Characteristics of Clinical Isolates of Streptococcus mutans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of S. mutans Strains
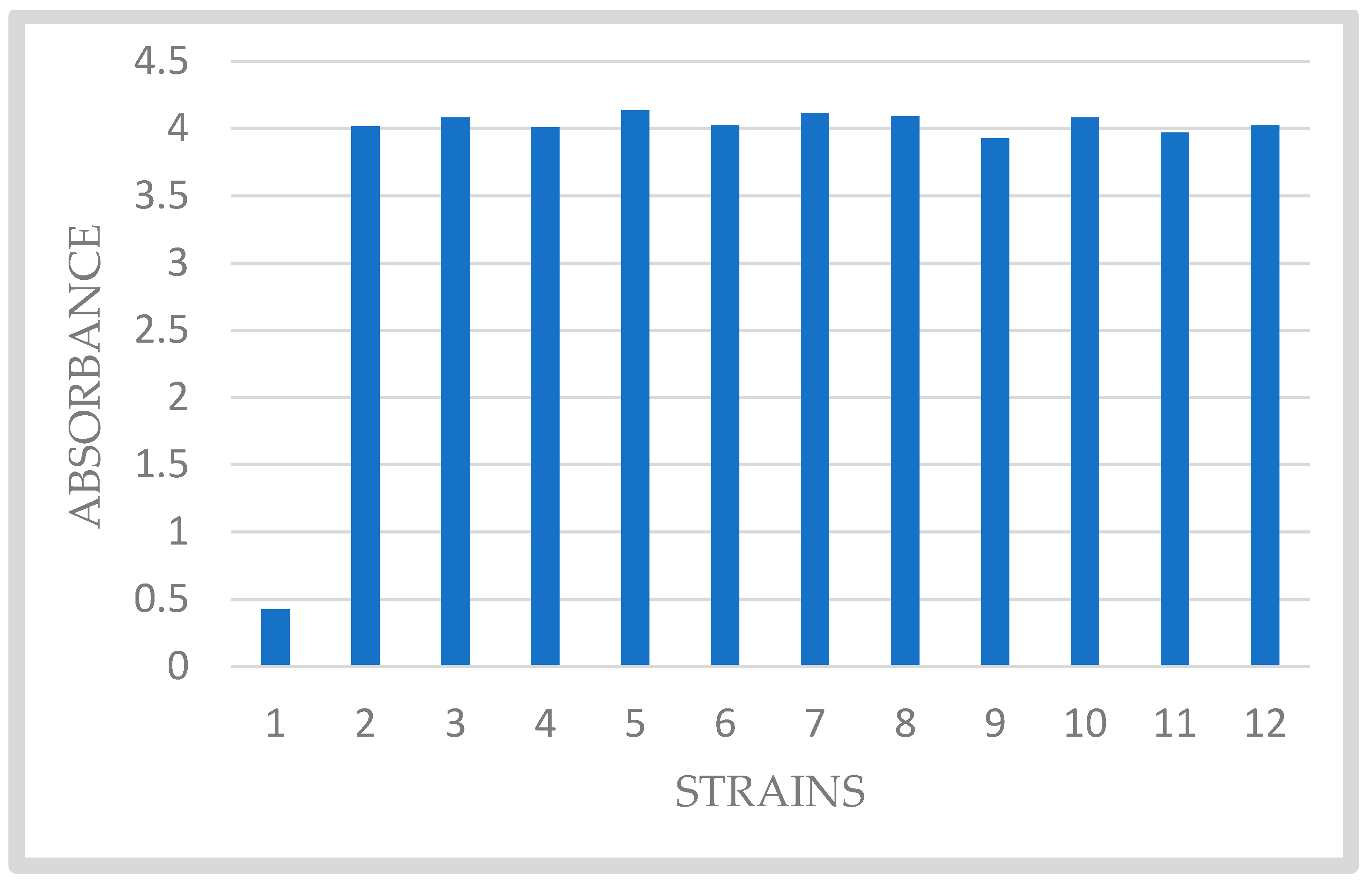
2.2. Biofilm Formation of Clinical Isolates of S. mutans
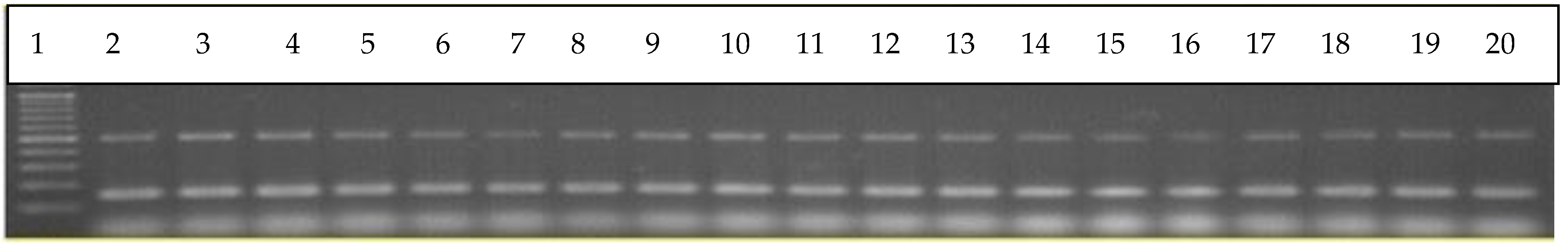
2.3. Binary Regulatory Systems of S. mutans Clinical Isolates
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Burmølle, M.; Webb, J.S.; Rao, D.; Hansen, L.H.; Sørensen, S.J.; Kjelleberg, S. Enhanced Biofilm Formation and Increased Resistance to Antimicrobial Agents and Bacterial Invasion Are Caused by Synergistic Interactions in Multispecies Biofilms. Appl. Environ. Microbiol. 2006, 72, 3916–3923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawada-Matsuo, M.; Komatsuzawa, H. Role of Streptococcus mutans two-component systems in antimicrobial peptide resistance in the oral cavity. JPN Dent. Sci. Rev. 2017, 53, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.T.; Ronson, C.W.; Ausubel, F.M. Two-component regulatory systems responsive to environmental stimuli share strongly conserved domains with the nitrogen assimilation regulatory genes ntrB and ntrC. Proc. Natl. Acad. Sci. USA 1986, 83, 7850–7854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 7. [Google Scholar] [CrossRef]
- Paśnik, U.; Brukwicka, I.; Błaszczak, B.; Kopański, Z.; Rowiński, J.; Strychar, J. Mikroflora jamy ustnej [Oral cavity microflora]. J. Clin. Healthc. 2017, 76, 05–09. [Google Scholar]
- Stużycka, I. The oral mikrobiome in dental caries. Pol. J. Microbiol. 2014, 63, 127–135. [Google Scholar] [CrossRef]
- He, X.S.; Shi, W.Y. Oral microbiology: Past, present and future. Int. J. Oral Sci. 2009, 1, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Strużycka, I.; Stępień, I. Biofilm nowy sposób rozumienia mikrobiologii. Nowa Stomatol. 2009, 3, 85–89. [Google Scholar]
- Strużycka, I. Biofilm- współczesne spojrzenie na etiologię próchnicy. Dental Forum 2010, 38, 73–79. [Google Scholar]
- Chałas, R.; Wójcik-Chęcińska, I.; Woźniak, M.J.; Grzonka, J.; Święszkowski, W.; Kurzydłowski, K.J. Płytka bakteryjna jako biofilm – zagrożenia w jamie ustnej oraz sposoby zapobiegania [Dental plaque as a biofilm—A risk in oral cavity and methods to prevent]. Postepy. Hig. Med. Dosw. 2015, 69, 1140–1148. (In Polish) [Google Scholar] [CrossRef]
- Fejerskov, O.; Kidd, E. Próchnica Zębów. Choroba Próchnicowa i Postępowanie Kliniczne; [Caries Disease and Clinical, Management]; Kaczmarek, U., Ed.; Urban&Partner: Wrocław, Poland, 2006. [Google Scholar]
- Dige, I.; Nyengaard, J.R.; Kilian, M.; Nyvad, B. Application of stereological principles for quantification of bacteria in intact dental biofilms. Oral Microbiol. Immunol. 2009, 24, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.S.; Kolenbrander, P.E. Development of a Multispecies Oral Bacterial Community in a Saliva-Conditioned Flow Cell. Appl. Environ. Microbiol. 2004, 70, 4340–4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritz, H. Microbial population shifts in developing human dental plaque. Arch. Oral Biol. 1967, 12, 1561–1568. [Google Scholar] [CrossRef]
- Berger, D.; Rakhamimova, A.; Pollack, A.; Loewy, Z. Oral Biofilms: Development, Control, and Analysis. High-Throughput 2018, 7, 24. [Google Scholar] [CrossRef]
- Pietruczuk-Padzik, A.; Stefańska, J.; Semczuk, K.; Dzierżanowska, D.; Tyski, S. Ocena Tworzenia biofilmu przez szczepy Staphylococcus aureus wyizolowane z plwociny pacjentów z mukowiscydozą. Med. Dośw. Mirobiol. 2010, 62, 1. [Google Scholar]
- Ajdic, D.; McShan, W.M.; McLaughlin, R.E.; Savic, G.; Chang, J.; Carson, M.B.; Primeaux, C.; Tian, R.; Kenton, S.; Jia, H.; et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 2002, 99, 14434–14439. [Google Scholar] [CrossRef] [Green Version]
- Krzyściak, W.; Kościelniak, D.; Papież, M.; Jurczak, A.; Vyhouskaya, P. Methods of Biotyping of Streptococcus mutans Species with the Routine Test as a Prognostic Value in Early Childhood Caries. Evid. Based Complement. Altern. Med. 2017, 2017, 6859543. [Google Scholar] [CrossRef] [Green Version]
- Beier, D.; Gross, R. Regulation of bacterial virulence by two-component systems. Curr. Opin. Microbiol. 2006, 9, 143–152. [Google Scholar] [CrossRef]
- Cheung, J.; Hendrickson, W.A. Sensor domains of two-component regulatory systems. Curr. Opin. Microbiol. 2010, 13, 116–123. [Google Scholar] [CrossRef] [Green Version]
- Galperin, M.Y. Diversity of structure and function of response regulator output domains. Curr. Opin. Microbiol. 2010, 13, 150–159. [Google Scholar] [CrossRef] [Green Version]
- Nowak, A.; Tyski, S. Dwuskładnikowe systemy regulacyjne ziarenkowców gram-dodatnich i ich rola w tworzeniu biofilmu. [Binary regulatory systems of gram-positive granulomas and their role in biofilm formation]. Post. Mikrobiol. 2012, 4, 265–276. [Google Scholar]
- Berkowitz, R.J. Mutans streptococci: Acquisition and transmission. Pediatr. Dent. 2006, 28, 106–109. [Google Scholar] [PubMed]
- Bryskier, A. Viridans group streptococci: A reservoir of resistant bacteria in oral cavities. Clin. Microbiol. Infect. 2002, 8, 65–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| AST-ST01 Streptococcus pneumoniae, Beta-haemolytic Streptococcus, Streptococcus viridans | |
|---|---|
| Antibiotic | MIC Scope |
| Ampicillin | 0.25–16 |
| Benzylpenicillin | 0.06–8 |
| Cefotaxime | 0.12–8 |
| Ceftriaxone | 0.12–8 |
| Clindamycin | 0.25–1 |
| Erythromycin | 0.12–8 |
| ICR S. agalactiae, S. pyogenes | NEG/POS |
| Levofloxacin | 0.25–16 |
| Linezolid | 2–8 |
| Tetracycline | 0.25–16 |
| Trimethoprim/Sulfameth | 10 (0.5/9.5)–320 (16/304) |
| Vancomycin | 0.12–8 |
| Primers/Multiplex | Product Size | TCS Name |
|---|---|---|
| SMU_1516 HK-F CGGCTTCTTGCCTTATCAAC SMU_1516 HK-R GTAAAGTCCTATTTAGAAGCTTTGGA SMU_1517 RR-F CCCCAAACCGTTTCAAGTAA SMU_1517 RR-R AAAATATTGAATCCGCAGTGG | 510 bp 211 bp | VicKR |
| SMU_1128 HK-F GTCTTGACGTCCAGCCAAAT SMU_1128 HK-R CGATTATTATCAGTGTGATGATTGTTT SMU_1129 RR-FGAAGAATTAAAAATGCGTATTCAGG SMU_1129 RR-R CAAAAATTTGTGACTTAGGTAAAATGA | 529 bp 211 bp | CiaHR |
| SMU_486 HK-F GCTGATTGGCTTGTTCTTGA SMU_486 HK-R TGAAAGTGTCTTTCCTTCTAATTCTG SMU_487 RR-F ATCGGTGAGGCTAGCAATG SMU_487 RR-R TAGAATTTTGGCTTCTTTCCAA | 520 bp 150 bp | LiaRS |
| SMU_1916 HK-F TCTTTGGTGGAATTCTGAATGA SMU_1916 HK-R AATGAGATAATGGCACAAAAGGA SMU_1917 RR-FATTGACCATTCTTCTGGCTGTT SMU_1917 RR-R TGAGTTTATGCCCCTCACTT | 500 bp 140 bp | ComDE |
| SMU_577 HK-FACCAGACGGTTGTTCCTTGA SMU_577 HK-R TGATGCCAACAAAGCTCGAT SMU_576 RR-F CTGCAGGAAATAATTGGTCTTG SMU_576 RR-R CAGCTACGACAGAAAAGAAAGG | 500 bp 200 bp | HKRR5 |
| SMU_660 HK-F AAAAACCTGCAGCAACAAGC SMU_660 HK-R AGCAGTTCCGTATTCCCTTT SMU_659 RR-F AGTTTTTGTCGGGACATTCG SMU_659 RR-R CCAGACTAGCATGGTGCTCA | 500 bp 199 bp | NsrRS |
| SMU_928 HK-FAAGGAGGTAGGAAATCGAGGA SMU_928 HK-R TGTTTCGCCAGTCATTAATTCTT SMU_927 RR-F ATGGACAAGATGCTATCGAAAAA SMU_927 RR-R TAATCATCAGCACCTGCCTCT | 501 bp 199 bp | HKRR7 |
| SMU_1009 HK-F CATTTTATACTGGCGGTTCCA SMU_1009 HK-R CCATCAATTGTCAAAGAAAGGTC SMU_1008 RR-F GCCCTATTTCAATGGCTTTT SMU_1008 RR-R TGCTTAGTGAACTCGTTAGCAC | 459 bp 210 bp | BceRS |
| SMU_1037 HK-F TCTCAGGATCTGTCTCAAATGG SMU_1037 HK-R CTCTGAGTCAACAGATTGAAGAAAA SMU_1038 RR-F GCTCTTTCCAAACCGATTCA SMU_1038 RR-R AAGCCACAATCCAGCAACTA | 495 bp 200 bp | HKRR9 |
| SMU_1145 HK-F TGGCATCACCCTTTACCAAT SMU_1145 HK-R TGTTCTTTTTAGTCATTCAAAGCTG SMU_1146 RR-F TGCAGACCCCAAACTTTTTC SMU_1146 RR-R TTTAAAAAGAGCCTATCCTGAAAA | 500 bp 200 bp | LcrRS |
| SMU_1548 HK-F CCCCACGTTTGATCGTAATC SMU_1548 HK-R GAACAGTATTGCTGTCTTTTTGATG SMU_1547 RR-F CACTAAGCGGATTGCTGTCA SMU_1547 RR-R GGATTCGTCAGCACCAAGAT | 551 bp 219 bp | HKRR11 |
| SMU_1814 HK-F CAAGCCCATACCGCTTCTT SMU_1814 HK-R CCTACTCTGGTTGAAAGTCACTACA SMU_1815 RR-F GCTTTGAGGAGTTTTTCTCGAT SMU_1815 RR-R CTGACCCCAACAGAAATTCA | 450 bp 175 bp | HKRR12 |
| SMU_1965 HK-F ATCATAATGCAGACTGACCTGTAGC SMU_1965 HK-R TCGCTCAATATCATTGTTTTTCT SMU_1964 RR-F AATCGCTAATTGCGTTCGAT SMU_1964 RR-R TCACAAATGGCGCAGTCTAA | 450 bp 216 bp | HKRR13 |
| AMY | + | PIPLC | − | dXYL | − | ADH1 | − | BGAL | − | AGLU | (−) |
| APPA | − | CDEX | − | AspA | − | BGAR | − | AMAN | − | PHOS | − |
| LeuA | (−) | ProA | − | BGURr | − | AGAL | − | Pyr A | − | BGUR | − |
| AlaA | + | TyrA | − | dSOR | + | URE | − | POLYB | + | dGAL | + |
| dRIB | − | ILATk | − | Lac | + | NAG | − | dMAL | + | BACI | + |
| NOVO | + | NC6.5 | − | dMAN | + | dMNE | + | MBdG | + | PUL | − |
| dRAF | + | O129R | − | SAL | + | SAC | + | dTRE | + | ADH2s | − |
| OPTO | + |
| Antimicrobial | MIC | Interpretation |
|---|---|---|
| Benzylpenicillin | <=0.06 | Sensitive |
| Ampicillin | <=0.25 | Sensitive |
| Cefotaxime | <=0.12 | Sensitive |
| Ceftriaxone | <=0.12 | Sensitive |
| Erythromycin | <=0.12 | Insufficient evidence that species is good target for therapy |
| Clindamycin | <=0.25 | Sensitive |
| Vancomycin | 1 | Sensitive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisarska, A.; Wolinowska, R.; Rudnicka, J.; Iwanicka-Grzegorek, E. Characteristics of Clinical Isolates of Streptococcus mutans. Appl. Sci. 2022, 12, 4579. https://doi.org/10.3390/app12094579
Pisarska A, Wolinowska R, Rudnicka J, Iwanicka-Grzegorek E. Characteristics of Clinical Isolates of Streptococcus mutans. Applied Sciences. 2022; 12(9):4579. https://doi.org/10.3390/app12094579
Chicago/Turabian StylePisarska, Aleksandra, Renata Wolinowska, Joanna Rudnicka, and Ewa Iwanicka-Grzegorek. 2022. "Characteristics of Clinical Isolates of Streptococcus mutans" Applied Sciences 12, no. 9: 4579. https://doi.org/10.3390/app12094579
APA StylePisarska, A., Wolinowska, R., Rudnicka, J., & Iwanicka-Grzegorek, E. (2022). Characteristics of Clinical Isolates of Streptococcus mutans. Applied Sciences, 12(9), 4579. https://doi.org/10.3390/app12094579





