The Benefit of Mycorrhizal Fungi and Beneficial Soil Bacteria in Drought Exposed Lettuce (Lactuca sativa var. capitata) Is Genotype and Environment Dependent
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Growth Condition
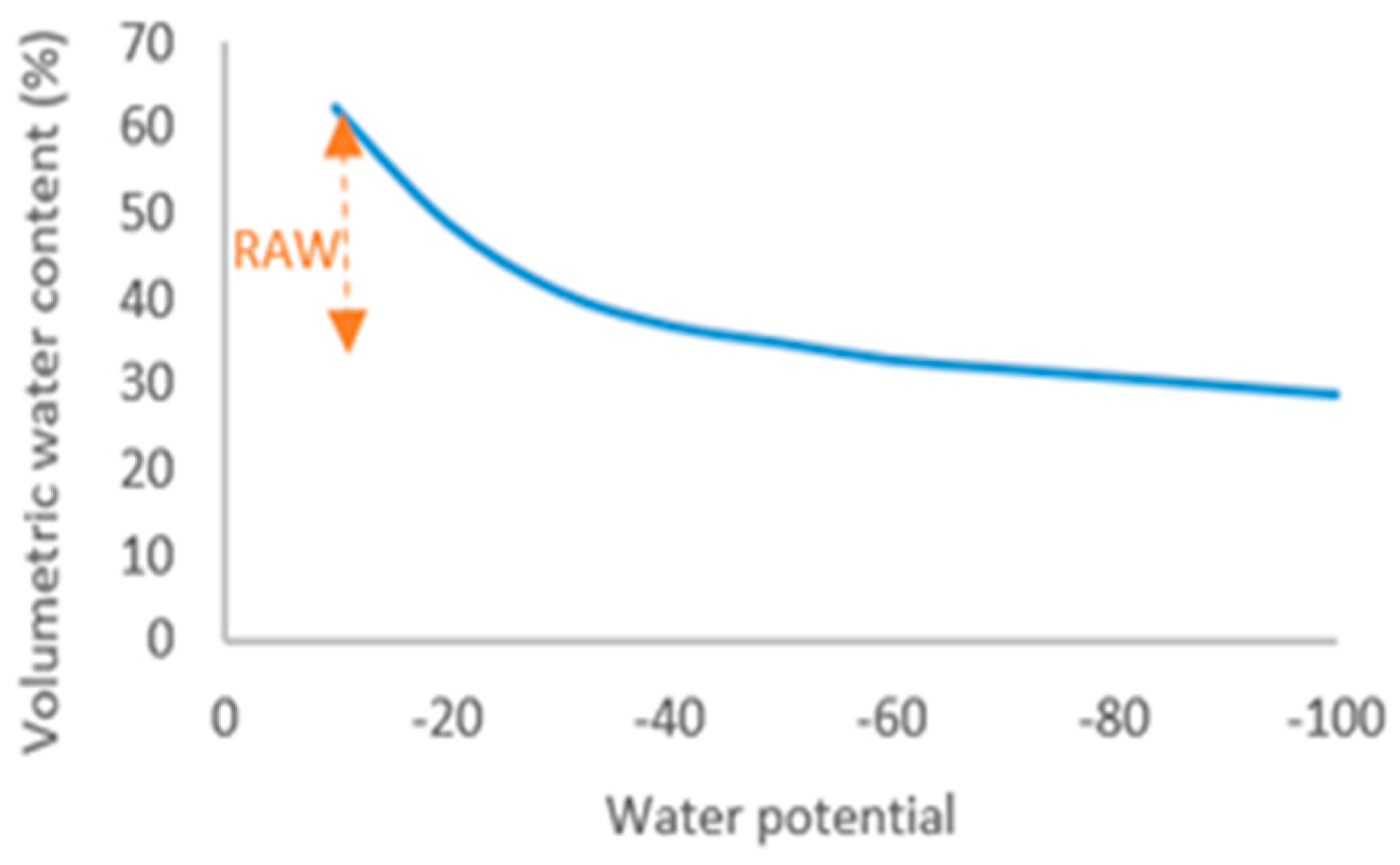
2.2. Irrigation and Mycorrhizal Treatment
2.3. Measurements of Morphological and Biochemical Attributes
2.4. Data Analysis
3. Results
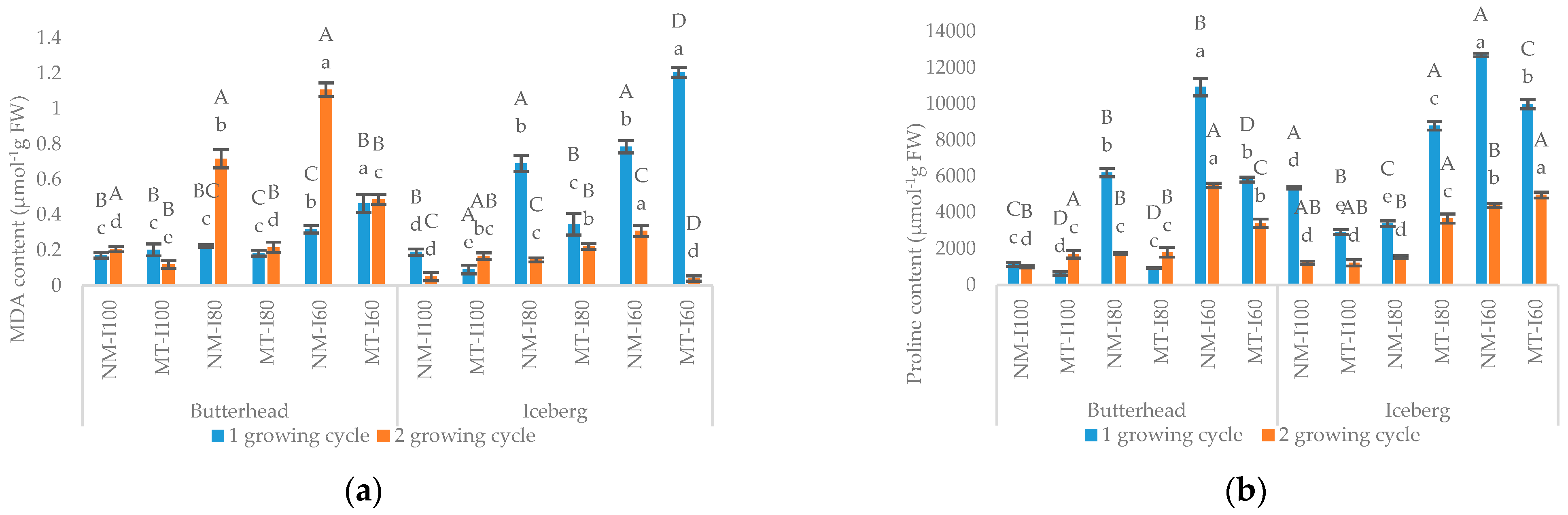
3.1. Morphometric and Biochemical Parameters in the First Growing Cycle
3.2. Morphometric and Biochemical Parameters in the Second Growing Cycle
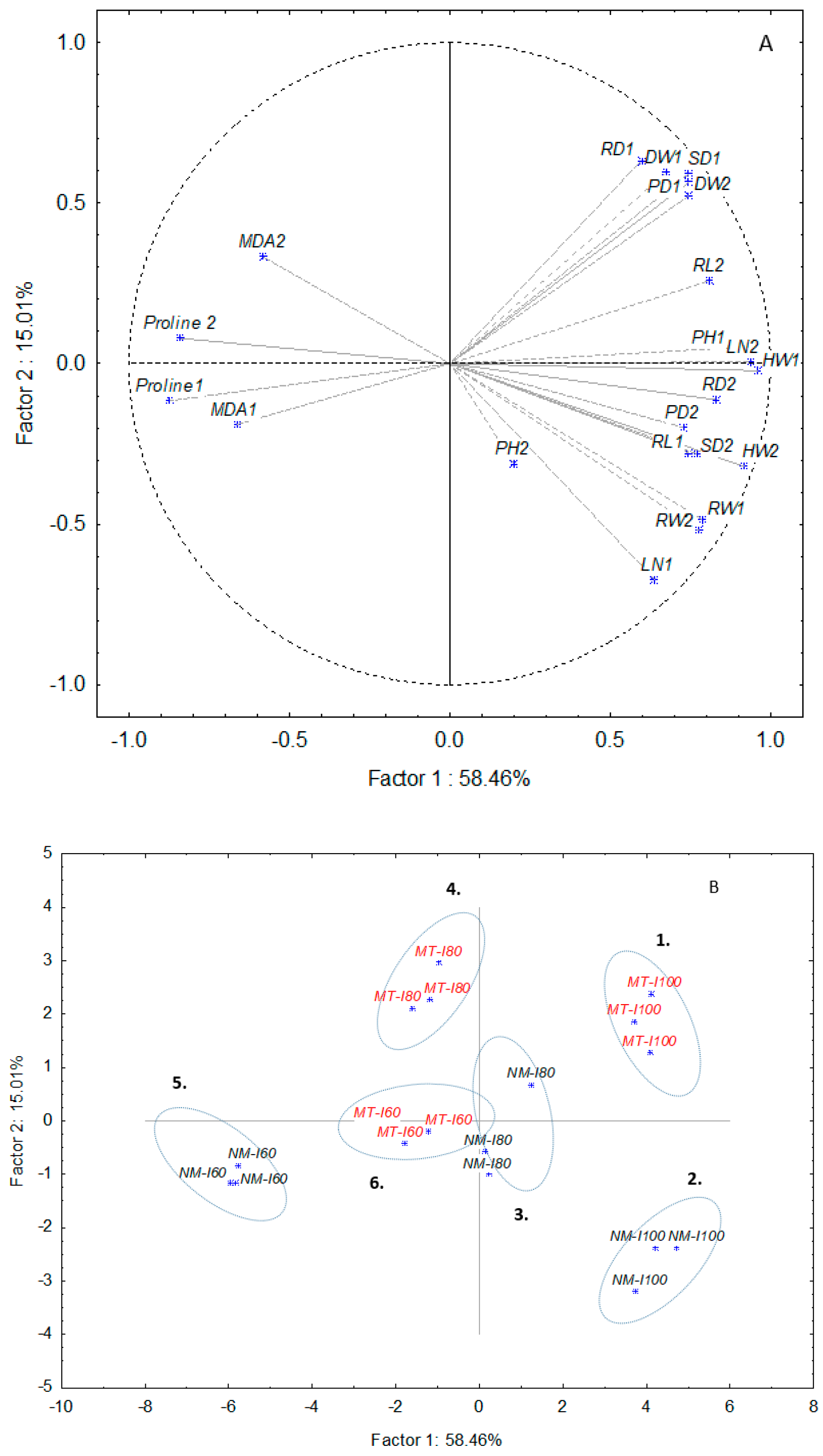
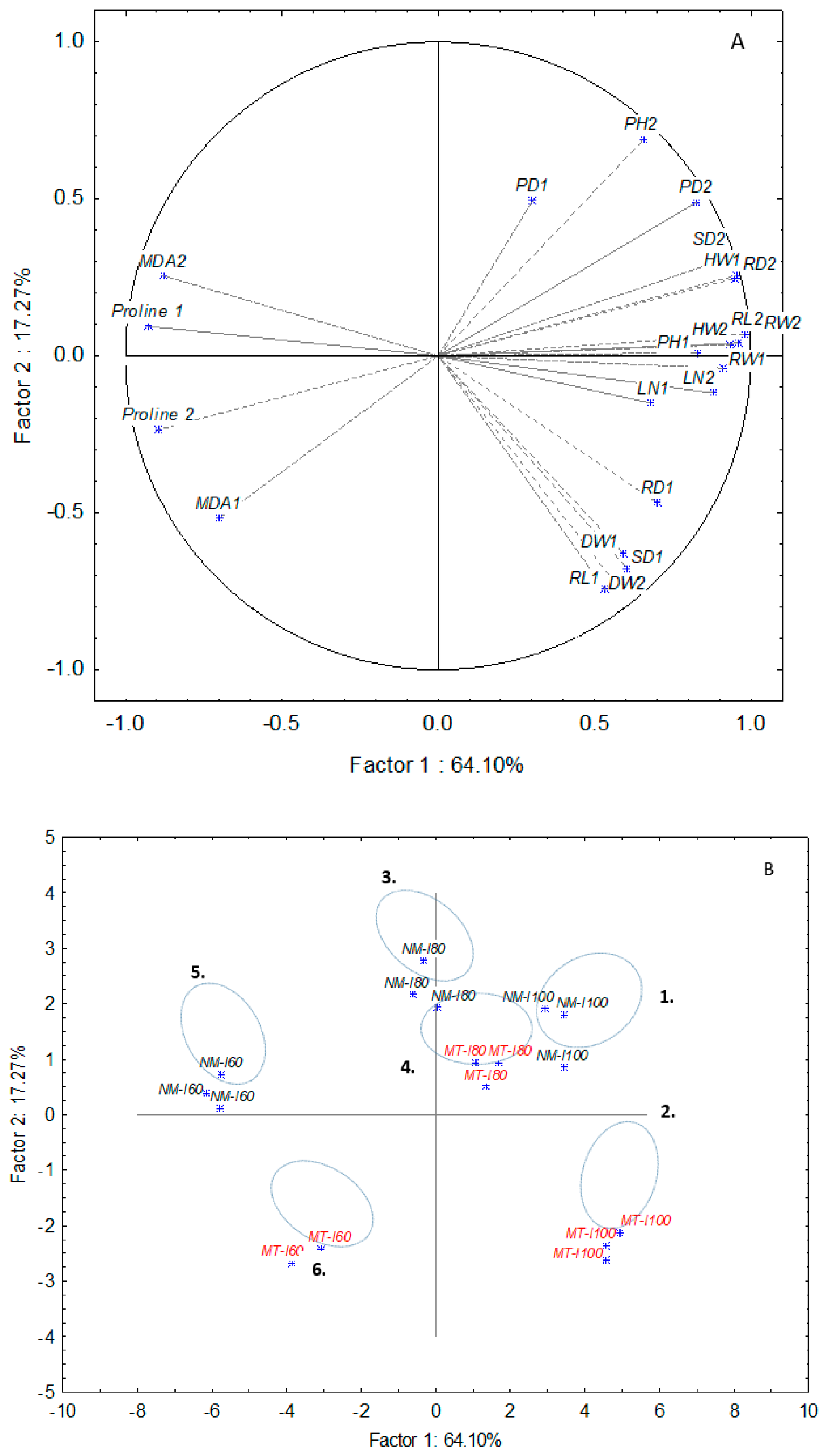
3.3. PCA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huey, C.J.; Gopinath, S.C.B.; Uda, M.N.A.; Zulhaimi, H.I.; Jaafar, M.N.; Kasim, F.H.; Yaakub, A.R.W. Mycorrhiza: A natural resource assists plant growth under varied soil conditions. 3 Biotech 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Shaffique, S.; Khan, M.A.; Imran, M.; Kang, S.M.; Park, J.S.; Wani, S.H.; Lee, I.J. Research Progress in the Field of Microbial Mitigation of Drought Stress in Plants. Front. Plant Sci. 2022, 13, 870626. [Google Scholar] [CrossRef] [PubMed]
- Dai, A. Drought under global warming: A review. Wiley Interdiscip. Rev. Clim. Chang. 2011, 2, 45–65. [Google Scholar] [CrossRef]
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate change impact and adaptation for wheat protein. Glob. Chang. Biol. 2018, 25, 155–173. [Google Scholar] [CrossRef]
- Marcinkowski, P.; Piniewski, M. Effect of climate change on sowing and harvest dates of spring barley and maize in Poland. Int. Agrophys. 2018, 32, 265–271. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; He, C.; Liu, D.L.; Feng, P.; Yao, N.; Zhang, R.; Xu, S.; Xue, J.; Feng, H.; et al. Optimizing Sowing Date and Planting Density Can Mitigate the Impacts of Future Climate on Maize Yield: A Case Study in the Guanzhong Plain of China. Agronomy 2021, 11, 1452. [Google Scholar] [CrossRef]
- Chhokar, R.S.; Sharma, R.K.; Kumar, N.; Singh, R.K.; Singh, G.P. Advancing Sowing Time and Conservation Tillage—The Climate-Resilient Approach to Enhance the Productivity and Profitability of Wheat. Int. J. Plant Prod. 2023, 17, 121–131. [Google Scholar] [CrossRef]
- Baye, K.N.; Melash, A.A.; Bogale, A.A. Role of Conservation Tillage as Climate Change Mitigation. Civ. Environ. Res. 2019, 11, 12–23. [Google Scholar]
- Sadiq, M.; Li, G.; Rahim, N.; Tahir, M.M. Sustainable Conservation Tillage Technique for Improving Soil Health by Enhancing Soil Physicochemical Quality Indicators under Wheat Mono-Cropping System Conditions. Sustainability 2021, 13, 8177. [Google Scholar] [CrossRef]
- Dalias, P.; Christou, A.; Neocleous, D. Adjustment of Irrigation Schedules as a Strategy to Mitigate Climate Change Impacts on Agriculture in Cyprus. Agriculture 2019, 9, 4. [Google Scholar] [CrossRef]
- El-Nashar, W.; Elyamany, A. Adapting Irrigation Strategies to Mitigate Climate Change Impacts. A Value Engineering Approach. Water Resour. Manag. 2022, 37, 2369–2386. [Google Scholar] [CrossRef]
- Rosa, L. Adapting agriculture to climate change via sustainable irrigation: Biophysical potentials and feedbacks. Environ. Res. Lett. 2022, 17, 063008. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Van Etten, J.; de Sousa, K.; Aguilar, A.; Steinke, J. Crop variety management for climate adaptation supported by citizen science. PNAS 2019, 116, 4194–4199. [Google Scholar] [CrossRef]
- Poudel, M.; Mendes, R.; Costa, L.A.S.; Bueno, C.G.; Meng, Y.; Folimonova, S.Y.; Garrett, K.A.; Martins, S.J. The Role of Plant-Associated Bacteria, Fungi, and Viruses in Drought Stress Mitigation. Front. Microbiol. 2021, 12, 743512. [Google Scholar] [CrossRef]
- Loo, W.T.; Chua, K.-O.; Mazumdar, P.; Cheng, A.; Osman, N.; Harikrishna, J.A. Arbuscular Mycorrhizal Symbiosis: A Strategy for Mitigating the Impacts of Climate Change on Tropical Legume Crops. Plants 2022, 11, 2875. [Google Scholar] [CrossRef]
- Douds, D.; Nagahashi, G.; Reider, C.; Hepperly, P. Inoculation with arbuscular mycorrhizal fungi increases the yield of potatoes in a high P soil. Biol. Agric. Hortic. 2007, 25, 67–78. [Google Scholar] [CrossRef]
- Almagrabi, O.; Abdelmoneim, T. Using of arbuscular mycorrhizal fungi to reduce the deficiency effect of phosphorous fertilization on maize plants (Zea mays L.). Life Sci. J. 2012, 9, 1648–1654. [Google Scholar]
- Sitoe, S.N.D.M.; Dames, J.F. Mitigating Climate Change: The Influence of Arbuscular Mycorrhizal Fungi on Maize Production and Food Security. In Arbuscular Mycorrhizal Fungi in Agriculture-New Insights; De Sousa, R.N., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Cui, N.; Shi, L.; Guo, J.; Zhang, T. Arbuscular mycorrhizal fungi alleviate elevated temperature and nitrogen deposition- induced warming potential by reducing soil N2O emissions in a temperate meadow. Ecol. Indic. 2021, 131, 108193. [Google Scholar] [CrossRef]
- Mathur, S.; Agnihotri, R.; Sharma, M.P.; Reddy, V.R.; Jajoo, A. Effect of High-Temperature Stress on Plant Physiological Traits and Mycorrhizal Symbiosis in Maize Plants. J. Fungi 2021, 7, 867. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, G.; Shagol, C.C.; Kim, K.; Han, S.; Sa, T. Spore associated bacteria regulates maize root K+/Na+ ion homeostasis to promote salinity tolerance during arbuscular mycorrhizal symbiosis. BMC Plant Biol. 2018, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, C.; Lu, T.; Zheng, Y. Cooperation between arbuscular mycorrhizal fungi and earthworms promotes the physiological adaptation of maize under a high salt stress. Plant and Soil. 2018, 423, 125–140. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Zahan, M.I.; Tahjib-Ul-Arif, M.; Akter, M.A.; Okazaki, S. Plant Salinity Tolerance Conferred by Arbuscular Mycorrhizal Fungi and Associated Mechanisms: A Meta-Analysis. Front. Plant Sci. 2022, 11, 1927. [Google Scholar] [CrossRef]
- Cosme, M.; Fernández, I.; Van der Heijden, M.G.; Pieterse, C.M. Non-mycorrhizal plants: The exceptions that prove the rule. Trends Plant Sci. 2018, 23, 577–587. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.N.; Wu, Q.S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular Mycorrhizal Fungi Alleviate Drought Stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) Grasses via Altering Antioxidant Enzyme Activities and Photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; El Modafar, C.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular Mycorrhizal Fungi Mediate Drought Tolerance and Recovery in Two Contrasting Carob (Ceratonia siliqua L.) Ecotypes by Regulating Stomatal, Water Relations, and (In)Organic Adjustments. Plants 2020, 9, 80. [Google Scholar] [CrossRef]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef]
- Hardie, K. The effect of removal the extraradical hyphae on water uptake by vesicular-arbuscular mycorrhyzal plant. New Phytol. 1985, 101, 677–684. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.J.; Fetene, M.; Bongers, F.; Kuyper, T.W. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 2012, 169, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Watts-Williams, S.J.; Cavagnaro, T.R.; Tyerman, S.D. Variable effects of arbuscular mycorrhizal fungal inoculation on physiological and molecular measures of root and stomatal conductance of diverse Medicago truncatula accessions. Plant Cell Environ. 2018, 42, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Saboor, A.; ArifAli, M.; Danish, S.; Ahmed, N.; Fahad, S.; Datta, R.; Ansari, M.J.; Nasif, O.; Rahman, M.R.; Glick, B.R. Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-defcient soils. Sci. Rep. 2021, 11, 18468. [Google Scholar] [CrossRef] [PubMed]
- Beltrano, J.; Ruscitti, M.; Arango, M.C.; Ronco, M. Effects of arbuscular mycorrhiza inoculation on plant growth, biological and physiological parameters and mineral nutrition in pepper grown under different salinity and p levels. J. Soil Sci. Plant Nutr. 2013, 13, 123–141. [Google Scholar] [CrossRef]
- Yan, Q.; Li, S.; Xiao, X.; Chen, J.; Liu, J.; Lin, C.; Guan, R.; Wang, D. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Cinnamomum migao by enhancing physio-biochemical responses. Ecol. Evol. 2022, 12, e9091. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, T.D.; Zou, X. Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ. 2012, 58, 186–191. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Q.; Sun, L.; Yang, X.; Yang, W.; Zhang, H. Stomatal Conductance and Morphology of Arbuscular Mycorrhizal Wheat Plants Response to Elevated CO2 and NaCl Stress. Front. Plant Sci. 2018, 19, 1363. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Qin, Q.Y.; Ma, W.Y.; Zhou, L.J.; Wu, Q.S.; Xu, Y.J.; Kuča, K.; Hashem, A.; Al-Arjani, A.B.F.; Almutairi, K.F.; et al. Metabolomics reveals arbuscular mycorrhizal fungi-mediated tolerance of walnut to soil drought. BMC Plant Biol. 2023, 23, 118. [Google Scholar] [CrossRef]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef]
- Sangwan, S.; Prasanna, R. Mycorrhizae Helper Bacteria: Unlocking Their Potential as Bioenhancers of Plant-Arbuscular Mycorrhizal Fungal Associations. Microb Ecol. 2022, 84, 1–10. [Google Scholar] [CrossRef]
- Abbaspour, H.; Saeidi-SarAfshari, S.; Abdel-Wahhab, M. Tolerance of mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Egamberdieva, D.; Alam, P.; Alyemeni, M.N.; Ashraf, M. Modification of osmolytes and antioxidant enzymes by epibrassinolide in chickpea seedlings under mercury (Hg) toxicity. J. Plant Growth Regul. 2018, 37, 309–322. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2018, 180, 1246–1250. [Google Scholar] [CrossRef]
- Bárzana, G.R.; Aroca, R.; Ruiz-Lozano, J.M. Localized and non-localized effects of arbuscular mycorrhizal symbiosis on accumulation of osmolytes and aquaporins and on antioxidant systems in maize plants subjected to total or partial Fernández-Lizarazo and Moreno-Fonseca: Mechanisms for tolerance to water-deficit stress in plants inoculated with arbuscular mycorrhizal fungi. A review root drying. Plant Cell Environ. 2015, 38, 1613–1627. [Google Scholar]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Wu, Q.S.; Cao, M.Q.; Zou, Y.N.; Wu, C.; He, X.H. Mycorrhizal colonization represents functional equilibrium on root morphology and carbon distribution of trifoliate orange grown in a split-root system. Sci. Hortic. 2016, 199, 95–102. [Google Scholar]
- Yooyongwech, S.; Samphumphuang, R.; Tisarum, C.; Theerawitaya, C.; Cha-Um, S. Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci. Hortic. 2016, 198, 107–117. [Google Scholar] [CrossRef]
- Wu, H.H.; Zou, Y.N.; Rahman Ni, Q.D.; Wu, Q.S. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017, 7, 42389. [Google Scholar] [CrossRef]
- Bozkurt, S.; Mansuroğlu, G.S.; Kara, M.; Önder, S. Responses of lettuce to irrigation levels and nitrogen forms. Afr. J. Agric. Res. 2009, 4, 1171–1177. [Google Scholar]
- Şenyiğit, U.; Kaplan, D. Impact of different irrigation water levels on yield and some quality parameters of lettuce (Lactuca sativa L. Var. Longifolia cv.) under unheated greenhouse condition. Infrastruct. Ecol. Rural Areas 2013, 2, 97–107. [Google Scholar]
- Acar, B.; Paksoy, M.; Türkmen, Ö.; Seymen, M. Irrigation and nitrogen level affect lettuce yield in greenhouse condition. IJIWM 2015, 2, 001–004. [Google Scholar]
- Acar, B. Water-yield relationships of lettuce plants for different irrigation strategies. Int. Sci. J. Mech. Agric. Conserv. Resour. 2020, 5, 177–180. [Google Scholar]
- Sesveren, S. Response of Lactuva Sativa Var. Crispa to deficit irrigation and leonardite treatments. All Life 2022, 15, 105–117. [Google Scholar] [CrossRef]
- Cantrell, I.C.; Linderman, R.G. Preinoculation of lettuce and onion with VA mycorrhizal fungi reduces deleterious effects of soil salinity. Plant Soil 2001, 233, 269–281. [Google Scholar] [CrossRef]
- Azcón, R.; Ambrosano, E.; Charest, C. Nutrient acquisition in mycorrhizal lettuce plants under different phosphorus and nitrogen concentration. Plant Sci. 2003, 165, 1137–1145. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Arbuscular Mycorrhizal Fungi (AMF) Improved Growth and Nutritional Quality of Greenhouse-Grown Lettuce. J. Agric. Food Chem. 2011, 59, 5504–5515. [Google Scholar] [CrossRef]
- Santander, C.; Sanhueza, M.; Olave, J.; Aroca, R.; Cumming, J.; Cornejo, P. Arbuscular Mycorrhizal Colonization Promotes the Tolerance to Salt Stress in Lettuce Plants through an Efficient Modification of Ionic Balance. J Soil Sci. Plant Nutr. 2019, 19, 321–331. [Google Scholar] [CrossRef]
- Epelde, L.; Urra, J.; Anza, M.; Gamboa, J.; Garbisu, C. Inoculation of arbuscular mycorrhizal fungi increases lettuce yield without altering natural soil communities. Arch. Agron. Soil Sci. 2020, 68, 413–430. [Google Scholar] [CrossRef]
- Rasouli, F.; Amini, T.; Asadi, M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Ercisli, S.; Skrovankova, S.; Mlcek, J. Growth and Antioxidant Responses of Lettuce (Lactuca sativa L.) to Arbuscular Mycorrhiza Inoculation and Seaweed Extract Foliar Application. Agronomy 2022, 12, 401. [Google Scholar] [CrossRef]
- Lowery, B.; Hickey, W.J.; Arshad, M.A.; Rattan, L. Soil water parameters and soil quality. Methods Assess. Soil Qual. 1996, 4, 143–155. [Google Scholar]
- Kumar, A.; Sharma, S.; Mishra, S. Influence of Arbuscular Mycorrhizal (AM) Fungi and Salinity on Seedling Growth, Solute Accumulation, and Mycorrhizal Dependency of Jatropha curcas L. J. Plant Growth Regul. 2010, 29, 297–306. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Pituch, K.A.; Stevens, J.P. Applied Multivariate Statistics for the Social Sciences, 6th ed.; Riegert, D., Ed.; Taylor and Francis: New York, NY, USA, 2016. [Google Scholar]
- Gutiérrez, D.A. Yield and Nitrogen Use Efficiency for Lettuce (Lactuca sativa) Grown with Airjection and Non-Aerated Irrigation. Master’s Thesis, Jordan College of Agricultural Sciences and Technology California State University, Fresno, CA, USA, 2020. [Google Scholar]
- Lee, J.S.; Chandra, D.; Son, J. Growth, Physicochemical, Nutritional, and Postharvest Qualities of Leaf Lettuce (Lactuca sativa L.) as Affected by Cultivar and Amount of Applied Nutrient Solution. Horticulturae 2022, 8, 436. [Google Scholar] [CrossRef]
- Kirova, E.B.; Geneva, M.P.; Kostadinov, K.; Filipov, S. Improving Yield and Quality-Related Physiological Characteristics of Lettuce by Integrated Inorganic and Organic Fertilizers Management. Agric. Conspec. Sci. 2021, 87, 127–134. [Google Scholar]
- Gholamhoseini, M.; Ghalavand, A.; Dolatabadian, A.; Jamshidi, E.; Khodaei-Joghan, A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013, 117, 106–114. [Google Scholar] [CrossRef]
- Bengough, A.G.; McKenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Kumari, J.; Rengel, Z. Root length and root lipid composition contribute to drought tolerance of winter and spring wheat. Plant Soil 2019, 439, 57–73. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L.; Yang, R.; Han, Y.; Hao, J.; Liu, C.; Fan, S. Effects of exogenous putrescine on the ultrastructure of and calcium ion flow rate in lettuce leaf epidermal cells under drought stress. Hortic. Environ. Biotechnol. 2019, 60, 479–490. [Google Scholar] [CrossRef]
- Nurliana, S.; Fachriza, S.; Hemelda, N.M.; Yuniati, R. Chitosan application for maintaining the growth of lettuce (Lactuca sativa) under drought condition. Earth Environ. Sci. 2021, 980, 012013. [Google Scholar] [CrossRef]
- Al-Karaki, G.; Mcmichael, B.; Zak, J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mychorrhiza 2004, 14, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.D.; Zhang, D.J.; Hu, X.C.; Wu, Q.S.; Jiang, C.J.; Xia, T.J.; Gao, X.B.; Kuča, K. Mycorrhiza-induced changes in root growth and nutrient absorption of tea plants. Plant Soil Environ. 2018, 64, 283–289. [Google Scholar] [CrossRef]
- Meddich, A.; Jaiti, F.; Bourzik, W.; El Asli, A.; Hafidi, M. Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Badvi, H.; Alemzade Ansari, N.; Mahmoodi Sorestani, M.; Eskandari, F. Effects of drought stress and mycorrhizal fungi on some morphophysiological characteristics of lettuce (Lactuca sativa L.). Plant Prod. 2015, 38, 27–39. [Google Scholar]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, A.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Gholinezhad, E.; Darvishzadeh, R.; Moghaddam, S.S.; Popović-Djordjević, J. Effect of mycorrhizal inoculation in reducing water stress in sesame (Sesamum indicum L.): The assessment of agrobiochemical traits and enzymatic antioxidant activity. Agric. Water Manag. 2020, 238, 106234. [Google Scholar] [CrossRef]
- Hazzoumi, Z.; Moustakime, Y.; Elharchli, E.; Joutei, K.A. Effect of arbuscular mycorrhizal fungi (AMF) and water stress on growth, phenolic compounds, glandular hairs, and yield of essential oil in basil (Ocimum gratissimum L.). Chem. Biol. Technol. Agric. 2015, 2, 10. [Google Scholar] [CrossRef]
- Abdi, N.; Van Biljon, A.; Steyn, C.; Labuschagne, M. Zn Fertilizer and Mycorrhizal Inoculation Effect on Bread Wheat Cultivar Grown under Water Deficit. Life 2023, 13, 1078. [Google Scholar] [CrossRef]
- Sium, A.; Shawon, A.; Swapan Kumar, R.; Sun Hee, W.; Kailas Dashrath, S.; Abdullah Mohammad, S. Effect of salinity on the morphological, physiological and biochemical properties of lettuce (Lactuca sativa L.) in Bangladesh. Open Agric. 2019, 4, 361–373. [Google Scholar]
- Ain-Lhout, F.; Zunzunegui, M.; Diaz Barradas, M.C.; Tirado, R.; Clavijo, A.; Garcia Novo, F. Comparison of proline accumulation in two mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil 2001, 230, 175–183. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wu, Q.S.; Huang, Y.M.; Ni, Q.D.; He, X.N. Mycorrhizal-Mediated Lower Proline Accumulation in Poncirus trifoliata under Water Deficit Derives from the Integration of Inhibition of Proline Synthesis with Increase of Proline Degradation. PlosOne 2013, 8, e80568. [Google Scholar] [CrossRef] [PubMed]
- Basahi, J.M.; Ismail, M.; Hassan, I.A. Effects of Enhanced UV-B Radiation and Drought Stress on Photosynthetic Performance of Lettuce (Lactuca sativa L. Romaine) Plants. Annu. Res. Rev. Biol. 2014, 4, 1739–1756. [Google Scholar] [CrossRef]
- Duangpan, S.; Sujitto, S.; Eksomtramage, T. Genotypic Variation in Proline Accumulation during Sequential Drought and Rewatering in Response to Drought Preconditioning. Int. J. Agric. Technol. 2017, 13, 927–940. [Google Scholar]
- Santos, R.C.; Godoy, J.I.; Fávero, A.P. Melhoramento do Amendoim. Santos, R.C. O Agronegócio do Amendoim no Brasil. Camp. Gd. Embrapa Algodão 2005, 4, 124–192. [Google Scholar]
- Yavuz, N.; Seymen, M.; Kal, Ü. Impacts of water stress and harvest time on physio-biochemical characteristics of lettuce. Int. J. Agric. Nat. Sci. 2021, 14, 61–77. [Google Scholar]
- Mombeini, M.; Alamzadeh Ansar, N.; Abdossi, V.H.; Naseri, A. Reducing destructive effects of drought stress on cucumber through seed priming with silicic acid, pyridoxine, and ascorbic acid along with foliar spraying with silicic acid. Agric. Conspec. Sci. 2021, 86, 35–49. [Google Scholar]
- Kiran, S. Effects of Vermicompost on Some Morphological, Physiological and Biochemical Parameters of Lettuce (Lactuca sativa var. crispa) under Drought Stress. Not. Bot. Horti Agrobo. 2019, 47, 352–358. [Google Scholar] [CrossRef][Green Version]
- Han, Y.; Fan, S.; Zhang, Q.; Wang, Y. Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings. Agric. Sci. 2013, 4, 112–115. [Google Scholar] [CrossRef]


| Units | Usual Range | Result | Unit | Usual Range | Result | ||
|---|---|---|---|---|---|---|---|
| Turbidity | 3 | Mg | me/L | 0–5 | 3.9 | ||
| pH | 6.5–8.4 | 7.4 | Ca | me/L | 0–20 | 6.8 | |
| EC | dS/m | 0–3 | 0.7 | K | me/L | 0–2 | 1.3 |
| Ions | |||||||
| Cl | mg/L | 0–30 | 3.4 | ||||
| B | mg/L | 0–2 | 0.6 | ||||
| Na | mg/L | 0–40 | 2.6 | ||||
| Second Growing Cycle | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Treatment | HW | PH | PD | LN | RW | RL | RD | SD | DW | MDA | Proline | |
| 1st growing cycle | Treatment | 1.00 | −0.24 ns | −0.41 ns | −0.08 ns | −0.47 * | −0.28 ns | −0.39 ns | −0.35 ns | −0.41 ns | −0.10 ns | 0.35 ns | 0.47 * |
| HW | −0.47 * | 1.00 | 0.52 * | 0.25 ns | 0.59 * | −0.02 ns | 0.24 ns | 0.20 ns | 0.61 * | 0.30 ns | −0.03 ns | −0.37 ns | |
| PH | −0.20 ns | 0.74 * | 1.00 | 0.32 ns | 0.28 ns | 0.53 * | 0.14 ns | 0.43 ns | 0.53 * | −0.36 ns | −0.42 ns | −0.34 ns | |
| PD | 0.14 ns | 0.52 * | 0.78 * | 1.00 | 0.72 * | 0.82 * | 0.68 * | 0.88 * | 0.76 * | 0.11 ns | −0.84 * | −0.71 * | |
| LN | −0.34 ns | 0.15 ns | −0.40 ns | −0.61 * | 1.00 | 0.44 ns | 0.80 * | 0.76 * | 0.89 * | 0.50 * | −0.57 * | −0.83 * | |
| RW | −0.28 ns | 0.35 ns | −0.17 ns | −0.43 ns | 0.90 * | 1.00 | 0.49 * | 0.88 * | 0.61 * | −0.20 ns | −0.93 * | −0.61 * | |
| RL | −0.59 * | 0.87 * | 0.70 * | 0.39 ns | 0.09 ns | 0.19 ns | 1.00 | 0.77 * | 0.72 * | 0.45 ns | −0.64 * | −0.88 * | |
| RD | −0.12 ns | 0.60 * | 0.69 * | 0.74 * | −0.54 * | −0.32 ns | 0.57 * | 1.00 | 0.82 * | 0.16 ns | −0.89 * | −0.85 * | |
| SD | −0.10 ns | 0.60 * | 0.66 * | 0.57 * | −0.40 ns | −0.20 ns | 0.55 * | 0.90 * | 1.00 | 0.32 ns | −0.65 * | −0.87 * | |
| DW | −0.21 ns | 0.72 * | 0.66 * | 0.63 * | −0.26 ns | −0.11 ns | 0.60 * | 0.75 * | 0.75 * | 1.00 | 0.09 ns | −0.36 ns | |
| MDA | 0.59 * | −0.86 * | −0.81 * | −0.55 * | 0.09 ns | −0.06 ns | −0.90 * | −0.60 * | −0.54 * | −0.64 * | 1.00 | 0.74 * | |
| Proline | 0.24 ns | −0.77 * | −0.45 ns | −0.48 * | −0.25 ns | −0.41 ns | −0.53 * | −0.48 * | −0.49 * | −0.64 * | 0.56 * | 1.00 | |
 Abbreviations: head weight (HW), plant height (PH), plant diameter (PD), leaf number (LN), root weight (RW), root length (RL), root diameter (RD), steam diameter (SD), dry weight (DW), malondialdehyde (MDA) content.
Abbreviations: head weight (HW), plant height (PH), plant diameter (PD), leaf number (LN), root weight (RW), root length (RL), root diameter (RD), steam diameter (SD), dry weight (DW), malondialdehyde (MDA) content.| Second Growing Cycle | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Treatment | HW | PH | PD | LN | RW | RL | RD | SD | DW | MDA | Proline | |
| 1st growing cycle | Treatment | 1.00 | −0.24 ns | −0.18 ns | −0.37 ns | −0.63 * | −0.59 * | −0.60 * | −0.34 ns | −0.29 ns | −0.52 * | 0.39 ns | 0.41 ns |
| HW | −0.46 ns | 1.00 | 0.53 * | 0.40 ns | 0.43 ns | 0.44 ns | 0.68 | −0.07 ns | 0.38 ns | 0.39 ns | −0.09 ns | −0.74 * | |
| PH | −0.39 ns | 0.67 * | 1.00 | 0.85 * | 0.13 ns | 0.81 * | 0.70 * | 0.16 ns | 0.23 ns | −0.33 ns | −0.46 ns | −0.32 ns | |
| PD | −0.52 * | 0.64 * | 0.36 ns | 1.00 | −0.01 ns | 0.89 * | 0.63 * | 0.18 ns | 0.26 ns | −0.29 ns | −0.72 * | −0.13 ns | |
| LN | −0.10 ns | −0.01 ns | −0.51 * | 0.12 ns | 1.00 | 0.29 ns | 0.57 * | 0.36 ns | 0.35 ns | 0.71 * | 0.06 ns | −0.78 * | |
| RW | −0.42 ns | 0.13 ns | −0.35 ns | 0.29 ns | 0.84 * | 1.00 | 0.80 * | 0.40 ns | 0.43 ns | −0.07 ns | −0.73 * | −0.34 ns | |
| RL | −0.14 ns | 0.31 ns | 0.75 * | 0.20 ns | −0.85 * | −0.70 * | 1.00 | 0.49 * | 0.70 * | 0.27 ns | −0.54 * | −0.64 * | |
| RD | −0.57 * | 0.74 * | 0.70 * | 0.43 ns | −0.28 ns | 0.03 ns | 0.51 * | 1.00 | 0.66 * | 0.08 ns | −0.42 ns | −0.32 ns | |
| SD | −0.54 * | 0.71 * | 0.74 * | 0.55 * | −0.47 ns | −0.22 ns | 0.72 * | 0.79 * | 1.00 | 0.34 ns | −0.49 * | −0.51 * | |
| DW | −0.55 * | 0.50 * | 0.70 * | 0.47 ns | −0.65 * | −0.32 ns | 0.77 * | 0.64 * | 0.81 * | 1.00 | 0.15 ns | −0.65 * | |
| MDA | 0.34 ns | −0.90 * | −0.59 * | −0.69 * | −0.12 ns | −0.16 ns | −0.19 ns | −0.51 * | −0.53 * | −0.37 ns | 1.00 | −0.06 ns | |
| Proline | 0.35 ns | −0.76 * | −0.88 * | −0.43 ns | 0.41 ns | 0.23 ns | −0.69 * | −0.71 * | −0.73 * | −0.71 * | 0.69 * | 1.00 | |
 Abbreviations: head weight (HW), plant height (PH), plant diameter (PD), leaf number (LN), root weight (RW), root length (RL), root diameter (RD), steam diameter (SD), dry weight (DW), malondialdehyde (MDA) content.
Abbreviations: head weight (HW), plant height (PH), plant diameter (PD), leaf number (LN), root weight (RW), root length (RL), root diameter (RD), steam diameter (SD), dry weight (DW), malondialdehyde (MDA) content.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kojić, A.; Marković, M.; Marček, T.; Velić, N.; Lojková, L.; Atilgan, A.; Japundžić-Palenkić, B. The Benefit of Mycorrhizal Fungi and Beneficial Soil Bacteria in Drought Exposed Lettuce (Lactuca sativa var. capitata) Is Genotype and Environment Dependent. Appl. Sci. 2023, 13, 12117. https://doi.org/10.3390/app132212117
Kojić A, Marković M, Marček T, Velić N, Lojková L, Atilgan A, Japundžić-Palenkić B. The Benefit of Mycorrhizal Fungi and Beneficial Soil Bacteria in Drought Exposed Lettuce (Lactuca sativa var. capitata) Is Genotype and Environment Dependent. Applied Sciences. 2023; 13(22):12117. https://doi.org/10.3390/app132212117
Chicago/Turabian StyleKojić, Antonija, Monika Marković, Tihana Marček, Natalija Velić, Lea Lojková, Atilgan Atilgan, and Božica Japundžić-Palenkić. 2023. "The Benefit of Mycorrhizal Fungi and Beneficial Soil Bacteria in Drought Exposed Lettuce (Lactuca sativa var. capitata) Is Genotype and Environment Dependent" Applied Sciences 13, no. 22: 12117. https://doi.org/10.3390/app132212117
APA StyleKojić, A., Marković, M., Marček, T., Velić, N., Lojková, L., Atilgan, A., & Japundžić-Palenkić, B. (2023). The Benefit of Mycorrhizal Fungi and Beneficial Soil Bacteria in Drought Exposed Lettuce (Lactuca sativa var. capitata) Is Genotype and Environment Dependent. Applied Sciences, 13(22), 12117. https://doi.org/10.3390/app132212117













