Tumor Volume Distributions Based on Weibull Distributions of Maximum Tumor Diameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Maximum Tumor Diameter Distribution
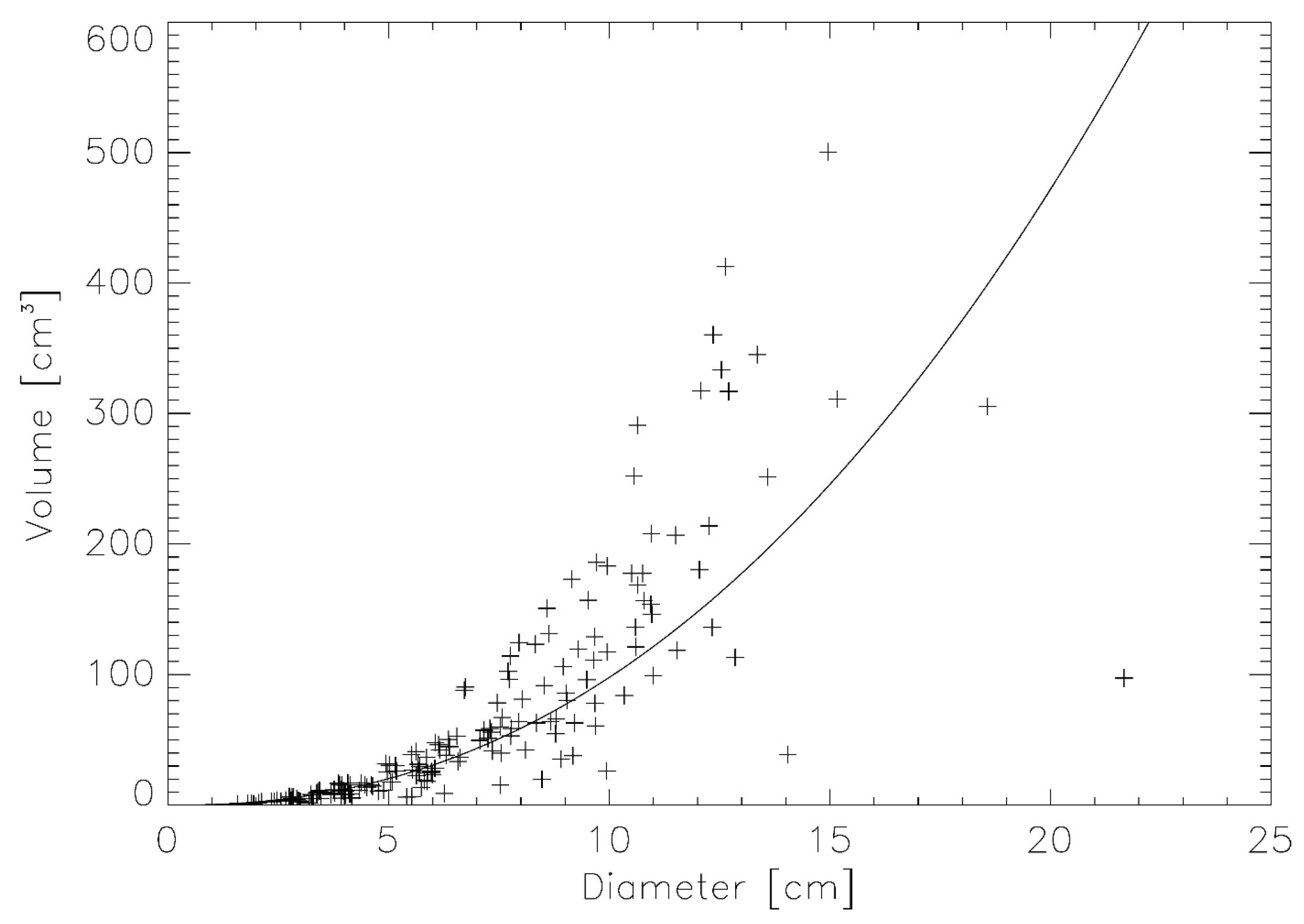
2.2. Verification Using Real Lung Tumor Volumes
2.3. Tumor Volume Distributions Determined from SEER for Breast, NSCLC and Liver
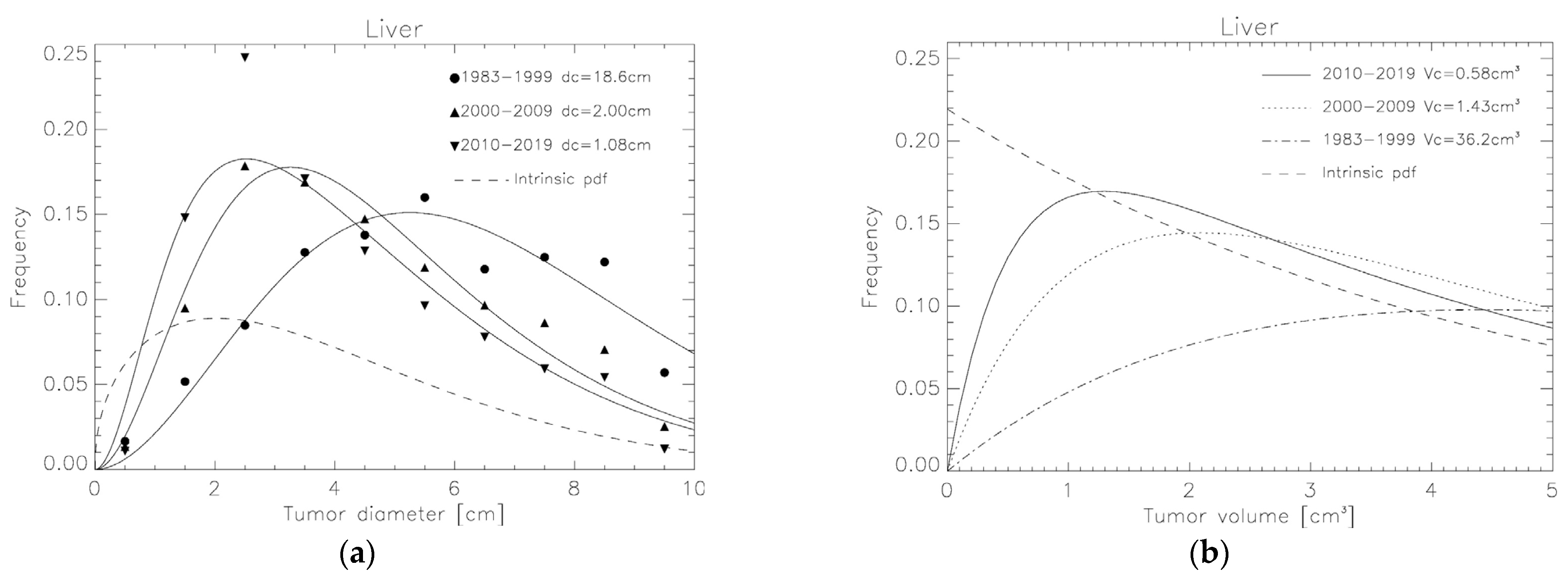
3. Results
3.1. Verification Using Real Lung Tumor Volumes
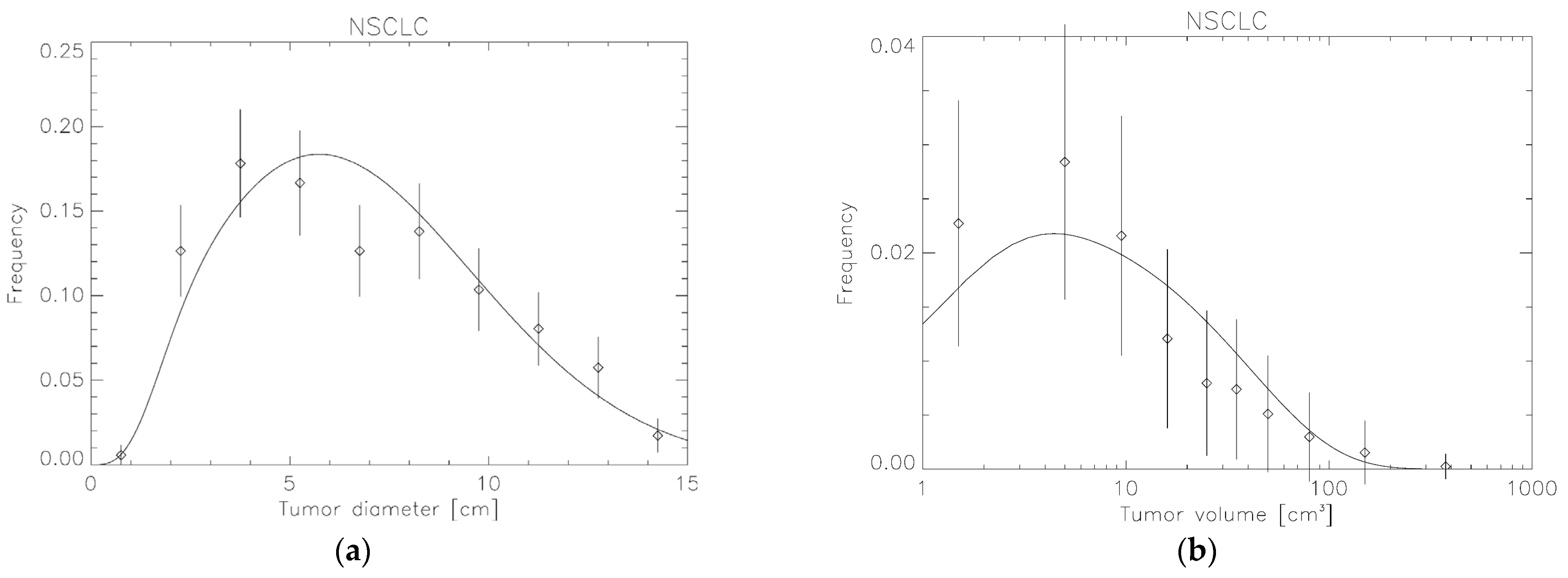
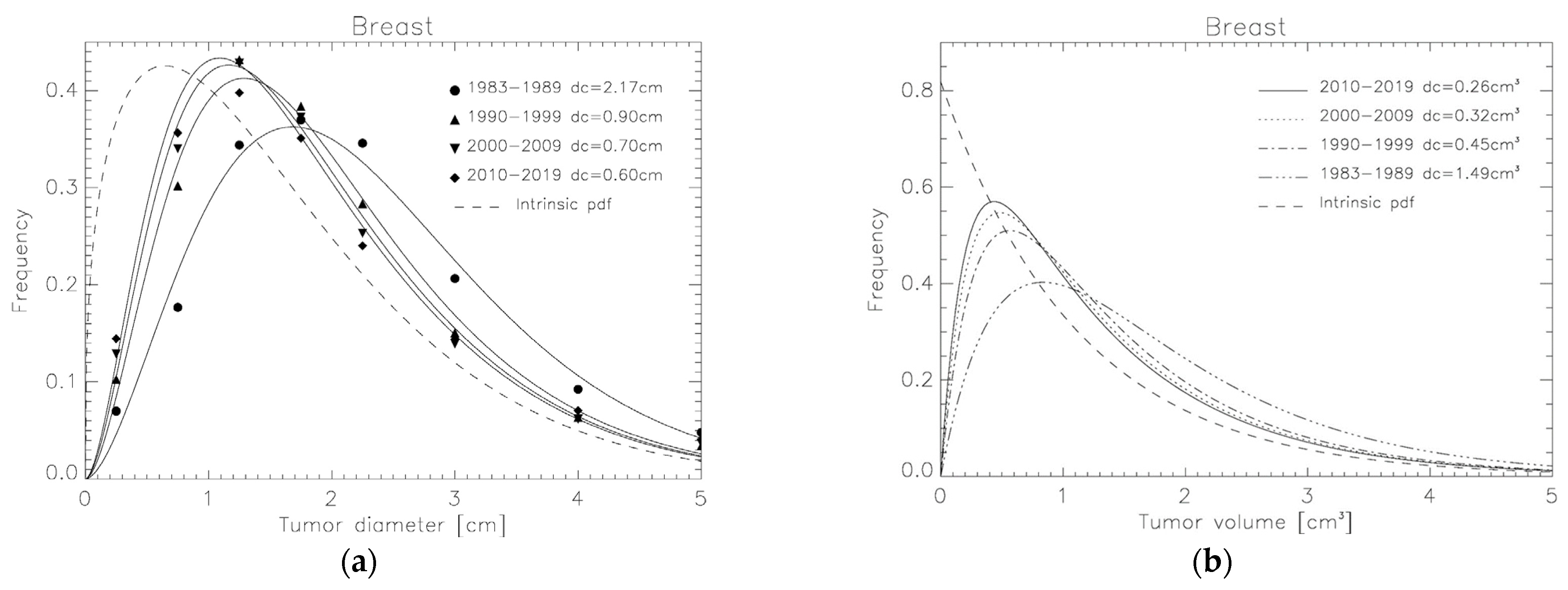
3.2. Tumor Volume Distributions Determined from SEER for Breast, NSCLC and Liver
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubben, H.H.; Thames, H.D.; Beck-Bornholdt, H.P. Tumor volume: A basic and specific response predictor in radiotherapy. Radiother. Oncol. 1998, 47, 167–174. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, W. Method of tumor volume evaluation using magnetic resonance imaging for outcome prediction in cervical cancer treated with concurrent chemotherapy and radiotherapy. Radiat. Oncol. J. 2012, 30, 70–77. [Google Scholar] [CrossRef]
- Kim, H.; Kim, W.; Lee, M.; Song, E.; Loh, J.J. Tumor volume and uterine body invasion assessed by MRI for prediction of outcome in cervical carcinoma treated with concurrent chemotherapy and radiotherapy. Jpn. J. Clin. Oncol. 2007, 37, 858–866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Munro, T.R.; Gilbert, C.W. The relation betweenvtumour lethal doses and the radiosensitivity of tumourvcells. Br. J. Radiol. 1961, 34, 246–251. [Google Scholar] [CrossRef]
- Webb, S.; Nahum, A.E. A model for calculating tumour control probability in radiotherapy including the effects of inhomogeneous distributions of dose and clonogenicvcell density. Phys. Med. Biol. 1993, 38, 653–666. [Google Scholar] [CrossRef]
- Nahum, A.E.; Sanchez-Nieto, B. Tumour control probability modelling: Basic principles and applications in treatment planning. Phys. Med. 2001, 17 (Suppl. 2), 13–23. [Google Scholar]
- Suit, H.; Skates, S.; Taghian, A.; Okunieff, P.; Efird, J.T. Clinical implications of heterogeneity of tumor response to radiation therapy. Radiother. Oncol. 1992, 25, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Bentzen, S.M.; Thames, H.D.; Overgaard, J. Does variation in the in vitro cellular radiosensitivity explain the shallow clinical dose-control curve for malignant melanoma? Int. J. Radiat. Biol. 1990, 57, 117–126. [Google Scholar] [CrossRef]
- Okunieff, P.; Morgan, D.; Niemierko, A.; Suit, H.D. Radiation dose-response of human tumors. Int. J. Radiat. Oncol. Biol. Phys. 1995, 32, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Hendry, J. Inter-tumour heterogeneity and tumour control. In Radiobiological Modelling in Radiation Oncology; Dale, R.G., Jones, B., Eds.; BIR Publications: London, UK, 2007; pp. 169–195. [Google Scholar]
- Schneider, U.; Besserer, J. Tumour volume distribution can yield information on tumour growth and tumour control. Z. Med. Phys. 2022, 32, 143–148. [Google Scholar] [CrossRef]
- Radonic, S.; Besserer, J.; Meier, V.; Bley, C.R.; Schneider, U. A Novel Analytical Population Tumor Control Probability Model Includes Cell Density and Volume Variations: Application to Canine Brain Tumor. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1530–1537. [Google Scholar] [CrossRef]
- Carlsson, G.; Gullberg, B.; Hafström, L. Estimation of liver tumor volume using different formulas—An experimental study in rats. J. Cancer Res. Clin. Oncol. 1983, 105, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Euhus, D.M.; Hudd, C.; LaRegina, M.C.; Johnson, F.E. Tumor measurement in the nude mouse. J. Surg. Oncol. 1986, 31, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef]
- Sorensen, A.G.; Patel, S.; Harmath, C.; Bridges, S.; Synnott, J.; Sievers, A.; Yoon, Y.H.; Lee, E.J.; Yang, M.C.; Lewis, R.F.; et al. Comparison of diameter and perimeter methods for volume calculation. J. Clin. Oncol. 2001, 19, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Mayr, N.A.; Taoka, T.; Yuh, W.T.; Denning, L.M.; Zhen, W.K.; Paulino, A.C.; Gaston, R.C.; Sorosky, J.I.; Meeks, S.L.; Walker, J.L.; et al. Method and timing of tumor volume measurement for outcome prediction in cervical cancer using magnetic resonance imaging. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Eichelberger, L.E.; Koch, M.O.; Daggy, J.K.; Ulbright, T.M.; Eble, J.N.; Cheng, L. Predicting tumor volume in radical prostatectomy specimens from patients with prostate cancer. Am. J. Clin. Pathol. 2003, 120, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Feldman, J.P.; Goldwasser, R.; Mark, S.; Schwartz, J.; Orion, I. A mathematical model for tumor volume evaluation using two-dimensions. J. Appl. Quant. Methods 2009, 4, 455–462. [Google Scholar]
- Güth, U.; Brenckle, D.; Huang, D.J.; Schötzau, A.; Viehl, C.T.; Dieterich, H.; Holzgreve, W.; Wight, E.; Singer, G. Three-dimensional pathological size assessment in primary breast carcinoma. Breast Cancer Res. Treat. 2009, 116, 257–262. [Google Scholar] [CrossRef]
- Thiel, D.D.; Jorns, J.; Lohse, C.M.; Cheville, J.C.; Thompson, R.H.; Parker, A.S. Maximum tumor diameter is not an accurate predictor of renal cell carcinoma tumor volume. Scand. J. Urol. 2013, 47, 472–475. [Google Scholar] [CrossRef]
- Kandethody, M.R.; Tsokos, C.P. Chapter 14—Some issues in statistical applications: An overview. In Mathematical Statistics with Applications in R, 3rd ed.; Ramachandran, K.M., Tsokos, C.P., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 569–613. ISBN 978-0-12-817815-7. [Google Scholar]
- Andea, A.A.; Bouwman, D.; Wallis, T.; Visscher, D.W. Correlation of tumor volume and surface area with lymph node status in patients with multifocal/multicentric breast carcinoma. Cancer 2004, 100, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://en.wikibooks.org/wiki/Probability/Transformation_of_Probability_Densities (accessed on 7 September 2023).
- Surveillance, Epidemiology, and End Results (Seer) Program. Seer*Stat Database: Incidence—Seer Research Data, 8 Registries, Nov 2021 Sub (1975–2019)—Linked to County Attributes—Time Dependent (1990–019) Income/Rurality, 1969–2020 Counties, National Cancer Institute, Dccps, Surveillance Research. Available online: www.seer.cancer.gov (accessed on 20 October 2022).
- National Cancer Institute. Seer*Stat Software, August 2019. Available online: https://seer.cancer.gov/seerstat/ (accessed on 20 October 2022).
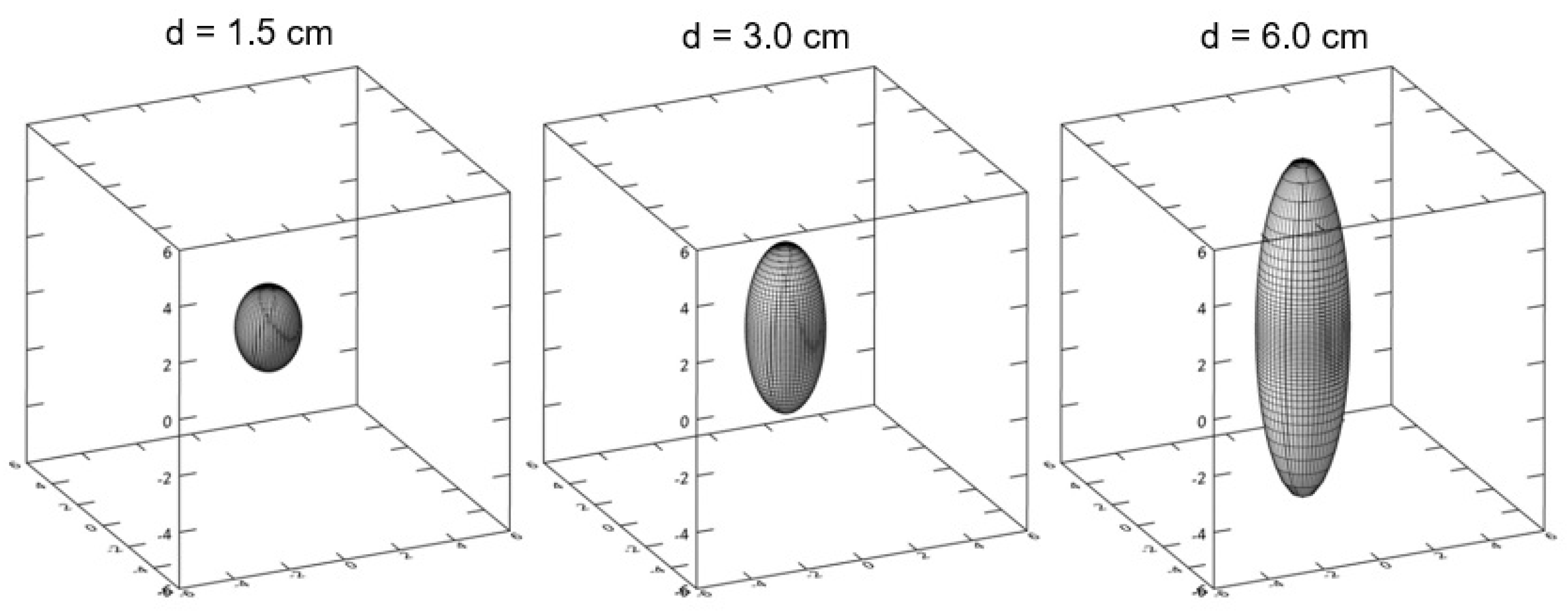

| Diameter Interval (cm) | Patient Number in Time Period | |||
|---|---|---|---|---|
| Breast Cancer | 1983–1989 | 1990–1999 | 2000–2009 | 2010–2019 |
| 0.0–0.5 | 2172 | 6165 | 9267 | 12,710 |
| 0.5–1.0 | 5481 | 18,122 | 24,458 | 31,366 |
| 1.0–1.5 | 10,668 | 25,907 | 30,770 | 34,998 |
| 1.5–2.0 | 11,471 | 23,032 | 26,788 | 30,885 |
| 2.0–2-5 | 10,726 | 17,013 | 18,191 | 21,124 |
| 2.5–3.5 | 12,802 | 18,069 | 20,012 | 25,335 |
| 3.5–4.5 | 5742 | 7636 | 8897 | 12,414 |
| 4.5–5.5 | 2979 | 4102 | 5342 | 7128 |
| NSCLC | 1983–1989 | 1990–1999 | 2000–2009 | 2010–2019 |
| 0.0–1.0 | 2134 | 2060 | 1705 | 2199 |
| 1.0–2.0 | 10,312 | 11,962 | 8825 | 11,586 |
| 2.0–3.0 | 18,678 | 17,578 | 10,293 | 11,132 |
| 3.0–4.0 | 18,809 | 15,894 | 8449 | 8663 |
| 4.0–5.0 | 15,007 | 12,410 | 6629 | 6670 |
| 5.0–6.0 | 12,021 | 9547 | 5066 | 4935 |
| 6.0–7.0 | 8206 | 6836 | 3845 | 3905 |
| 7.0–8.0 | 5468 | 4802 | 2770 | 2934 |
| 8.0–9.0 | 4011 | 3318 | 1964 | 2026 |
| 9.0–10.0 | 1794 | 1142 | 473 | 412 |
| Liver | 1983–1999 | 2000–2009 | 2010–2019 | |
| 0.0–1.0 | 47 | 98 | 159 | |
| 1.0–2.0 | 147 | 693 | 2179 | |
| 2.0–3.0 | 241 | 1304 | 3566 | |
| 3.0–4.0 | 363 | 1234 | 2516 | |
| 4.0–5.0 | 392 | 1076 | 1893 | |
| 5.0–6.0 | 455 | 867 | 1418 | |
| 6.0–7.0 | 335 | 705 | 1146 | |
| 7.0–8.0 | 355 | 630 | 872 | |
| 8.0–9.0 | 347 | 515 | 798 | |
| 9.0–10.0 | 162 | 185 | 176 | |
| Fit of Modified Weibull to Maximum Diameter (Equation (1)) | Results for Exponential Distribution of Volumes | ||||
|---|---|---|---|---|---|
| dc (cm) | λ | k | Vc (cm3) | (cm3) | |
| Breast cancer | 0.57 ± 0.04 | 1.35 ± 0.05 | 1.12 ± 0.02 | ||
| 1983–1989 | 2.17 ± 0.24 | 1.49 ± 0.23 | |||
| 1990–1999 | 0.90 ± 0.09 | 0.45 ± 0.06 | |||
| 2000–2009 | 0.70 ± 0.09 | 0.32 ± 0.05 | |||
| 2010–2019 | 0.60 ± 0.10 | 0.26 ± 0.10 | |||
| NSCLC | 0.25 ± 0.07 | 1.49 ± 0.03 | 4.26 ± 0.004 | ||
| 1983–1989 | 2.04 ± 0.13 | 1.52 ± 0.15 | |||
| 1990–1999 | 1.48 ± 0.10 | 0.94 ± 0.09 | |||
| 2000–2009 | 0.98 ± 0.13 | 0.51 ± 0.10 | |||
| 2010–2019 | 0.70 ± 0.21 | 0.31 ± 0.14 | |||
| Liver | 0.22 ± 0.02 | 1.45 ± 0.09 | 4.70 ± 0.01 | ||
| 1983–1999 | 18.6 ± 16 | 36.3 ± 47 | |||
| 2000–2009 | 2.00 ± 0.33 | 1.43 ± 0.35 | |||
| 2010–2019 | 1.08 ± 0.50 | 0.59 ± 0.38 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneider, U.; Radonic, S.; Besserer, J. Tumor Volume Distributions Based on Weibull Distributions of Maximum Tumor Diameters. Appl. Sci. 2023, 13, 10925. https://doi.org/10.3390/app131910925
Schneider U, Radonic S, Besserer J. Tumor Volume Distributions Based on Weibull Distributions of Maximum Tumor Diameters. Applied Sciences. 2023; 13(19):10925. https://doi.org/10.3390/app131910925
Chicago/Turabian StyleSchneider, Uwe, Stephan Radonic, and Jürgen Besserer. 2023. "Tumor Volume Distributions Based on Weibull Distributions of Maximum Tumor Diameters" Applied Sciences 13, no. 19: 10925. https://doi.org/10.3390/app131910925
APA StyleSchneider, U., Radonic, S., & Besserer, J. (2023). Tumor Volume Distributions Based on Weibull Distributions of Maximum Tumor Diameters. Applied Sciences, 13(19), 10925. https://doi.org/10.3390/app131910925







