Abstract
The ice plant (Mesembryanthemum crystallinum L.) is a type of succulent known to contain various minerals and beneficial compounds and is enriched in compounds exhibiting a diversity of bioactivity. This study aims to determine the potential antioxidant and anti-inflammatory effects of the ice plant by organs (cotyledon, stem, and leaf). The ice plant ethanol extract showed that antioxidant activity, measured by the DPPH radical scavenging ability (51.79 ± 4.18%), and hydroxyl radical scavenging activities (6.57 ± 0.29%) had lower levels than those of control BHT, but had higher antioxidant activity in the leaves of the ice plant, while ABTS+ (58.91 ± 7.23%) and Fe2+ chelating activity (16.89 ± 1.68%) showed high antioxidant activity in the cotyledon. Total polyphenols (115.43 ± 0.47 mg QE/g) and flavonoid contents (1218.07 ± 1.00 mg GAE/g) were notably high in the cotyledon. UHPLC-TOF/HRMS analyses identified 17 polyphenolic compounds of ice plants. The main constituents of the extracts obtained in this study were flavonoids, including their O-glycosides, and compounds not previously described were found. The production of NO as a major indicator of the inflammatory response was found to decrease at 100 and 300 μg/mL (p < 0.05). The levels of the inflammatory cytokines TNF-α in 10 μg/mL (p < 0.05), 50, 100, and 300 μg/mL (p < 0.000); IL-6 in 100 and 300 μg/mL (p < 0.01); and IL-1β in 50, 100 μg/mL (p < 0.01), and 300 μg/mL (p < 0.05) confirmed that the anti-inflammatory effect was exhibited by inhibiting the production of inflammatory cytokines according to the concentration of each organ of the ice plant.
1. Introduction
The ice plant (Mesembryanthemum crystallinum L.) is actually a succulent perennial plant, not an annual herbaceous plant. Additionally, it is rather in the genus Mesembryanthemum, which is in the family Aizoaceae. The plant is also referred to as the common ice plant or crystal ice plant, with the name ice plant being generically used to represent the resemblance of the stems and leaves to ice crystals. Bladder cells have ice crystal-like shapes on the stems and leaves. They contain various minerals and beneficial compounds such as inositol and β-carotene [1,2]. Ice plants germinate, photosynthesize, and grow as C3 plants. They then convert to CAM-type plants, where growth as succulents involves seed germination, followed by the formation of cotyledons with four or five leaves, and subsequent stem growth leading to shoot formation [3]. Nam et al. 2017 [4] verified the antioxidant, antidiabetic, and hepatoprotective effects of ice plants after fermentation. Kang et al. [5] reported on the angiotensin-converting enzyme inhibition in 3T3-L1 preadipocyte differentiation, whereas Lee et al. [6] investigated the bioactivities of ice plant extracts. It is common for various plants to exhibit highly varied compositions and beneficial bioactivities in relation to the cultivation conditions, harvest period, and plant parts. Several studies have reported the various functional ingredients from different plant parts, such as antioxidant activity from Kang et al. [7], physiological activity from parts of the Mulberry plant [8], and antioxidant and phytochemical activity from extracts from different parts of Kim et al. [9]. However, no study has yet investigated the varied functionality of the different parts of ice plants, such as the cotyledon, stem, and leaf, regarding nutritional composition and mineral analyses to utilize their functionality efficiently. In crassulacean acid metabolism (CAM) plants, CO2 is reduced to C4 by phosphoenolpyruvate carboxylase (PEPcase) and stored before it is fixed as glucose via the C3 pathway. Therefore, there is an increasing possibility that the C4 compounds of ice plants, which are CAM plants, can be used as biochemical pathway precursors in the production of phenolic compounds and flavonoids. In addition, in the area of a plant that acts as a source, the generation pathway of such an antioxidant may operate smoothly compared to a plant that acts as a sink.
This study aimed to characterize the nutritional and biochemical composition in plant organs of ice plant, such as the cotyledon, stem, and leaf. There is limited information regarding the phytochemistry and bioactivity of M. crystallinum L. Therefore, this study evaluated their cytotoxicity, antioxidation, and anti-inflammation activities. In this study, ethanol extracts were first prepared for each organ according to the growth, cotyledon, stem, and leaf of the ice plant. First, the antioxidant activity was analyzed for each extract, and anti-inflammatory experiments were conducted by inducing a strong inflammatory response in macrophages and measuring the release of cytokines and nitric oxide. Furthermore, the murine macrophage-derived cell line RAW264.7 became multinuclear through cell–cell fusion after incubation with purified LPS [10]. This led to anti-inflammatory effects on LPS-activated RAW 264.7 cells, as evidenced by a reduction in cytokine release and nitric oxide production.
2. Materials and Methods
2.1. Sample Preparation
The ice plants used in this study were purchased from the Yesan Ice Plant Signal Agricultural Corporation in the geographical location of Republic of Korea, postal code 32446. They were Grown in October 2022. They were separated into different parts, including the apical part of the first and second leaf pair, the stem apex, and the basal bud. After purchase, the plants were separated into cotyledons, stems, and shoots (Figure 1). Preserved plant samples with unique voucher sample numbers (C1, S1, ss) are stored in room 402 of Sookmyung Women’s University Soonheon Hall for reference. The ice plants were freeze-dried for 72 h using the MCFD 8505 machine from Ilshin Lab Co. in Seoul, Korea, and then, ground to obtain a powder. Extracts were concentrated using a rotary evaporator (N-1000) from Eyela Co. in Tokyo, Japan.

Figure 1.
Photographs of the ice plant (Mesembryanthemum crystallinum) to characterize the properties of biochemical function and contents: (a) whole plant, (b) coleoptile, (c) stem, and (d) leaf. The leaf and stem are separated into apex and basal parts.
2.2. Total Phenolic Content
The total phenolic content of each extract was determined by the Folin–Ciocalteau method [11]. The appropriate dilutions of extracts were oxidized with Folin−Ciocalteu reagent, and the reaction was neutralized with sodium carbonate. Absorbance of the resulting blue color was measured at 570 nm after 60 min using a microplate reader (Molecular Devices, Sunnyvale, CA, USA). Using gallic acid as standard, the total phenolic content was expressed as micromoles of gallic acid equivalent per gram for each organ. Data are reported as mean ± S.D. for at least three replications.
2.3. Total Flavonoid Content
The total flavonoid content was measured using the method reported by Ghasemzadeh et al. [12] after modifications. First, 0.5 mL ethanol extracts from each organ of M. crystallinum L. were briefly mixed with 1.5 mL of 95% ethanol, 0.1 mL of 10% aluminum chloride hexahydrate, 0.1 mL of 1 M potassium acetate, and 2.8 mL of deionized water (DW). After incubation at room temperature for 60 min, the absorbance of the mixture was measured at 415 nm against a distilled water (DW) blank on a visible microplate reader.
2.4. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
The DPPH radical scavenging activity was measured according to the method by Kim et al. [13]. An amount of 1 mL of a 0.1 mM solution of DPPH (Sigma Aldrich, Bengaluru, India) in ethanol was mixed with extracts at different concentrations. The mixture was then incubated at room temperature for 30 min in the dark. The control was prepared by mixing 1 mL of DPPH solution with double DW. The absorbance was measured against a blank at 517 nm using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
2.5. 2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS+) Radical Scavenging Activity
The ABTS+ radical scavenging activity was measured using the method in Re et al. [14]. A solution of ABTS+ was prepared through dilution until the absorbance at 734 nm reached ≤ 1.5. An amount of 1 mL of the reaction mixture contained 10 µL of ethanol extract from each organ, a 20 µL solution of myoglobin from M. crystallinum, and 150 µL ABTS+ reagent (10 mL ABTS and 25 µL 3% H2O2). The mixture was kept at room temperature in the dark, and after 10 min, the optical density was measured using a microplate reader (Molecular Devices, Sunnyvale, CA, USA).
2.6. Fe2+ Chelating Activity
The Fe2+ chelating activity was analyzed using the method in Le et al. [15], after modifications. Samples were mixed in 96-well microplates with pH 4.9, Na acetate buffer and 30 μL of a FeCl2 solution (0.1 mg/mL water). After 30 min, 12.5 μL of ferrozine solution (40 mM in water) was added. The absorbance and color changes of the Fe2+/ferrozine in the solution were measured using a microplate reader at 562 nm using an Infinite 200pro (Tecan, PerkinElmer, Männedorf, canton of Zürich, Switzerland).
2.7. UHPLC-TOF/HRMS Analysis of Phenolic Compounds
Freeze-dried samples were weighed to 0.1 g each, and 5 mL of 80% methanol aqueous solution was used to extract these samples using sonication for 1 h followed by filtering with a 0.2 μm membrane filter. An Ultimate 3000 RSLC UHPLC system (Thermo Fisher Scientific Inc. Waltham, MA, USA) was used to analyze the phenolic compounds for profiling of the ice plant (Mesembryanthemum crystallinum L.) for this study. The system consisted of an autosampler, a column oven, an ultrahigh-pressure solvent delivery pump, and an automatic degasser. Chromatographic separation of the samples was performed using a Waters Cortects T3 (150 × 2.1 mm, 1.6 μm, Waters Co. US-MA). The column temperature was set to 45 °C. The injection volume was 1 μL with a flow rate of 0.4 mL/min. The mobile phases (0.1% formic acid in DW, A; 0.1% formic acid in ACN B) was eluted with the linear gradient programmed as follows: (i) 0–1 min, 3% B; (ii) 1–8 min, from 3% to 10% B; (iii) 30 min, from 10% to 95% B; (iv) 35–35.1 min, from 95–3% B. MS and MS/MS detection were conducted using a Q-Exactive Orbitrap mass spectrometer (Thermo Scientific, Horsham and Loughborough, England, and Inchinnan, Scotland.) operating in a positive and negative electrospray ionization mode. The mass scan was used in full scan mode and data-dependent mass spectra (DDMS2) mode with the mass range set at 100–1500 m/z. The mass spectrometry conditions were set as follows: ion spray voltage of 3.5 kV in positive mode and 3.0 kV in negative mode; ion source type of H-ESI; sheath gas of 50 (Arb); the aux gas pressure of 10 (Arb); sweep gas of 1 (Arb); and source temperature of 320 °C. During the profiling analysis, the phenolic compounds were identified based on their accurate mass (m/z) and molecular (m/z) ion fragmentation pattern using Scafford Elements version 2.2.1. Mass spectral libraries were used including the NIST Library 2017 and the MassBank of North America (MoNA) library database [16,17].
2.8. Viability and Proliferation of Raw 264.7 Cells
Raw 264.7 (2 × 104 cells/well) cells from a murine macrophage cell line were obtained from the Korean Cell Line Bank (KCLB40071). Absorbance was measured using the Multi Detection Reader (Infinite 200, TECAN Group Ltd., Männedorf, Switzerland). The control group was treated solely with a solvent of dissolved samples in the same concentration as the high-concentration test group. Cell viability was calculated using the following formula:
Cell viability (%) = (Absorbance of the treatment group/Absorbance of the control group) × 100.
To monitor the anti-inflammatory effects of LPS treatment, Raw 264.7 cells were aliquoted into a 96-well plate at 2 × 104 cells/80 μL/well for 24 h. The cells were then treated with 1 μg/mL LPS at 0, 10, 30, 50, 100, and 300 μg/mL sample concentrations and incubated for 24 h at 37 °C in a 5% CO2 incubator. Next, 10 μL of WST-1 reagent was added for 1 h and the absorbance was measured.
2.9. NO (Nitric Oxide) Production
To measure NO (nitric oxide) production, Raw 264.7 cells were aliquoted into a 48-well plate at 4 × 106 cells/mL for 24 h. Then, 100 μL of the culture supernatant was mixed with G1 μg/mL of LPS Griess reagent of equal volume. The absorbance was measured at 548 nm. The concentration of the produced NO was calculated using the standard curve of NaNO2 dissolved in Dulbecco’s Modified Eagle medium.
2.10. Cytokine (TNF-α, IL-6, IL-1β) Analysis
The levels of TNF-α (BMS607HS, Invitrogen., Waltham, MA, USA), IL-6 (M6000B, R&D system, Minneapolis, MN, USA), and IL-1β (DY401-05, R&D system., Walsh Ave, Santa Clara, CA, USA) were measured in the culture supernatant by ELISA.
2.11. Statistical Analysis
For statistical analysis, one-way analysis of variance and Duncan post hoc tests were performed. A p-value of <0.05 was considered to be statistically significant between and among parameters (SPSS v26. SPSS Inc., Chicago, IL, USA).
Raw chromatographic data for the identification of phenolic compounds were processed using Scafford Elements version 2.2.1 with automatic peak detection. Mass spectrum deconvolutions were performed with references to the NIST (2017) [16,17].
3. Results and Discussion
3.1. Total Polyphenol and Flavonoid Contents
The phenolic compounds are plant-origin metabolites with a phenolic hydroxyl group that tend to bind with macromolecules such as proteins. They exhibit various bioactivities including antioxidant activity, which removes radicals produced in the body, as well as anticancer, anti-inflammatory, and anti-allergy effects. They are also used as indirect indicators of antioxidant activity based on a positive correlation [12]. The total polyphenol content (in mg GAE/g) in each organ of the ice plant was the highest in the cotyledon (115.43 ± 0.47), followed by the shoot (78.90 ± 0.27) and the stem (78.83 ± 0.40) (Table 1). In the report of Kang et al. [18], the total polyphenol content in an ethanol extract of M. crystallinum was measured as 23.70 ± 0.72 mg GAE/g. In a study by Ibtisseme et al. [19], the total polyphenol content in a heated extract of M. crystallinum was measured as 23.89 ± 0.27 mg GAE/g. The results show that the polyphenol level in the cotyledon was high. Polyphenol is created by plant photosynthesis; therefore, the more chlorophyll a plant has, the higher the level of polyphenol. This was also the case in the Kang et al. [5] study, where the cotyledon with the highest levels of chlorophyll also had the highest levels of polyphenol. Since this is the organs of the leaf with the majority of the chlorophyll, it can be considered that cotyledon extracts show the best activity among the plant organs.

Table 1.
Contents of total polyphenols and total flavonoids of ethanol extracts from different organs of ice plant. (M. crystallinum).
Flavonoids are representative of the antioxidant compounds found in various plants and have a diversity of bioactivities and an ability to suppress the production of lipid peroxides and reactive oxygen species [19,20]. The total flavonoid content (in mg QE/g) was the highest in the cotyledon (1218.07 ± 1.00), followed by the stem (703.97 ± 0.25) and the shoot (671.29 ± 0.63) (Table 1). The content was higher than that measured for the heated extract of M. crystallinum in the Ibtisseme et al. study (4.85 ± 0.9 μg/mg) [19]. The reason for the difference in the results can be attributed to the different methods of extraction. Since flavonoids are fat-soluble antioxidants, ethanol extraction may have had a better yield than hydrothermal extraction. Therefore, comparing these results also shows the differences between the two extracting methods. Flavonoids are also polyphenol compounds commonly found in plant leaves [21], likely to account for the high flavonoid content in the cotyledon of ice plants.
The radical scavenging activity of ice plant (M. crystallinum) extracts was compared with those of butylated hydroxy toluene (BHT) at the same concentration and expressed as a percentage of inhibition against DPPH and ABTS+, respectively (Table 2). The DPPH assay has been reported to allow the analysis of the anti-radical activity in a relatively short time [22]. As the radicals are scavenged by the antioxidants, a color change from purple to yellow reflects a fall in absorbance, while hydrogen forms a stable DPPH-H molecule [20]. In this study, the level of DPPH scavenging in varying concentrations of the three different parts of the ice plant was the highest in the leaves, followed by the stem and cotyledon. The antioxidant effect was lower than that of control BHT.

Table 2.
DPPH and ABTS+ radical scavenging activities with mean ± S.D. (%), ranging from 62.5 μg/mL to 1000 μg/mL of ethanol extract from the cotyledon, stem, and leaves of ice plant (M. crystallinum).
However, the shoot parts showed higher antioxidant activity than BHT in 62.5 ppm in Table 2. For the heated extract of M. crystallinum [17], the level of DPPH scavenging was reported as 75.5 ± 1.07% at 2 mg/mL. In contrast, the DPPH scavenging of 400 μg/mL of ethanol extract of M. crystallinum was 51.3% in the study by Lee et al. [6]. The discrepancy is presumed to be due to the ice plant cultivation conditions, harvest period, and extraction conditions of the ice plant. ABTS+ radical scavenging activity is also a commonly used method of measuring antioxidant activity with DPPH. It is used to measure activation on the principle that ABTS changes from cyan to colorless by reacting with antioxidants [14]. Based on studies showing the correlation between ABTS+ radical scavenging activity and total polyphenol content, ABTS+ scavenging activity in different organs of the ice plant was measured. The results show that the higher the polyphenol content, the higher the antioxidant activity; in Table 2, it is shown in the order of cotyledon > shoot > stem. These results support the results of Lee et al. [6], who tested the antioxidant and anti-inflammatory activity of extracts from red beetroot [23]. In addition, it was consistent with the results of a study by Arnao [24], which reported that ABTS+ radical erasure activity was higher than that of DPPH radical erasure activity when measuring the same sample. This is because ABTS+ produces radicals by enzyme reaction that dissolve in hydrophilic and organic solvents, and DPPH can be dissolved in free radicals in organic solvents to measure the antioxidant activity of hydrophilic and hydrophobic substances.
3.2. Hydroxyl Radical Scavenging Activity and Fe2+ Chelating Activity
Among the oxidizing agents, hydroxyl radicals have the highest toxicity. Since they are generated in the Fenton reaction between Fe2+ and hydrogen peroxides, they serve as important biomarkers to assess the effects of antioxidant activity on candidate materials; this particularly regards to the effects of antioxidant activity on the reduction of oxidative stress, which is induced by reactive oxygen species that have a high level of reactivity. In this study, the hydroxyl radical scavenging activity in different organs of the ice plant was lower compared to the reference material: L-ascorbic acid at 1 mg/mL. Meanwhile, the activity was higher than that of the 16.0% inhibition, caused by 1 mg/mL of the ethanol extract of a fresh sample of Salicornia herbacea, a similar halophyte, in the study by Kim [25]. This suggests that hydroxyl radical scavenging could vary across plants with similar characteristics. The hydroxyl radical scavenging activity in this study was the highest in the leaves, followed by the stem and cotyledon, indicating that for ice plant extracts, despite a relatively low activity, the effect on and removal of hydroxyl radicals varies according to the plant organs (Table 3).

Table 3.
Hydroxyl radical scavenging activity and FE2+ chelating activity with mean ± S.D. (%) ranging from 62.5 μg/mL to 1000 μg/mL of ethanol extract from the cotyledon, stem, and leaf of M. crystallinum L.
The Fe2+ chelating activity leads to the generation of hydroxyl radicals caused by the reduction processes of reactive oxygen species and involves the transfer of hydrogen peroxide. Thus, the chelating of Fe2+ indicates the level of inhibition of free radicals generated via the Fenton reaction. In this study, the Fe+ chelating activity was not high in each organ of the ice plant; however, significant differences were observed in activity, with the highest activity in the cotyledon, followed by the leaves and stems.
3.3. Identification of Phenolic Compounds by UHPLC-TOF/HRMS
A targeted analysis, based on UHPLC-TOF/HRMS (ultra-high-performance liquid chromatography–time-of-flight high-resolution mass spectrometry), was conducted to identify the phenolic compounds present in the ethanol extracts, since the phenolic composition of M. crystallinum is still not reported in the literature. Table 4 shows that the phenolic profile was diverse among the cotyledon, stem, and shoots of M. crystallinum. The 17 compounds detected in extracts of each organ of M. crystallinum were salicylaldehyde, cinnanate, feruloyl hexoside, sinapyl alcohol, sinapic acid, sinapyl alcohol, mellein, roseoside, 2-O-p-coumaroyl-d-glucose, 4′-O-b-d-glucosyl-5-O-methylvisamminol, cinncassiol A 19-glucoside, blumenol C glucoside, NNAL-N-glucuronide, isochinomin, 2,2′-dihydroxy-4′,6′-dimethoxychalcone, 2′-hydroxy-3′,4′-dimethoxychalcone, and cinncassiol D2 glucoside. The main constituents of extracts obtained in this study were flavonoids, including their O-glycosides; nevertheless, compounds that have not been previously described were found in this study. The mass spectrometry stage analyses were performed in the positive and negative ionization modes.

Table 4.
Distribution of (tentatively) identified metabolites in different organs of the ice plant (Mesembryanthemum crystallinum).
Interestingly, phenolic acid and flavonoid compounds contained in the different organs of M. crystallinum L. were not previously reported. Heim et al. [26] analyzed the polyphenol compounds in the plants of the Mesembryanthemum genus and reported the detection of phloretin, quercitrin, avicularin, phloretin xyloglucoside, and rutin. Their identification in the cotyledon and stem extracts of the ice plant could encourage further investigation of promising sources of natural, biological, and active molecules. With the 17 compounds identified within the units, it would be advisable, using quantitative analysis, to select the organs of M. crystallinum that provide the most nutritional content for the development of foods whose components fully demonstrate the body’s regulatory functions related to biological defense.
Among the three organs of M. crystallinum observed during the research process, the organs consisting of the most flavonoids were the cotyledon and stem. The main compound classes, those that were detected only in the cotyledon and stem of the M. crystallinum extracts, were epigallocatechin and 1-O-feruloylglucose.
Epigallocatechin (otherwise known as green tea), or particularly, its major polyphenolic constituent, epigallocatechin-3-gallate (EGCG), has been shown to possess remarkable cancer chemopreventive and therapeutic potential against various cancer sites in animal tumor bioassay systems and some human epidemiologic studies [27]. The flavenoid, 1-O-Feruloylglucose (FG), is a new flavonoid that has been identified from chromatography of the ethanolic extract of R. officinalis (or rosemary) [28]. Feruloyl and their glucosides were identified as the major phenolic constituents of defatted safflower seed extract (SSE), respectively [29]. FG is a phenolic compound isolated from the corks of Euonymus alatus (Thunb.) [25]. FG inhibits adipogenesis in 3T3-L1 adipocytes by inhibiting the expression of PPARγ and FABP4. The compound detected solely in M. crystallinum, from cotyledon extracts, was caffeoyl putrescin. Phenolic acids found in potatoes, which were subsequently analyzed, contained caffeoyl-putrescine [27]. Cinnamate groups display strong absorption of UV radiation due to the conjugated structure of the benzene ring and adjacent carbon double bonds [30,31]. The statement is saying that Methyl cinnamate and ethyl cinnamate, which are types of cinnamic acid, are naturally found in many plants or fruits, such as cinnamon and strawberries, and are nontoxic and biocompatible [32]. Putrescine is also a type of polyamine, a class of organic compounds that are involved in the regulation of cell growth and division, as well as other cellular processes. The metabolite [6-[3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy-3,4,5-trihydroxyoxan-2-yl]methyl (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enoate is a type of secondary metabolite. Secondary metabolites are compounds that are not directly involved in the primary processes of life, such as growth, reproduction, and maintenance of cellular functions. Instead, they play a role in other functions, such as defense against predators or competition for resources; the presence of hydroxyl (OH) and methoxy (OCH3) groups suggests that it may have antioxidant or antimicrobial properties. Caffeic acid is a type of polyphenol that is found in a variety of plants, including coffee beans, and is known for its antioxidant and anti-inflammatory properties. Feruloyl hexoside specifically refers to a type of ester compound that is formed from the condensation of ferulic acid and a hexose sugar, such as glucose. Ferulic acid is a type of polyphenol that is found in a variety of plants, including coffee beans and cereal grains, and is known for its antioxidant and anti-inflammatory properties. Sinapyl alcohol specifically refers to a type of phenolic alcohol that is found in the lignin of plant cell walls. Procyanidin B2 specifically refers to a type of polyphenol that is found in many plants, including grapes, apples, and cocoa. Polyphenols are a diverse class of compounds that have been shown to have a number of health benefits, including antioxidant, anti-inflammatory, and antiproliferative effects. Sinapic acid specifically refers to a type of phenolic acid that is widely distributed in the plant kingdom and has been shown to have a number of health benefits, including antioxidant, anti-inflammatory, and antiproliferative effects. Catechin specifically refers to a type of flavanol, which is a type of polyphenol that is widely distributed in the plant kingdom. Catechins are known for their high antioxidant activity and have been shown to have a number of health benefits, including anti-inflammatory, anti-carcinogenic, and anti-viral effects. Mellein is a type of anthraquinone, which is a group of natural pigments that are widely distributed in the plant kingdom. These compounds are known for their strong red–blue color and have been used in the dyeing of textiles and other materials. Roseoside is a type of flavonoid, which is a group of polyphenolic compounds that are widely distributed in the plant kingdom. Flavonoids have a variety of functions in plants, including protection against environmental stress, attracting pollinators, and defense against herbivores and pathogens. The compound 2-O-p-Coumaroyl-d-glucose is a type of phenolic compound, which is a group of naturally occurring compounds that contain a phenol functional group. Feruloyl dopamine is a type of phenolic compound, which is a group of naturally occurring compounds that contain a phenol functional group. Nodakenin is a type of flavonoid, which is a group of naturally occurring compounds that are widely distributed in the plant kingdom. It is also known for their antioxidant properties, which can contribute to their protective effects against oxidative stress and other forms of cellular damage. L-DOPA is a neurotransmitter and a metabolic precursor to the neurotransmitter dopamine. It is commonly used in the treatment of Parkinson’s disease, a degenerative disorder of the nervous system, because it can increase the levels of dopamine in the brain. Kaempferol is a type of flavonoid; they are also known for their antioxidant properties. Isochinomin is a natural product isolated from the roots of several plant species, including some species of the genus Asarum and Isatis. It has been shown to have anti-inflammatory and anti-cancer properties in vitro and in animal studies, making it a potential candidate for development as a therapeutic agent. Negletein is a natural product isolated from various plant species, including some species of the genus Helminthostachys and Allium. It has been shown to have several biological activities, including anti-inflammatory, anti-tumor, and anti-viral effects, although more research is needed to fully understand its properties and potential therapeutic applications. Cinncassiol D2 is a natural product isolated from various plant species, including some species of the genus Cassia. It has been shown to have several biological activities, including anti-inflammatory effects. The following compounds play crucial roles in primary processes of life, such as growth, reproduction, and maintenance of cellular functions: sugars, amino acids, and nucleotides. In contrast, compounds that are foreign to the organism and are not naturally produced by the cells, such as drugs, pollutants, and other chemical agents, are considered exogenous compounds. The body produces and metabolizes compounds, known as endocannabinoids, which are involved in regulating physiological processes. The most well-known endocannabinoids are anandamide and 2-arachidonoylglycerol. Lastly, compounds involved in communication between neurons in the nervous system, such as dopamine, serotonin, and norepinephrine, are referred to as neurotransmitters.
Various effects of functional materials for these flavonoids have been revealed. Future studies using quantitative analysis are required to elucidate the precise components.
3.4. Cell Proliferation of Raw 264.7 Cells
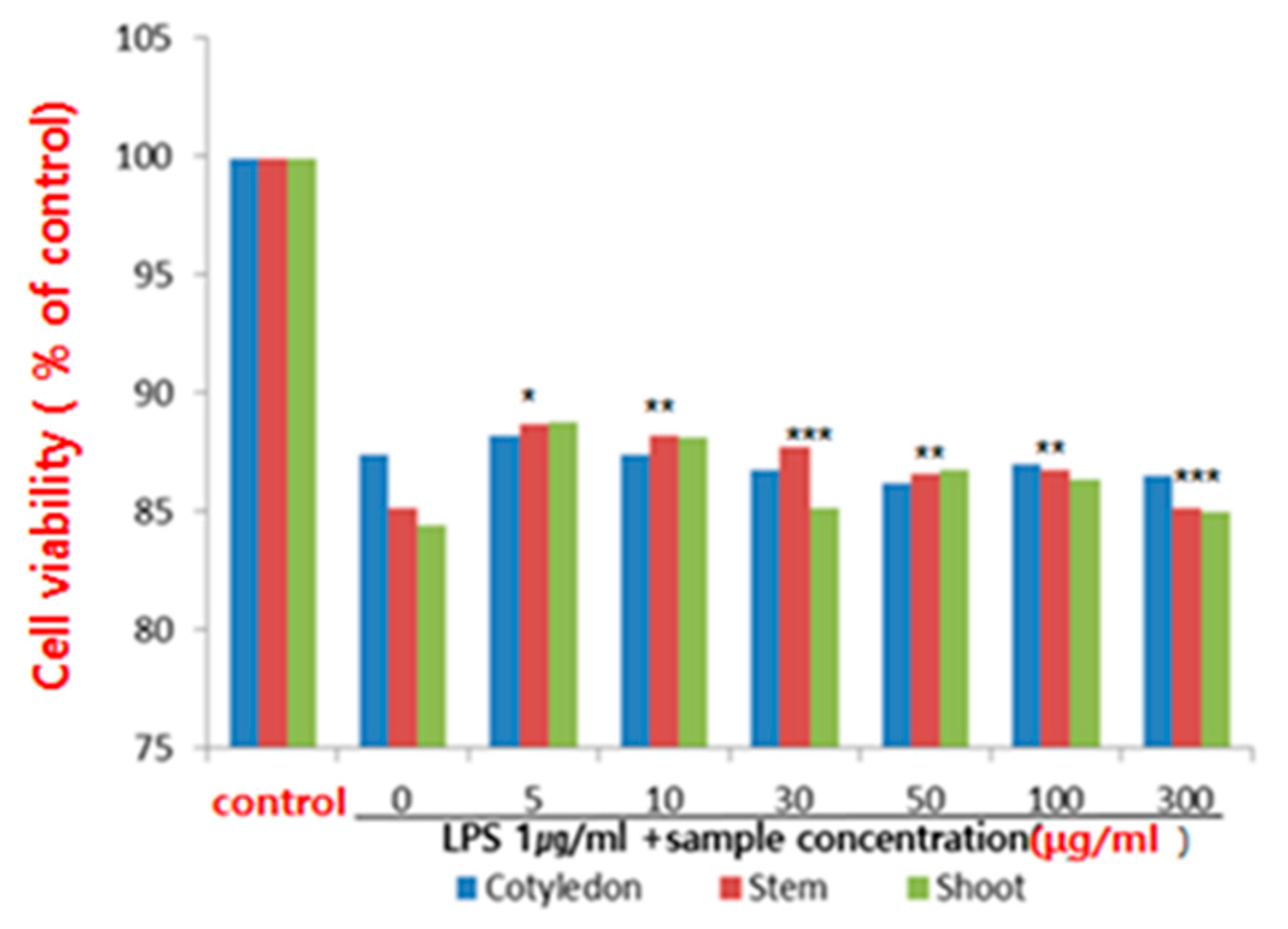
Macrophages are immune cells that are found throughout the human body and serve as the primary defense mechanism for innate immunity. These cells are capable of phagocytosis and play a crucial role in the immune response to antigens by secreting cytokines and immune modulators to find and eliminate bacteria and foreign substances [28]. The RAW 264.7 cell line, a type of macrophage, was induced with LPS in this study and the production of cytokines TNF-α, IL-1β, and IL-6 were measured to assess the cell’s ability to proliferate and respond to antigens. The results showed that ethanol extracts from various parts of the ice plant had no toxic effect on the survival of the RAW 264.7 macrophages, with concentrations up to 5–300 μg/mL not having any detrimental impact. In this study, RAW 264.7 cells, a macrophage cell line, were induced with LPS, macrophage-related cytokines, tumor necrosis factor-alpha (TNF-α), IL-1β (IL-1β), and IL-6(Figure 2).
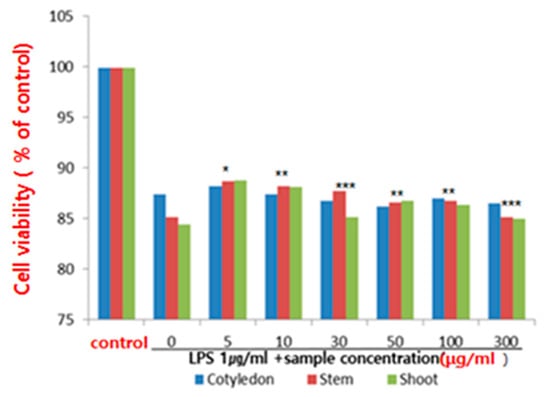
Figure 2.
Effect of ethanol extract of the ice plant (M. crystallinum) on cell viability in lipopolysaccharide (LPS)-treated RAW 264.7 cells (* p < 0.5, ** p < 0.01, *** p < 0.001 compared to the control by ANOVA).
M. crystallinum, also known as the Ice plant, has been studied for its potential health benefits, including its anti-inflammatory properties. This study investigated the effect of an ethanol extract of the Ice plant on cell viability in lipopolysaccharide (LPS)-treated RAW 264.7 cells, which are a type of immune cell often used in studies of inflammation. LPS is a component of the outer membrane of certain types of bacteria, and it is commonly used in studies to induce an immune response and inflammation. In this study, the researchers treated RAW 264.7 cells with LPS to induce inflammation, and then they treated the cells with different concentrations of the ice plant extract to determine its effect on cell viability. The results showed that the ice plant extract was able to increase cell viability in a dose-dependent manner, meaning that higher concentrations of the extract led to greater increases in cell viability. The researchers also found that the Ice plant extract was able to decrease the production of pro-inflammatory cytokines, which are molecules that promote inflammation and can contribute to cell damage and death. Based on these findings, concluded that the ethanol extract of the ice plant may have anti-inflammatory and cytoprotective effects on immune cells, which could potentially have therapeutic benefits in treating inflam-matory conditions. However, more research is needed to fully understand the mechanisms un-derlying these effects and to determine the optimal dosages and delivery methods for potential therapeutic use.
3.5. NO (Nitric Oxide) Assay
NO is a modulator with an important role in both its physiological and pathological states. The overproduction of NO induces inflammation and apoptosis, while NO, as an inflammatory factor, causes various chronic inflammatory diseases [33,34]. LPS from Escherichia coli induces oxidative stress to stimulate immune cells, thereby increasing the production of nitric oxide synthase (iNOS) and prostaglandin G2 (PGE2) [35]. In this study, anti-inflammatory activity was measured for each part of the ice plant using LPS-treated macrophages. In the experiment to measure NO production after LPS treatment, the group treated solely with LPS led to NO production of 13.81 ± 0.55 μM, a level higher than that of the control (1.99 ± 0.28) with uninduced LPS, indicating an adequate level of LPS-induced inflammatory response. As shown in Table 5, it was found that the extracts from different parts of the ice plant could reduce excess NO production in LPS-induced macrophages, indicating an effect as immune response modulators. In a previous study, the LPS-induced NO production could be reduced in a concentration-dependent manner by the extracts of plebeian sage leaves [36]. Another study showed the NO inhibitory activity of the extract fractions of northern bamboo leaves [37]. The trend agreed with a study reporting the change in NO production caused by the extracts of lemon myrtle leaves [38].

Table 5.
Effect of ethanol extract on nitric oxide (NO) production with mean ± S.D. from the cotyledon, stem, and leaf of the ice plant.
3.6. Cytokine Secretion (Production)
Cytokines are proteins secreted by immune cells, such as macrophages, that mediate immune cell proliferation, activation, and differentiation to promote inflammation. The immune response modulators TNF-α, IL-1β, and IL-6 are representative pro-inflammatory cytokines [37,39]. LPS-induced Raw 264.7 cells were treated with varying concentrations of ethanol extracts from different parts of the ice plant, and TNF-α (Table 6), IL-1β (Table 7), and IL-6 (Table 8) production were measured to determine the inhibitory effects of each extract on inflammatory cytokines.

Table 6.
Effects of ethanol extract according to each organ of the ice plant on TNF-a levels in LPS-induced RAW 264.7 cells.

Table 7.
Effects of ethanol extract according to each ice plant organs on the IL-6 levels in LPS-induced RAW 264.7 cells.

Table 8.
Effects of ethanol extract according to the organ of the ice plant on IL-1β levels in LPS-induced RAW 264.7 cells.
3.6.1. TNF-α (Tumor Necrosis Factor-α Assay)
TNF-α is a pro-inflammatory cytokine known to be secreted from the T cells of the immune system. Using signaling via two transmembrane receptors, TNFR1 and TNFR2, cell proliferation, survival, differentiation, and apoptosis are modulated by TNF-α. When immune cells are stimulated with a high-concentration of LPS, the secretion of inflammatory mediators such as TNF-α and NO is promoted, causing a fatal effect on the host [40]. The TNF-α production in macrophages treated with 1 μg/mL of LPS was 252.50 ± 4.5 pg/mL. This showed a significant increase compared to 158.04 ± 3.0 pg/mL of the non-treated control group. As shown in Table 6, at different concentrations of each part of the ice plant, TNF-α production was found to be inhibited by approximately 100 μg/mL of the stem and 300 μg/mL of the cotyledon and shoot. TNF-α production was inhibited by approximately 36%, 27%, and 35%, respectively. The leaf extracts of the plants also showed a 35% inhibitory ability of TNF-α production in Shin & Jung’s [41] study, which supports the results of this research.
3.6.2. IL-6 (Interleukin-6 Assay)
IL-6 is a representative inflammatory cytokine produced by macrophages and has been reported to play a critical role in the pathogenesis of various inflammatory diseases. It is generated rapidly, but transiently, in response to infection and tissue damage to contribute to the host defense through an acute phase response, as well as hematosis and immune response stimulation. The expression of IL-6 is strictly controlled by the transcription and post-transcription mechanisms, while it is known to have a pathological effect on chronic inflammation and autoimmunity. The level of IL-6 has been reported to increase in inflammatory lesions without exception [41,42]. In this study, IL-6 production in macrophages treated with 1 μg/mL of LPS was 95.11 ± 2.79 μg/mL, a significant increase compared to 1.66 ± 0.34 μg/mL of the non-treated control group after 24 h. IL-6 production was found to be inhibited from 50 μg/mL to 300 μg/mL at each part of the ice plant. An anti-inflammatory effect can be considered to be present because of the reduced amount of IL-1β production for each part. Lee and Kang’s [43] study showed an inhibition of IL-6 when an ethanol sage extract was induced using LPS.
3.6.3. IL-1β
IL-1β is a multifunctional cytokine and an inflammatory modulator alongside IL-6 and TNF-α. At a low concentration, IL-1β engages in cell growth or homeostasis in the body, with a central role in the immune defense of the host and the response to wounds. IL-1β is mostly produced by macrophages, and IL-1β overproduction causes various diseases. LPS-treated Raw 264.7 cells showed an increase in the production of TNFα, IL-6, and IL-1β, while the treatment with varying concentrations at 0, 30, 50, 100, and 300 μg/mL) of each organ of the ice plant led to a concentration-dependent inhibition of IL-1β production (Table 8). A study by Jang et al. [44] confirmed that the concentration of IL-1β, from ethanol extracts from water parsley, decreased significantly depending on from concentrations of 62.5 μg/mL. However, in this study, IL-1β production was shown to be inhibited at 300 μg/mL by approximately 31% and 40% from the stem and shoot of the ice plant, respectively, and by 13% of the cotyledon stimulated macrophages. In addition, at a concentration of 300 μg/mL, IL-6 production was inhibited by approximately 35%, 15%, and 11% for the cotyledon, stem, and shoot, respectively.
Results indicated that the inhibition of TNF-α and IL-1β was the highest at 300 μg/mL concentration of the ice plant shoot, whereas the inhibition of IL-6 was the highest at 300 μg/mL concentration of the ice plant cotyledon. The inflammatory cytokines IL-6 and TNF-α exhibit a diversity of immune functions and interact with the target cells [45] while mediating and modulating inflammation. Thus, the anti-inflammatory effects of the three different organs of the ice plant were verified by the concentration inhibition of the production of inflammatory cytokines.
4. Conclusions
In this study, the antioxidant and anti-inflammatory effects of different organs of the ice plant (cotyledon, stem, and shoot) were determined. For assessing the antioxidant effects, total polyphenols and flavonoids contents; DPPH, ABTS+, and hydroxyl radical scavenging activities; and Fe2+ chelating activity were evaluated. As flavonoids and polyphenols are abundant in plants, their total contents were high in each organ of the ice plant investigated in this study, with notably high contents in the cotyledon. It was confirmed that DPPH radical scavenging ability and Fe2+ chelating activity had lower levels than the control BHT, but had higher antioxidant activity in the shoot area of the ice plant, while ABTS+ and hydroxyl radical scavenging activities showed high antioxidant activity in the cotyledon. Physiological activity measurements in the three sections of the ice plant showed significant differences in all parameters determined. In this study, identifications were conducted to confirm the physiologically active substances in M. crystallinum L. based on different plant organs, and a total of 17 peaks were identified. These 17 peaks showed significant differences in terms of the chemical properties of M. crystallinum L. and its different organs. The phenolic compounds, according to the different organs of M. crystallinum, were identified and the major substances among them that contribute to antioxidant properties were analyzed.
Furthermore, Raw 264.7 cells were used to assess the anti-inflammatory effects of the three different organs of the ice plant. The cell viability showed no significant variation (500 μg/mL for the cotyledon, and 300 μg/mL for the stem and the shoot) (p < 0.001) to indicate the lack of cytotoxicity. The production of NO, as a major indicator of the inflammatory response, was found to decrease in 100 and 300 μg/mL. The levels of the inflammatory cytokines TNF-α, IL-6, and IL-1β confirmed that the anti-inflammatory effect was exhibited by inhibiting the production of inflammatory cytokines according to the concentration of each organ of the ice plant. Therefore, it can be declared that LPS-induced macrophages have anti-inflammatory activity, and it can be expected that they can be used as basic data when developing foods with physiological effects that improve health by using ice plants in the future.
Author Contributions
Conceptualization, Y.-W.K.; methodology, Y.-W.K.; software, Y.-W.K.; validation, Y.-W.K. and N.-M.J.; formal analysis, Y.-W.K.; investigation, Y.-W.K.; resources, Y.-W.K.; data curation, Y.-W.K.; writing—original draft preparation, Y.-W.K.; writing—review and editing, Y.-W.K. and N.-M.J.; visualization, Y.-W.K.; supervision, Y.-W.K. and N.-M.J.; project administration, Y.-W.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Agarie, S.; Kawaguchi, A.; Kodera, A.; Sunagawa, H.; Kojima, H.; Nose, A.; Nakahara, T. Potential of the Common Ice Plant, Mesembryanthemum crystallinum as a New High-Functional Food as Evaluated by Polyol Accumulation. Plant Prod. Sci. 2009, 12, 37–46. [Google Scholar] [CrossRef]
- Hamed, A.I.; Said, R.B.; Kontek, B.; Al-Ayed, A.S.; Kowalczyk, M.; Moldoch, J.; Stochmal, A.; Olas, B. LC–ESI-MS/MS profile of phenolic and glucosinolate compounds in samh flour (Mesembryanthemum forsskalei Hochst. ex Boiss) and the inhibition of oxidative stress by these compounds in human plasma. Food Res. Int. 2016, 85, 282–290. [Google Scholar] [CrossRef]
- Adams, P.; Nelson, D.E.; Yamada, S.; Chmara, W.; Jensen, R.G.; Bohnert, H.J.; Griffiths, H. Growth and development of Mesembryanthemum crystallinum (Aizoaceae). N. Phytol. 1998, 138, 171–190. [Google Scholar] [CrossRef]
- Nam, S.; Kang, S.; Kim, S.; Ko, K. Effect of fermented ice plant (Mesembryanthemum crystallinum L.) extracts against antioxidant, antidiabetic and liver protection. J. Life Sci. 2017, 27, 909–918. [Google Scholar] [CrossRef]
- Kang, S.M.; Kim, S.J.; Nam, S. Inhibitory effect of cell differentiation against 3T3-L1 pre-adipocytes and angiotensin converting enzyme (ACE) activity of ice plant (Mesembryanthemum crystallinum). J. Korean Soc. Food Sci. Nutr. 2017, 46, 1012–1017. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, H.D.; Yu, S.N.; Kim, S.H.; Park, S.K.; Ahn, S.C. Biological activities of Mesembryanthemum crystallinum (ice plant) extract. J. Life Sci. 2015, 25, 638–645. [Google Scholar] [CrossRef]
- Kang, D.-G.; Lee, Y.-J.; Kim, J.S.; Sohn, H.-Y. An Evaluation of the Anti-thrombosis and Antioxidant Activities of Different Parts of Dystaenia takeshimana. J. Life Sci. 2022, 32, 303–309. [Google Scholar] [CrossRef]
- Park, Y.J.; Jeong, H.Y.; Kang, K.O.; Heo, B.G. Chemical Composition and Physiological Activity of Mulberry Plant Parts. J. People Plants Environ. 2012, 15, 249–256. [Google Scholar]
- Kim, J.-S. Antioxidant activity and phytochemical contents of the extracts from different parts of Moringa oleifera. J. Plant Biotechnol. 2020, 47, 248–253. [Google Scholar] [CrossRef]
- Nakanishi-Matsui, M.; Yano, S.; Matsumoto, N.; Futai, M. Lipopolysaccharide induces multinuclear cell from RAW264.7 line with increased phagocytosis activity. Biochem. Biophys. Res. Commun. 2012, 425, 144–149. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Antioxidant activities, total phenolics and flavonoids content in two varieties of Malaysia young ginger (Zingiber officinale Roscoe). Molecules 2010, 15, 4324–4333. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.-H.; Park, S.-D.; Choi, S.-Y.; Seong, J.-H.; Moon, K.-D. Preparation and antioxidant activity of health drink with extract powders from safflower (Carthamus tinctorius L.) seed. Korean J. Food Sci. Technol. 2002, 34, 617–624. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Le, K.; Chiu, F.; Ng, K. Identification and quantification of antioxidants in Fructus lycii. Food Chem. 2007, 105, 353–363. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Kumar, B. Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal. 2017, 7, 214–222. [Google Scholar] [CrossRef]
- Budak, H.N.; Guzel-Seydim, Z.B. Antioxidant activity and phenolic content of wine vinegars produced by two different techniques. J. Sci. Food Agric. 2010, 90, 2021–2026. [Google Scholar] [CrossRef]
- Kang, S.; Kim, S.; Ha, S.; Lee, C.; Nam, S. Biochemical components and physiological activities of ice plant (Mesembryanthemum crystallinum). J. Korean Soc. Food Sci. Nutr. 2016, 45, 1732–1739. [Google Scholar] [CrossRef]
- Ibtissem, B.; Abdelly, C.; Sfar, S. Antioxidant and antibacterial properties of Mesembryanthemum crystallinum and Carpobrotus edulis extracts. Adv. Chem. Eng. Sci. 2012, 2, 359–365. [Google Scholar] [CrossRef]
- Hanen, F.; Riadh, K.; Samia, O.; Sylvain, G.; Christian, M.; Chedly, A. Interspecific variability of antioxidant activities and phenolic composition in Mesembryanthemum genus. Food Chem. Toxicol. 2009, 47, 2308–2313. [Google Scholar] [CrossRef]
- Bandonien, D.; Murkovic, M. The detection of radical scavenging compounds in crude extract of borage (Borago officinalis L.) by using an on-line HPLC-DPPH method. J. Biochem. Biophys. Methods 2002, 53, 45–49. [Google Scholar] [CrossRef]
- Pourmorad, F.; Hosseinimehr, S.; Shahabimajd, N. Antioxidant activity, phenol and flavonoid contents of some selected Iranian medicinal plants. Afr. J. Biotechnol. 2006, 5. [Google Scholar] [CrossRef]
- Yi, M.-R.; Kang, C.-H.; Bu, H.-J. Antioxidant and anti-inflammatory activity of extracts from red beet (Beta vulagaris) root. Korean J. Food Preserv. 2017, 24, 413–420. [Google Scholar] [CrossRef]
- Arnao, M.B. Some methodological problems in the determination of antioxidant activity using chromogen radicals: A practical case. Trends Food Sci. Technol. 2000, 11, 419–421. [Google Scholar] [CrossRef]
- Kim, I. Antioxidant and Pro-oxidant Activities of Hamcho (Salicornia herbacea L.). Korean J. Food Nutr. 2015, 28, 40–46. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Lai, C.-S.; Shao, X.; Pan, M.-H.; Ho, C.-T. Flavonoids and Phenolic Compounds from Rosmarinus officinalis. J. Agric. Food Chem. 2010, 58, 5363–5367. [Google Scholar] [CrossRef]
- Koyama, N.; Kuribayashi, K.; Seki, T.; Kobayashi, K.; Furuhata, Y.; Suzuki, K.; Arisaka, H.; Nakano, T.; Amino, Y.; Ishii, K. Serotonin Derivatives, Major Safflower (Carthamus tinctorius L.) Seed Antioxidants, Inhibit Low-Density Lipoprotein (LDL) Oxidation and Atherosclerosis in Apolipoprotein E-Deficient Mice. J. Agric. Food Chem. 2006, 54, 4970–4976. [Google Scholar] [CrossRef]
- Kwak, S.H.; Kim, Y.H. Anti-adipogenic effect of 1-O-feruloyl-β-D-glucose on 3T3-L1 preadipocytes. Korean J. Food Preserv. 2018, 25, 689–695. [Google Scholar] [CrossRef]
- Kita, A.; Rytel, E.; Miedzianka, J.; Turski, W.A.; Wicha-Komsta, K.; Kucharska, A.Z.; Lenartowicz, T. The content of biologically active compounds in potato tubers of Ismena (yellow flesh) and Provita (purple flesh) varieties–A comparison. J. Food Compos. Anal. 2023, 115, 104898. [Google Scholar] [CrossRef]
- Baker, L.A.; Marchetti, B.; Karsili, T.N.V.; Stavros, V.G.; Ashfold, M.N.R. Photoprotection: Extending lessons learned from studying natural sunscreens to the design of artificial sunscreen constituents. Chem. Soc. Rev. 2017, 46, 3770–3791. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992, 6, 3051–3064. [Google Scholar] [CrossRef] [PubMed]
- Boscá, L.; Zeini, M.; Través, P.G.; Hortelano, S. Nitric oxide and cell viability in inflammatory cells: A role for NO in macrophage function and fate. Toxicology 2005, 208, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.-M.; Kim, J.-E.; Bae, E.-Y.; Kim, K.-A.; Ly, S.Y. Anti-inflammatory effects of fruit and leaf extracts of Lycium barbarum in lipopolysaccharide-stimulated RAW264.7 cells and animal model. J. Nutr. Health 2019, 52, 129–138. [Google Scholar] [CrossRef]
- Jeong, H.-R.; Sung, M.-S.; Kim, Y.-H.; Ham, H.-M.; Choi, Y.-M.; Lee, J.-S. Anti-Inflammatory Activity of Salvia Plebeia R. Br. Leaf through Heme Oxygenase-1 Induction in LPS-Stimulated RAW264.7 Macrophages. J. Korean Soc. Food Sci. Nutr. 2012, 41, 888–894. [Google Scholar] [CrossRef]
- Blay, M.; Espinel, A.E.; Delgado, M.A.; Baiges, I.; Bladé, C.; Arola, L.; Salvadó, J. Isoflavone effect on gene expression profile and biomarkers of inflammation. J. Pharm. Biomed. Anal. 2010, 51, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.K.; Jung, K.I.; Choi, Y.J.; Gal, S.W. Anti-inflammatory Effects of Lemon Myrtle (Backhousia citriodora) Leaf Extracts in LPS-induced RAW 264.7 Cells. J. Life Sci. 2017, 27, 986–993. [Google Scholar] [CrossRef]
- Lendlein, A.; Jiang, H.; Jünger, O.; Langer, R. Light-induced shape-memory polymers. Nature 2005, 434, 879–882. [Google Scholar] [CrossRef]
- Ha, Y.B.; Park, J.H.; Jang, J.W.; Lim, D.W.; Kim, J.E. Comparative Study for Anti-inflammatory and Anti-obesity Effect of Fractions from Leaf and Stem of Sasa Borealis. J. Physiol. Pathol. Korean Med. 2016, 30, 2288–2529. [Google Scholar] [CrossRef]
- Shin, J.A.; Jeong, J.-M. Anti-Inflammatory Effects of BENDU381 in Lipopolysaccharide-Stimulated RAW264.7 Cells. J. Korean Soc. Food Sci. Nutr. 2020, 49, 1202–1211. [Google Scholar] [CrossRef]
- Delgado, A.V.; McManus, A.T.; Chambers, J.P. Production of Tumor Necrosis Factor-alpha, Interleukin 1-beta, Interleukin 2, and Interleukin 6 by rat leukocyte subpopulations after exposure to Substance P. Neuropeptides 2003, 37, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-Y.; Kang, S.A. Antioxidative and Anti-Inflammatory Activities of Salvia plebeia R. Br Extracts. Korean J. Food Nutr. 2020, 33, 483–492. [Google Scholar] [CrossRef]
- Jang, J.-H.; Cho, H.-W.; Lee, B.-Y.; Yu, K.-Y.; Yoon, J.-Y. Anti-Inflammatory Effects of Oenanthe javanica Ethanol Extract and Its Fraction on LPS-Induced Inflammation Response. J. Korean Soc. Food Sci. Nutr. 2016, 45, 1595–1603. [Google Scholar] [CrossRef]
- Namkoong, S.; Jang, S.-A.; Sohn, E.-H.; Bak, J.P.; Sohn, E.; Koo, H.J.; Yoon, W.-J.; Kwon, J.-E.; Jeong, Y.J.; Meng, X.; et al. Comparative Study of Litsea japonica Leaf and Fruit Extract on the Anti-inflammatory Effects. Korean J. Plant Res. 2015, 28, 145–152. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).