Abstract
To provide a summary and overview of the use of Hybrid Assistive Limb in spinal cord injured patients over the past 10 years. A review of the literature was performed via Web of Science and PubMed using the search terms “Hybrid assistive limb” or “HAL“ or “wearable robot“ or “exoskeleton” and “SCI” or “spinal cord injury” by two of the authors. Relevant articles were then studied in full text. Our review of the literature found 21 articles that met the inclusion criteria of this narrative review including 344 participants. Articles were sorted into two general categories: (1) clinical trials, and (2) single-case or two-case reports. The vast majority of patients improved functionally, showing increased walking distances, walking speeds, and endurance. In addition, a variety of other advances were described, such as temporary decrease in spasticity, improvement in bladder and bowel management, pain reduction, and change in muscle activity. Even though there is no uniform application of HAL training in people living with SCI the current study situation suggests that many patients could benefit from this innovative training within their means.
1. Introduction
In 2016, globally, there were 0.93 million new cases of spinal cord injury (SCI) with age-standardized incidence rates of 13 per 100,000 [1]. The number of prevalent cases of SCI was 27.04 million [1] and thus represents a substantial portion of the global injury burden. Individuals who have experienced SCI find themselves in a situation where even previously simple things become insuperable barriers. Fortunately, a lot has happened in the treatment of SCI in recent years. Robotic therapy approaches and exoskeletons have increasingly found their way into SCI centers around the world. Most exoskeletons have a similar appearance from the outside. There is an external support frame along the lower extremities, a type of hip belt or attachment to the hip, and a shoe device, either for use with the patient’s own shoes or over shoes belonging to the system. However, the systems differ in their application and clinical objective. Some systems are assistive, and some are rehabilitative. There is also a difference in the control mechanism. Here there is joystick control or posture control and in one case, electromyography (EMG) based support. Our narrative review focuses on this EMG controlled system, the Hybrid Assistive Limb (HAL; Cyberdyne, Inc., Tsukuba, Japan). The HAL is a wearable robot suit that senses a patient’s voluntary actions, such as real-time myoelectric potential, foot pressure, and joint angle, and assists hip and knee joint movement [2]. Figure 1 shows the HAL. Initially, HAL was developed in order to physically support a wearer’s daily activities and heavy work [3] but it has also been used from the earliest times for welfare and patient training. The HAL was used in the rehabilitation of acute [4] and chronic [5] stroke patients, patients with neuromuscular diseases [6], and SCI [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. To the best of our knowledge, there is currently no paper that concisely presents the previous studies in patients with SCI and helps to provide an overview of the different training approaches, patient collectives and the results achieved. Therefore, the purpose of this paper is to summarize and present a review of previous studies on HAL training in patients with SCI.
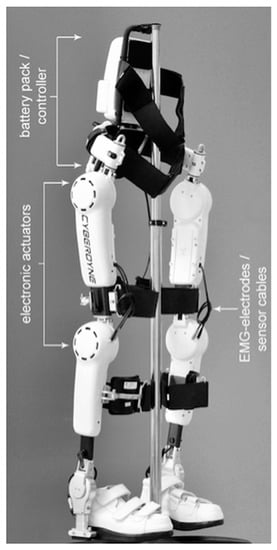
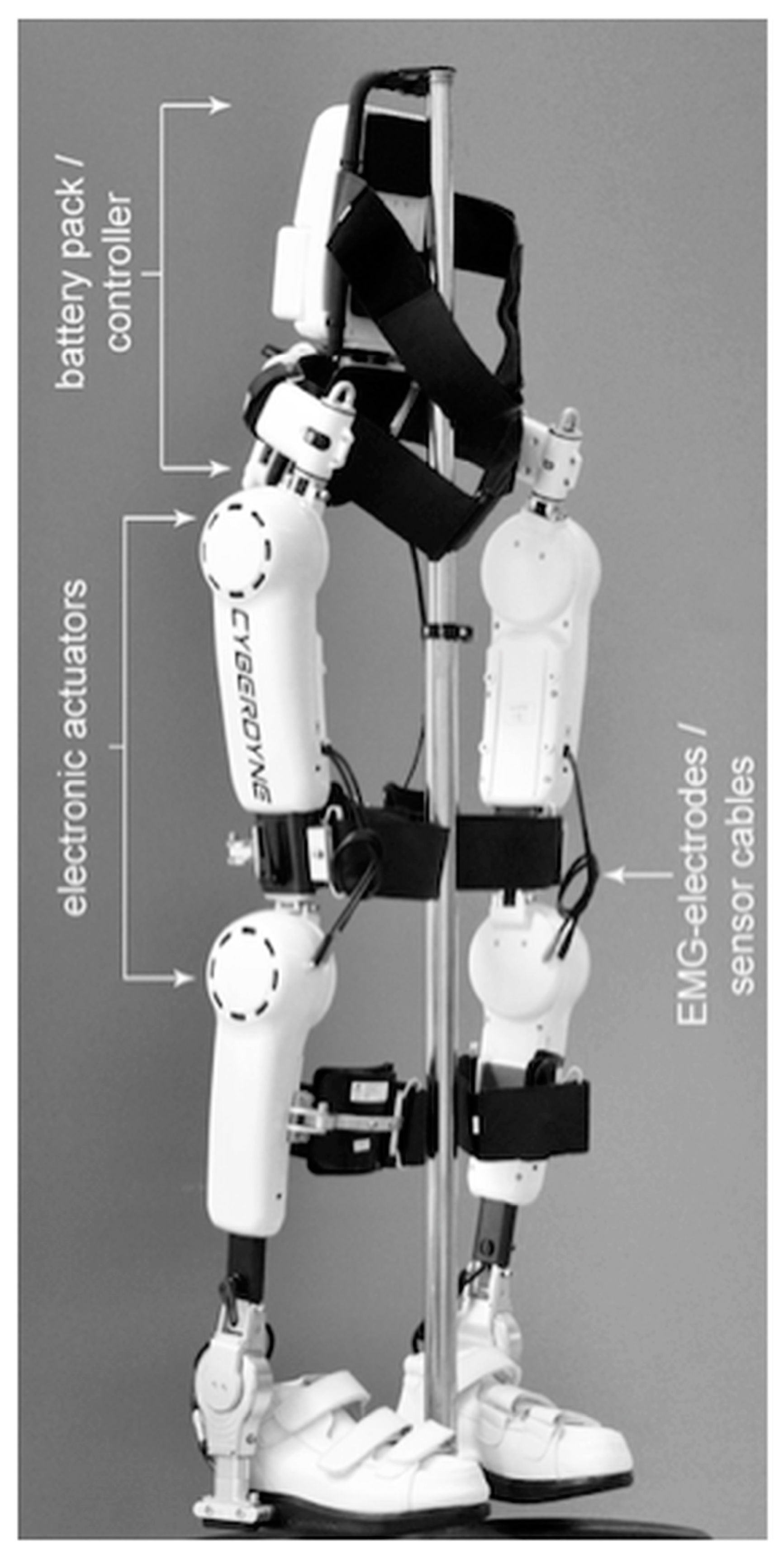
Figure 1.
HAL®—Hybrid assistive limb exoskeleton (Cyberdyne Inc.).
2. Materials and Methods
A narrative review of the literature was conducted with electronic searches for eligible studies using the databases Web of Science, PubMed, and Scopus on 15 September 2022 and 6 January 2023 using the following search term combinations in the titles: “Hybrid assistive limb” or “HAL“ AND “SCI”, “Hybrid assistive limb” or “HAL“ AND “spinal cord injury”, “Hybrid assistive limb” or “wearable robot“ AND “SCI”, “Hybrid assistive limb” or “wearable robot” AND “spinal cord injury”, “Hybrid assistive limb” or “exoskeleton“ AND “SCI”, “Hybrid assistive limb” or “exoskeleton” AND “spinal cord injury”, “HAL” or “wearable robot“ AND “SCI”, “HAL” or “wearable robot” AND “spinal cord injury”, “HAL” or “exoskeleton“ AND “SCI”, “HAL” or “exoskeleton” AND “spinal cord injury”. The search was limited to articles that were published in (or subsequently translated to) English or German. No restriction on date published was enforced. All study designs were included. Two of the authors screened the titles and then abstracts independently and studies were considered relevant if they addressed any clinical application of the HAL system in people living with SCI. In case of disagreement, a discussion was held to reach consensus on which studies should be reviewed in full text. If necessary, a third author was consulted to make the final decision. Studies reporting the use of HAL in other conditions, such as post-stroke or multiple sclerosis, were excluded as well as studies reporting use of other exoskeleton systems. Reference lists of the involved studies were manually searched for further articles. A narrative review rather than a systematic review was undertaken to report a broad overview of the use of the HAL system in the field of rehabilitative SCI treatment. Although the system has been available for a long time, there are currently no randomized controlled trials, which the authors believe are essential for a systematic review. Articles were sorted into 2 general categories for discussion: (1) clinical trials, and (2) single-case or two-case reports.
3. Results
In their paper from 2005 Suzuki et al. [7] proposed for the first time an algorithm to estimate human intentions related with walking in order to comfortably and safely support a paraplegia patient’s walk with the HAL robot suit. This work presents mainly technical data of algorithm development and therefore was not considered further in this review. Nevertheless, it should be mentioned here, as it has set the stage for further application studies.
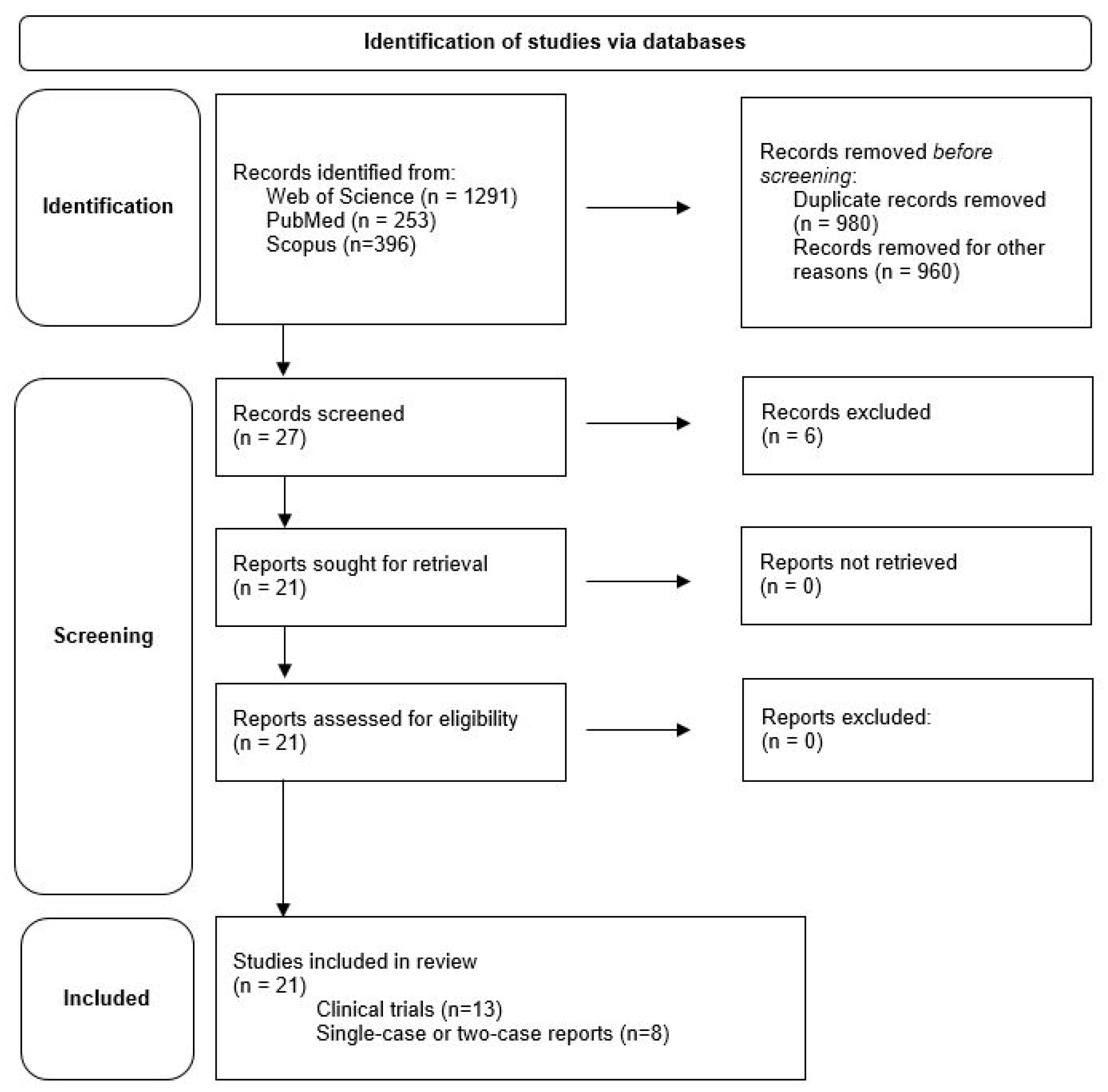
A total of 1940 studies were found using the search criteria described above. After excluding studies that had a completely different topic but were still listed, either used exoskeletons other than the HAL system, or used the HAL device for other conditions, and after removing all duplicates, 27 published articles were identified. Studies only reporting technology data (3) and reviews on general HAL application (3) were excluded. Twenty-one relevant articles, as deemed so by the authors, were then studied in full text (Figure 2). Overall, the studies discuss results from 344 participants, with some of the same subjects included in different studies.
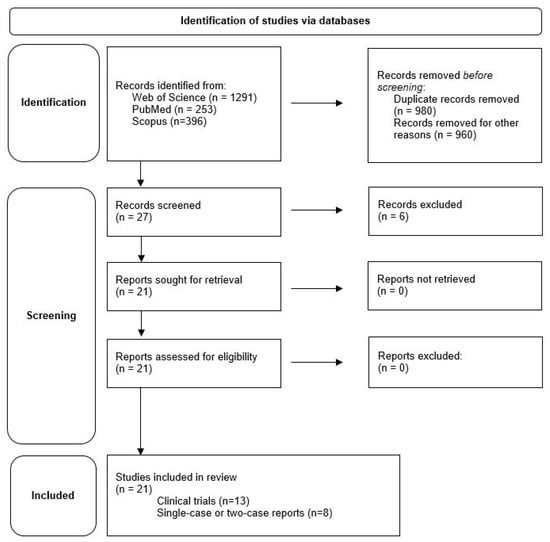
Figure 2.
Presentation of the results of the literature search.
Of these 21 articles, 13 are reports about clinical trials using HAL [8,9,10,11,12,13,14,15,16,17,18,19,20] and eight are single-case [2,22,23,24,25] or two-case reports [21,26,27]. Ten articles originate from Germany [9,10,11,12,13,16,17,20,21,22], ten from Japan [2,8,14,15,18,19,23,24,26,27], and one from the USA [25]. Years of publication of these articles range from 2013 to 2021. The underlying experimental series started in part as early as 2012, which means that we present a survey of the last ten years in this review. To the best of our knowledge this is the first ever review summarizing experiences with HAL in people living with SCI.
Study protocols differed in terms of training type (treadmill or walker), training time (20 to 40 min of net HAL walking training), time period over which training was performed (2 to 52 weeks), number of training sessions per week (2 to 5), resulting total training sessions (8 to approximately 180), and assessments performed. The patients included in these studies represent the full range of spinal cord injuries. There are traumatic and non-traumatic injuries, chronic and acute patients, complete and incomplete paralysis, lesion levels from C2 to L4, American spinal injury association impairment scale (AIS) A to D, and patients with and without spastic motor behavior. The age of the participants varies from 16 to 74 years. Some studies conducted by the same research groups (Ruhr-University Bochum Group [9,10,11,12,13,16,17,20,21,22], Keio University School of Medicine Group [15,18,19], University of Tsukuba and University of Tsukuba Hospital Group [8,14,23,24,26,27]) have partial overlap in their study populations.
3.1. Clinical Trials
In 2013, Kubota et al. [8] published a paper summarizing findings of previous studies on robotic devices used in clinical settings with the conclusion that robot-assisted rehabilitation is controversial. They introduced the newly developed HAL and had the intention to investigate the feasibility of HAL rehabilitation training for patients with limited mobility. The study collective consisted of 12 stroke patients, four patients with musculoskeletal diseases, eight SCI patients, and 14 other diseases. The SCI patients were all in a chronic stage (1–6 years since SCI) and included four paraplegia as well as four tetraplegia. Four of them were incomplete, one complete, one had spina bifida, one central cervical cord injury, and one spinal dural arteriovenous fistula. Patients performed 16 HAL sessions in eight weeks using a walking device or treadmill for 90 min (net walking time 20 min). One of these patients dropped out in course of the study due to neuropathic pain and for one patient the baseline measurement was not assessed, as he was unable to ambulate with any assistance. Therefore, only six SCI patients were included in the statistical analysis. In this study, the functional ambulation results were presented for the whole group of participants included in the statistical analysis (n = 27). Since the informative value about this diverse patient collective, in the view of the authors of the review, is very limited, we refrain from presenting it here in detail. However, significant improvements in gait speed, number of steps, and cadence were observed, as assessed by the 10-Meter-walking-test (10MWT). Improved Timed-Up-and-Go test (TUG) and Berg balance scale (BBS) results were also observed, but they were not statistically significant [8]. The only SCI specific result that was given is a large effect size in gait speed (Cohan d = 0.78), which leads the authors to conclude that training effectiveness in SCI patients can be expected. The conclusion of this study states that HAL training is feasible, safe and effective in patients with limited mobility [8].
Results that are more detailed are provided by a study from Aach et al. [9] involving eight patients in chronic stage (1–19 years since SCI) after traumatic SCI. Included patients had incomplete paraplegia (AIS B/C/D, n = 4) or complete paraplegia (AIS A, n = 4) with zones of partial preservation (ZPP) and performed treadmill training using HAL five times per week for 90 days (mean number of sessions 51.75 ± 5.6). Treadmill associated results improved over all patients for walking speed and walking time, but no information was provided on the statistical significance except for ambulated distance, which increased from 195.88 ± 166.71 m to 954.13 ± 380.35 m (p < 0.05). Functional assessments were the 10MWT, TUG, 6-Minute-Walk-Test (6MWT), walking index for SCI II (WISCI II) score, and lower extremity motor score (LEMS). The authors state that although the WISCI II score did not improve statistically, three patients showed functional improvements in gait abilities. For the 10MWT a significant increase in gait speed similar to Kubota et al. [8] and decrease in number of steps were found as well as a decreased time for the TUG and increased LEMS. Similarly, they reported significant improvement for the 6MWT, although it should be noted that only three participants were able to take part in the baseline measurement (with an average walking distance of 187 ± 162.2 m) and the five others who did not participate (scored as 0 m) decimated the total baseline data to 70.1 ± 130 m. However, the subgroup who performed the 6MWT at baseline improved their walking distance to 287.3 ± 229.4 [9]. In this study, information on statistical significance was not given in detail, it was only stated that the p value is <0.05. Thus, it remains unknown whether the improvements were highly significant or whether the results were just at the threshold of significance. In addition to these results, the authors reported on seven participants with increased muscle volume as measured by the circumferences 10/20 cm cranial of the inner knee joint gap and 15 cm distal of it. One patient lost muscle volume. Furthermore, one participant with spinal spasticity was able to reduce Ashworth scale from four to two for a few hours after training. One patient switched from AIS B to C after treatment.
Seven of these eight patients from the Aach et al. [9] study were also part of a study by Sczesny-Kaiser et al. [10] to investigate whether body weight supported treadmill training with HAL affects cortical excitability in the primary somatosensory cortex (S1) in SCI patients, as measured by paired-pulse somatosensory evoked potentials (ppSEP) stimulated above the level of injury. These seven patients were supplemented by four additional patients (8.5 months to 17 years since SCI) performing the same intervention protocol as described for a total of eleven participants. No electrophysiological results of these eleven patients were published until then. Nerve stimulation was conducted successively on both sides with a block electrode placed on the wrist. ppSEP was assessed before and after the HAL training period and compared with a healthy control group (n = 11). Additionally, electroneurography (ENG), somatosensory evoked potentials (SEP) of the tibial nerves, and motor evoked potentials (MEP) of anterior tibial muscles were performed before and after the training period. The authors present a statistical difference between SCI patients and healthy control group at baseline. After intervention, this difference was no longer present. Comparison of patient’s data pre- and post-training showed a significant statistical training effect. ENG, SEP of tibial nerves and MEP showed no significant differences after training. Functional outcomes consequently to the previous study from Aach et al. [9] were statistical significance for 10MWT-speed, 6MWT, TUG, LEMS. These results show that SCI patients had increased cortical excitability in the hand area of S1 prior to training and that this was normalized following 12 weeks HAL training accompanied by significant improvements in walking abilities [10]. However, a correlation analysis revealed no relationship between the extent of changes in excitability and time since injury, functional walking parameters, or LEMS. The authors, anyway, conclude that walking improvements may be related to a renewed S1 and M1 representation of impaired lower extremities likely related to the recruitment and more effective use of remaining somatosensory afferent pathways and corticospinal tracts [10]. The missing correlation between functional abilities and changes of excitability in S1 might indicate that either other cortical areas or even a complex supraspinal network is required for walking rehabilitation [10].
In 2017, Jansen et al. [11] published a proof of concept to the pilot study from Aach et al. [9] and expanded the collective to 21 chronic SCI (1–19 years since SCI) patients. All of them improved significantly in respect to treadmill associated data (walking distance, speed) and in functional tests assessed by standardized over-ground walking tests (10MWT, TUG, 6MWT) without HAL, which therefore confirms findings of the pilot study [9,11]. In addition to these results, this study revisits the issue of spasticity and states that in eight subjects with spastic motor behavior intervention led to a temporary relief. This was already discussed for one patient in the pilot study [9]. Furthermore, the authors indicate that 18 of 21 patients reported improvements of their bowel and bladder dysfunctions after the training period with two of them able to discontinue self-catheterization [11]. However, both spasticity and bowel and bladder improvements were not supported by statistical data.
A question often discussed in the SCI literature is in which patient population improvements are most likely to occur and in which less so. According to Grasmücke et al. [12] it is mainly postulated that the neurological and ambulatory recovery depends on a patient’s lesion level and age, as well as their initial AIS grade whereby low AIS grade and age ≥ 50 years seem to be negative predictors for functional recovery following SCI. In order to investigate this in relation to HAL training a study was set up with the aim to compare the functional outcomes of patients with chronic SCI (1 to 20 years since SCI) in four injury-level groups and additionally analyze results as a function of age (<50 years vs. ≥50 years) [12]. The four groups were classified as (1) incomplete tetraplegia (n = 13, C2–8, AIS C and D), (2) incomplete paraplegia with spastic motor behavior (n = 15, T2–12, AIS C and D), (3) complete paraplegia with an absence of spastic motor behavior (n = 18, T11–L4, AIS A and ZPP from L3 to S1), and (4) incomplete paraplegia with an absence of spastic motor behavior (n = 9, T12–L3, AIS C and D) [12]. A total of 55 participants were involved. As part of the Ruhr-University Bochum group, the same training program (12 weeks, 5 units/week) was completed and the results of the initial eight patients from Aach et al. [9] and in part from Jansen et at. [11] were also included. As seen in the other studies [9,10,11], the cohort improved in treadmill related and functional parameters. In contrast, however, to the assumptions made in the literature, the lesion level and patients’ age were not significantly associated with training-related functional improvements. No significant differences between subgroups were found. Subgroup 2 showed slightly less improvement in treadmill walking speed, treadmill distance and 6MWT, but these differences were statistically not significant [12]. Concerning patients’ age only 10MWT-speed showed a significantly larger improvement in the younger group compared to the older group. Another interesting finding of this study was that most improvements occurred during the 4th and 10th week of the training period [12]. From the results demonstrated here, the authors conclude that older age or lesion level seem to be less important in terms of the extent of functional improvement following HAL training. Nevertheless, incomplete SCI lesions of the thoracic spine, including spastic motor behavior, appear to be a non-significant negative predictor for training-related improvements [12].
To examine long-term data of variable-frequency HAL training after the initial training period, it was again Jansen et al. [13] who followed up the pilot study of Aach et al. [9] with one year of HAL training. All eight chronic SCI participants continued HAL training after their initial training period of 12 weeks and were divided into two groups. Group 1 (n = 4) continued with 3–5 training sessions per week for a further 40 weeks and group 2 (n = 4) continued with one session per week. In the first 12 weeks, the participants had 51.75 ± 5.6 training sessions on average. During the second training period, the mean number of training sessions in group 1 was 126.8 ± 7.9 and 32.3 ± 3.3 in group 2. Results were given both for the first training period (repetition of Aach et al. [9] results) and after one year of treatment. In order not to be redundant, we here only discuss new results after one year. Concerning treadmill related parameters (speed, walking time, and distance) no significant improvements were demonstrated between 12 and 52 weeks results for evaluation as a total group (n = 8), as individual groups (n = 4/4), and for comparison between subgroups. The same was seen in the functional testing. Considering all eight patients together, irrespective of continuous training frequency during the following 40 weeks, there were no significant changes for 10MWT, cadence, number of steps, step length, TUG, 6MWT, or WISCI II. Taking into consideration the two subgroups separately, there was no difference between 12 and 52 weeks and no difference between the groups. These certainly surprising results indicate that the initial functional gain after 3 months of daily HAL training was stable and consistent over a 1-year period [13].
A completely new approach to HAL therapy was described by Shimizu et al. [14] in their 2017 study. HAL support is predominantly based on EMG signals from the lower extremity or weight shift over the sensor shoes. Since such muscle signals are usually undetectable in patients with complete paraplegia or tetraplegia and controlled loading of the feet is rare, the researchers decided to use upper extremity muscle activity as a trigger of lower extremity movement. This is based on the assumption that the lower extremities move synchronously and almost simultaneously with the contralateral upper extremities during natural locomotion [14]. The authors hypothesized that triggering lower limb motion by upper limb muscle activity was feasible to generate voluntary gait and furthermore, that this may activate paralyzed lower limb muscles. Muscle activities from the anterior and posterior deltoid were used for contralateral hip flexion and extension and activities from the biceps and triceps brachii for contralateral knee flexion and extension [14]. Patients underwent ten sessions of walker assisted HAL training protocol for 60–90 min (net HAL training time 30 min) once or twice a month (n = 2) or twice a week (n = 2). In two patients who were able to flex their hips (before or along the intervention) a knee extension training using hip flexor activation was performed additionally in order to regain active knee extension. EMG was used to evaluate muscle activity of the tensor fasciae latae (TFL) and quadriceps femoris (Quad) [14]. Prior to intervention none of the participants showed apparent activation in Quad and only one in TFL. However, gait phase dependent activity of the lower limb muscles was seen during voluntarily triggered ambulation driven by upper limb muscle activities and in some cases active contraction in Quad was observed after knee extension session using the hip flexor as trigger [14]. Therefore, the authors conclude that this method is a feasible option for patients with severe paraplegia or tetraplegia. Although these results are certainly exciting, it is difficult to generalize. In this study, only four patients were examined and they had different training frequencies. Furthermore, in two of the four patients, the upper body controlled training was started after three and five sessions, respectively, and another mode of the HAL accompanied with heavy assistance from three therapists was used beforehand to familiarize the patients with the use of such a device. One of the four patients received additional physiotherapy during the training phase. Nevertheless, with this study the authors contribute to the understanding of possible applications of the HAL even in severely injured patients. In addition to the results already mentioned, the patients improved their walking distance and three out of four patients showed improvements in Ashworth score, which is in line with the results of other studies [9,11].
A question similar in approach to Grasmücke et al. [12] was pursued by Okawara et al. [15], who asked whether HAL training is appropriate for all severities of SCI. They stated that in most of the preliminary studies patients already had a certain walking function before the treatment participated. They set out to investigate the effect of training in relation to the severity of SCI. For this purpose, 20 chronic patients were recruited and divided into a low walking ability group (n = 8) and a high walking ability group (n = 12) based on their WISCI II scores at baseline. Twenty training sessions with a frequency of 2–5 per week were performed on the treadmill. Gait performance on treadmill (speed, distance, and time) was significantly lower in the low walking ability group while perceived exertion was higher. Functional testings’ (10MWT time, 10MWT-speed, 10MWT number of steps, TUG) improved in the higher walking ability group significantly while in the low walking ability group none of the participants were able to complete the assessments at either point. BBS score improved significantly following training in the high walking ability group but not in the low walking ability group. Barthel Index (BI) and Functional Independence Measure (FIM) as assessment of activities of daily living (ADL) were unchanged for both groups, indicating that there was no effect of training regardless of baseline walking ability [15]. The authors summed up the results such that patients with a WISCI II score below six barely benefited from training. They contrast these results with Jansen et al. [11] in whose collective, which improved significantly, there was also one patient with a WISCI II score of one. In Jansen’s study, however, individual results were not reported, so it is not clear whether this patient also improved. Interestingly, despite the sparse results in the slow walking ability group, 19 of the 20 patients reported subjective improvement after training and all patients were either fully or slightly satisfied.
The issue of bladder and bowel dysfunctions raised by Jansen et al. [11] is revisited by Brinkemper et al. [16] in a retrospective study. Here, 35 patients with acute (n = 13) or chronic (n = 22) SCI completed two standardized and established gastroenterological questionnaires (Cleveland Clinic Constipation Scoring System (CCCS), Wexner Score) and a self-developed questionnaire asking for bladder function before and after completing the training. The study protocol corresponds to that of the other Ruhr-University Bochum studies and functional results of the patients were already published. Comparisons were made for all patients and the group was divided into subgroups of acute and chronic patients. Within these subgroups, further subgroups were formed according to high or low baseline scores. Wexner Score, a questionnaire to assess the severity of fecal incontinence, over all patients and for the group of chronic patients decreased significantly. For the group of acute patients Wexner Score decreased insignificantly. Patients from both groups with higher baseline scores could decrease significantly. CCCS, a questionnaire to assess the severity of constipation, reduced non-significantly for all patients, the group of acute, and the group of chronic patients. For the subgroup of chronic patients with higher baseline scores, CCCS decreased missing out significance. The self-developed questionnaire showed an improvement in bladder function in 28.24% of all patients, 31.43% of chronic patients, and 23.08% of acute patients. The authors conclude that there is a trend toward improvement in bladder and bowel function after HAL training, with patients with higher baseline scores in Wexner Score and CCCS seeming to benefit more and see this as confirming the results of studies [28,29,30,31] using other exoskeletons. However, the mixed population of acute and chronic patients and different injury levels as well as the retrospective study approach remains a limitation of this study.
Another study based on the Ruhr-University Bochum protocol was published by Brinkemper et al. [17]. This time, however, the focus was not on functional improvements of the patients but on whether the functional improvements were accompanied by physiological improvements in gait. For this purpose, a 3D gait analysis was performed on 15 patients (acute n = 5, chronic n = 10) using an inertial measurement unit sensor system. While all functional parameters improved as expected, physiological differences were also found in some phases of gait cycle showing improved knee extension during initial contact and increased maximum hip extension prior to swing phase. Furthermore, all joint angles showed a larger range of motion and those findings were accompanied by significant improvement in all spatiotemporal and gait phase parameters. Thus, according to the authors, this study was the first to demonstrate improved gait physiology after HAL training in SCI patients.
In addition to the frequently addressed ability to walk in patients with SCI, the ability to maintain a seated position for an extended period of time also plays an important role. To evaluate the effect of HAL training on trunk muscle strength, Okawara et al. [18] conducted a study in nine chronic (1–10 years since SCI) patients. The HAL intervention consisted of 20 training sessions of approximately 40 min net HAL training time each. Trunk muscle strength was defined as the ability to maintain a seated posture in four directions (anterior, posterior, left lateral, and right lateral) and measured pre- and post-training [18]. An examiner pushed the participant’s torso using a dynamometer until the participant could no longer maintain the seated posture. The patients were part of the collective from Okawara et al. [15] where the functional recovery results have already been published. Trunk muscle strengths from baseline to after 20 training sessions improved in all directions, however, statistical significance was only shown in the change of lateral trunk muscle strength. Surprisingly for the authors, the changes in anterior, posterior, and lateral trunk muscle strength showed significant positive correlations with age at baseline, indicating that older adult subjects with chronic SCI experienced greater improvements in trunk muscle strength [18]. This contrasts somewhat with the results of Grasmücke et al. [12] who induced a lower level of improvement in gait function (10MWT-speed) in older adult patients than in younger patients during the chronic phase of SCI.
A study by Sawada et al. [19], which is based on the same study population as Okawara et al. [15], deals with the influence of HAL training on quality of life (QOL). Since paraplegia affects one’s entire life, QOL suffers from it and requires follow-up in the course of rehabilitation. Patients performed the already known 20 sessions HAL protocol and were assessed by short Form−36 questionnaire Japanese version (SF-36v2), WISCI II, FIM motor score, and the Neuropathic Pain Symptom Inventory (NPSI) self-questionnaire. As with Okawara et al. [15] patients were divided into a low walking ability group and a high walking ability group. Baseline values in SF-36v2 of the participants were lower compared to healthy individuals and were not improved in the course of the training. Likewise, WISCI II, FIM motor score, and NPSI did not show significant differences, irrespective of baseline walking abilities. However, a correlation analysis showed positive correlation between FIM motor score and some subscales of SF-36v2 and a negative correlation between NPSI and the change in subscale Vitality and Mental Health, leading the authors to conclude that in their protocol those with higher functional independence and lower pain at pre intervention were more likely to improve [19]. The authors see an explanation for the generally low improvement in QOL in the unchanged pain score, which in turn can be explained by an already low pain prevalence at baseline.
The largest study population in a clinical trial with HAL to date is provided by the study of Zieriacks et al. [20]. This involved 121 patients with acute (n = 47) and chronic (n = 74) SCI. Partial results of this collective have been published previously [9,10,11,12,13,16,17]. The aim was to assess whether HAL training is advantageous for acute and chronic participants and if length of time post injury affects the outcome. Significant improvements in treadmill-related parameters (time, distance) and all functional parameters (10MWT, 6MWT, WISCI II, LEMS) were reported for the whole group as well as for the subgroups of acute and chronic patients. However, significant differences between acute and chronic participants’ outcomes were found in 6MWT, LEMS, and WISCI II, showing that chronic participants improved significantly less than acute participants did. Nevertheless, since both subgroups showed significant improvements individually, the authors concluded that HAL training is advantageous for both acute and chronic patients and that there is no time related cut-off threshold following SCI for effectiveness [20]. Table 1 summarizes the clinical trials studies details and results.

Table 1.
Spinal cord injury clinical trials studies characteristics.
3.2. Single-Case or Two-Case Reports
In addition to clinical trials in more or less large collectives, there are some publications that are single case or two case reports on selected issues.
For example, Cruciger et al. [21] published the pain-related data of two subjects from the collective of the Aach et al. [9] study individually. Those chronic patients (both AIS A with ZPP, 10 and 19 years since SCI) had therapy-resistant chronical neuropathic pain. During the course of HAL training, pain symptoms improved in both patients to the extent that medication could be reduced. A brief increase in pain in response to the lower dosage was followed by a renewed reduction in pain during training. Patients changed from a permanent medication to an on-demand medication. The average pain intensity, measured by numerical pain rating scale (NRS-11; 0 = no pain, 10 = worst pain possible), decreased from 4.3 at the first training week to 0.6 after 12 weeks. Furthermore, an improvement on health related QOL in the SF-36 questionnaire was found reflecting the impact of the intervention. According to the authors in a one-year follow up, both patients reported neither recurrence of pain nor need for medication [21]. These results are in contrast to those of Sawada et al. [19] who found no changes in pain and QOL in their study. However, the collective in Sawada et al. [19] also showed no motor functional improvements while in the study by Cruciger et al. [21], two patients improved in terms of 10MWT and LEMS. This could potentially have had an impact on the QOL data.
It was also Cruciger et al. [22] who first reported the use of HAL training in an acutely injured SCI patient in a single case report. The patient had an incomplete motor T10 lesion (AIS C) and began training 77 days after the accident. After 12 weeks HAL training there was recovery of motor functions and walking abilities as shown by increase in WISCI II from 8 to 18 and conversion to AIS D.
Ikumi et al. [23] wanted to find out if HAL application was possible in a severely affected chronic complete tetraplegic patient and subjected him to 10 HAL trainings in 5 weeks. To perform the treadmill training, two physicians and one therapist were needed. Walking distance and time increased from 25.2 m and 7.6 min to 148.3 m and 15 min and the modified Ashworth scale decreased from 15.3 to 5.75 points after HAL training and lasted for up to 30 min [23]. Therefore, the authors conclude feasibility and efficiency of HAL rehabilitation for tetraplegic patients.
Another special case is reported by Shimizu et al. [24] in their presentation of a man whose condition deteriorated due to a spinal dural arteriovenous fistula 19 years after the initial SCI. He underwent surgery and his neurological as well as motor status improved again. Six months post-operatively his improvement had plateaued. This was the point where HAL training started for ten sessions during three months. The patient improved in terms of gait speed and cadence in 10MWT, International Standards for Neurological and Functional Classification of Spinal Cord Injury (ISNSCI) motor score (14 to 16), WISCI II (7 to 12), manual muscle testing and to some extent in muscle activities measured via EMG. Even though the improvements shown here are not enormous, they do show a further development after the performance increase had initially reached a plateau.
The very first application of HAL in the USA is described by Yilmaz et al. [25]. This involved six patients with neurologic motor deficits of various etiologies who underwent 60 HAL sessions in 12 weeks. Among others, one patient was post spinal cord infarction following pulmonary embolism. The aim of this study was to investigate the impact of HAL training on QOL. The spinal cord infarction patient showed improvements in distance on treadmill and in a physical function mobility score. However, she showed a worse QOL after training in a six months follow up. The authors note that this is probably due to the very high expectations for the novel therapy, which is an extremely interesting point that has not been mentioned in previous studies. In the other patients of the collective, there was no noteworthy improvement in QOL despite good mobility improvements, which suggests that QOL is determined by more diverse factors [25]. This result contrasts with that of Cruciger et al. [21], whose two patients improved in health related QOL, but is confirmed by the study of Sawada et al. [19].
HAL training was completed as a contiguous training phase in most studies. Kanazawa et al. [2], however, chose an alternating design of conventional gait training (A) and HAL training (B) in their single-case study of a patient with chronic thoracic SCI. A similar protocol has already been practiced in stroke patients. The patient completed a total 25-week program with a switch from conventional gait training to HAL training every 5 weeks. In total, 15 weeks of conventional gait training and 10 weeks of HAL (A1-B1-A2-B2-A3) were performed, with three sessions per week. Accompanying physiotherapy took place permanently. Global parameters such as 2-min-walk-test, WISCI II, and BBS improved from the beginning to 25 weeks while spasticity did not. Walking speed, stride length, and cadence improved after phase A but not B [2]. During phase B a decrease in speed was seen, which could, according to the authors, reflect fatigue and changes in lower limb spasticity after using HAL. However, the alternating training program is considered potentially successful, although it remains unclear which phases have which share in the overall result.
As in Yilmaz et al. [25] a two-case study by Watanabe et al. [26] included patients after spinal cord infarction. Both patients were in a very early acute phase (14 and 7 days after infarction) at the start of training. In addition to conventional physical therapy, they received 7–8 HAL sessions 3–4 times per week. Improvements in LEMS, WISCI II, comfortable gait speed, stride, cadence, BI, FIM, and further assessments were observed in both study participants [26]. Therefore, the authors state that gait treatment using HAL in people with acute spinal cord infarction is possible and may be beneficial.
Soma et al. [27] presents in their study a severely affected case with a patient who was diagnosed with cervical spondylotic myelopathy, underwent surgery, and was rediagnosed with traumatic cervical SCI (incomplete tetraplegic, AIS C) after a fall. He received HAL training 2–3 times per week for 13 sessions and additional physical therapy. Improvements were observed in gait speed, step length, and cadence based on a 10MWT, ISNCSCI motor score, and WISCI II score [27]. The authors state, as Okawara et al. [15] did before, that in other studies, such as that of Aach et al. [9], only patients who were already able to perform high-performance testing at baseline, such as the 6MWT, participated and it can be seen from their results that even severely affected patients benefit from HAL training. However, it must be noted that also in the mentioned Aach et al. [9] study only three out of eight patients were able to perform the 6MWT at baseline. After the training, all eight were able to do so. Regardless of this, a notable improvement of a severely affected patient is reported here, who, beyond the functional tests, also showed kinematic improvements, e.g., in hip extension, which is consistent with the results of Brinkemper et al. [17]. Table 2 summarizes single-case or two-case reports studies details and results.

Table 2.
Spinal cord injury single-case or two-case reports studies characteristics.
4. Discussion
The studies shown in this review on the use of HAL in patients with SCI demonstrate a wide range of use from acute patients to chronic patients and from patients with well-preserved residual functionality to severely affected patients. With the exception of skin reddening at the site of the electrodes, leg cuffs or shoes, no other adverse events or injuries were reported. The vast majority of patients improved functionally, showing increased walking distances, walking speeds, and endurance. In addition, a variety of other advances were described, such as temporary decrease in spasticity, improvement in bladder and bowel management, pain reduction, and change in muscle activity. Nevertheless, no clear recommendation can be made as to which patient population is most likely to benefit from this innovative training. It seems that in principle its application is feasible and effective in many types of SCI, but whether it is superior to other training and therapies remains unclear. To date, there are no published controlled randomized trials with firstly, other similar exoskeleton systems and secondly, conventional therapy of the same extent and frequency. This is imperative to differentiate the potential benefits of HAL therapy from others and should be done in the future.
Accessibility to HAL training is mentioned in only a few studies. Okawara et al. [18] states that in Germany, HAL training is only funded for patients within the scope of the employers’ liability insurance association, and in Japan, government support is provided for patients with neuromuscular diseases. Thus, access to training for a wide range of patients still seems to be a problem which should be solved. In addition to the insurance and thus financial hurdle, the availability for people of different constitutions must also be mentioned. Limitations for the application of this exoskeletal system, as with other manufacturers, are restrictions on patient’s height and weight, contractures, insufficient cardiopulmonary capacity, and spasticity that does not decrease with the use of the system.
The following limitations of the present work should be considered. The underlying selective literature search remains incomplete, and a possible bias cannot be excluded with certainty. Some of the authors of the review are also authors of included studies. The studies found are partly limited in their methodological quality. HAL was developed in Japan and is most widely used there. There might be studies published in Japanese journals that were not included in this review. Seven of the included studies [8,9,14,23,24,26,27] list Professor Yoshiyuki Sankai, the inventor of HAL and CEO of Cyberdyne Inc. as a co-author. However, most state that Professor Sankai and Cyberdyne were not involved in conducting the study, data collection and analysis, writing, or submission. Different versions of HAL may have been used in the early studies than in the later studies. It was not always documented which other therapies were made and if medication was changed in the course of the training. Many of the studies presented have mixed collectives including chronic, acute, incomplete, and complete SCI.
5. Conclusions
Currently, there is no uniform application regarding the appropriate collective, as well as the type and frequency of training. Nevertheless, the current study situation suggests that many patients with paraplegia could benefit from qualified use within their means. Although exoskeletal training in SCI patients is still controversial, it offers unprecedented opportunities for patients and therapists. Future studies should focus on a comparison of HAL with other therapies as mentioned above and develop an optimal standardized therapy in terms of training weeks, training units per week, and training duration per unit.
Author Contributions
Conceptualization, A.B., D.G. and E.Y.; methodology, A.B., D.G. and E.Y.; formal analysis, A.B. and D.G.; data curation, A.B. and M.A.; writing—original draft preparation, A.B.; writing—review and editing, D.G. and E.Y.; visualization, A.B.; supervision, T.A.S. and M.A.; project administration, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge support by the Open Access Publication Funds of the Ruhr-Universität Bochum.
Conflicts of Interest
The authors declare no conflict of interest.
References
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the global burden of disease study. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, A.; Yoshikawa, K.; Koseki, K.; Takeuchi, R.; Mutsuzaki, H. A Consecutive 25-Week Program of Gait Training, Using the Alternating Hybrid Assistive Limb (HAL®) Robot and Conventional Training, and its Effects on the Walking Ability of a Patient with Chronic Thoracic Spinal Cord Injury: A Single Case Reversal Design. Medicina 2019, 55, 746. [Google Scholar] [CrossRef] [PubMed]
- Okamura, J. EMG-based prototype powered assistive system for walking aid. In Proceedings of the Asian Symposium on Industrial Automation and Robotics, Bangkok, Thailand, 6–7 May 1999; pp. 229–234. [Google Scholar]
- Ueba, T.; Hamada, O.; Ogata, T.; Inoue, T.; Shiota, E.; Sankai, Y. Feasibility and Safety of Acute Phase Rehabilitation After Stroke Using the Hybrid Assistive Limb Robot Suit. Neurol. Med. Chir. 2013, 53, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, H.; Kamibayashi, K.; Nakata, Y.; Yamawaki, K.; Ariyasu, R.; Sankai, Y.; Sakane, M.; Eguchi, K.; Ochiai, N. Pilot study of locomotion improvement using hybrid assistive limb in chronic stroke patients. BMC Neurol. 2013, 13, 141. [Google Scholar] [CrossRef] [PubMed]
- Sczesny-Kaiser, M.; Kowalewski, R.; Schildhauer, T.; Aach, M.; Jansen, O.; Grasmücke, D.; Güttsches, A.-K.; Vorgerd, M.; Tegenthoff, M. Treadmill Training with HAL Exoskeleton—A Novel Approach for Symptomatic Therapy in Patients with Limb-Girdle Muscular Dystrophy—Preliminary Study. Front. Neurosci. 2017, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kawamura, Y.; Hayashi, T.; Sakurai, T.; Hasegawa, Y.; Sankai, Y. Intention-Based Walking Support for Paraplegia Patient. In Proceedings of the 2005 IEEE International Conference on Systems, Man and Cybernetics, Waikoloa, HI, USA, 10–12 October 2005; Volume 3, pp. 2707–2713. [Google Scholar]
- Kubota, S.; Nakata, Y.; Eguchi, K.; Kawamoto, H.; Kamibayashi, K.; Sakane, M.; Sankai, Y.; Ochiai, N. Feasibility of Rehabilitation Training With a Newly Developed Wearable Robot for Patients With Limited Mobility. Arch. Phys. Med. Rehabil. 2013, 94, 1080–1087. [Google Scholar] [CrossRef] [PubMed]
- Aach, M.; Cruciger, O.; Sczesny-Kaiser, M.; Höffken, O.; Meindl, R.C.; Tegenthoff, M.; Schwenkreis, P.; Sankai, Y.; Schildhauer, T. Voluntary driven exoskeleton as a new tool for rehabilitation in chronic spinal cord injury: A pilot study. Spine J. 2014, 14, 2847–2853. [Google Scholar] [CrossRef]
- Sczesny-Kaiser, M.; Höffken, O.; Aach, M.; Cruciger, O.; Grasmücke, D.; Meindl, R.; Schildhauer, T.A.; Schwenkreis, P.; Tegenthoff, M. HAL® exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. J. Neuroeng. Rehabil. 2015, 12, 68. [Google Scholar] [CrossRef]
- Jansen, O.; Grasmuecke, D.; Meindl, R.C.; Tegenthoff, M.; Schwenkreis, P.; Sczesny-Kaiser, M.; Wessling, M.; Schildhauer, T.; Fisahn, C.; Aach, M. Hybrid Assistive Limb Exoskeleton HAL in the Rehabilitation of Chronic Spinal Cord Injury: Proof of Concept; the Results in 21 Patients. World Neurosurg. 2018, 110, e73–e78. [Google Scholar] [CrossRef]
- Grasmücke, D.; Zieriacks, A.; Jansen, O.; Fisahn, C.; Sczesny-Kaiser, M.; Wessling, M.; Meindl, R.C.; Schildhauer, T.A.; Aach, M. Against the odds: What to expect in rehabilitation of chronic spinal cord injury with a neurologically controlled Hybrid Assistive Limb exoskeleton. A subgroup analysis of 55 patients according to age and lesion level. Neurosurg. Focus 2017, 42, E15. [Google Scholar] [CrossRef]
- Jansen, O.; Schildhauer, T.; Meindl, R.C.; Tegenthoff, M.; Schwenkreis, P.; Sczesny-Kaiser, M.; Grasmücke, D.; Fisahn, C.; Aach, M. Functional Outcome of Neurologic-Controlled HAL-Exoskeletal Neurorehabilitation in Chronic Spinal Cord Injury: A Pilot With One Year Treatment and Variable Treatment Frequency. Glob. Spine J. 2017, 7, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Kadone, H.; Kubota, S.; Suzuki, K.; Abe, T.; Ueno, T.; Soma, Y.; Sankai, Y.; Hada, Y.; Yamazaki, M. Voluntary Ambulation by Upper Limb-Triggered HAL® in Patients with Complete Quadri/Paraplegia Due to Chronic Spinal Cord Injury. Front. Neurosci. 2017, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Okawara, H.; Sawada, T.; Matsubayashi, K.; Sugai, K.; Tsuji, O.; Nagoshi, N.; Matsumoto, M.; Nakamura, M. Gait ability required to achieve therapeutic effect in gait and balance function with the voluntary driven exoskeleton in patients with chronic spinal cord injury: A clinical study. Spinal Cord 2020, 58, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Brinkemper, A.; Grasmücke, D.; Yilmaz, E.; Reinecke, F.; Schildhauer, T.A.; Aach, M. Influence of Locomotion Therapy With the Wearable Cyborg HAL on Bladder and Bowel Function in Acute and Chronic SCI Patients. Global Spine J. 2021; Epub ahead of print. [Google Scholar] [CrossRef]
- Brinkemper, A.; Aach, M.; Grasmücke, D.; Jettkant, B.; Rosteius, T.; Dudda, M.; Yilmaz, E.; Schildhauer, T.A. Improved Physiological Gait in Acute and Chronic SCI Patients After Training With Wearable Cyborg Hybrid Assistive Limb. Front. Neurorobot. 2021, 15, 116. [Google Scholar] [CrossRef]
- Okawara, H.; Tashiro, S.; Sawada, T.; Sugai, K.; Matsubayashi, K.; Kawakami, M.; Nori, S.; Tsuji, O.; Nagoshi, N.; Matsumoto, M.; et al. Neurorehabilitation using a voluntary driven exoskeletal robot improves trunk function in patients with chronic spinal cord injury: A single-arm study. Neural Regen. Res. 2022, 17, 427–432. [Google Scholar] [CrossRef]
- Sawada, T.; Okawara, H.; Matsubayashi, K.; Sugai, K.; Kawakami, M.; Tashiro, S.; Nori, S.; Tsuji, O.; Nagoshi, N.; Matsumoto, M.; et al. Influence of body weight-supported treadmill training with voluntary-driven exoskeleton on the quality of life of persons with chronic spinal cord injury: A pilot study. Int. J. Rehabilitation Res. 2021, 44, 343–349. [Google Scholar] [CrossRef]
- Zieriacks, A.; Aach, M.; Brinkemper, A.; Koller, D.; Schildhauer, T.A.; Grasmücke, D. Rehabilitation of Acute Vs. Chronic Patients With Spinal Cord Injury With a Neurologically Controlled Hybrid Assistive Limb Exoskeleton: Is There a Difference in Outcome? Front. Neurorobot. 2021, 15, 728327. [Google Scholar] [CrossRef]
- Cruciger, O.; Schildhauer, T.A.; Meindl, R.C.; Tegenthoff, M.; Schwenkreis, P.; Citak, M.; Aach, M. Impact of locomotion training with a neurologic controlled hybrid assistive limb (HAL) exoskeleton on neuropathic pain and health related quality of life (HRQoL) in chronic SCI: A case study. Disabil. Rehabil. Assist. Technol. 2016, 11, 529–534. [Google Scholar] [CrossRef]
- Cruciger, O.; Tegenthoff, M.; Schwenkreis, P.; Schildhauer, T.A.; Aach, M. Locomotion training using voluntary driven exoskeleton (HAL) in acute incomplete SCI. Neurology 2014, 83, 474. [Google Scholar] [CrossRef]
- Ikumi, A.; Kubota, S.; Shimizu, Y.; Kadone, H.; Marushima, A.; Ueno, T.; Kawamoto, H.; Hada, Y.; Matsumura, A.; Sankai, Y.; et al. Decrease of spasticity after hybrid assistive limb® training for a patient with C4 quadriplegia due to chronic SCI. J. Spinal Cord Med. 2017, 40, 573–578. [Google Scholar] [CrossRef]
- Shimizu, Y.; Nakai, K.; Kadone, H.; Yamauchi, S.; Kubota, S.; Ueno, T.; Marushima, A.; Hiruta, K.; Endo, A.; Kawamoto, H.; et al. The Hybrid Assistive Limb® intervention for a postoperative patient with spinal dural arteriovenous fistula and chronic spinal cord injury: A case study. J. Spinal Cord Med. 2018, 41, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Schmidt, C.K.; Mayadev, A.; Tawfik, T.; Kobota, K.; Cambier, Z.; Norvell, D.D.; Chapman, J. Does treadmill training with Hybrid Assistive Limb (HAL) impact the quality of life? A first case series in the United States. Disabil. Rehabil. Assist. Technol. 2019, 14, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Marushima, A.; Kawamoto, H.; Kadone, H.; Ueno, T.; Shimizu, Y.; Endo, A.; Hada, Y.; Saotome, K.; Abe, T.; et al. Intensive Gait Treatment Using a Robot Suit Hybrid Assistive Limb in Acute Spinal Cord Infarction: Report of Two Cases. J. Spinal Cord Med. 2019, 42, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Soma, Y.; Kubota, S.; Kadone, H.; Shimizu, Y.; Takahashi, H.; Hada, Y.; Koda, M.; Sankai, Y.; Yamazaki, M. Hybrid Assistive Limb Functional Treatment for a Patient with Chronic Incomplete Cervical Spinal Cord Injury. Int. Med. Case Rep. J. 2021, 14, 413–420. [Google Scholar] [CrossRef]
- Baunsgaard, C.; Nissen, U.; Brust, A.; Frotzler, A.; Ribeill, C.; Kalke, Y.; León, N.; Gómez, B.; Samuelsson, K.; Antepohl, W.; et al. Exoskeleton gait training after spinal cord injury: An exploratory study on secondary health conditions. J. Rehabil. Med. 2018, 50, 806–813. [Google Scholar] [CrossRef]
- Hubscher, C.H.; Herrity, A.N.; Williams, C.S.; Montgomery, L.R.; Willhite, A.M.; Angeli, C.A.; Harkema, S.J. Improvements in bladder, bowel and sexual outcomes following task-specific locomotor training in human spinal cord injury. PLoS ONE 2018, 13, e0190998. [Google Scholar] [CrossRef]
- Juszczak, M.; Gallo, E.; Bushnik, T. Examining the Effects of a Powered Exoskeleton on Quality of Life and Secondary Impairments in People Living with Spinal Cord Injury. Top. Spinal Cord Inj. Rehabil. 2018, 24, 336–342. [Google Scholar] [CrossRef]
- Chun, A.; Asselin, P.K.; Knezevic, S.; Kornfeld, S.; Bauman, W.A.; Korsten, M.A.; Harel, N.Y.; Huang, V.; Spungen, A.M. Changes in bowel function following exoskeletal-assisted walking in persons with spinal cord injury: An observational pilot study. Spinal Cord 2020, 58, 459–466. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).