Effects of Protein Structure Changes on Texture of Scallop Adductor Muscles under Ultra-High Pressure
Abstract
:1. Introduction
2. Experimental Materials and Test Methods
2.1. Experimental Materials
2.2. Sample Processing and Preparation
2.3. Experimental Reagents and Main Equipment
2.3.1. Experimental Reagents
2.3.2. Main Instruments and Equipment of the Experiment
2.4. Experimental Methods
2.4.1. Determination of Musculature of Adductor of Scallops (Texture Profile Analysis, TPA)
2.4.2. Raman Spectrum Analysis
2.4.3. (Scanning Electron Microscopy) SEM Analysis
2.4.4. Analysis of Sensory Evaluation
2.4.5. Statistic Analysis
3. Influence of Ultra-High Pressure Packaging Technology on Protein Structure of Scallop Adductor Muscle
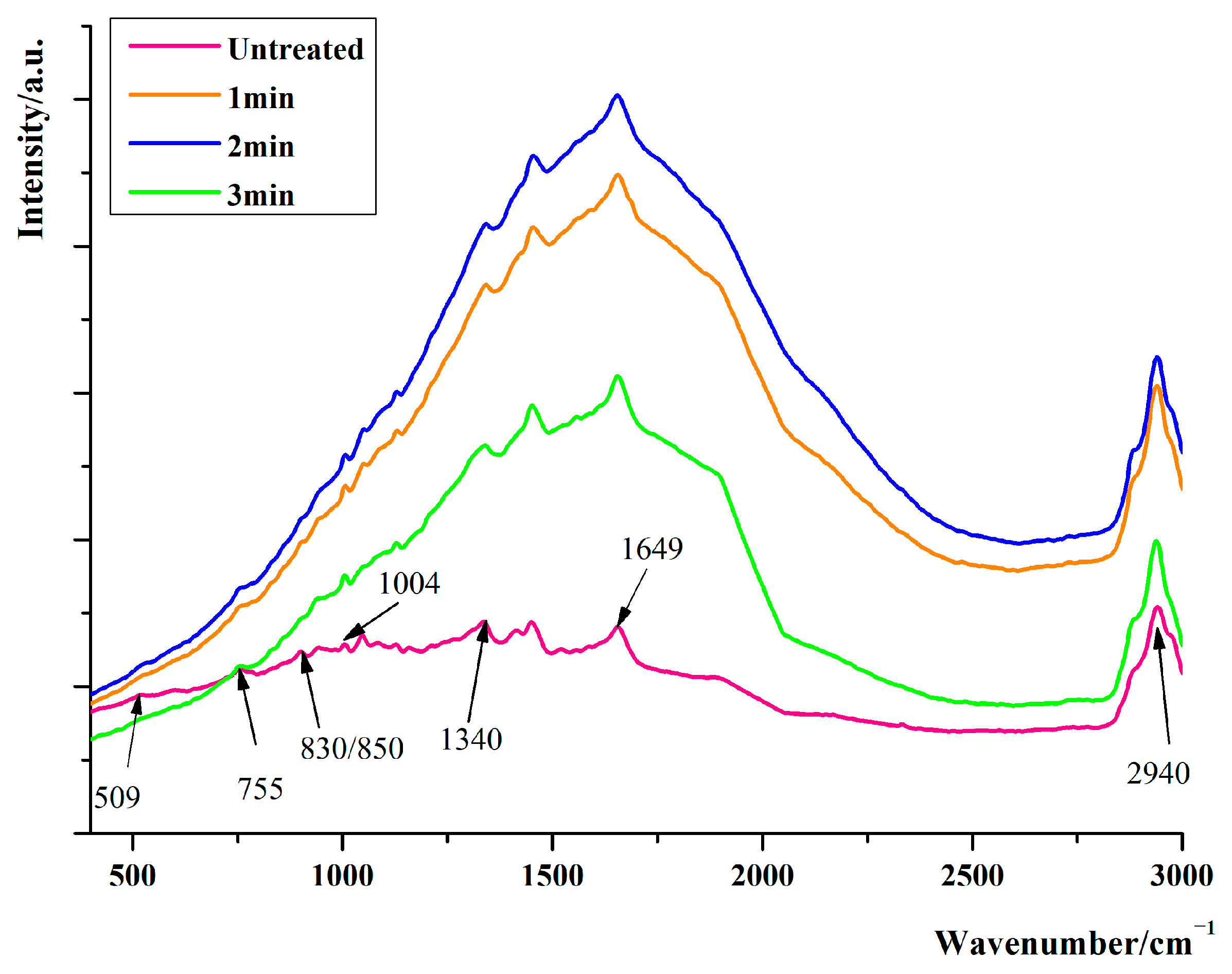
3.1. Raman Spectroscopic Analysis of Proteins in Adductor Muscle of Scallops under Ultra-High Pressure with Different Holding Times
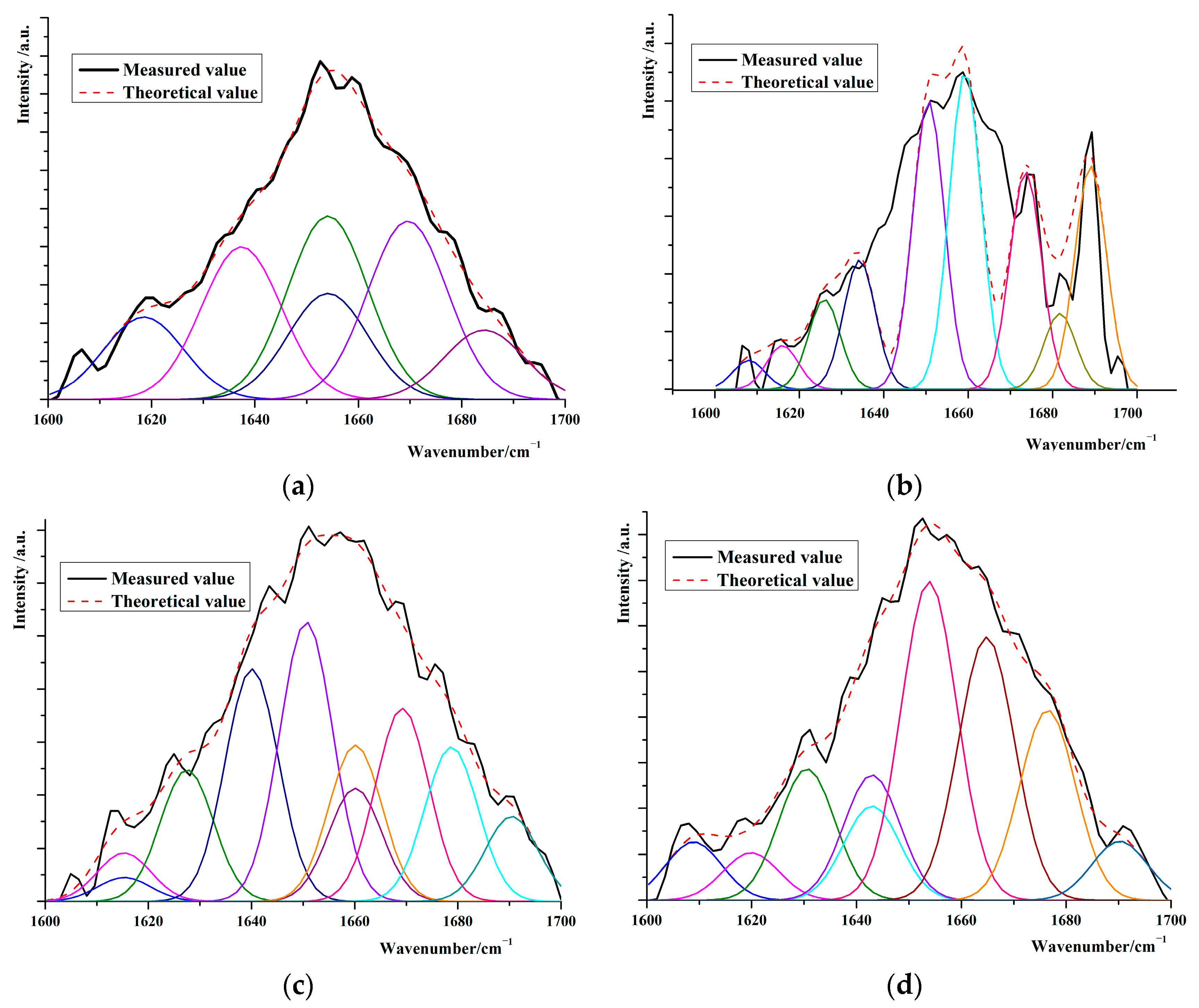
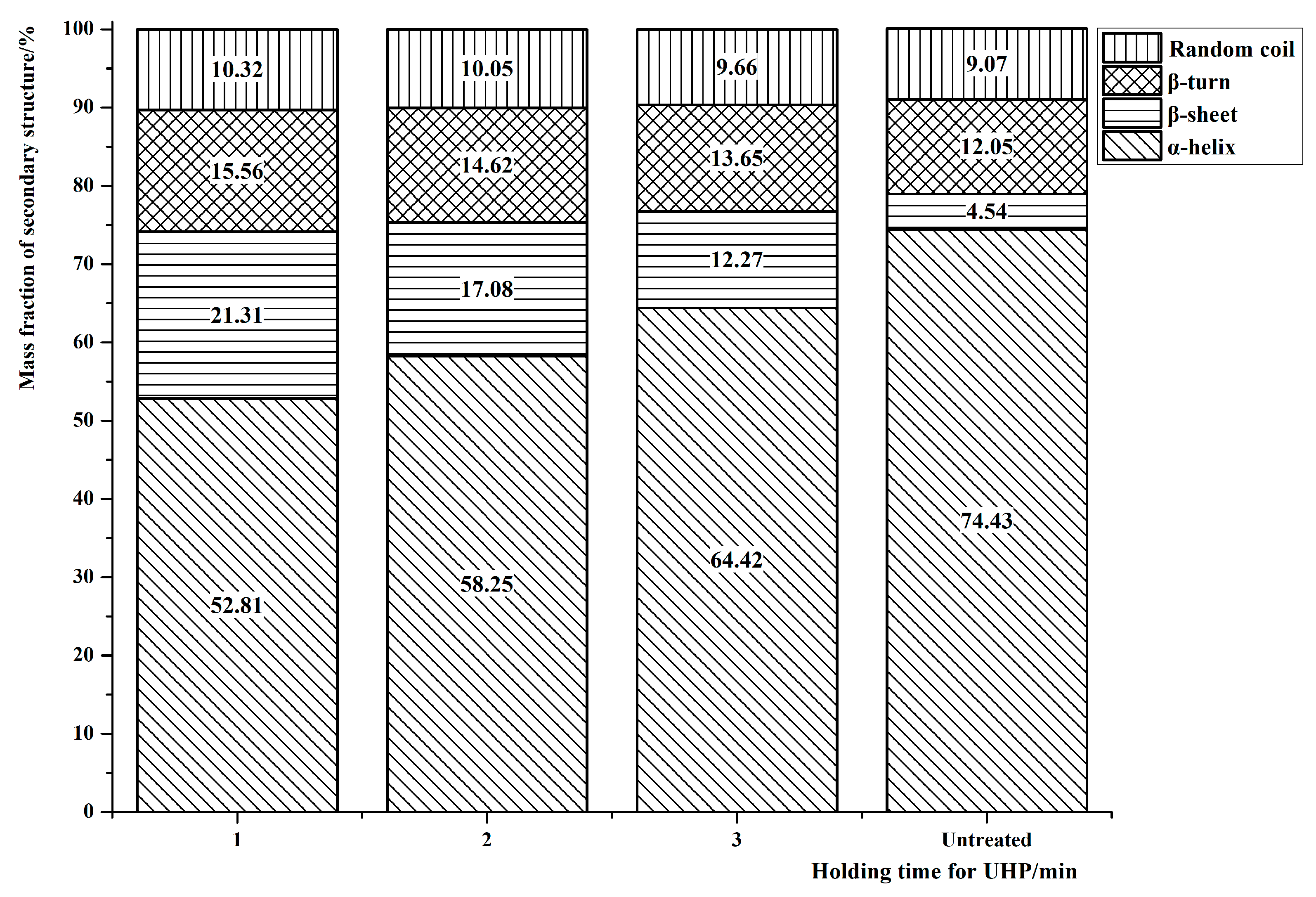
3.2. Changes in the Secondary Structure of Protein in the Interfacial Adductor Muscle of Scallops
3.3. Influence of Pressure Holding Time on Side Chain Conformation of Protein in Adductor Muscle at the Interface of Scallops
3.3.1. Influence of Pressure Holding Time on Tyrosine Residues in Proteins
3.3.2. Changes of Tryptophan Residues in Adductor Muscle Proteins at the Interface of Scallops
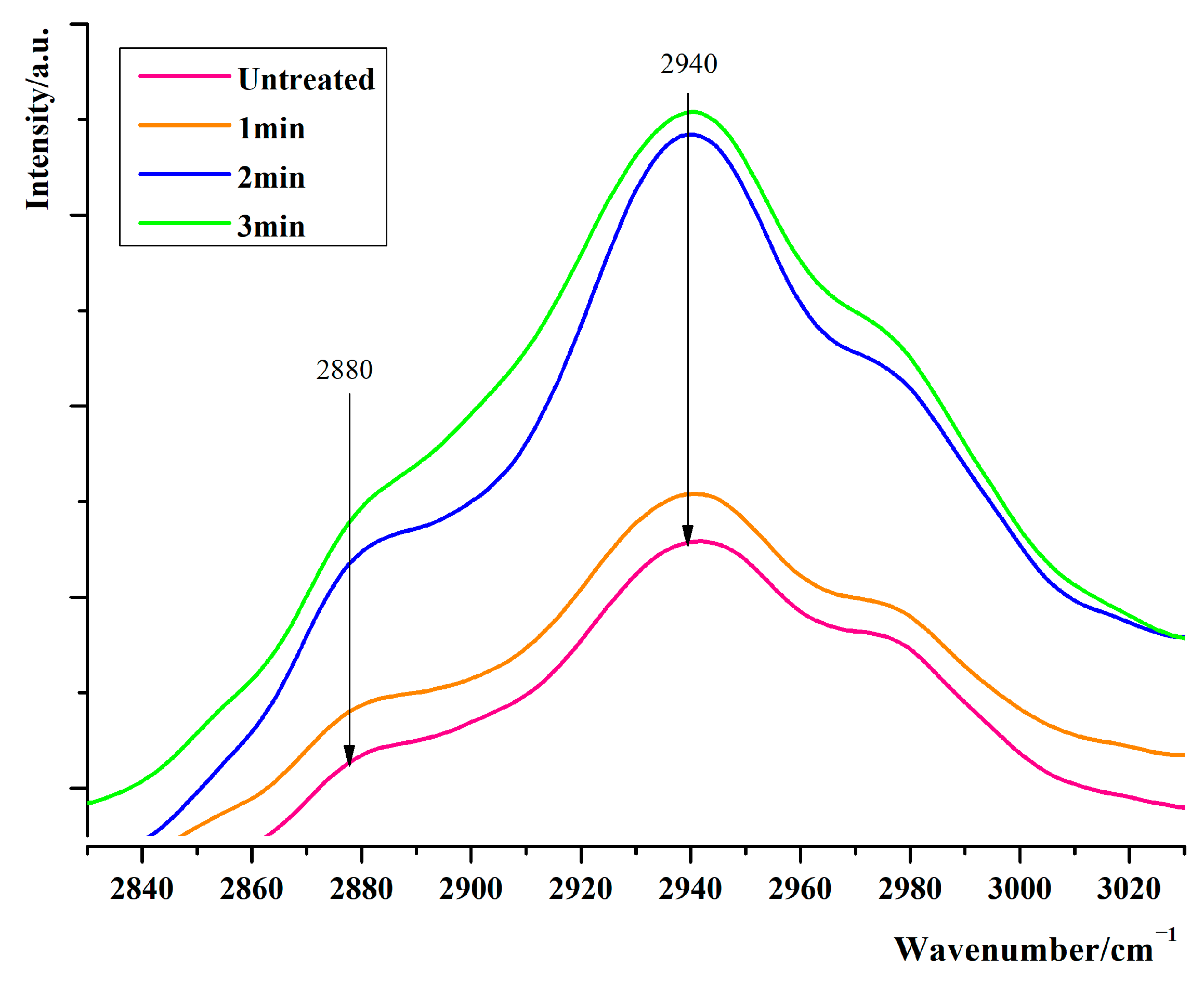
3.3.3. Influence of Pressure Holding Time on the Vibration of C-H Bond of Protein in Adductor Muscle at the Interface of Scallops
3.3.4. Influence of Pressure Holding Time on Protein Disulfide Bond
4. Scanning Electron Microscope Analysis of the Effect of Ultra-High Pressure Treatment on the Protein of the Adductor Muscle at the Interface of Scallops
5. Effect of Pressure Holding Time on Musculature of Adductor of Scallops
6. Sensory Evaluation of the Adductor Muscle of Scallops under Ultra-High Pressure
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Naciye, K.; Ravi, P.; Irem, S.; Aybike, K.; Aybike, S.; Anjineyulu, K. Impact of different microwave treatments on food texture. J. Texture Stud. 2021, 53, 710–736. [Google Scholar]
- Muhammad, A.K.; Sher, A.; Yang, H.J.; Asghar, A.K.; Zulfiqar, A.; Ronald, K.T.; Zhou, G.H. Improvement of color, texture and food safety of ready-to-eat high pressure-heat treated duck breast. Food Chem. 2019, 277, 646–654. [Google Scholar]
- Szczeshiak, A.S. Texture: Is it still an overlooked food attribute. Food Technol. 1990, 44, 86–95. [Google Scholar]
- Tong, S.N.; Liu, R.; Wang, W.; Lv, X.R.; Xu, Y.X.; Mi, H.B.; Li, J.R.; Yi, S.M.; Li, X.P. Synergistic effect of ultrahigh pressure and allicin on gel properties, flavor characteristics, and myosin structure of the obturator muscle of the scallop (Patinopecten yessoensis). Food Sci. 2023, 88, 3007–3021. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.X.; Ou, Y.J.; Xu, W.Q.; Ni, J.; Tan, J.Y.; Shen, J. Effects of storage conditions on nutrition and quality of live Patinopecten yessoensis. Fish. Mod. 2019, 46, 83–88. [Google Scholar]
- Xuan, X.T.; Cui, Y.; Lin, X.D.; Yu, J.F.; Liao, X.J.; Ling, J.G.; Shang, H.T. Impact of high hydrostatic pressure on the shelling efficacy, physicochemical properties, and microstructure of fresh razor clam (Sinonovacula constricta). J. Food Sci. 2018, 83, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.C.; Liu, C.; Fu, Q. The differences of bacterial communities in the tissues between healthy and diseased Yesso scallop (Patinopecten yessoensis). AMB Express 2019, 9, 148. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.X.; Li, M.; Sun, J.X. Characterization of bacterial community associated with four organs of the yesso scallop (Patinopecten yessoensis) by high-throughput sequencing. J. Ocean. Univ. China 2019, 18, 493–500. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Wu, Z.X.; Zhao, G.H.; Li, D.Y.; Liu, X.Y.; Qin, L.J.; Jiang, P.F.; Zhou, D.Y. Effect of air frying and baking on physicochemical properties and digestive properties of scallop (Patinopecten yessoensis) adductor muscle. Food Biosci. 2023, 52, 102460. [Google Scholar] [CrossRef]
- Cui, Y.; Lin, X.D.; Kang, M.L.; Yu, J.F.; Guo, R.X.; Ling, J.G. Advances in application of ultra high pressure for preservation and processing of aquatic products. Food Sci. 2016, 37, 291–299. [Google Scholar]
- Zhang, B.; Fang, C.D.; Hao, G.J. Effect of kappa-carrageen an oligosaccharides on myofibrillar protein oxidation in peeled shrimp (Litopenaeus vannamei) during long-term frozen storage. Food Chem. 2018, 245, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Yang, W.G.; Zhou, G.; Xu, D.L.; Lou, Q.M.; Zhang, J.J.; Cui, Y. Shelling of Solenocera melantho Using Ultra High Pressure and Its Effect on the Quality of Muscle. Food Sci. 2017, 38, 43–48. [Google Scholar]
- Kong, N.; Zhao, J.Y.; Zhao, B.; Liu, J.Y.; Li, F.Z.; Wang, L.L.; Song, L.S. Effects of high temperature stress on the intestinal histology and microbiota in Yesso scallop Patinopecten yessoensis. Mar. Environ. Res. 2023, 185, 105881. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.H.; Chen, Q.H.; Xuan, X.T.; Cui, Y.; Shang, H.T.; Ling, J.G.; Chen, X.E.; Fang, X.B. Effects of ultra-high pressure treatment on shucking and quality of Patinopecten yessoensis. J. Zhejiang Ocean Univ. 2020, 39, 117–122. [Google Scholar]
- Yue, J.; Zhang, Y.F.; Jin, Y.F.; Deng, Y.; Zhao, Y.Y. Impact of high hydrostatic pressure on non-volatile and volatile compounds of squid muscles. Food Chem. 2016, 194, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.M.; Chi, H.Y.; Hsu, K.C. High-pressure treatment for shelf-life extension and quality improvement of oysters cooked in a traditional Taiwanese oyster omelet. J. Food Prot. 2010, 73, 53–61. [Google Scholar] [CrossRef]
- Shi, Y.M.; Li, Y.Y.; Wu, D.D.; Wang, P.; Zhang, D.K.; Lou, Y.H. Effects of different freezing methods on quality of Penaeus vannameiv. Food Ferment. Ind. 2019, 45, 94–100. [Google Scholar]
- Dong, X.H.; Zhao, M.M.; Jiang, Y.M. Application of ultra—High pressure technique in protein food industry. Food Ind. Technol. 2012, 33, 451–454. [Google Scholar]
- Chen, Q.M.; Xie, Y.F.; Guo, Y.F.; Cheng, Y.L.; Yao, W.R. Application of Raman spectroscopy in a correlation study between protein oxidation/denaturation and conformational changes in beef after repeated freeze–thaw. Int. J. Food Sci. Technol. 2021, 57, 719–727. [Google Scholar] [CrossRef]
- Yan, L.X.; Tian, Y.Y.; Jiang, M.H.; Liu, J.R.; Xu, T.Y. Characteristics changes in activity and taste properties in Yesso Scallop Patinopecten yessoensis: From waterless transportation to wet storage selling. Fish. Sci. 2022, 41, 44–51. [Google Scholar]
- Bryant, R.N.; Pasteris, J.D.; Fike, D.A. Express: Variability in the Raman spectrum of unpolished growth and fracture surfaces of pyrite due to laser heating and crystal orientation. Appl. Spectrosc. 2018, 72, 37–47. [Google Scholar] [CrossRef]
- Liang, Z.W.; Du, Z.F.; Li, C.L.; Wang, M.X.; Wang, B.; Zhang, X. Confocal raman micro-spectroscopy analysis of mussel foot. Spectrosc. Spectr. Anal. 2020, 40, 755–759. [Google Scholar]
- Sun, W.Z.; Zhao, Q.Z.; Zhao, M.M. Structural evaluation of myofibrillar proteins during processing of Cantonese sausage by Raman spectroscopy. J. Agric. Food Chem. 2011, 59, 11070–11077. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.H.; Yang, X.S.; Xu, Z.; Guo, Q.Y.; Li, X.Y. Effect of moisture content on the texture and chroma of lightly baked scallop. Food Mach. 2010, 26, 47–50. [Google Scholar]
- Lu, B.; Li, M.D.; Zhang, Y.F.; Niu, X.C.; Wang, Z.J.; Jing, L.Z.; Liu, J. Raman spectroscopic characterization of the structural changes in soybean protein isolate induced by low pressure homogenization. Mod. Food Sci. Technol. 2018, 34, 58–63. [Google Scholar]
- Ramirez-Suarez, J.C.; Morrissey, M.T. Effect of high pressure processing (HPP) on shelf life of albacoretuna (Thunnus alalunga) minced muscle. Innov. Food Sci. Emerg. Technol. 2006, 7, 19–27. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, H.X.; Li, J.R. Effects of ultra-high pressure treatments on the qualities of Litopenaeus vannamei. Food Res. Dev. 2010, 31, 1–6. [Google Scholar]
- Ranman, M.S.; Al-farsi, S.A. Instru mental texture profile analysis (TPA) of date flesh as a function of moisture content. J. Food Eng. 2005, 66, 505–511. [Google Scholar] [CrossRef]
- Zhang, G.N.; Hu, b.; Wu, F.R. Effects of Angelica sinensis on the microstructure of myogenic fibronectin in snakehead mullet. J. Huazhong Agric. Univ. 2018, 37, 117–122. [Google Scholar]
- Jiang, L.T. The relation of chemical component to texture of white sufu. Mod. Food Sci. Technol. 2010, 26, 797–800. [Google Scholar]
- Jia, Y.; Hu, Z.H.; Wang, X.L.; Chen, S.H.; Liu, X.J.; Xia, L. Effect of ultra-High Pressure Treatment on Shucking and Processing Properties of Squilla. Food Sci. 2015, 36, 47–52. [Google Scholar]
- Gong, X.; Chang, J.; Zhang, Y.L.; Li, D.T.; Xia, N.; Wang, J.; Sun, Z.H. Structural Changes of the Interface Material of Scallop Adductor under Ultra-High Pressure. Processes 2023, 11, 521. [Google Scholar] [CrossRef]
- Zeng, X.Y.; Jiao, D.X.; Yu, X.N.; Chen, L.H.; Chen, L.H.; Sun, Y.; Guo, A.R.; Zhu, C.; Wu, J.S.; Liu, J.S.; et al. Effect of ultra-high pressure on the relationship between endogenous proteases and protein degradation of Yesso scallop (Mizuhopecten yessoensis) adductor muscle during iced storage. Food Chem. 2022, 15, 100438. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Gong, X.; Zhang, Y.L.; Sun, Z.L.; Xia, N.; Zhang, H.J.; Wang, J.; Zhang, X. Simulation analysis of organic-inorganic interface failure of scallop under ultra-high pressure. Coatings 2022, 12, 963. [Google Scholar] [CrossRef]




| Sensory Attributes | State | Score |
|---|---|---|
| Appearance | The shell meat is milky white with a bright luster | 3 |
| The shell meat is light yellow and shiny in water | 2 | |
| The shell meat is dark yellow and lacks luster | 1 | |
| Odor | Fresh and sweet without obvious fishy taste | 3 |
| average and slightly fishy | 2 | |
| Mild freshness and strong fishy taste | 1 | |
| State | Fresh and sweet | 3 |
| Moderate sweetness | 2 | |
| Mild sweetness | 1 | |
| Texture | Good elasticity without obvious fiber or stickiness | 3 |
| Moderate elasticity and slight stickiness | 2 | |
| Poor elasticity and obvious fiber sensation | 1 |
| Wave Number/cm−1 | Band Attribution Information |
|---|---|
| 509 | Stretching vibration of S-S |
| 755 | Stretching vibration of Tryptophan |
| 830/853 | The fundamental and overtone Fermi resonances of tyrosine |
| 1004 | Respiratory vibration of Phenylalanine ring |
| 1333 | III band of amide |
| 1657 | I band of amide |
| 2945 | Telescopic vibration of C-H |
| Number | Holding Time /min | Texture Analyzer | |||
|---|---|---|---|---|---|
| Hardness/N | Elasticity/N | Cohesiveness/% | Chewiness/N | ||
| 1 | untreated | 1.23 ± 0.01 d | 7.16 ± 0.02 a | 11.00 ± 0.04 d | 1.08 ± 0.01 d |
| 2 | 1 | 1.49 ± 0.03 c | 5.06 ± 0.07 d | 11.16 ± 0.19 c | 7.52 ± 0.12 a |
| 3 | 2 | 1.53 ± 0.02 b | 5.58 ± 0.24 c | 19.71 ± 0.07 b | 6.30 ± 0.16 b |
| 4 | 3 | 1.70 ± 0.02 a | 6.17 ± 0.36 b | 20.21 ± 0.10 a | 3.18 ± 0.08 c |
| Sensory Attributes | Holding Time /min | Appearance | Odor | State | Texture | |
|---|---|---|---|---|---|---|
| Number | ||||||
| 1 | untreated | 2.38 ± 0.03 | 2.91 ± 0.01 | 2.48 ± 0.11 | 2.24 ± 0.04 | |
| 2 | 1 | 2.64 ± 0.02 | 2.89 ± 0.03 | 2.65 ± 0.02 | 2.47 ± 0.07 | |
| 3 | 2 | 2.73 ± 0.05 | 2.84 ± 0.06 | 2.94 ± 0.02 | 2.89 ± 0.02 | |
| 4 | 3 | 2.81 ± 0.08 | 2.72 ± 0.05 | 2.83 ± 0.08 | 2.51 ± 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, X.; Chang, J.; Wang, J.; Zhang, Y.; Li, D.; Liu, C.; Hou, L.; Xia, N. Effects of Protein Structure Changes on Texture of Scallop Adductor Muscles under Ultra-High Pressure. Appl. Sci. 2023, 13, 13247. https://doi.org/10.3390/app132413247
Gong X, Chang J, Wang J, Zhang Y, Li D, Liu C, Hou L, Xia N. Effects of Protein Structure Changes on Texture of Scallop Adductor Muscles under Ultra-High Pressure. Applied Sciences. 2023; 13(24):13247. https://doi.org/10.3390/app132413247
Chicago/Turabian StyleGong, Xue, Jiang Chang, Jing Wang, Yinglei Zhang, Danting Li, Chai Liu, Lida Hou, and Ning Xia. 2023. "Effects of Protein Structure Changes on Texture of Scallop Adductor Muscles under Ultra-High Pressure" Applied Sciences 13, no. 24: 13247. https://doi.org/10.3390/app132413247
APA StyleGong, X., Chang, J., Wang, J., Zhang, Y., Li, D., Liu, C., Hou, L., & Xia, N. (2023). Effects of Protein Structure Changes on Texture of Scallop Adductor Muscles under Ultra-High Pressure. Applied Sciences, 13(24), 13247. https://doi.org/10.3390/app132413247







