Radiomic Analysis for Human Papillomavirus Assessment in Oropharyngeal Carcinoma: Lessons and Pitfalls for the Next Future
Abstract
:Featured Application
Abstract
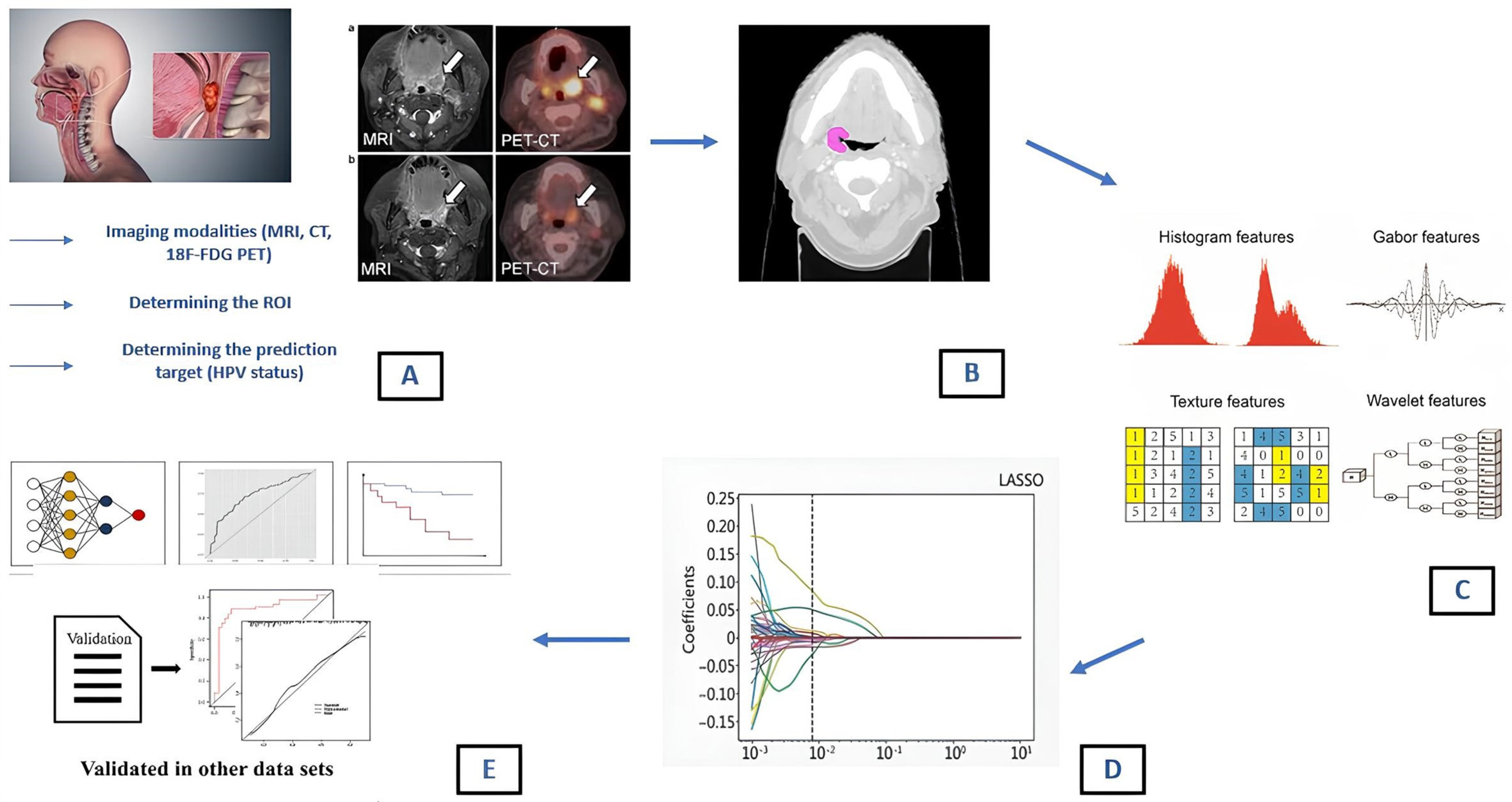
1. Introduction
- -
- Shape features: these are related to the geometric properties of the ROI/VOI (volume, diameter, sphericity and compacity);
- -
- First order (histogram) features: these describe the distribution of voxel intensities within the image region (mean, median, skewness, kurtosis);
- -
- Second order texture features: these consider the statistical relationship between neighboring voxels or groups of voxels within the segmented lesion. Many matrices, such as the Gray-Level Co-occurrence Matrix (GLCM), Gray-Level Run Length Matrix (GLRLM) and Neighborhood Gray-Level Different Matrix (NGLDM), can be used for feature extraction [21]. GLCM provides information about pixel pair distribution within the image; GLRLM identifies the length of consecutive voxels with the same intensity in a pre-set direction in the image; NGLDM analyzes the difference between a gray value and the average gray value of its neighbors within a certain distance [22].
- -
- Higher-order texture features: additional mathematical transformations are used to highlight specific aspects of the ROI. This can lead to a virtually endless number of features among which fractals, SUV metric for PET specific applications [23,24], Minkowski functionals, wavelet transform and Laplacian transforms of Gaussian-filtered images are included [25,26].
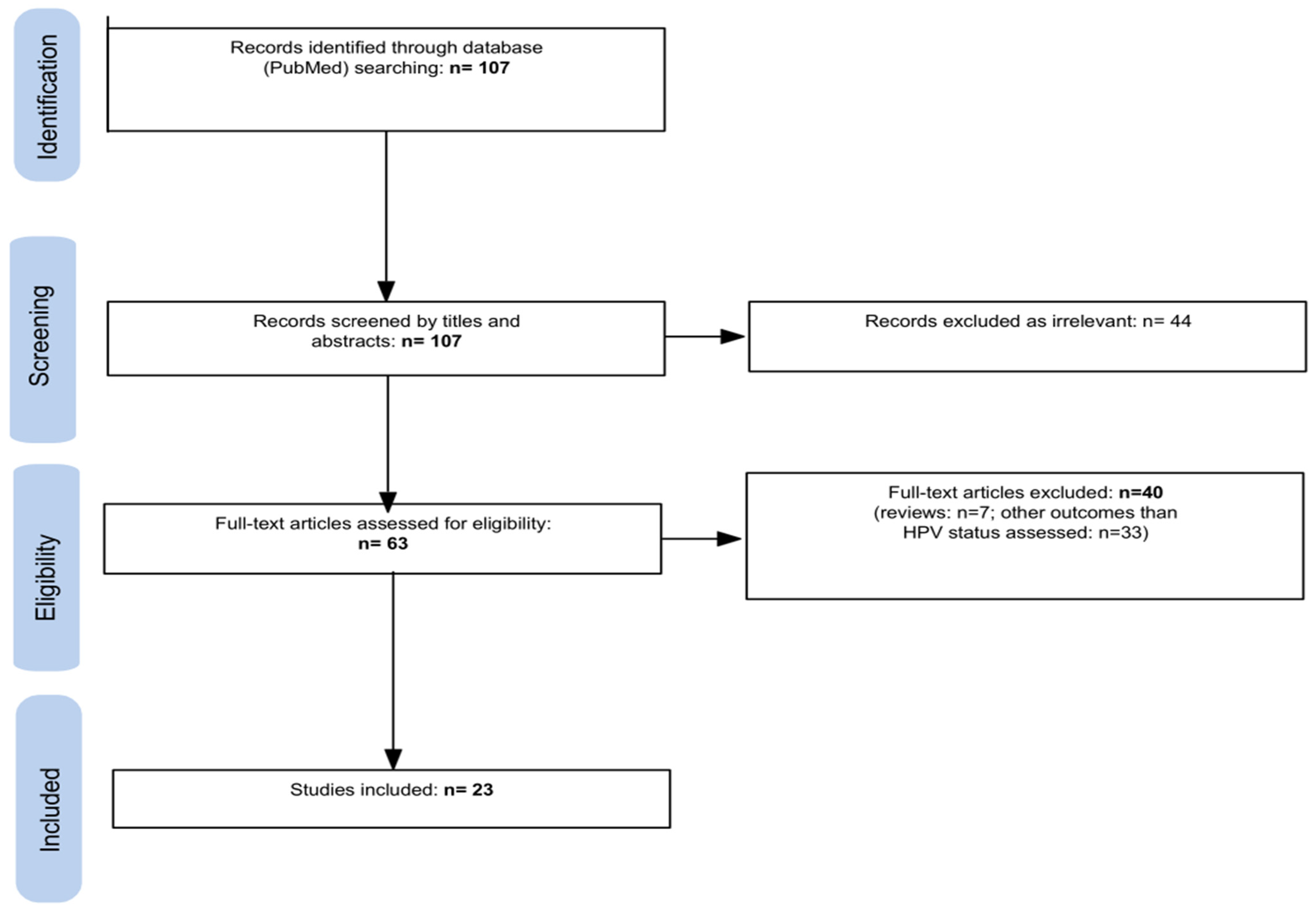
2. Materials and Methods
3. Results
4. Discussion
4.1. MRI-Based Radiomic Features (Table 1)
| Study | Year | Imaging Modality | No. of Patients | HPV Status | Enrollment Period | Prospective | Multicenter | Predictive Performances | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Giannitto C et al. [31] | 2020 | MRI | 32 | HPV+: 20 HPV−: 9 NA: 3 | 2008–2016 | No | No | Higher values of GOH/10Percentile (p = 0.03) and lower values of GOH/90Percentile (p = 0.03) for HPV+ | MRI-based radiomics is a feasible and promising approach for the prediction of tumor phenotype |
| Park YM et al. [32] | 2022 | MRI | 155 | HPV+: 136 HPV−: 19 | November 2005–December 2015 | No | No | AUC 0.792 | MRI radiomics showed satisfactory performance in predicting HPV status |
| Sohn B et al. [33] | 2021 | MRI | 62 | HPV+: 52 HPV−: 10 | July 2012–June 2018 | No | No | AUC 0.982 (training set); AUC 0.744 (test set) | Radiomics-based MRI regarded as a potential imaging biomarker for the assessment of HPV status |
| Li Q et al. [34] | 2023 | MRI | 141 | HPV+: 78 HPV−: 63 | January 2011–December 2020 | No | Yes | AUC 0.91 | PT-LN fusion model based on multisequence MRI could serve as a noninvasive method for assessing HPV status in OPSCC |
| Boot PA et al. [35] | 2023 | MRI | 249 | HPV+: 91 HPV−: 158 | 2008–2018 | No | No | AUC 0.79 (radiomic model only); AUC 0.89 (radiomic and clinical model combination) | MR-radiomic features can predict HPV status with sufficient accuracy, supporting the role of MRI-based radiomics as a potential imaging biomarker |
| Bos P et al. [11] | 2021 | MRI | 153 | HPV+: 76 HPV−: 77 | January 2010–December 2015 | No | No | AUC 0.764 (radiomic only); AUC 0.871 (radiomic and clinical model combination) | Models based on clinical variables and/or radiomic tumor features can predict HPV status in OPSCC patients |
| Suh CH et al. [36] | 2020 | MRI | 60 | HPV+: 48 HPV−: 12 | April 2012–November 2017 | No | No | AUC 0.77 (logistic regression); AUC 0.76 (random forest); AUC 0.71 (XG boost) | MRI radiomic signature can guide classification of HPV status |
| Ravanelli M et al. [37] | 2018 | MRI | 59 | HPV+: 28 HPV−: 31 | March 2010–April 2017 | No | No | Sensitivity of 83.3% and specificity of 92.6% | ADC is significantly lower in OPSCC HPV+ compared with HPV− OPC |
| Bos P et al. [40] | 2022 | MRI | 153 | HPV+: 76 HPV−: 77 | January 2010–December 2015 | No | No | AUC 0.84 | Labor- and time-consuming full tumor delineations may be substituted from alternative delineations in a model that predicts HPV status in OPSCC |
4.2. CT-Based Radiomic Features (Table 2)
| Study | Year | Imaging Modality | No. of Patients | HPV Status | Enrollment Period | Prospective | Multicenter | Predictive Performances | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Buch K et al. [15] | 2015 | CT | 40 | HPV+: 29 HPV−: 11 | December 2009–October 2013 | No | No | Statistically significant differences between HPV-positive and HPV-negative tumors (p = 0.006) | Texture analysis may be considered for the evaluation of HPV status |
| Mungai F et al. [16] | 2019 | CT | 50 | HPV+: 35 HPV−: 15 | October 2014–October 2017 | No | No | Decrease in NGLDM contrast and busyness associated with increased likelihood of HPV+ status | Texture analysis of CT images of the primary OPSCC can distinguish between HPV-related and HPV-negative lesions and predict the HPV status of the tumor |
| Ranjbar S et al. [41] | 2018 | CT | 107 | HPV+: 92 HPV−: 15 | 01 January 2010–31 December 2014 | No | No | Accuracy of 75.7% | HPV infection can be inferred from the CT appearance of OPSCC beyond what is apparent to the trained human eye |
| Choi Y et al. [47] | 2020 | CT | 86 | HPV+: 53 HPV−: 33 | January 2009–September 2019 | No | No | AUC 0.865 (training set); AUC 0.747 (test set) | CT-based radiomics may be useful in predicting HPV status in OPSCC |
| Ou D et al. [48] | 2017 | CT | 120 | HPV+: 27 HPV−: 74 NA: 19 | June 2006–October 2012 | No | No | AUC 0.78 (radiomics) vs. AUC 0.64 (p16) | Radiomics signature provides additional information to HPV/p16 status |
| Song B et al. [42] | 2021 | CT | 582 | HPV+: 457 HPV−: 125 | 2005–2010 | No | No | AUC 0.70 (validation cohort); AUC 0.89 (training cohort) | Intratumoral and peritumoral radiomic features can predict HPV status of OPSCC patients |
| Bagher-Ebadian H et al. [43] | 2020 | CT | 187 | HPV+: 116 HPV−: 71 | NA | No | No | AUC 0.878 | Radiomic features associated with spatial arrangement and morphological appearance of the tumor CT datasets may be exploited for classification of HPV status |
| Bagher-Ebadian H et al. [44] | 2022 | CT | 128 | HPV+: 60 HPV−: 68 | NA | No | No | AUC 0.789 (radiomic model); AUC 0.895 (radiomic and clinical model combination) | Radiomics-based classifier enables better prediction of HPV than clinical factors; the combination of both yields even higher accuracy |
| Bogowicz M et al. [45] | 2017 | CT | 149 | HPV+: 62 HPV−: 87 | 2003–2013 | No | No | AUC 0.85 (training set); AUC 0.78 (validation set) | Heterogeneity of HNSCC tumor density is associated with HPV status and local control after radical chemoradiation |
| Leijenar RT et al. [46] | 2018 | CT | 778 | HPV+: 426 HPV−: 352 | NA | No | Yes | AUC 0.76 (all training data); AUC 0.73 (training pts without artifacts) | Standard medical images can provide molecular information; radiomics can serve as an imaging biomarker of HPV status |
4.3. 18F-Fluorodeoxyglucose Positron-Emission Tomography (18F-FDG PET)-Based Radiomic Features (Table 3)
| Study | Year | Imaging Modality | No. of Patients | HPV Status | Enrollment Period | Prospective | Multicenter | Predictive Performances | Main Findings |
|---|---|---|---|---|---|---|---|---|---|
| Fujima N et al. [14] | 2020 | 18F-FDG PET/CT | 120 | HPV+: 70 HPV−: 50 | January 2010–June 2019 | No | No | AUC 0.83 (deep learning diagnostic model) | Deep learning diagnostic model with FDG-PET imaging data can be useful for determining the HPV status in patients with OPSCC |
| Haider SP et al. [50] | 2020 | 18F-FDG PET/CT | 435 | HPV+: 315 HPV−: 120 | 2009–2019 | No | No | AUC 0.78 (cross-validation); AUC 0.77 (independent validation) | Potential added value from combining PET- and CT-based radiomics for prediction of HPV status |
| Lv W et al. [51] | 2022 | 18F-FDG PET/CT | 806 | HPV+: 115 HPV−: 86 | NA | No | Yes | AUC 0.653 (FusedImg model) | Radiomics score can be used as a surrogate for HPV status |
| Woo C et al. [52] | 2023 | 18F-FDG PET/CT | 126 | HPV+: 103 HPV−: 23 | January 2012–February 2020 | No | Yes | AUC (PET+ clinical) 0.78 | An HPV status classifier was developed by combining metabolic parameters derived from 18F-FDG PET/CT and clinical parameters in OPSCC |
5. Major Limitations of Radiomic Analysis in Clinical Practice and New Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Elrefaey, S.; Massaro, M.A.; Chiocca, S.; Chiesa, F.; Ansarin, M. HPV in oropharyngeal cancer: The basics to know in clinical practice. Acta Otorhinolaryngol. Ital. 2014, 34, 299–309. [Google Scholar] [PubMed]
- Ragin, C.C.R.; Taioli, E. Survival of squamous cell carcinoma of the head and neck in relation to human papillomavirus infection: Review and meta-analysis. Int. J. Cancer 2007, 121, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Golusinski, P.; Corry, J.; Poorten, V.V.; Simo, R.; Sjögren, E.; Mäkitie, A.; Kowalski, L.P.; Langendijk, J.; Braakhuis, B.J.M.; Takes, R.P.; et al. De-escalation studies in HPV-positive oropharyngeal cancer: How should we proceed? Oral Oncol. 2021, 123, 105620. [Google Scholar] [CrossRef]
- Machiels, J.-P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef]
- Rahimi, S.; Akaev, I.; Brennan, P.A.; Virgo, A.; Marani, C.; Gomez, R.S.; Yeoh, C.C. A proposal for classification of oropharyngeal squamous cell carcinoma: Morphology and status of HPV by immunohistochemistry and molecular biology. J. Oral Pathol. Med. 2020, 49, 110–116. [Google Scholar] [CrossRef]
- Prigge, E.-S.; Arbyn, M.; von Knebel Doeberitz, M.; Reuschenbach, M. Diagnostic accuracy of p16INK4a immunohistochemistry in oropharyngeal squamous cell carcinomas: A systematic review and meta-analysis. Int. J. Cancer 2017, 140, 1186–1198. [Google Scholar] [CrossRef]
- Spadarella, G.; Ugga, L.; Calareso, G.; Villa, R.; D’Aniello, S.; Cuocolo, R. The impact of radiomics for human papillomavirus status prediction in oropharyngeal cancer: Systematic review and radiomics quality score assessment. Neuroradiology 2022, 64, 1639–1647. [Google Scholar] [CrossRef]
- Bos, P.; van den Brekel, M.W.M.; Gouw, Z.A.R.; Al-Mamgani, A.; Waktola, S.; Aerts, H.J.W.L.; Beets-Tan, R.G.H.; Castelijns, J.A.; Jasperse, B. Clinical variables and magnetic resonance imaging-based radiomics predict human papillomavirus status of oropharyngeal cancer. Head Neck 2021, 43, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, S.C.; Peck, B.W.; Li, G.; Wei, Q.; Sturgis, E.M.; Ginsberg, L.E. Differences in imaging characteristics of HPV-positive and HPV-Negative oropharyngeal cancers: A blinded matched-pair analysis. AJNR Am. J. Neuroradiol. 2013, 34, 2005–2009. [Google Scholar] [CrossRef] [PubMed]
- Tahari, A.K.; Alluri, K.C.; Quon, H.; Koch, W.; Wahl, R.L.; Subramaniam, R.M. FDG PET/CT imaging of oropharyngeal squamous cell carcinoma: Characteristics of human papillomavirus-positive and -negative tumors. Clin. Nucl. Med. 2014, 39, 225–231. [Google Scholar] [CrossRef]
- Fujima, N.; Homma, A.; Harada, T.; Shimizu, Y.; Tha, K.K.; Kano, S.; Mizumachi, T.; Li, R.; Kudo, K.; Shirato, H. The utility of MRI histogram and texture analysis for the prediction of histological diagnosis in head and neck malignancies. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2019, 19, 5. [Google Scholar] [CrossRef] [PubMed]
- Buch, K.; Fujita, A.; Li, B.; Kawashima, Y.; Qureshi, M.M.; Sakai, O. Using Texture Analysis to Determine Human Papillomavirus Status of Oropharyngeal Squamous Cell Carcinomas on CT. AJNR Am. J. Neuroradiol. 2015, 36, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Mungai, F.; Verrone, G.B.; Pietragalla, M.; Berti, V.; Addeo, G.; Desideri, I.; Bonasera, L.; Miele, V. CT assessment of tumor heterogeneity and the potential for the prediction of human papillomavirus status in oropharyngeal squamous cell carcinoma. Radiol. Med. 2019, 124, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer Oxf. Engl. 1990 2012, 48, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Bibault, J.-E.; Xing, L.; Giraud, P.; El Ayachy, R.; Giraud, N.; Decazes, P.; Burgun, A.; Giraud, P. Radiomics: A primer for the radiation oncologist. Cancer Radiother. J. Soc. Fr. Radiother. Oncol. 2020, 24, 403–410. [Google Scholar] [CrossRef]
- Lambin, P.; Leijenaar, R.T.H.; Deist, T.M.; Peerlings, J.; de Jong, E.E.C.; van Timmeren, J.; Sanduleanu, S.; Larue, R.T.H.M.; Even, A.J.G.; Jochems, A.; et al. Radiomics: The bridge between medical imaging and personalized medicine. Nat. Rev. Clin. Oncol. 2017, 14, 749–762. [Google Scholar] [CrossRef]
- Nardone, V.; Boldrini, L.; Grassi, R.; Franceschini, D.; Morelli, I.; Becherini, C.; Loi, M.; Greto, D.; Desideri, I. Radiomics in the Setting of Neoadjuvant Radiotherapy: A New Approach for Tailored Treatment. Cancers 2021, 13, 3590. [Google Scholar] [CrossRef]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.T.; Lee, H.D.; Spolaôr, N.; Coy, C.S.R.; Wu, F.C. Prototype system for feature extraction, classification and study of medical images. Expert Syst. Appl. 2016, 63, 267–283. [Google Scholar] [CrossRef]
- Cusumano, D.; Dinapoli, N.; Boldrini, L.; Chiloiro, G.; Gatta, R.; Masciocchi, C.; Lenkowicz, J.; Casà, C.; Damiani, A.; Azario, L.; et al. Fractal-based radiomic approach to predict complete pathological response after chemo-radiotherapy in rectal cancer. Radiol. Med. 2018, 123, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Barucci, A.; Farnesi, D.; Ratto, F.; Pelli, S.; Pini, R.; Carpi, R.; Esposito, M.; Olmastroni, M.; Romei, C.; Taliani, A.; et al. Fractal-Radiomics as Complexity Analysis of CT and MRI Cancer Images. In Proceedings of the IEEE Workshop on Complexity in Engineering (COMPENG), Florence, Italy, 10–12 October 2018; pp. 1–5. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Avery, E.; Sanelli, P.C.; Aboian, M.; Payabvash, S. Radiomics: A Primer on Processing Workflow and Analysis. Semin. Ultrasound CT MRI 2022, 43, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Steyerberg, E.W.; Harrell, F.E.; Borsboom, G.J.; Eijkemans, M.J.; Vergouwe, Y.; Habbema, J.D. Internal validation of predictive models: Efficiency of some procedures for logistic regression analysis. J. Clin. Epidemiol. 2001, 54, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Elmahdy, M.; Sebro, R. Radiomics analysis in medical imaging research. J. Med. Radiat. Sci. 2023, 70, 3–7. [Google Scholar] [CrossRef]
- Caprini, E.; D’Agnese, G.; Brennan, P.A.; Rahimi, S. Human papilomaviru-related oropharyngeal squamous cell carcinoma and radiomics: A new era? J. Oral Pathol. Med. 2023, 52, 300–304. [Google Scholar] [CrossRef]
- Giannitto, C.; Marvaso, G.; Botta, F.; Raimondi, S.; Alterio, D.; Ciardo, D.; Volpe, S.; De Piano, F.; Ancona, E.; Tagliabue, M.; et al. Association of quantitative MRI-based radiomic features with prognostic factors and recurrence rate in oropharyngeal squamous cell carcinoma. Neoplasma 2020, 67, 1437–1446. [Google Scholar] [CrossRef]
- Park, Y.M.; Lim, J.-Y.; Koh, Y.W.; Kim, S.-H.; Choi, E.C. Machine learning and magnetic resonance imaging radiomics for predicting human papilloma virus status and prognostic factors in oropharyngeal squamous cell carcinoma. Head Neck 2022, 44, 897–903. [Google Scholar] [CrossRef]
- Sohn, B.; Choi, Y.S.; Ahn, S.S.; Kim, H.; Han, K.; Lee, S.-K.; Kim, J. Machine Learning Based Radiomic HPV Phenotyping of Oropharyngeal SCC: A Feasibility Study Using MRI. Laryngoscope 2021, 131, E851–E856. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, T.; Gong, J.; Xiang, S.; Shen, C.; Zhou, X.; Hu, C.; Wu, B.; Lu, X. Applying multisequence MRI radiomics of the primary tumor and lymph node to predict HPV-related p16 status in patients with oropharyngeal squamous cell carcinoma. Quant. Imaging Med. Surg. 2023, 13, 2234–2247. [Google Scholar] [CrossRef] [PubMed]
- Boot, P.A.; Mes, S.W.; de Bloeme, C.M.; Martens, R.M.; Leemans, C.R.; Boellaard, R.; van de Wiel, M.A.; de Graaf, P. Magnetic resonance imaging based radiomics prediction of Human Papillomavirus infection status and overall survival in oropharyngeal squamous cell carcinoma. Oral Oncol. 2023, 137, 106307. [Google Scholar] [CrossRef]
- Suh, C.H.; Lee, K.H.; Choi, Y.J.; Chung, S.R.; Baek, J.H.; Lee, J.H.; Yun, J.; Ham, S.; Kim, N. Oropharyngeal squamous cell carcinoma: Radiomic machine-learning classifiers from multiparametric MR images for determination of HPV infection status. Sci. Rep. 2020, 10, 17525. [Google Scholar] [CrossRef] [PubMed]
- Ravanelli, M.; Grammatica, A.; Tononcelli, E.; Morello, R.; Leali, M.; Battocchio, S.; Agazzi, G.M.; Buglione di Monale E Bastia, M.; Maroldi, R.; Nicolai, P.; et al. Correlation between Human Papillomavirus Status and Quantitative MR Imaging Parameters including Diffusion-Weighted Imaging and Texture Features in Oropharyngeal Carcinoma. AJNR Am. J. Neuroradiol. 2018, 39, 1878–1883. [Google Scholar] [CrossRef] [PubMed]
- Driessen, J.P.; Caldas-Magalhaes, J.; Janssen, L.M.; Pameijer, F.A.; Kooij, N.; Terhaard, C.H.J.; Grolman, W.; Philippens, M.E.P. Diffusion-weighted MR imaging in laryngeal and hypopharyngeal carcinoma: Association between apparent diffusion coefficient and histologic findings. Radiology 2014, 272, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Westra, W.H. The morphologic profile of HPV-related head and neck squamous carcinoma: Implications for diagnosis, prognosis, and clinical management. Head Neck Pathol. 2012, 6 (Suppl. S1), 48–54. [Google Scholar] [CrossRef]
- Bos, P.; van den Brekel, M.W.M.; Taghavi, M.; Gouw, Z.A.R.; Al-Mamgani, A.; Waktola, S.; J W L Aerts, H.; Beets-Tan, R.G.H.; Castelijns, J.A.; Jasperse, B. Largest diameter delineations can substitute 3D tumor volume delineations for radiomics prediction of human papillomavirus status on MRI’s of oropharyngeal cancer. Phys. Medica 2022, 101, 36–43. [Google Scholar] [CrossRef]
- Ranjbar, S.; Ning, S.; Zwart, C.M.; Wood, C.P.; Weindling, S.M.; Wu, T.; Mitchell, J.R.; Li, J.; Hoxworth, J.M. Computed Tomography-Based Texture Analysis to Determine Human Papillomavirus Status of Oropharyngeal Squamous Cell Carcinoma. J. Comput. Assist. Tomogr. 2018, 42, 299–305. [Google Scholar] [CrossRef]
- Song, B.; Yang, K.; Garneau, J.; Lu, C.; Li, L.; Lee, J.; Stock, S.; Braman, N.M.; Koyuncu, C.F.; Toro, P.; et al. Radiomic Features Associated With HPV Status on Pretreatment Computed Tomography in Oropharyngeal Squamous Cell Carcinoma Inform Clinical Prognosis. Front. Oncol. 2021, 11, 744250. [Google Scholar] [CrossRef] [PubMed]
- Bagher-Ebadian, H.; Lu, M.; Siddiqui, F.; Ghanem, A.I.; Wen, N.; Wu, Q.; Liu, C.; Movsas, B.; Chetty, I.J. Application of radiomics for the prediction of HPV status for patients with head and neck cancers. Med. Phys. 2020, 47, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Bagher-Ebadian, H.; Siddiqui, F.; Ghanem, A.I.; Zhu, S.; Lu, M.; Movsas, B.; Chetty, I.J. Radiomics outperforms clinical factors in characterizing human papilloma virus (HPV) for patients with oropharyngeal squamous cell carcinomas. Biomed. Phys. Eng. Express 2022, 8, 045010. [Google Scholar] [CrossRef]
- Bogowicz, M.; Riesterer, O.; Ikenberg, K.; Stieb, S.; Moch, H.; Studer, G.; Guckenberger, M.; Tanadini-Lang, S. Computed Tomography Radiomics Predicts HPV Status and Local Tumor Control After Definitive Radiochemotherapy in Head and Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Leijenaar, R.T.; Bogowicz, M.; Jochems, A.; Hoebers, F.J.; Wesseling, F.W.; Huang, S.H.; Chan, B.; Waldron, J.N.; O’Sullivan, B.; Rietveld, D.; et al. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: A multicenter study. Br. J. Radiol. 2018, 91, 20170498. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Nam, Y.; Jang, J.; Shin, N.-Y.; Ahn, K.-J.; Kim, B.-S.; Lee, Y.-S.; Kim, M.-S. Prediction of Human Papillomavirus Status and Overall Survival in Patients with Untreated Oropharyngeal Squamous Cell Carcinoma: Development and Validation of CT-Based Radiomics. AJNR Am. J. Neuroradiol. 2020, 41, 1897–1904. [Google Scholar] [CrossRef] [PubMed]
- Ou, D.; Blanchard, P.; Rosellini, S.; Levy, A.; Nguyen, F.; Leijenaar, R.T.H.; Garberis, I.; Gorphe, P.; Bidault, F.; Ferté, C.; et al. Predictive and prognostic value of CT based radiomics signature in locally advanced head and neck cancers patients treated with concurrent chemoradiotherapy or bioradiotherapy and its added value to Human Papillomavirus status. Oral Oncol. 2017, 71, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Wijsman, R.; Kaanders, J.H.; Oyen, W.J.; Bussink, J. Hypoxia and tumor metabolism in radiation oncology: Targets visualized by positron emission tomography. Q. J. Nucl. Med. Mol. Imaging 2013, 57, 244–256. [Google Scholar]
- Haider, S.P.; Mahajan, A.; Zeevi, T.; Baumeister, P.; Reichel, C.; Sharaf, K.; Forghani, R.; Kucukkaya, A.S.; Kann, B.H.; Judson, B.L.; et al. PET/CT radiomics signature of human papilloma virus association in oropharyngeal squamous cell carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2978–2991. [Google Scholar] [CrossRef]
- Lv, W.; Xu, H.; Han, X.; Zhang, H.; Ma, J.; Rahmim, A.; Lu, L. Context-Aware Saliency Guided Radiomics: Application to Prediction of Outcome and HPV-Status from Multi-Center PET/CT Images of Head and Neck Cancer. Cancers 2022, 14, 1674. [Google Scholar] [CrossRef]
- Woo, C.; Jo, K.H.; Sohn, B.; Park, K.; Cho, H.; Kang, W.J.; Kim, J.; Lee, S.-K. Development and Testing of a Machine Learning Model Using 18F-Fluorodeoxyglucose PET/CT-Derived Metabolic Parameters to Classify Human Papillomavirus Status in Oropharyngeal Squamous Carcinoma. Korean J. Radiol. 2023, 24, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Narita, N.; Kanno, M.; Takabayashi, T.; Fujieda, S.; Okazawa, H. Role of PET/MRI in oral cavity and oropharyngeal cancers based on the 8th edition of the AJCC cancer staging system: A pictorial essay. Ann. Nucl. Med. 2018, 32, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Freihat, O.; Tóth, Z.; Pintér, T.; Kedves, A.; Sipos, D.; Cselik, Z.; Lippai, N.; Repa, I.; Kovács, Á. Pre-treatment PET/MRI based FDG and DWI imaging parameters for predicting HPV status and tumor response to chemoradiotherapy in primary oropharyngeal squamous cell carcinoma (OPSCC). Oral Oncol. 2021, 116, 105239. [Google Scholar] [CrossRef] [PubMed]
- Samolyk-Kogaczewska, N.; Sierko, E.; Dziemianczyk-Pakiela, D.; Nowaszewska, K.B.; Lukasik, M.; Reszec, J. Usefulness of Hybrid PET/MRI in Clinical Evaluation of Head and Neck Cancer Patients. Cancers 2020, 12, 511. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-I.; Cheon, G.J.; Kang, S.Y.; Paeng, J.C.; Kang, K.W.; Lee, D.S.; Chung, J.-K. Prognostic value of simultaneous 18F-FDG PET/MRI using a combination of metabolo-volumetric parameters and apparent diffusion coefficient in treated head and neck cancer. EJNMMI Res. 2018, 8, 2. [Google Scholar] [CrossRef]
- Ibrahim, A.; Primakov, S.; Beuque, M.; Woodruff, H.C.; Halilaj, I.; Wu, G.; Refaee, T.; Granzier, R.; Widaatalla, Y.; Hustinx, R.; et al. Radiomics for precision medicine: Current challenges, future prospects, and the proposal of a new framework. Methods 2021, 188, 20–29. [Google Scholar] [CrossRef]
- Song, J.; Yin, Y.; Wang, H.; Chang, Z.; Liu, Z.; Cui, L. A review of original articles published in the emerging field of radiomics. Eur. J. Radiol. 2020, 127, 108991. [Google Scholar] [CrossRef]
- Tortora, M.; Gemini, L.; Scaravilli, A.; Ugga, L.; Ponsiglione, A.; Stanzione, A.; D’Arco, F.; D’Anna, G.; Cuocolo, R. Radiomics Applications in Head and Neck Tumor Imaging: A Narrative Review. Cancers 2023, 15, 1174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morelli, I.; Becherini, C.; Banini, M.; Valzano, M.; Bertini, N.; Loi, M.; Francolini, G.; Meattini, I.; Salvestrini, V.; Bonomo, P.; et al. Radiomic Analysis for Human Papillomavirus Assessment in Oropharyngeal Carcinoma: Lessons and Pitfalls for the Next Future. Appl. Sci. 2023, 13, 12942. https://doi.org/10.3390/app132312942
Morelli I, Becherini C, Banini M, Valzano M, Bertini N, Loi M, Francolini G, Meattini I, Salvestrini V, Bonomo P, et al. Radiomic Analysis for Human Papillomavirus Assessment in Oropharyngeal Carcinoma: Lessons and Pitfalls for the Next Future. Applied Sciences. 2023; 13(23):12942. https://doi.org/10.3390/app132312942
Chicago/Turabian StyleMorelli, Ilaria, Carlotta Becherini, Marco Banini, Marianna Valzano, Niccolò Bertini, Mauro Loi, Giulio Francolini, Icro Meattini, Viola Salvestrini, Pierluigi Bonomo, and et al. 2023. "Radiomic Analysis for Human Papillomavirus Assessment in Oropharyngeal Carcinoma: Lessons and Pitfalls for the Next Future" Applied Sciences 13, no. 23: 12942. https://doi.org/10.3390/app132312942
APA StyleMorelli, I., Becherini, C., Banini, M., Valzano, M., Bertini, N., Loi, M., Francolini, G., Meattini, I., Salvestrini, V., Bonomo, P., Livi, L., & Desideri, I. (2023). Radiomic Analysis for Human Papillomavirus Assessment in Oropharyngeal Carcinoma: Lessons and Pitfalls for the Next Future. Applied Sciences, 13(23), 12942. https://doi.org/10.3390/app132312942







