokinetic and conventional resistance training. Clin. Physiol. Funct. Imaging 2017, 37, 137–142. Available online: https://pubmed.ncbi.nlm.nih.gov/26184103/ (accessed on 8 December 2022). [CrossRef]
- Worsley, P.R.; Kitsell, F.; Samuel, D.; Stokes, M. Validity of measuring distal vastus medialis muscle using rehabilitative ultrasound imaging versus magnetic resonance imaging. Man. Ther. 2014, 19, 259–263. [Google Scholar] [CrossRef]
- Balshaw, T.G.; Fry, A.; Maden-Wilkinson, T.M.; Kong, P.W.; Folland, J.P. Reliability of quadriceps surface electromyography measurements is improved by two vs. single site recordings. Eur. J. Appl. Physiol. 2017, 117, 1085–1094. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5427161/ (accessed on 11 December 2022). [CrossRef]
- Wang, F.-Z.; Sun, H.; Zhou, J.; Sun, L.-L.; Pan, S.-N. Reliability and Validity of Abdominal Skeletal Muscle Area Measurement Using Magnetic Resonance Imaging. Acad. Radiol. 2021, 28, 1692–1698. [Google Scholar] [CrossRef]
- Fernández Carnero, S.; Arias Buria, J.L.; Cuenca Zaldívar, J.N.; Leal Quiñones, A.; Calvo-Lobo, C.; Martin Saborido, C. Rehabilitative Ultrasound Imaging Evaluation in Physiotherapy: Piloting a Systematic Review. Appl. Sci. 2019, 9, 181. Available online: www.mdpi.com/journal/applsci (accessed on 11 December 2022). [CrossRef]
- Teyhen, D.; Koppenhaver, S. Rehabilitative ultrasound imaging. J. Physiother. 2011, 57, 196. Available online: https://pubmed.ncbi.nlm.nih.gov/21843838/ (accessed on 11 December 2022). [CrossRef] [PubMed]
- Fernández-Carnero, S.; Calvo-Lobo, C.; Garrido-Marin, A.; Arias-Buría, J.L. 2nd Rehabilitative Ultrasound Imaging Symposium in Physical Therapy, Madrid, Spain, 3–5 June 2016. Br. J. Sports Med. 2018, 52 (Suppl. S2), A1–A4. Available online: https://bjsm.bmj.com/content/52/Suppl_2/A1 (accessed on 11 December 2022).
- Mendis, M.D.; Wilson, S.J.; Stanton, W.; Hides, J.A. Validity of Real-Time Ultrasound Imaging to Measure Anterior Hip Muscle Size: A Comparison With Magnetic Resonance Imaging. J. Orthop. Sports Phys. Ther. 2010, 40, 577–581. Available online: https://pubmed.ncbi.nlm.nih.gov/20479536/ (accessed on 10 December 2022). [CrossRef] [PubMed]
- Mechelli, F.; Arendt-Nielsen, L.; Stokes, M.; Agyapong-Badu, S. Validity of Ultrasound Imaging Versus Magnetic Resonance Imaging for Measuring Anterior Thigh Muscle, Subcutaneous Fat, and Fascia Thickness. Methods Protoc. 2019, 2, 58. Available online: https://pubmed.ncbi.nlm.nih.gov/31295936/ (accessed on 10 December 2022). [CrossRef]
- Giles, L.S.; Webster, K.E.; McClelland, J.A.; Cook, J. Can ultrasound measurements of muscle thickness be used to measure the size of individual quadriceps muscles in people with patellofemoral pain? Phys. Ther. Sport. 2015, 16, 45–52. Available online: https://pubmed.ncbi.nlm.nih.gov/24894764/ (accessed on 10 December 2022). [CrossRef]
- Miyachi, R.; Yamazaki, T.; Ohno, N.; Miyati, T. Relationship between Muscle Cross-Sectional Area by MRI and Muscle Thickness by Ultrasonography of the Triceps Surae in the Sitting Position. Healthcare 2020, 8, 166. Available online: https://pubmed.ncbi.nlm.nih.gov/32532123/ (accessed on 10 December 2022). [CrossRef]
- Högelin, E.R.; Thulin, K.; von Walden, F.; Fornander, L.; Michno, P.; Alkner, B. Reliability and Validity of an Ultrasound-Based Protocol for Measurement of Quadriceps Muscle Thickness in Children. Front. Physiol. 2022, 13, 1268. [Google Scholar] [CrossRef]
- El-Ansary, D.; Marshall, C.J.; Farragher, J.; Annoni, R.; Schwank, A.; McFarlane, J.; Bryant, A.; Han, J.; Webster, M.; Zito, G.; et al. Architectural anatomy of the quadriceps and the relationship with muscle strength: An observational study utilising real-time ultrasound in healthy adults. J. Anat. 2021, 239, 847–855. Available online: https://onlinelibrary.wiley.com/doi/10.1111/joa.13497 (accessed on 12 December 2022). [CrossRef]
- Chauhan, B.; A Hamzeh, M.; I Cuesta-Vargas, A. Prediction of muscular architecture of the rectus femoris and vastus lateralis from EMG during isometric contractions in soccer players. Springerplus 2013, 2, 548. Available online: https://link.springer.com/articles/10.1186/2193-1801-2-548 (accessed on 10 December 2022). [CrossRef]
- Dieterich, A.V.; Pickard, C.M.; Deshon, L.E.; Strauss, G.R.; Gibson, W.; Davey, P.; McKay, J. M-mode ultrasound used to detect the onset of deep muscle activity. J. Electromyogr. Kinesiol. 2015, 25, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, A.; Deshon, L.; Strauss, G.R.; McKay, J.; Pickard, C.M. M-mode ultrasound reveals earlier gluteus minimus activity in individuals with chronic hip pain during a step-down task. J. Orthop. Sport. Phys. Ther. 2016, 46, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Romero-Morales, C.; Calvo-Lobo, C.; Navarro-Flores, E.; Mazoteras-Pardo, V.; García-Bermejo, P.; López-López, D.; Martínez-Jiménez, E.M.; De-la-Cruz-Torres, B. M-Mode Ultrasound Examination of Soleus Muscle in Healthy Subjects: Intra- and Inter-Rater Reliability Study. Healthcare 2020, 8, 555. Available online: https://pubmed.ncbi.nlm.nih.gov/33322505/ (accessed on 12 December 2022). [CrossRef] [PubMed]
- Hernández-Socorro, C.R.; Saavedra, P.; López-Fernández, J.C.; Ruiz-Santana, S. Assessment of Muscle Wasting in Long-Stay ICU Patients Using a New Ultrasound Protocol. Nutrients 2018, 10, 1849. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6316061/ (accessed on 11 December 2022). [CrossRef]
- Ma, A.J.T.; Tenan, M.S.; Haynes, C.A. Differences in muscle contraction onset as determined by ultrasound and electromyography. Muscle Nerve 2019, 59, 494–500. Available online: https://pubmed.ncbi.nlm.nih.gov/30536792/ (accessed on 11 December 2022).
- Petraş, A.; Drăgoi, R.G.; Pupazan, V.; Drăgoi, M.; Popa, D.; Neagu, A. Using portable ultrasound to monitor the neuromuscular reactivity to low-frequency electrical stimulation. Diagnostics 2021, 11, 65. Available online: https://www.researchgate.net/publication/348202327_Using_Portable_Ultrasound_to_Monitor_the_Neuromuscular_Reactivity_to_Low-Frequency_Electrical_Stimulation (accessed on 11 December 2022). [CrossRef]
- Buelga-Suarez, J.; Alba-Martin, P.; Cuenca-Zaldívar, N.; García-Escudero, M.; Bierge-Sanclemente, P.; Almazán-Polo, J.; Fernández-Carnero, S.; Pecos-Martín, D. Test-Retest Reliability of Ultrasonographic Measurements from the Rectus Femoris Muscle 1–5 Years after Anterior Cruciate Ligament Reconstruction in the Ipsilateral and Contralateral Legs: An Observational, Case-Control Study. J. Clin. Med. 2022, 11, 1867. Available online: https://www.mdpi.com/2077-0383/11/7/1867/htm (accessed on 5 December 2022). [CrossRef]
- Neelly, K.; Wallmann, H.W.; Backus, C.J. Validity of measuring leg length with a tape measure compared to a computed tomography scan. Physiother. Theory Pract. 2013, 29, 487–492. Available online: https://www.researchgate.net/publication/234068603_Validity_of_measuring_leg_length_with_a_tape_measure_compared_to_a_computed_tomography_scan (accessed on 22 December 2022). [CrossRef]
- Kesemenli, C.C.; Sarman, H.; Baran, T.; Memisoglu, K.; Binbir, I.; Savas, Y.; Isik, C.; Boyraz, I.; Koc, B. A new isometric quadriceps-strengthening exercise using EMG-biofeedback. Int. J. Clin. Exp. Med. 2014, 7, 2651–2655. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4211772/ (accessed on 22 December 2022).
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3576830/ (accessed on 23 December 2022).
- Takahashi, Y.; Fujino, Y.; Miura, K.; Toida, A.; Matsuda, T.; Makita, S. Intra- and inter-rater reliability of rectus femoris muscle thickness measured using ultrasonography in healthy individuals. Ultrasound J. 2021, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Filippo, M.; Lars, A.-N.; Maria, S.; Sandra, A.-B. Inter-rater and intra-rater reliability of ultrasound imaging for measuring quadriceps muscle and non-contractile tissue thickness of the anterior thigh. Biomed. Phys. Eng. Express 2019, 5, 037002. Available online: https://iopscience.iop.org/article/10.1088/2057-1976/ab102f (accessed on 27 December 2022). [CrossRef]
- Strasser, E.M.; Draskovits, T.; Praschak, M.; Quittan, M.; Graf, A. Association between ultrasound measurements of muscle thickness, pennation angle, echogenicity and skeletal muscle strength in the elderly. Age 2013, 35, 2377–2388. Available online: https://pubmed.ncbi.nlm.nih.gov/23456136/ (accessed on 27 December 2022). [CrossRef] [PubMed]
- Lee, J.H.; Cheon, S.; Jun, H.P.; Huang, Y.L.; Chang, E. Bilateral Comparisons of Quadriceps Thickness after Anterior Cruciate Ligament Reconstruction. Medicina 2020, 56, 335. Available online: https://www.mdpi.com/1648-9144/56/7/335/htm (accessed on 7 February 2023). [CrossRef] [PubMed]
- Sicilia-Gomez, C.; Fernández-Carnero, S.; Martin-Perez, A.; Cuenca-Zaldívar, N.; Naranjo-Cinto, F.; Pecos-Martín, D.; Cervera-Cano, M.; Nunez-Nagy, S. Abdominal and Pelvic Floor Activity Related to Respiratory Diaphragmatic Activity in Subjects with and without Non-Specific Low Back Pain. Diagnostics 2022, 12, 2530. [Google Scholar] [CrossRef]
- Calvo-Lobo, C.; Almazán-Polo, J.; Becerro-de-Bengoa-Vallejo, R.; Losa-Iglesias, M.E.; Palomo-López, P.; Rodrí-guez-Sanz, D.; López-López, D. Ultrasonography comparison of diaphragm thickness and excursion between athletes with and without lumbopelvic pain. Phys. Ther. Sport 2019, 37, 128–137. Available online: https://pubmed.ncbi.nlm.nih.gov/30954705/ (accessed on 27 December 2022). [CrossRef]
- Silva, S.B.; De Abreu, L.C.; Valenti, V.E.; Nogueira, D.V.; Moraes, É.R.; Natividade, V.; Gallo, P.R.; Gallo, D.; Zacaro, P. Verbal and visual stimulation effects on rectus femoris and biceps femoris muscles during isometric and concentric. Int. Arch. Med. 2013, 6, 38. Available online: https://intarchmed.biomedcentral.com/articles/10.1186/1755-7682-6-38 (accessed on 27 December 2022). [CrossRef]
- Johansson, C.A.; Kent, B.E.; Shepard, K.F. Relationship Between Verbal Command Volume and Magnitude of Muscle Contraction. Phys. Ther. 1983, 63, 1260–1265. Available online: https://academic.oup.com/ptj/article/63/8/1260/2727605 (accessed on 27 December 2022). [CrossRef]
- Ema, R.; Wakahara, T.; Mogi, Y.; Miyamoto, N.; Komatsu, T.; Kanehisa, H.; Kawakami, Y. In vivo measurement of human rectus femoris architecture by ultrasonography: Validity and applicability. Clin. Physiol. Funct. Imaging 2013, 33, 267–273. Available online: https://pubmed.ncbi.nlm.nih.gov/23692615/ (accessed on 28 December 2022). [CrossRef]
- Akima, H.; Saito, A. Activation of quadriceps femoris including vastus intermedius during fatiguing dynamic knee extensions. Eur. J. Appl. Physiol. 2013, 113, 2829–2840. [Google Scholar] [CrossRef]
- Xerogeanes, J.W.; Mitchell, P.M.; Karasev, P.A.; Kolesov, I.A.; Romine, S.E. Anatomic and Morphological Evaluation of the Quadriceps Tendon Using 3-Dimensional Magnetic Resonance Imaging Reconstruction. Am. J. Sports Med. 2013, 41, 2392–2399. Available online: https://journals.sagepub.com/doi/10.1177/0363546513496626?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed (accessed on 28 December 2022). [CrossRef] [PubMed]
- Aitken, S.A. Normative Values for Femoral Length, Tibial Length, and the Femorotibial Ratio in Adults Using Standing Full-Length Radiography. Osteology 2021, 1, 86–91. Available online: https://www.researchgate.net/publication/351568076_Normative_Values_for_Femoral_Length_Tibial_Length_and_the_Femorotibial_Ratio_in_Adults_Using_Standing_Full-Length_Radiography (accessed on 27 December 2022). [CrossRef]
- Ando, R.; Saito, A.; Umemura, Y.; Akima, H. Local architecture of the vastus intermedius is a better predictor of knee extension force than that of the other quadriceps femoris muscle heads. Clin. Physiol. Funct. Imaging 2015, 35, 376–382. Available online: https://pubmed.ncbi.nlm.nih.gov/24915999/ (accessed on 28 December 2022). [CrossRef] [PubMed]
- Valera-Calero, J.A.; Fernández-De-las-peñas, C.; Varol, U.; Ortega-Santiago, R.; Gallego-Sendarrubias, G.M.; Arias-Buría, J.L. Ultrasound Imaging as a Visual Biofeedback Tool in Rehabilitation: An Updated Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 7554. Available online: https://pubmed.ncbi.nlm.nih.gov/34300002/ (accessed on 8 February 2023). [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).