Oral Cavity Beta-Defensin Levels Are Regulated Differently during Radiotherapy in Head and Neck Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
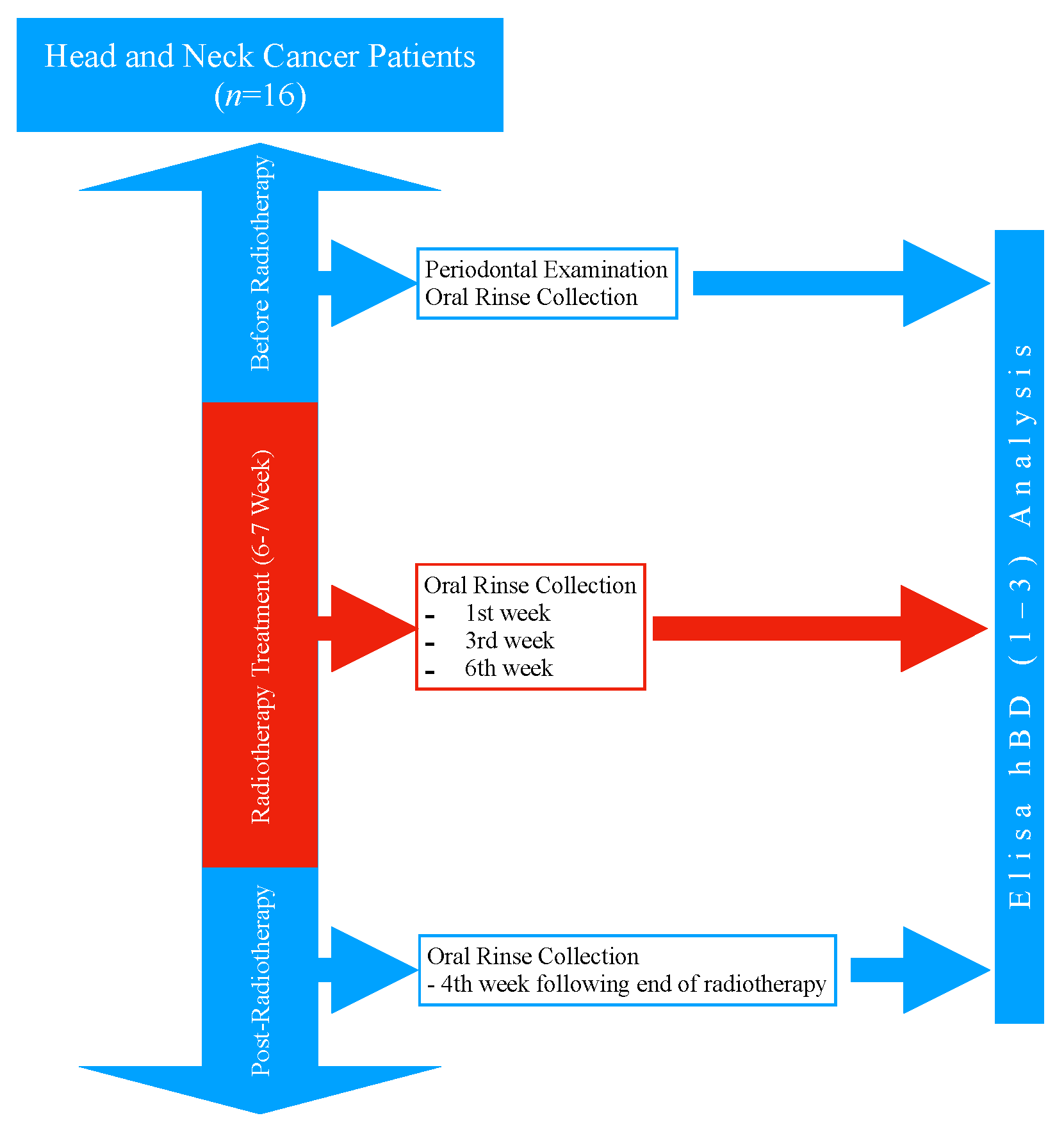
2.1. Study Design and Population
2.2. Periodontal Examination Procedure
2.3. Oral Rinse Collection Procedure
2.4. The Radiotherapy Treatment Procedure
2.5. Beta-Defensin Analysis
2.6. Statistical Analyses
3. Results
3.1. The Demographic and Periodontal Characteristics of the Patients
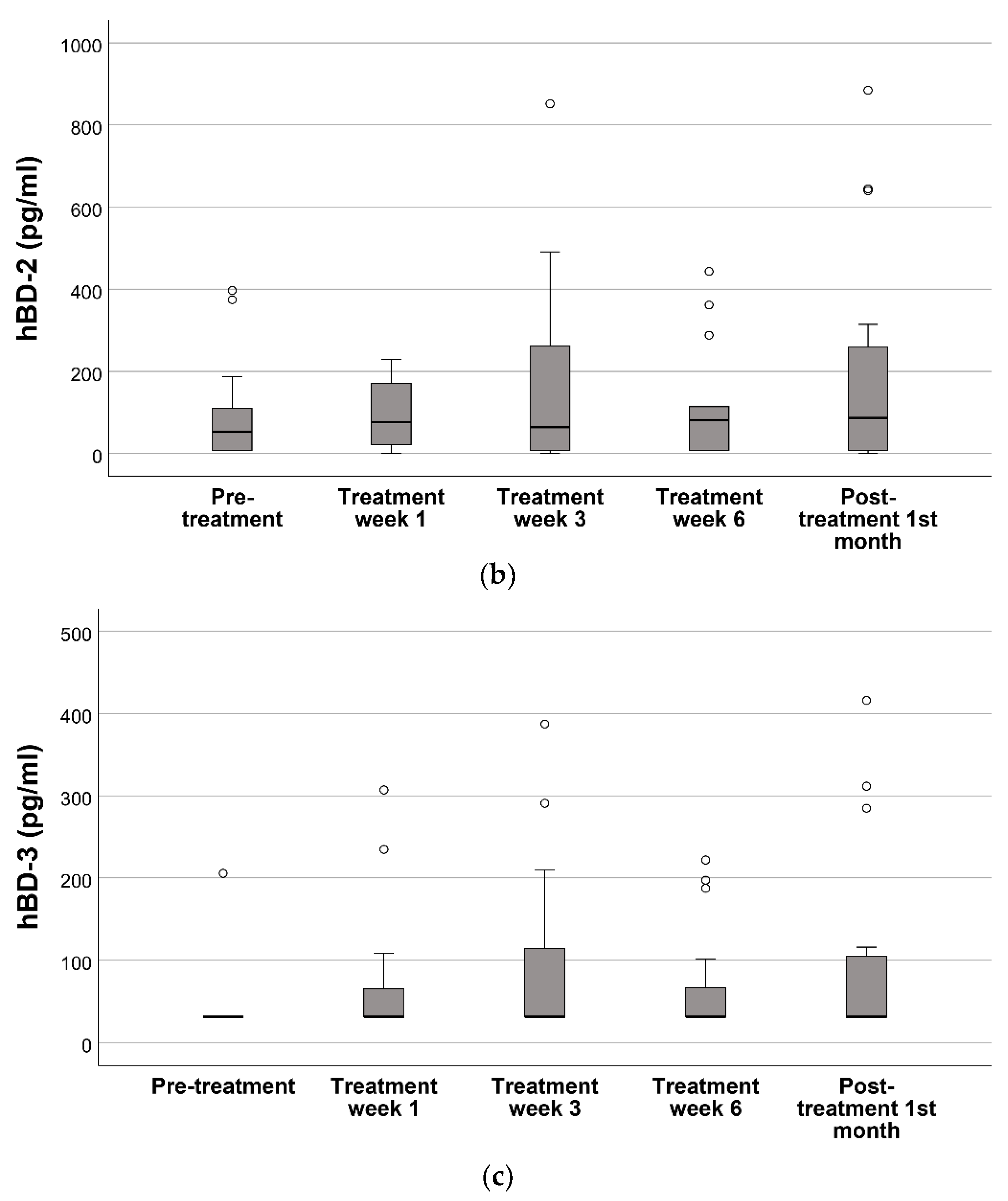
3.2. Oral hBD-1, hBD-2, and hBD-3 Levels during and after the Radiotherapy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gursoy, U.K.; Könönen, E. Understanding the roles of gingival beta-defensins. J. Oral Microbiol. 2012, 4, 15127. [Google Scholar] [CrossRef]
- Bonass, W.A.; High, A.S.; Owen, P.J.; Devine, D.A. Expression of beta-defensin genes by human salivary glands. Oral Microbiol. Immunol. 1999, 14, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Dunsche, A.; Açil, Y.; Siebert, R.; Harder, J.; Schröder, J.-M.; Jepsen, S. Expression profile of human defensins and antimicrobial proteins in oral tissues. J. Oral Pathol. Med. 2001, 30, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Schibli, D.J.; Hunter, H.N.; Aseyev, V.; Starner, T.D.; Wiencek, J.M.; McCray, P.B., Jr.; Tack, B.F.; Vogel, H.J. The Solution Structures of the Human β-Defensins Lead to a Better Understanding of the Potent Bactericidal Activity of HBD3 against Staphylococcus aureus. J. Biol. Chem. 2002, 277, 8279–8289. [Google Scholar] [CrossRef]
- Ji, S.; Hyun, J.; Park, E.; Lee, B.-L.; Kim, K.-K.; Choi, Y. Susceptibility of various oral bacteria to antimicrobial peptides and to phagocytosis by neutrophils. J. Periodontal Res. 2007, 42, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Ouhara, K.; Komatsuzawa, H.; Yamada, S.; Shiba, H.; Fujiwara, T.; Ohara, M.; Sayama, K.; Hashimoto, K.; Kurihara, H.; Sugai, M. Susceptibilities of periodontopathogenic and cariogenic bacteria to antibacterial peptides, β-defensins and LL37, produced by human epithelial cells. J. Antimicrob. Chemother. 2005, 55, 888–896. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jun, H.-K.; Lee, H.-R.; Chung, C.-P.; Choi, B.-K. Antibacterial and lipopolysaccharide (LPS)-neutralising activity of human cationic antimicrobial peptides against periodontopathogens. Int. J. Antimicrob. Agents 2010, 35, 138–145. [Google Scholar] [CrossRef]
- Williams, W.M.; Torres, S.; Siedlak, S.L.; Castellani, R.J.; Perry, G.; Smith, M.A.; Zhu, X. Antimicrobial peptide β-defensin-1 expression is upregulated in Alzheimer’s brain. J. Neuroinflammation 2013, 10, 898. [Google Scholar] [CrossRef]
- Ganz, T. Defensins: Antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 2003, 3, 710–720. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef]
- Kim, J.; Yang, Y.L.; Jang, S.-H.; Jang, Y.-S. Human β-defensin 2 plays a regulatory role in innate antiviral immunity and is capable of potentiating the induction of antigen-specific immunity. Virol. J. 2018, 15, 124. [Google Scholar] [CrossRef]
- Agarwal, S.; Chauhan, A.; Singh, K.; Kumar, K.; Kaur, R.; Masih, M.; Gautam, P.K. Immunomodulatory effects of β-defensin 2 on macrophages induced immuno-upregulation and their antitumor function in breast cancer. BMC Immunol. 2022, 23, 53. [Google Scholar] [CrossRef]
- Swanson, K.; Gorodetsky, S.; Good, L.; Davis, S.; Musgrave, D.; Stelwagen, K.; Farr, V.; Molenaar, A. Expression of a β-Defensin mRNA, Lingual Antimicrobial Peptide, in Bovine Mammary Epithelial Tissue Is Induced by Mastitis. Infect. Immun. 2004, 72, 7311–7314. [Google Scholar] [CrossRef]
- Agier, J.; Brzezińska-Błaszczyk, E.; Różalska, S.; Wiktorska, M.; Wawrocki, S.; Żelechowska, P. β-Defensin Strengthens Antimicrobial Peritoneal Mast Cell Response. J. Immunol. Res. 2020, 2020, 5230172. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.Q.; Arnold, R.; Fernandez-Golarz, C.; Parrish, A.B.; Almekinder, T.; He, J.; Ho, S.-M.; Svoboda, P.; Pohl, J.; Marshall, F.F.; et al. Human β-Defensin-1, a Potential Chromosome 8p Tumor Suppressor: Control of Transcription and Induction of Apoptosis in Renal Cell Carcinoma. Cancer Res. 2006, 66, 8542–8549. [Google Scholar] [CrossRef] [PubMed]
- Bullard, R.S.; Gibson, W.; Bose, S.K.; Belgrave, J.K.; Eaddy, A.C.; Wright, C.J.; Hazen-Martin, D.J.; Lage, J.M.; Keane, T.E.; Ganz, T.A.; et al. Functional analysis of the host defense peptide Human Beta Defensin-1: New insight into its potential role in cancer. Mol. Immunol. 2008, 45, 839–848. [Google Scholar] [CrossRef]
- Han, Q.; Wang, R.; Sun, C.; Jin, X.; Liu, D.; Zhao, X.; Wang, L.; Ji, N.; Li, J.; Zhou, Y.; et al. Human Beta-Defensin-1 Suppresses Tumor Migration and Invasion and Is an Independent Predictor for Survival of Oral Squamous Cell Carcinoma Patients. PLoS ONE 2014, 9, e91867. [Google Scholar] [CrossRef]
- Bindra, G.K.; Williams, S.A.; Lay, F.T.; Baxter, A.A.; Poon, I.K.H.; Hulett, M.D.; Phan, T.K. Human β-Defensin 2 (HBD-2) Displays Oncolytic Activity but Does Not Affect Tumour Cell Migration. Biomolecules 2022, 12, 264. [Google Scholar] [CrossRef]
- Kesting, M.R.; Loeffelbein, D.J.; Hasler, R.J.; Wolff, K.-D.; Rittig, A.; Schulte, M.; Hirsch, T.; Wagenpfeil, S.; Jacobsen, F.; Steinstraesser, L. Expression Profile of Human Beta-Defensin 3 in Oral Squamous Cell Carcinoma. Cancer Investig. 2009, 27, 575–581. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, B.; Liao, C.; Zhang, W.; Wang, W.; Chang, Y.; Shao, Y. Human beta-defensin 3 contributes to the carcinogenesis of cervical cancer via activation of NF-κB signaling. Oncotarget 2016, 7, 75902–75913. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Irani, S.; Barati, I.; Badiei, M. Periodontitis and oral cancer-current concepts of the etiopathogenesis. Oncol. Rev. 2020, 14, 465. [Google Scholar] [CrossRef]
- Karam, S.D.; Raben, D. Radioimmunotherapy for the treatment of head and neck cancer. Lancet Oncol. 2019, 20, e404–e416. [Google Scholar] [CrossRef] [PubMed]
- Keskin, M.; Lähteenmäki, H.; Rathnayake, N.; Räisänen, I.T.; Tervahartiala, T.; Pärnänen, P.; Şenışık, A.M.; Karaçetin, D.; Balkanay, A.Y.; Heikkilä, P.; et al. Active matrix metalloproteinase-8 and interleukin-6 detect periodontal degeneration caused by radiotherapy of head and neck cancer: A pilot study. Expert Rev. Proteom. 2020, 17, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Keskin, M.; Kompuinen, J.; Harmankaya, I.; Karaçetin, D.; Nissilä, V.; Gürsoy, M.; Sorsa, T.; Gürsoy, U.K. Oral Cavity Calprotectin and Lactoferrin Levels in Relation to Radiotherapy. Curr. Issues Mol. Biol. 2022, 44, 4439–4446. [Google Scholar] [CrossRef] [PubMed]
- Abiko, Y.; Mitamura, J.; Nishimura, M.; Muramatsu, T.; Inoue, T.; Shimono, M.; Kaku, T. Pattern of expression of beta-defensins in oral squamous cell carcinoma. Cancer Lett. 1999, 143, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hussaini, H.M.; Lim, P.K.; Rahman, R.A.; Abraham, T.; Cheong, C.S.; Zain, R.B.; Ismail, M.S.; Nam, A.N. Expression of human b-defensin-3 in oral squamous cell carcinoma using tissue microarray—A preliminary study. J. Oral Pathol. Med. 2006, 35, 432–433. [Google Scholar]
- Wenghoefer, M.; Pantelis, A.; Dommisch, H.; Reich, R.; Martini, M.; Allam, J.-P.; Novak, N.; Bergé, S.; Jepsen, S.; Winter, J. Decreased gene expression of human β-defensin-1 in the development of squamous cell carcinoma of the oral cavity. Int. J. Oral Maxillofac. Surg. 2008, 37, 660–663. [Google Scholar] [CrossRef]
- Joly, S.; Compton, L.M.; Pujol, C.; Kurago, Z.B.; Guthmiller, J.M. Loss of human β-defensin 1, 2, and 3 expression in oral squamous cell carcinoma. Oral Microbiol. Immunol. 2009, 24, 353–360. [Google Scholar] [CrossRef]
- Shuyi, Y.; Feng, W.; Jing, T.; Hongzhang, H.; Haiyan, W.; Pingping, M.; Liwu, Z.; Zwahlen, R.A.; Hongyu, Y. Human beta-defensin-3 (hBD-3) upregulated by LPS via epidermal growth factor receptor (EGFR) signaling pathways to enhance lymphatic invasion of oral squamous cell carcinoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2011, 112, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Kamino, Y.; Kurashige, Y.; Uehara, O.; Sato, J.; Nishimura, M.; Yoshida, K.; Arakawa, T.; Nagayasu, H.; Saitoh, M.; Abiko, Y. hBD-2 is downregulated in oral carcinoma cells by DNA hypermethylation, and increased expression of hBD-2 by DNA demethylation and gene transfection inhibits cell proliferation and invasion. Oncol. Rep. 2014, 32, 462–468. [Google Scholar] [CrossRef]
- Gao, C.; Yue, W.; Tian, H.; Li, L.; Li, S.; Si, L. Human beta-defensin 2 promotes the proliferation of lung cancer cells through ATP-binding cassette transporter G2. Int. J. Clin. Exp. Pathol. 2016, 9, 5944–5949. [Google Scholar]
- Ghosh, S.K.; McCormick, T.S.; Weinberg, A. Human Beta Defensins and Cancer: Contradictions and Common Ground. Front. Oncol. 2019, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network® (NCCN®) Guideline (V.1.2021). Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwin-q-Zvtb8AhVrQ_EDHS0IByoQFnoECBUQAQ&url=https%3A%2F%2Fwww.nccn.org%2Fguidelines%2Fguidelines-process%2Ftransparency-process-and-recommenda-tions%2FGetFileFromFileManager%3FfileManagerId%3D11180&usg=AOvVaw2gJrBKrcFg5BTpeJRmkcvn (accessed on 16 December 2022).
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Lubin, J.H.; Colt, J.S.; Camann, D.; Davis, S.; Cerhan, J.; Severson, R.K.; Bernstein, L.; Hartge, P. Epidemiologic Evaluation of Measurement Data in the Presence of Detection Limits. Environ. Health Perspect. 2004, 112, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.-P.; Wang, L.-F.; Chiang, F.-Y.; Lee, K.-W.; Kuo, P.-L.; Liang, C.-H. IL-8 promotes HNSCC progression on CXCR1/2-meidated NOD1/RIP2 signaling pathway. Oncotarget 2016, 7, 61820–61831. [Google Scholar] [CrossRef]
- DasGupta, T.; Nweze, E.I.; Yue, H.; Wang, L.; Jin, J.; Ghosh, S.K.; Kawsar, H.I.; Zender, C.; Androphy, E.J.; Weinberg, A.; et al. Human papillomavirus oncogenic E6 protein regulates human β-defensin 3 (hBD3) expression via the tumor suppressor protein p53. Oncotarget 2016, 7, 27430–27444. [Google Scholar] [CrossRef]
- Donald, C.D.; Sun, C.Q.; Lim, S.D.; Macoska, J.; Cohen, C.; Amin, M.B.; Young, A.N.; Ganz, T.A.; Marshall, F.F.; Petros, J.A. Cancer-Specific Loss of β-Defensin 1 in Renal and Prostatic Carcinomas. Lab. Investig. 2003, 83, 501–505. [Google Scholar] [CrossRef]
- Güncü, G.N.; Yilmaz, D.; Könönen, E.; Gursoy, U.K. Salivary Antimicrobial Peptides in Early Detection of Periodontitis. Front. Cell. Infect. Microbiol. 2015, 5, 99. [Google Scholar] [CrossRef]
- Chambers, M.S.; Tomsett, K.L.; Artopoulou, I.I.; Garden, A.S.; El-Naggar, A.K.; Martin, J.W.; Keene, H.J. Salivary flow rates measured during radiation therapy in head and neck cancer patients: A pilot study assessing salivary sediment formation. J. Prosthet. Dent. 2008, 100, 142–146. [Google Scholar] [CrossRef]
- Rodriguez-Carlos, A.; Miramontes, C.E.V.; Marin-Luevano, P.; González-Curiel, I.; Enciso-Moreno, J.A.; Rivas-Santiago, B. Metformin promotes Mycobacterium tuberculosis killing and increases the production of human β-defensins in lung epithelial cells and macrophages. Microbes Infect. 2020, 22, 111–118. [Google Scholar] [CrossRef]
- Koerdt, S.; Steinstraesser, L.; Stoeckelhuber, M.; Wales, C.; Rohleder, N.; Babaryka, G.; Steiner, T.; Wolff, K.-D.; Loeffelbein, D.; Muecke, T.; et al. Radiotherapy for oral cancer decreases the cutaneous expression of host defence peptides. J. Cranio-Maxillofacial Surg. 2016, 44, 882–889. [Google Scholar] [CrossRef]
- Chieosilapatham, P.; Ogawa, H.; Niyonsaba, F. Current insights into the role of human β-defensins in atopic dermatitis. Clin. Exp. Immunol. 2017, 190, 155–166. [Google Scholar] [CrossRef]
- Seo, S.J.; Ahn, S.W.; Hong, C.K.; Ro, B.I. Expressions of β-defensins in human keratinocyte cell lines. J. Dermatol. Sci. 2001, 27, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Horrell, E.W.; D’Orazio, J. UV-independent induction of beta defensin 3 in neonatal human skin explants. F1000Research 2015, 3, 288. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Rho, Y.K.; Lee, H.I.; Jeong, M.S.; Li, K.; Seo, S.J.; Kim, M.N.; Hong, C.K. The Effect of Calcipotriol on the Expression of HumanβDefensin-2 and LL-37 in Cultured Human Keratinocytes. J. Immunol. Res. 2009, 2009, 645898. [Google Scholar] [CrossRef]
- Sun, C.Q.; Arnold, R.S.; Hsieh, C.-L.; Dorin, J.R.; Lian, F.; Li, Z.; Petros, J.A. Discovery and mechanisms of host defense to oncogenesis: Targeting the β-defensin-1 peptide as a natural tumor inhibitor. Cancer Biol. Ther. 2019, 20, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, D.; Yilmaz, N.; Polat, R.; Nissilä, V.; Aydın, E.G.; Rautava, J.; Gürsoy, M.; Gürsoy, U.K. Salivary levels of hBDs in children and adolescents with type 1 diabetes mellitus and gingivitis. Clin. Oral Investig. 2022, 26, 4897–4904. [Google Scholar] [CrossRef]
- Yilmaz, D.; Topcu, A.O.; Akcay, E.U.; Altındis, M.; Gursoy, U.K. Salivary human beta-defensins and cathelicidin levels in relation to periodontitis and type 2 diabetes mellitus. Acta Odontol. Scand. 2020, 78, 327–331. [Google Scholar] [CrossRef]
- Pereira, A.G.; Costa, L.C.M.; Soldati, K.R.; De Abreu, M.H.N.G.; Costa, F.O.; Zandim-Barcelos, D.L.; Cota, L.O.M. Gingival Crevicular Fluid Levels of Human Beta-defensin 2 and 3 in Healthy and Diseased Sites of Individuals with and without Periodontitis. J. Int. Acad. Periodontol. 2020, 22, 90–99. [Google Scholar]
- Pereira, A.L.; Franco, G.C.; Cortelli, S.C.; Aquino, D.R.; Costa, F.; Raslan, S.A.; Cortelli, J.R. Influence of Periodontal Status and Periodontopathogens on Levels of Oral Human β-Defensin-2 in Saliva. J. Periodontol. 2013, 84, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Vardar-Sengul, S.; Demirci, T.; Sen, B.H.; Erkizan, V.; Kurulgan, E.; Baylas, H. Human β defensin-1 and -2 expression in the gingiva of patients with specific periodontal diseases. J. Periodontal Res. 2007, 42, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Atalay, N.; Balci, N.; Toygar, H.U.; Yardimci, G.; Gürsoy, U.K. Serum, saliva, and gingival tissue human β-defensin levels in relation to retinoic acid use. J. Periodontol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Komesu, M.C.; Ribeiro, A.E.R.A.; Lourenco, A.G.; Motta, A.C.F. Influence of periodontal condition on levels of human beta defensins 1 and 2 in saliva. J. Microbiol. Exp. 2018, 6, 40–44. [Google Scholar] [CrossRef]
- Xiao, Z.; Ding, W.; Wen, C.; Ge, C.; Liu, L.; Xu, K.; Cao, S. Correlation between salivary HBD-2 and LL-37 expression levels with blood glucose and periodontal status in patients with type 2 diabetes mellitus. Am. J. Transl. Res. 2022, 14, 3286–3291. [Google Scholar]
- Young, A.N.; Amin, M.B.; Moreno, C.S.; Lim, S.D.; Cohen, C.; Petros, J.A.; Marshall, F.F.; Neish, A.S. Expression Profiling of Renal Epithelial Neoplasms: A Method for Tumor Classification and Discovery of Diagnostic Molecular Markers. Am. J. Pathol. 2001, 158, 1639–1651. [Google Scholar] [CrossRef]
- Meyer, J.E.; Harder, J.; Görögh, T.; Weise, J.B.; Schubert, S.; Janssen, D.; Maune, S. Human beta-defensin-2 in oral cancer with opportunistic Candida infection. Anticancer. Res. 2004, 24, 1025–1030. [Google Scholar]
- Wang, X.; Jiang, W.; Duan, N.; Qian, Y.; Zhou, Q.; Ye, P.; Jiang, H.; Bai, Y.; Zhang, W.; Wang, W. NOD1, RIP2 and Caspase12 are potentially novel biomarkers for oral squamous cell carcinoma development and progression. Int. J. Clin. Exp. Pathol. 2014, 7, 1677–1686. [Google Scholar]
- Li, X.; Song, W.; Zhang, M.; Zhao, P. Human β-defensin 1 Functions as a Tumor Suppressor via ER Stress-triggered JNK pathway in Hepatocellular Carcinoma. J. BUON 2021, 26, 1365–1372. [Google Scholar]
- Gambichler, T.; Skrygan, M.; Huyn, J.; Bechara, F.G.; Sand, M.; Altmeyer, P.; Kreuter, A. Pattern of mRNA expression of β-defensins in basal cell carcinoma. BMC Cancer 2006, 6, 163. [Google Scholar] [CrossRef]
- Scola, N.; Gambichler, T.; Saklaoui, H.; Bechara, F.; Georgas, D.; Stücker, M.; Gläser, R.; Kreuter, A. The expression of antimicrobial peptides is significantly altered in cutaneous squamous cell carcinoma and precursor lesions. Br. J. Dermatol. 2012, 167, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.-M.; Chen, J.-Y.; Guo, L.; Wang, C.-Y.; Tan, W.-T.; Wen, Q.; Zhang, S.-D.; Deng, G.-H.; Lin, Y.; Kwok, H.F. β-defensin 1 expression in HCV infected liver/liver cancer: An important role in protecting HCV progression and liver cancer development. Sci. Rep. 2017, 7, 13404. [Google Scholar] [CrossRef] [PubMed]
- Krisanaprakornkit, S.; Weinberg, A.; Perez, C.N.; Dale, B.A. Expression of the Peptide Antibiotic Human β-Defensin 1 in Cultured Gingival Epithelial Cells and Gingival Tissue. Infect. Immun. 1998, 66, 4222–4228. [Google Scholar] [CrossRef]
- Bonamy, C.; Sechet, E.; Amiot, A.; Alam, A.; Mourez, M.; Fraisse, L.; Sansonetti, P.J.; Sperandio, B. Expression of the human antimicrobial peptide β-defensin-1 is repressed by the EGFR-ERK-MYC axis in colonic epithelial cells. Sci. Rep. 2018, 8, 18043. [Google Scholar] [CrossRef]
- Shi, N.; Jin, F.; Zhang, X.; Clinton, S.K.; Pan, Z.; Chen, T. Overexpression of human β-defensin 2 promotes growth and invasion during esophageal carcinogenesis. Oncotarget 2014, 5, 11333–11344. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Gibson, W.; Bullard, R.S.; Donald, C.D. PAX2 oncogene negatively regulates the expression of the host defense peptide human beta defensin-1 in prostate cancer. Mol. Immunol. 2009, 46, 1140–1148. [Google Scholar] [CrossRef]

| Patient Characteristics | ||
|---|---|---|
| Age (in years) | Mean ± SD | 51.81 ± 14.03 |
| Gender | ||
| Female | 3 (19%) | |
| Male | 13 (81%) | |
| Systemic Condition n (%) | ||
| Healthy | 7 (44%) | |
| Type II Diabetes | 4 (25%) | |
| Chronic Obstructive Pulmonary Disease | 3 (19%) | |
| Cardiovascular Diseases | 2 (12%) | |
| Hypothyroidism | 2 (12%) | |
| Medication n (%) | ||
| No Medication | 7 (44%) | |
| Metformin HCl | 4 (25%) | |
| ASA | 3 (19%) | |
| Ipratropium bromide | 3 (19%) | |
| Levoythroxine Sodium | 2 (12%) | |
| Atorvastatin | 1 (6%) | |
| Metoprolol | 1 (6%) | |
| Smoking (≥10 cigarettes a day, more than 5 years) | ||
| Yes | 16 (100%) | |
| No | 0 (0%) | |
| Primary Tumor Types n (%) | ||
| Nasopharynx CA | 8 (50%) | |
| Oral CA | 3 (19%) | |
| Larynx CA | 3 (19%) | |
| Parotid CA | 2 (12%) | |
| Chemotherapy n (%) | ||
| Yes | 8 (50%) | |
| No | 8 (50%) | |
| Total radiotherapy dose (cGy) | Mean ± SD | 6481.75 ± 567.21 |
| Number of Teeth mean (SD) | 21 (±6.7) |
| Stage of Periodontitis | |
| Stage I | 1 |
| Stage II | 4 |
| Stage III | 7 |
| Stage IV | 4 |
| Grade of Periodontitis | |
| Grade A | 0 |
| Grade B | 0 |
| Grade C | 16 |
| Bleeding on Probing (%) mean (SD) | 48 (±20) |
| 88 (±13) | |
| Plaque Index (%) mean (SD) | |
| Probing Depth (mm) mean (SD) Clinical Attachment Level (mm) mean (SD) 2.68 (±1.3) | 3.72 (±1.44) |
| Clinical Attachment Level (%) at least one tooth | |
| ≥4 mm | 13 |
| ≥6 mm | 7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keskin, M.; Suomi, E.; Harmankaya, İ.; Karaçetin, D.; Sorsa, T.; Gürsoy, U.K. Oral Cavity Beta-Defensin Levels Are Regulated Differently during Radiotherapy in Head and Neck Cancer Patients. Appl. Sci. 2023, 13, 2056. https://doi.org/10.3390/app13042056
Keskin M, Suomi E, Harmankaya İ, Karaçetin D, Sorsa T, Gürsoy UK. Oral Cavity Beta-Defensin Levels Are Regulated Differently during Radiotherapy in Head and Neck Cancer Patients. Applied Sciences. 2023; 13(4):2056. https://doi.org/10.3390/app13042056
Chicago/Turabian StyleKeskin, Mutlu, Emma Suomi, İlknur Harmankaya, Didem Karaçetin, Timo Sorsa, and Ulvi Kahraman Gürsoy. 2023. "Oral Cavity Beta-Defensin Levels Are Regulated Differently during Radiotherapy in Head and Neck Cancer Patients" Applied Sciences 13, no. 4: 2056. https://doi.org/10.3390/app13042056
APA StyleKeskin, M., Suomi, E., Harmankaya, İ., Karaçetin, D., Sorsa, T., & Gürsoy, U. K. (2023). Oral Cavity Beta-Defensin Levels Are Regulated Differently during Radiotherapy in Head and Neck Cancer Patients. Applied Sciences, 13(4), 2056. https://doi.org/10.3390/app13042056






