Abstract
Morphological and biochemical responses were assessed in an iceberg (GIL) and butterhead (GBL) lettuce (Lactuca sativa var. capitata) treated with commercially available soluble preparation of mycorrhizal fungi and beneficial soil bacteria (MT) grown in three irrigation treatments considered in greenhouse (continental Croatia, 2022): I100—control treatment (100% volumetric water content—VWC); I80—80% VWC, moderate drought and I60—60% VWC, severe drought), in two growing cycles. MT was applied during lettuce drought-sensitive stages, i.e., transplanting and heading. Study results show that MT improved lettuce growth-related traits, yet the results are genotype and growing-cycle dependent. The beneficial effect of MT was also noted for root length, weight, and diameter which confirms the mycorrhizal role in improving the plant water uptake. Both lettuce genotypes responded to water deficit by overproduction of MDA and proline content, whereby the response of tested variables was growing cycle and genotype-specific. Both genotypes in severe drought treatment (MT-I60) responded with higher MDA in the first growing cycle and lower MDA content in the second growing cycle. MT-I60 treatment reduced proline accumulation in GBH in both growing cycles, while increased accumulation in GIL during the second growing cycle. The responses of lettuce to MT are genotype-specific and shaped by environmental conditions.
1. Introduction
Drought is one of the major abiotic stresses that cause significant reductions in crop yield and quality [1,2] and is expected to be more severe since the occurrence of drought is increased due to alteration in the evapotranspiration (ET) as well as unfavorable rainfall distribution caused by global warming [3,4]. Several study results have shown that the negative consequences of drought can be overcome by adaptation of different strategies to improve the climate resilience of agriculture, i.e., sowing time [5,6,7], tillage systems [8,9], irrigation [10,11,12], selection of crop and varieties [13,14] and use of beneficial soil bacteria and mycorrhizal fungi [15,16]. The latter was reported in the literature as a complementary measure that can mitigate the effects of biotic and abiotic stresses in crop production [16].
The literature review shows that mycorrhizal fungi can improve nutrient uptake [17,18,19] and increase the tolerance of crops against various abiotic stresses, i.e., high-temperature stress [20,21,22], salinity [23,24,25], and drought stress. The ability of mycorrhizal fungi to mitigate the negative effects of drought has been explored in prior studies by Cosme et al. [26], Zhang et al. [27], Li et al. [28], Boutaskint et al. [29], and Huang et al. [30].
An earlier observation was given by Hardie [31]. The author emphasizes that the major problem in investigating the role of fungus in plant water relations is to distinguish between indirect effects such as enhanced nutrition and growth (e.g., greater plant mass, reduced root:shoot ratio) and direct effects of fungal hyphae. Recent research showed that Mycorrhizal plants increase stomatal conductance [30,32,33,34,35], leaf area [30,32,36,37], transpiration rate [30], leaf water potential [38,39,40], net photosynthetic rate [30,37] and relative water content [37,41]. The development and effectiveness of mycorrhiza are closely related to the activity of beneficial soil bacteria, whereby the role is manifested in triggering various plant growth factors, leading to better availability of nutrients in the soil and uptake by plants [42].
In drought conditions, as well as other abiotic stress caused by environmental factors, plants produce antioxidants as a protector against oxidative damage. A series of recent studies have indicated higher superoxide dismutase (SOD) [30,37], peroxidase (POD) [30,37,43,44], and catalase (CAT) [30,37,44] in drought-stressed mycorrhizal plants, regardless of plant species. Furthermore, excessive production of reactive oxygen species (ROS) in stressed conditions causes malondialdehyde (MDA) accumulation, which leads to cell membrane damage and, ultimately, cell death [45]. As stated by Morales and Munné-Bosch [46] MDA is one of the final products of polyunsaturated fatty acid peroxidation in the cells and for this reason, it is a widely used and reliable marker for determining the degree of injury to a stressed plant. Previous studies have shown decreased MDA content, regardless of plant species subjected to drought stress and mycorrhizae [45,47,48,49]. Proline is an osmolyte that provides plants with an osmotic mechanism to maintain a favorable osmotic potential for water uptake, therefore, alleviating the injury of drought stress [49]. The impact of mycorrhizae on proline content subjected to drought has been explored in prior studies by Abbaspour et al. [43], Yooyongwech et al. [50], Wu et al. [51], Huang et al. [30], Yan et al. [37]. In general, proline content was decreased in drought-stressed mycorrhizal plants.
Studies on the negative impact of drought stress on lettuce yield and quality are well documented, it is also well acknowledged that the lettuce’s highest yield, leaf number, plant weight, height and diameter, root weight, length, and diameter were obtained on no stressed treatments, that is on treatment with the highest irrigation rate [52,53,54,55,56]. The beneficial effects of mycorrhizae on the growth-related traits and nutritional quality of lettuce plants were previously reported by many authors as well [57,58,59,60,61]. Rasouli et al. [62] noted that higher lettuce head diameter, head fresh and dry weight, and root fresh and dry weight were achieved by applying mycorrhizae.
While the literature regarding the beneficial effect of mycorrhizae on plant growth is quite large, the available information on the interaction of the mycorrhizal fungi and beneficial soil microorganisms in terms of crop production; i.e., overcoming abiotic stress is limited. For that reason, the complex interactions of the crop, mycorrhiza fungi, and beneficial microorganisms in the soil rhizosphere are nowadays considered a key role in overcoming abiotic stress. Furthermore, the synergistic relationship between mycorrhizae and beneficial microorganisms was the main focus in planning this research, since the mechanisms by which beneficial bacteria stimulate mycorrhizal effectiveness are still poorly understood.
Considering the increasing drought stress concerns upon crop productivity, this study aimed to investigate the effects of mycorrhizal treatment (MT) on the morphological and biochemical response of butterhead and iceberg lettuce exposed to drought treatments. We hypothesized that (1) MT would improve plant growth by affecting lettuce head weight (HW) and growth parameters (plant height (PH), plant diameter (PD), root length (RL), weight (RW), and diameter (RD) and leaf number (LN) in moderate and severe drought conditions; (2) MT would affect MDA and proline accumulation in moderate and severe drought conditions; and (3) the contribution of MT to drought resistance would be higher in severe drought treatment.
2. Materials and Methods
2.1. Study Site and Growth Condition
The study was conducted under greenhouse conditions in the continental region of Croatia (2022, N45°09′ E18°1′) in two growing cycles with the same study treatments and methods. The greenhouse area is 240 m2 (8 m × 30 m), positioned in a south-north direction with an automatized ventilation system. Lettuce seedlings (Lactuca sativa L.) were grown from conventional seeds in seedling containers made of styrofoam, dimensions 537 × 328 × 65 mm. Containers are disinfected before use. One seed was sown in each section, which enabled uniform germination and sprouting as well. In this part of the study, the plants were watered with the same amount of water to enable the initial growth to be as uniform as possible.
The containers were filled with commercially available substrate (Klasmann potgrond H, Klasmann-Deilmann GmbH, Geeste, Germany), a mixture of frozen black and fine white sphagnum peat with added water-soluble nutrients and microelements. Peat has a fine structure (≤7 mm) with a 6.0 pH reaction (H2O) and the following composition: 210 mg/L N, 150 mg/L P2O5, 270 mg/L K2O, 150 mg/L S, and 100 mg/L Mg. Total porosity (82 ± 2%) was measured indirectly via saturation during moisture release curve development. Two widespread types of lettuce (G), that is iceberg lettuce (GIL) and butterhead lettuce (GBL) were used in the study. Three-week-old seedlings were transplanted into growing pots with a capacity of 3 L, in the first growing cycle on 17 March, and in the second growing cycle on 28 April 2022. After transplanting, the plants in all treatments were watered with equal irrigation rates (0.29 L/pot) to enable good rooting and uniform initial growth. Lettuce was fertilized with organo-mineral foliar with following composition: N (6.6 g/L), P2O5 (13.3 g/L), K2O (13.3 g/L), Ca (2 g/L), Mg (4 g/L), Fe (17.2 g/L), Zn (26.5 g/L), Mn (13.3 g/L) and Cu (13.3 g/L). Lettuce was fertilized on 28 March and 11 and 25 April in the first growing cycle, and 20 April and 30 and 10 May in the second growing cycle. Lettuce protection was achieved by the application of pesticides. During the study, air temperature and humidity were recorded every ten minutes (Data logger, Axiomet AX-DT200).
The 3-factor study was set up in three replications with five plants within the treatment and with the following study factors: G—genotype: GIL = iceberg lettuce (Great lakes), GBL = butterhead lettuce (Trocadero Marta), I—irrigation rate: I100—control treatment (100% water holding capacity (WHC); I80—80% WHC and I60—60% WHC, and M—mycorrhizae: NM—control treatment (nonmycorrhizal, without mycorrhizae), MT—mycorrhizal treatment (added mycorrhizae). In total, there were 180 plants, or containers per cycle (360 in research). Two weeks after transplanting, the plants were watered according to predetermined irrigation treatments.
2.2. Irrigation and Mycorrhizal Treatment
The substrate water content (SWC) of the air-dried substrate (%) was determined by the gravimetric method since the air-dried substrate contains hygroscopic moisture, that is, a certain amount of water that should be considered when determining the irrigation rate. The SWC was determined by weighing 100 g of air-dried substrate, which constitutes the weight of the wet sample. The sample of air-dried substrate was dried in a dryer in a paper bag at 70 °C, i.e., a lower temperature than usual (105 °C) due to the high content of organic matter. After a constant weight is reached, the sample of the dry substrate is weighed again, which gives the weight of the dry sample. Afterward, the SWC is determined by the gravimetric method according to Lowery et al. [63]:
SWC = (Swet − Sdry)/Sdry × 100
The SWC represents substrate water content (%), Swet (g), and Sdry (g) is the weight of wet and dry substrate, respectively. The Hyprop (Hydraulic PROPerty analyzer, Decagon Devices, Inc., Pullman, WA, USA) instrument was used to develop a moisture release curve (Figure 1) that represents the relationship between the water potential and volumetric water content (%).
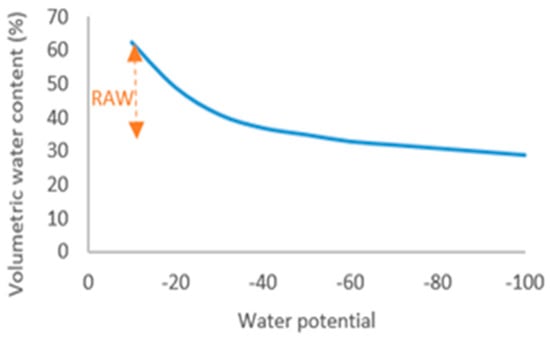
Figure 1.
Substrate moisture release curve.
The readily available water (RAW, 29.2%) or the amount of water that is easily extracted by the plants was determined as the difference between water retention capacity (−1 to −5 kPa, WRC, 62.3%) and refill point (−5 to −10 kPa, 33.1%). The irrigation rate corresponds to RAW which means that in this study, an irrigation rate of 0.29 L/pot is sufficient to fill the water content from the refill point to the WRC in the I100 irrigation treatment. Consequently, the irrigation rate on I80 and I60 irrigation treatments was 0.18 and 0.14 L/pot, respectively. The irrigation rates were calculated to meet irrigation thresholds. Plants were watered with water from a well of satisfactory quality, i.e., without restrictions on use (Table 1).

Table 1.
Results of chemical analysis of irrigation water.
The well is 3 m away from the greenhouse and 20 m deep. Before watering, the water was previously aged to settle larger particles and to heat the water to an optimal 20–25 °C. The irrigation time was monitored by the AT sensor (Delta-T Devices Ltd., Cambridge, Great Britain) with substrate moisture output as volumetric water content (VWC, %). VWC was calculated using a calibration curve provided by a manufacturer for soilless peat-based substrates. Accordingly, the thresholds for irrigation events were 45% (I100), 38% (I80), and 30% (I60). Each container was irrigated by hand so that minimum drainage water occurred.
The commercial mycorrhizal preparation used was Mykorrhiza Soluble (Tyroler Glückspilze, Innsbruck, Austria) and consisted of four species of endomycorrhizal fungi (Glomus intraradices, Glomus mosseae, Glomus aggregatum, and Glomus etunicatum), five species of ectomycorrhizal fungi (Pisolithus tinctorius, Rhizopogon villosulus, Rhizopogon luteolus, Rhizopogon fulvigleba, and Rhizopogon amylopogon), and beneficial microorganism (Bacillus subtilis, B. licheniformis, B. azotoformans, B. megaterium, B. coagulans, B. pumilus, B. thuringiensis, B. stearothermophilus, Paenibacillus polymyxa, P. durum, P. fluorescens, P. gordonae, Azotobacter polymyxa, A. chroococcum, Saccharomyces cerevisiae, Streptomyces griseus, S. lydicus, Pseudomonas aureofaciens, Deinococcus erythromyxa). The acting mechanism of beneficial soil microorganisms in this study is assessed by promoting the functionality of MT. The mycorrhizal treatment was carried out by adding 1 tablespoon in 7 L of irrigation water at the most sensitive stages of lettuce development, after transplanting and heading the growth stage (MT study treatment).
The estimation of the plant response to mycorrhizal colonization in terms of biomass enhancement was calculated for I60 and I80 irrigation treatment by using the following formula provided by Kumar et al. [64]:
where MD (%) is mycorrhizal dependency, DWm is the dry weight of the mycorrhizal plant, and DWnm is the dry weight of the non-mycorrhizal plant.
MD = (DWm − DWnm)/DWm × 100
2.3. Measurements of Morphological and Biochemical Attributes
The lettuce’s fresh head weight (HW, g), plant diameter (PD, cm), and plant height (PH, cm) were measured at harvest, that is 52 (first growing cycle) and 53 (second growing cycle) days after planting. Furthermore, root weight (RW, g), root diameter (RD, mm) and root length (RL, cm), steam diameter (SD, cm), and leaf number (LN, n) as well. At the same time, the fresh leaf samples were taken and placed in a freezer at −80 °C for determination of biochemical attributes. All measurements were taken on the same plant. For the proline and MDA determination, five leaves were taken from each group treatment, joined, and ground in liquid nitrogen using a pestle and mortar. Around 100 mg of fine powder was transferred into Eppendorf tubes. Each treatment had three repetitions.
The extent of membrane damage was verified by malondialdehyde (MDA) determination using thiobarbituric acid (TBA) reaction [65]. Leaves (100 mg) were homogenized in 1.2 mL of reaction solution [0.3% (w/v) (TBA) in 10% (w/v) trichloroacetic acid (TCA)] and heated at 85 °C for 45 min. The reaction was stopped by cooling in an ice bath for 20 min, and the mixture was centrifuged (20,000× g for 20 min). Absorbance was read from the supernatant at 532 nm and the corrections for the non-specific turbidity were made by taking away the absorbance at 600 nm. TBA in 10% TCA solution represented a blank. The concentration of MDA was expressed in micromols per gram of fresh weight [µmol g−1 FW] using an extinction coefficient of 155 mM−1 cm−1).
Leaf tissue (100 mg) was homogenized in 2 mL of 3% (w/v) sulfosalicylic acid and the supernatant was obtained by centrifugation at 15,000× g for 20 min. Acid ninhydrin and glacial acetic acid were joined to the supernatant and the reaction mixture was heated at 100 °C for 1 h. The reaction was interrupted by cooling in an ice bath. After the addition of toluene, the absorbance of free proline separated in the upper phase at 520 nm was recorded [66]. Proline concentration was determined using a calibration curve in the range of 25–300 µM and expressed as micromols per gram of fresh weight [µmol g−1 FW].
2.4. Data Analysis
For the data analyses, Statistica 14.0.0.15 (TIBCO Software Inc., Palo Alto, CA, USA) was used. The results were presented as the mean value ± standard deviation. The variability of the results was checked using factorial analysis of variance (ANOVA) and the interactions of genotype (G), irrigation (I), mycorrhizae (M), combined M × I, M × G, I × G, and M × I × G, respectively. Differences among the variables (M, I, G) were compared using Fisher’s least significant difference (LSD) test. To check the associations between variables, Spearman’s rank order coefficients were calculated for both growing cycles, for nonmycorrhizal and mycorrhizal groups, separately. Principal component analysis (PCA) was done to reveal the variations in morphometric and biochemical response (of two lettuce genotypes under different treatment combinations. Factor loadings were done to verify the proportion of total variance with different principal components (PCs). Values higher than (>0.71) were considered as strong [67].
3. Results
The average air temperature and air humidity during the first growing cycle were 22.6 °C and 76%, respectively, while the average air temperature during the second growing cycle was 1.3 °C higher compared to the first growing cycle. At the beginning of May, extremely high daily air temperatures occurred (38 °C) which affected the water regime, i.e., increased the plants’ water need in second growing season. Consequently, the net irrigation rate in the first growing cycle ranged from 0.7 L/pot (I60) to 1.15 L/pot (I100), and from 1.4 L/pot (I60) to 2.3 L/pot (I100) in the second growing cycle.
3.1. Morphometric and Biochemical Parameters in the First Growing Cycle
A separate analysis of variance revealed significant effects (p ≤ 0.05) of genotype (G: GIL, GBL), irrigation treatments (I: I100, I80, I60), and mycorrhizae (M: NM and MT) and their interactions for morphometric and biochemical parameters in the first growing cycle (Supplement Table S1). On average across treatments, uniform results were obtained for irrigation (I) and lettuce genotype (G) regardless of the growing cycle. The irrigation rate linearly increased all tested variables in both growing cycles, meaning that the highest values were recorded on the I100 treatment. The highest HW in both growing cycles was recorded for butterhead lettuce (GBL).
Significant interactions were noticed among the main effects of all study factors in most of the tested variables. Exceptions were HW, PH, PD, RW, and MDA (for MT); RD and DW (I × MT); HW, PH, SD, and MDA (for MT × G); PH and RD (I × G) while RD, DW, and proline showed non-significant interaction for G × I × M. Results revealed the reduction of lettuce growth with the increasing drought intensity in both genotypes within NM treatment. The negative impact was observed for the HW, PH, PD, LN, RW, RL, SD, and RD, respectively (Figure 2a–h).
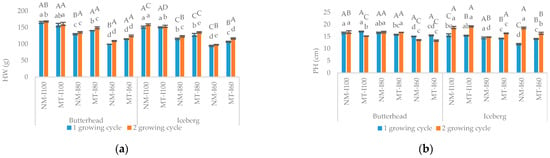
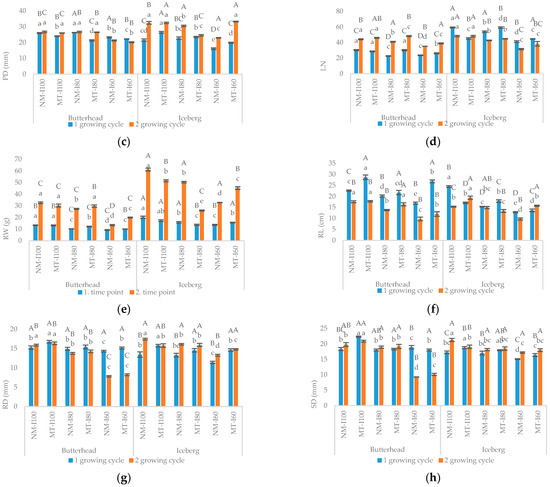
Figure 2.
The effect of mycorrhizal (NM—nonmycorrhizal, MT—mycorrhizal treatment) and irrigation (I100—control treatment (100% water holding capacity (WHC); I80—80% WHC and I60—60% WHC) treatments on head weight (HW, (a)), plant height (PH, (b)), plant diameter (PD, (c)), leaf number (LN, (d)), root weight (RW, (e)), root length (RL, (f)), rood diameter (RD, (g)) and steam diameter (SD, (h)). Upper case letters indicate significant differences between genotypes under same treatment, whilst lower case letters show differences between treatments within each genotype for different growing cycle (p ≤ 0.05, LDS test).
In iceberg lettuce, MT had a beneficial effect on PD while in butterhead PD values were significantly lower than in the non-mycorrhizal group (Figure 2c). In butterhead, mycorrhizal treatment promoted root growth parameters (RL and RD) but showed an inhibitory effect on RL in iceberg suggesting that the success of mycorrhizal application depends on genotype properties (Figure 2f,g). The inhibitory effect of drought on most of the morphometric parameters (HW, PH, PD, LN, RD, and SD) was also noticeable in an MT group (Figure 2a–h). The exception was the butterhead in which there were no differences in SD between control (I100) and drought stress groups (I80 and I60, Figure 2h). The MT under moderate water stress (I80) stimulated HW and LN in both lettuce genotypes (Figure 2a,d). The positive effect of MT at the severe drought treatment (I60) was noticed for HW, LN, and RD in both varieties (Figure 2a,d,g). Additionally, MT on I60 treatment improved PH and PD values in the iceberg and RL values in butterhead compared to nonmycorrhizal treatment (Figure 2a–c). On the other hand, butterhead showed better lateral growth (PD) in the nonmycorrhizal treatment (I60) than in the mycorrhizal treated group (Figure 2c). Opposite, in butterhead, the mycorrhizal treatment seemed to be more effective in root growth promotion (RL) (Figure 2f). On the other hand, at the same drought intensity (I80) butterhead lettuce was higher (PH) than iceberg plants meaning that mycorrhizal treatment in this variety induces stem elongation (Figure 2b). At the highest drought intensity (I60) the differences between varieties in mycorrhizal treatment were not detected. The response of GIL to mycorrhizal colonization, i.e., mycorrhizal dependence (MD) ranged from 0.76% (I80) to 11.89% (I60). The MD of GBH ranged from 7.17% (I80) to 10.23% (I60).
Mycorrhizal treatment also modified biochemical response. Both lettuce varieties responded to water deficit by overproduction of MDA and proline content (Figure 3a,b). The proline accumulation under medium drought stress (I80) was from 46% (in butterhead) to 200% (in iceberg) higher than under optimal conditions (I100) while severe drought stress (I60) resulted in 813% (butterhead) and 242% (iceberg) proline increase. Exposure to drought stress increased the proline (I80) and MDA content (I80 and I60) in an iceberg and MDA content in butterhead (I60) compared to the nonmycorrhizal group (NM) indicating that this may relate to abiotic stress impact on lettuce varieties. Compared to butterhead, the MT stimulated lateral growth of stem (PD) and MDA production in iceberg lettuce under water optimal conditions.
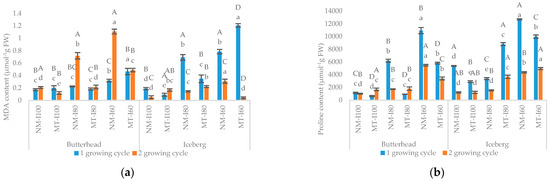
Figure 3.
The effect of mycorrhizal (NM—nonmycorrhizal, MT—mycorrhizal treatment) and irrigation (I100—control treatment (100% water holding capacity (WHC); I80—80% WHC and I60—60% WHC) treatments on MDA (a) and proline (b) content in butterhead and iceberg lettuce. Upper case letters indicate significant differences between genotypes under same treatment, whilst lower case letters show differences between treatments within each genotype for different growing cycle (p ≤ 0.05, LDS test).
The highest drought level (I60) caused the greatest membrane damage, especially in butterhead while iceberg variety at both drought levels (I80 and I60) showed increased MDA content compared to the control group (I100, Figure 2a). Similarly, proline accumulation in icebergs and butterheads significantly increased because of the water-limited conditions (Figure 2b). In water optimal conditions (I100), the MT decreases proline content compared to the NM group (Figure 2b).
Comparative analysis also showed a positive association between proline and MDA content in NM (r = 0.56) and MT (r = 0.69) groups (Table 2 and Table 3).

Table 2.
Spearman’s correlation coefficients among morphometric and biochemical parameters under nonmycorrhizal treatment in first and second growing cycles, respectively (* significant at 0.05 level, ns = not significant).

Table 3.
Spearman’s correlation coefficients among morphometric and biochemical parameters under mycorrhizal treatment in the first and second growing cycles, respectively (* significant at 0.05 level, ns = not significant).
3.2. Morphometric and Biochemical Parameters in the Second Growing Cycle
A separate analysis of variance revealed significant effects (p ≤ 0.05) of genotype (G: GIL, GBL), irrigation treatments (I: I100, I80, I60), and mycorrhizae (M: NM and MT) and their interactions for morphometric and biochemical parameters in the second growing cycle (Supplement Table S2). Mycorrhizal treatment had a significant impact on HW, PH, RW, RL, DW, MDA, and proline, respectively. Irrigation treatment as well as I × G interaction affected all tested variables. The genotype effect was significant for most of the variables (except RL). On the other hand, M × I interactions were noticed for HW, PH, RL, LN, RW, RD, MDA, and proline, while the significant effects for M × G were found for HW, PD, RW, SD, MDA, and proline, respectively. Finally, the impact of M × I × G was visible for PH, LN, RW, RL, RD, SD, MDA, and proline, respectively.
In the second growing cycle the reduction of growth (HW, PH, PD, LN, RW, RL, and RD), was noticed in non-mycorrhizal treatment for iceberg and butterhead lettuce with the increasing drought level (Figure 2a–h). Furthermore, MDA and proline content showed a tendency to rise under water deficit (I80 and I60) compared to control (Figure 3a,b). A comparative analysis also confirmed a positive association (r = 0.74) between proline and MDA content in the non-mycorrhizal treated group (Table 2 and Table 3).
Under optimal water conditions (I100), the root length (RL) of the iceberg was higher in the mycorrhizal group compared to the non-mycorrhizal group while in the butterhead the positive effect was notable for the aboveground growth parameters (Figure 2e,h). The application of beneficial microorganisms under medium drought level (I80) increased HW (Figure 2a), RW (Figure 2e), and RL (Figure 2f) in butterhead (Figure 2d). At the same drought treatment (I80), mycorrhizal treated icebergʾs plants were higher (PH) than untreated although showed root growth inhibition (RW, RL), and decreased plant lateral growth (PD) (Figure 2c). A comparative analysis for nonmycorrhizal treatment showed a negative connection (p < 0.05) between irrigation treatment and LN, RW, and RL, respectively (Table 2). Interestingly, exposure to mycorrhizal treatment under the highest drought intensity (I80) increased RW, and RL in both lettuce varieties compared to I60 suggesting the biostimulatory role of mycorrhizal treatment (Figure 2a,f). In the second growing season, the MD of GIL ranged from 0.47% (I80) to 8.10% (I60), while the MD of GBH ranged from 0.53% (I80) to 5.96% (I60).
Proline accumulation in iceberg was significantly higher in the mycorrhizal treated group than in the untreated under both drought levels (I80 and I60) while in untreated mycorrhizal butterhead plants, proline biosynthesis under severe drought stress (I60) was 61% higher than in mycorrhizal group (Figure 3b). The mycorrhizal application resulted in lower membrane damage in both iceberg and butterhead plants under both water scarcity treatments (Figure 3a). MDA content in the non-mycorrhizal group (I60) was 126% (butterhead) and 660% (iceberg) higher compared to mycorrhizal treated group. Correlation analysis revealed a strong negative correlation (p < 0.05) between proline content and most growth parameters (HW, PD, PH, RL, RD, SD, LN) in non-mycorrhizal and mycorrhizal treated groups in both growing cycles (Table 2 and Table 3). Comparing the impact of mycorrhizal treatment between lettuce genotypes, the application of mycorrhizal mixture promoted icebergʾs root growth (RL) and proline accumulation in both irrigation treatments (I80 and I60) compared to butterhead. On the other hand, butterhead lettuce had a higher stem lateral growth (SD) underwater optimal conditions (I100) and larger root weight (RW) than iceberg under medium drought stress (I80) (Figure 2e,h). In general, mycorrhizal dependency of both lettuce genotypes increased as water stress levels increased. In both growing cycles, the highest MD (17.4% and 31.3%) was recorded for GIL on severe drought treatment (MT-GIL-I60).
3.3. PCA Analysis
PCA analysis for lettuce varieties was done separately for both lettuce varieties and both growing cycles (Figure 4 and Figure 5). The total variation characterized by PC1 and PC2 explains 73.47% of the variation for the iceberg and 81.37% for the butterhead, respectively. In the iceberg, six clusters were notable (Figure 4). According to the loading cases, mycorrhizal treatment (MT) promoted dry weight (DW1, DW2), stem diameter (SD1), and plant diameter (PD1) (Cluster 1) while the untreated group (NM) showed improved SD2, HW2, and RW2 values at the second growing cycle (Cluster 2) (Supplement Table S3). Moderate drought level (I80) caused the separation of cluster 3 (80) and cluster 4 (G-I80) based on morphological and biochemical parameters. Mycorrhizal treatment promoted root length (RL), MDA, and proline accumulation in the second growing cycle. Unlike cluster 4, group 3 showed higher values of head weight (HW2), plant height (PH1), plant diameter (PD2), leaf number (LN1, LN2), and root weight (RW1). The most intense drought (I80) split two treatment categories on cluster 5 (60) and cluster 6 (G-I60) according to different biochemical responses (MDA and proline content) in the first and second growing cycles. In butterhead, the score plot also distinguished six groups (Figure 5). The first cluster denotes control (C) which is separated from the second cluster (G) based on lower stem diameter (SD1, SD2), root diameter (RD1), head weight (HW2), and root length (RL1), respectively. Mycorrhizal treated plants grown under medium drought (G-I80) were clustered (cluster 4) according to higher head weight (HW2), leaf number (LN1), root weight (RW1, RW2), and root length (RL2) compared to non-mycorrhizal group (80) (cluster 3) (Supplement Table S4). Finally, mycorrhizal application at the highest drought level (I60, G-60) reduced proline accumulation in both time points (proline 1, proline 2).
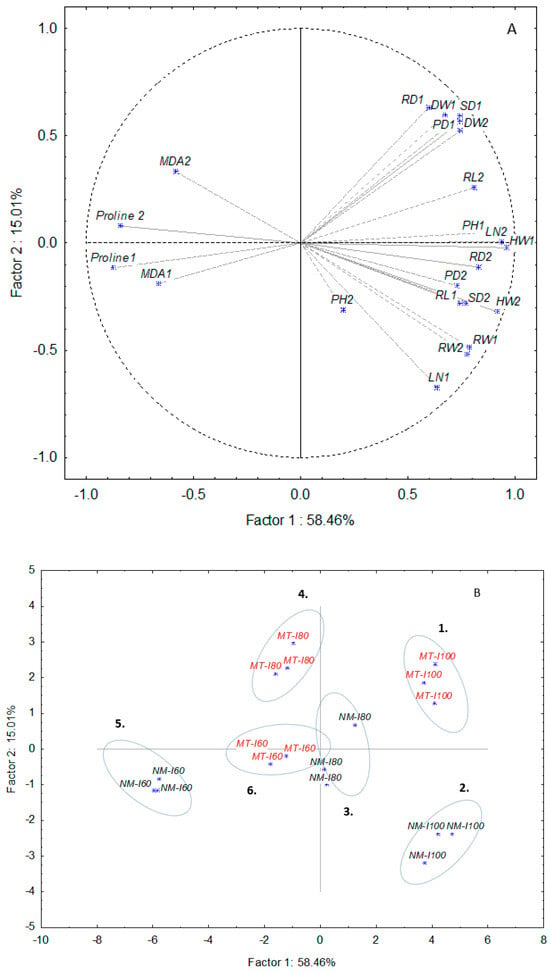
Figure 4.
Principal component analysis of data sets of morphological and biochemical parameters for iceberg in non-mycorrhizal and mycorrhizal (MT) treatments under control (C) and drought (60, 80) conditions. Head weight (HW), plant height (PH), plant diameter (PD), leaf number (LN), root weight (RW), root length (RL), root diameter (RD), stem diameter (SD), dry weight (DW), malondialdehyde (MDA), and proline. Factor loadings (A) and scores (B) of the first two factors. The number beside each variable represents the day of treatment. Black letters present non-mycorrhizal treatment and red marks mycorrhizal treatment.
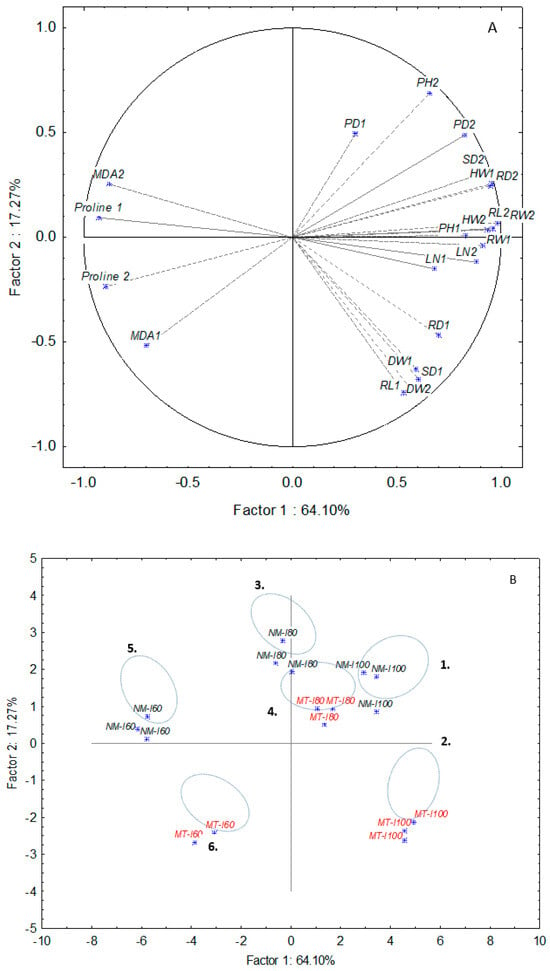
Figure 5.
Principal component analysis of data sets of morphological and biochemical parameters for butterhead in non-mycorrhizal and mycorrhizal (MT) treatments under control (C) and drought (60, 80) conditions. Head weight (HW), plant height (PH), plant diameter (PD), leaf number (LN), root weight (RW), root length (RL), root diameter (RD), stem diameter (SD), dry weight (DW), malondialdehyde (MDA), and proline. Factor loadings (A) and scores (B) of the first two factors. The number beside each variable represents the day of treatment. Black letters present non-mycorrhizal treatment and red marks mycorrhizal treatment.
4. Discussion
In line with previous studies [52,53,54,55,56] drought treatments (I80 and I60) reduced lettuce head weight (HW) as well as growth parameters (PH, PD, RL, RD, RW, and SD), regardless of the lettuce genotype and growing cycles. On average across treatments, the highest stability was shown by the butterhead genotype (G-BL) with the highest HW in both growing cycles. This is an important finding in the understanding of the genetic advance for the most important yield-forming growth parameter, and the beneficial effect of MT on crop growth. The genetic dependence of different lettuce cultivars has also been shown to be significant in many previous studies regardless of the study factors, such as irrigation method [68,69], fertilizers management [70], and nutrient solution [68]. Similar results are obtained for the MT × I × G effect, whereby in severe drought treatment (MT-GBL-I60), better response to MT showed butterhead lettuce in 1st growing cycle, i.e., the highest HW, PH, RL, RD, and SD. In the second growing cycle, on severe water stress treatment (I60) the highest HW, PH, HD, RW, RL, RD, and SD were recorded for iceberg lettuce (MT-GIL-I60). Gholamhoseini et al. [71] claim that MT can effectively improve plant productivity under various water deficit stresses. In this study, the results showed the beneficial effect of MT on lettuce grown in moderate (I80) and severe (I60) drought stress, whereby a more pronounced HW increase in iceberg lettuce was recorded in a second growing cycle; i.e., for 41% (MT-I60) and 31% higher (MT-I60) compared to control treatment (NM-GIL-I100).
As previously stated by Bengough et al. [72] plants with deeper root systems extract water from deeper soil layers and help the plants to avoid drought stress. Therefore, drought stress reduces root length [73,74,75] which is also confirmed in this study (Figure 2f) where the beneficial effect of MT on root growth was shown in both drought treatments (I80, I60). On average, the root elongation (RL) ranged from 11.7% (MT-I80) to 35.7% (MT-I60), while the RD increase ranged from 6.2% (MT-I80) to 11.5% (MT-I60) compared to NM treatment. Therefore, the more pronounced effect of MT is on severe drought treatments. This result ties well with previous studies wherein the symbiotic relationship with mycorrhizae may improve root size and efficiency, under drought-exposed crops [76,77]. We showed that MT increases drought resistance by providing lettuce with increased water uptake. The improved drought resistance comes because of symbiotic relations due to which developed mycorrhizal mycelia proliferate deeper and form a network specialized in the acquisition of water [78]. Also, the more pronounced effect of MT on severe drought treatments was for RW, whereby an increase in RW (41%) is visible only in the severe drought treatment (MT-I60), compared to the NM treatment (NM-I60). In moderate water stress (MT-I80) and optimal water treatment (MT-I100), mycorrhizae reduced RW. The same conclusion was reached by Badvi et al. [79] who reported that the application of mycorrhizal fungal inoculum was significant on all lettuce traits, except for root fresh weight. The higher root system attributes (RL, RW, and RD); i.e., the larger root system confirms that plants in MT have better adaptation in drought-stressed conditions, while the differences in lettuce genotypes point to better genetic potential in adaptation to abiotic stress; in this case the adaptation to drought, and heat stress for GIL. In this study, MD is more pronounced in treatments with increased water stress (I60), regardless of a growing cycle or lettuce genotype, aiming that both genotypes benefited from MT. However, in both growing cycles GIL showed higher mycorrhizal response. The limited number of published research results on MD in lettuce grown in different drought treatments indicates the need for further research. Furthermore, some research results are contradictory to ours. For example, Ruiz-Lozano et al. [80] state that the highest MD was recorded in treatments with a larger amount of irrigation water.
Also, the results of previous studies have shown that MT has a positive impact on plant-water relationships, with a beneficial effect in growing of drought exposed tomato [80], sesame [81], basil [82], orange [51], wheat [83], and lettuce [84]. Regardless of tested plant species, authors have shown that MT alleviates the negative effects of induced drought, i.e., higher proline accumulation in the drought-stressed plants, suggesting that proline accumulation is of great importance in plant responses to drought stress [80,85,86,87]. When comparing our results to those studies, it must be pointed out that the results are genotype-dependent. Those differences are related to the genetic potential of lettuce cultivars to stress tolerance; that is the enhanced proline accumulation in the drought-sensitive genotype (IG). As far as butterhead lettuce is concerned, the results are in accordance with the aforementioned studies, i.e., the accumulation of proline in drought treatments (MT-I80 and MT-I60) is lower in MT compared to NM treatment. In this study, moderate drought (I80) in iceberg lettuce triggered the overaccumulation of proline compared to butterhead, indicating that MT promotes the activation of antioxidative routes in the iceberg (Figure 3b). Duangpan et al. [88] stated that proline accumulation in response to drought stress in oil palm was genotype-dependent and drought-preconditioning enhanced proline accumulation in the sensitive genotype, but not in the tolerant genotype. The statement is confirmed by research on peanuts, in which the author stated that the stress tolerance in peanuts is genotype-dependent [89]. Similarly, to the findings of Yavuz et al. [90] who reported that MDA content was irrigation, i.e., drought level and harvest time-dependent, we found significant differences in irrigation treatment in both growing cycles, yet the results are not uniform. In the first growing cycle, the MDA reduction was noted on moderate stress treatment (MT-I80), while MDA incensement was noted on severe drought treatment (MT-I60). In the second growing season, only MDA incensement was noted on mild water stress (GIL-MT-I80). The study conducted on cucumber with moderate (80–85% Field Capacity, FC) and severe drought stress (60–65% FC), shows the increased MDA content in both drought treatments [91]. Another study conducted on lettuce confirmed MDA increasing in response to increasing drought stress levels [92], although it should be emphasized that more intensive drought treatments (50% and 20% of FC) were applied in the research compared to our study.
As it seems, the ability of mycorrhizal fungi to promote drought resistance appears to be limited to plant genotype, that is, genotypic specificity in terms of drought tolerance level, but also other abiotic factors. The differences in this study obtained in the first and second growing cycles are probably caused by extremely high air temperatures during the second growing cycle. Iceberg lettuce variety (Great Lakes) clearly showed better adaptability and tolerance to extremely high air temperatures, i.e., heat stress that occurred at the beginning of May (lettuce maturation phase). This statement is based on the results of present research, which clearly indicate that a higher RW is recorded for IG in heat stress conditions regardless of the MT or I treatment (Figure 2e). This indicates that RW can be considered as a main root growth attribute that increases a plant’s ability to tolerate high air temperatures. Current studies do not provide a sufficiently detailed insight into the importance of heat stress when growing lettuce, but studies in this context are more related to combined water stress in relation to heat stress.
As for MDA accumulation in heat-stressed lettuce, a previous study by Han et al. [93] revealed that high heat-resistant lettuce varieties were lower in MDA content than the low heat-resistant varieties. A similar pattern of results was obtained in this study (Figure 3a) since the lower MDA content in the second growing season was recorded in iceberg lettuce, especially on NM drought treatments (I80 and I60). The same author claims that the proline content and growth were higher in heat-resistant varieties which is in accordance with our study results (Figure 3b).
The main challenge in using commercial preparations is to find the most suitable preparation, i.e., mycorrhizae and beneficial microorganisms’ combination for the target crop and production system. Given that the results of the research depend considerably on the growing cycle under controlled conditions, further research is needed to assess the effectiveness of the preparation used in open field conditions, as it can be assumed that the effectiveness of the preparation will depend on several agroecological, i.e., environmental factors.
5. Conclusions
The tested morphological and biochemical traits of lettuce genotypes considerably varied across mycorrhizal and drought treatments. As expected, drought stress reduced lettuce growth-related parameters regardless of genotype or growing cycles. The differences in the mycorrhizal treatment efficiency between the first and second growing cycles are because of differences in environmental conditions, where the influence of heat stress in the second growing cycle stands out. The increased cell membrane damage (MDA, µmol g−1 FW) was noticed for both lettuce genotypes in the first growing cycle; 0.32 (NM-I60) to 0.47 (MT-I60) for butterhead lettuce and 0.79 (NM-I60) to 1.21 (MT-I60) for iceberg lettuce. In heat-stressed conditions the MDA content was reduced in both lettuce genotypes; 1.12 (NM-I60) to 0.49 (MT-I60) for butterhead lettuce and 0.31 (NM-I60) to 0.04 (MT-I60). The results are consistent with the proline content in the first growing cycle, where a reduced content was recorded in MT for both genotypes; 5492 (NM-I60) to 3413 (MT-I60) for butterhead lettuce and 12,705 (NM-I60) to 9991 (MT-I60) for iceberg lettuce. Lettuce genotypes reacted differently to heat stress in the second growing cycle whereby the proline content (µmol g−1 FW) in butterhead lettuce was reduced in MT; 5492 (NM-I60) to 3413 (MT-I60) and increased in iceberg lettuce; 4385 (NM-I60) to 4963 (MT-I60). Further studies are needed to test different heat stress in combination with drought treatments to receive more precise insights into the beneficial effects of mycorrhizae in different environmental conditions and varieties of lettuce genotypes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app132212117/s1, Table S1: Mean squares and significant test after analysis of variance for morphological and biochemical traits of two lettuce genotypes evaluated across two pretreatments and three water regimes for the first growing cycle; Table S2: Mean squares and significant test after analysis of variance for morphological and biochemical traits of two lettuce genotypes evaluated across two pretreatments and three water regimes for the second growing cycle; Table S3: Factor loadings of morphological and biochemical parameters for iceberg in non-mycorrhizal and mycorrhizal (G) treatments under control (C) and drought (I60, I80) conditions. Head weight (HW), plant height (PH), plant diameter (PD), leaf number (LN), root weight (RW), root length (RL), root diameter (RD), stem diameter (SD), dry weight (DW), malondialdehyde (MDA), and proline. The number beside each variable represents the growing cycle; Table S4: Factor loadings of morphological and biochemical parameters for butterhead in non-mycorrhizal and mycorrhizal treatments (G) under control (C) and drought (60, 80) conditions. Head weight (HW), plant height (PH), plant diameter (PD), leaf number (LN), root weight (RW), root length (RL), root diameter (RD), stem diameter (SD), dry weight (DW), malondialdehyde (MDA), and proline. Number beside each variable represents the time point.
Author Contributions
Conceptualization, N.V. and M.M.; methodology, M.M., A.K. and B.J.-P.; formal analysis, T.M., L.L., A.A. and B.J.-P.; investigation, M.M. and B.J.-P.; data curation, T.M.; writing—original draft preparation, M.M. and T.M.; writing—review and editing, M.M. and A.K.; visualization, T.M. and A.A.; supervision, N.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Mushroom Production Center GmbH, Karmelitergasse 21, 6020 Innsbruck, Austria (www.gluckspilze.com accessed on 1 November 2023), which kindly provided the soluble mycorrhizal preparation used for this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huey, C.J.; Gopinath, S.C.B.; Uda, M.N.A.; Zulhaimi, H.I.; Jaafar, M.N.; Kasim, F.H.; Yaakub, A.R.W. Mycorrhiza: A natural resource assists plant growth under varied soil conditions. 3 Biotech 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Shaffique, S.; Khan, M.A.; Imran, M.; Kang, S.M.; Park, J.S.; Wani, S.H.; Lee, I.J. Research Progress in the Field of Microbial Mitigation of Drought Stress in Plants. Front. Plant Sci. 2022, 13, 870626. [Google Scholar] [CrossRef] [PubMed]
- Dai, A. Drought under global warming: A review. Wiley Interdiscip. Rev. Clim. Chang. 2011, 2, 45–65. [Google Scholar] [CrossRef]
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate change impact and adaptation for wheat protein. Glob. Chang. Biol. 2018, 25, 155–173. [Google Scholar] [CrossRef]
- Marcinkowski, P.; Piniewski, M. Effect of climate change on sowing and harvest dates of spring barley and maize in Poland. Int. Agrophys. 2018, 32, 265–271. [Google Scholar] [CrossRef]
- Xu, F.; Wang, B.; He, C.; Liu, D.L.; Feng, P.; Yao, N.; Zhang, R.; Xu, S.; Xue, J.; Feng, H.; et al. Optimizing Sowing Date and Planting Density Can Mitigate the Impacts of Future Climate on Maize Yield: A Case Study in the Guanzhong Plain of China. Agronomy 2021, 11, 1452. [Google Scholar] [CrossRef]
- Chhokar, R.S.; Sharma, R.K.; Kumar, N.; Singh, R.K.; Singh, G.P. Advancing Sowing Time and Conservation Tillage—The Climate-Resilient Approach to Enhance the Productivity and Profitability of Wheat. Int. J. Plant Prod. 2023, 17, 121–131. [Google Scholar] [CrossRef]
- Baye, K.N.; Melash, A.A.; Bogale, A.A. Role of Conservation Tillage as Climate Change Mitigation. Civ. Environ. Res. 2019, 11, 12–23. [Google Scholar]
- Sadiq, M.; Li, G.; Rahim, N.; Tahir, M.M. Sustainable Conservation Tillage Technique for Improving Soil Health by Enhancing Soil Physicochemical Quality Indicators under Wheat Mono-Cropping System Conditions. Sustainability 2021, 13, 8177. [Google Scholar] [CrossRef]
- Dalias, P.; Christou, A.; Neocleous, D. Adjustment of Irrigation Schedules as a Strategy to Mitigate Climate Change Impacts on Agriculture in Cyprus. Agriculture 2019, 9, 4. [Google Scholar] [CrossRef]
- El-Nashar, W.; Elyamany, A. Adapting Irrigation Strategies to Mitigate Climate Change Impacts. A Value Engineering Approach. Water Resour. Manag. 2022, 37, 2369–2386. [Google Scholar] [CrossRef]
- Rosa, L. Adapting agriculture to climate change via sustainable irrigation: Biophysical potentials and feedbacks. Environ. Res. Lett. 2022, 17, 063008. [Google Scholar] [CrossRef]
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Nat. Clim. Chang. 2015, 5, 143–147. [Google Scholar] [CrossRef]
- Van Etten, J.; de Sousa, K.; Aguilar, A.; Steinke, J. Crop variety management for climate adaptation supported by citizen science. PNAS 2019, 116, 4194–4199. [Google Scholar] [CrossRef]
- Poudel, M.; Mendes, R.; Costa, L.A.S.; Bueno, C.G.; Meng, Y.; Folimonova, S.Y.; Garrett, K.A.; Martins, S.J. The Role of Plant-Associated Bacteria, Fungi, and Viruses in Drought Stress Mitigation. Front. Microbiol. 2021, 12, 743512. [Google Scholar] [CrossRef]
- Loo, W.T.; Chua, K.-O.; Mazumdar, P.; Cheng, A.; Osman, N.; Harikrishna, J.A. Arbuscular Mycorrhizal Symbiosis: A Strategy for Mitigating the Impacts of Climate Change on Tropical Legume Crops. Plants 2022, 11, 2875. [Google Scholar] [CrossRef]
- Douds, D.; Nagahashi, G.; Reider, C.; Hepperly, P. Inoculation with arbuscular mycorrhizal fungi increases the yield of potatoes in a high P soil. Biol. Agric. Hortic. 2007, 25, 67–78. [Google Scholar] [CrossRef]
- Almagrabi, O.; Abdelmoneim, T. Using of arbuscular mycorrhizal fungi to reduce the deficiency effect of phosphorous fertilization on maize plants (Zea mays L.). Life Sci. J. 2012, 9, 1648–1654. [Google Scholar]
- Sitoe, S.N.D.M.; Dames, J.F. Mitigating Climate Change: The Influence of Arbuscular Mycorrhizal Fungi on Maize Production and Food Security. In Arbuscular Mycorrhizal Fungi in Agriculture-New Insights; De Sousa, R.N., Ed.; IntechOpen: London, UK, 2022. [Google Scholar]
- Duc, N.H.; Csintalan, Z.; Posta, K. Arbuscular mycorrhizal fungi mitigate negative effects of combined drought and heat stress on tomato plants. Plant Physiol Biochem. 2018, 132, 297–307. [Google Scholar] [CrossRef]
- Cui, N.; Shi, L.; Guo, J.; Zhang, T. Arbuscular mycorrhizal fungi alleviate elevated temperature and nitrogen deposition- induced warming potential by reducing soil N2O emissions in a temperate meadow. Ecol. Indic. 2021, 131, 108193. [Google Scholar] [CrossRef]
- Mathur, S.; Agnihotri, R.; Sharma, M.P.; Reddy, V.R.; Jajoo, A. Effect of High-Temperature Stress on Plant Physiological Traits and Mycorrhizal Symbiosis in Maize Plants. J. Fungi 2021, 7, 867. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, G.; Shagol, C.C.; Kim, K.; Han, S.; Sa, T. Spore associated bacteria regulates maize root K+/Na+ ion homeostasis to promote salinity tolerance during arbuscular mycorrhizal symbiosis. BMC Plant Biol. 2018, 18, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, C.; Lu, T.; Zheng, Y. Cooperation between arbuscular mycorrhizal fungi and earthworms promotes the physiological adaptation of maize under a high salt stress. Plant and Soil. 2018, 423, 125–140. [Google Scholar] [CrossRef]
- Dastogeer, K.M.G.; Zahan, M.I.; Tahjib-Ul-Arif, M.; Akter, M.A.; Okazaki, S. Plant Salinity Tolerance Conferred by Arbuscular Mycorrhizal Fungi and Associated Mechanisms: A Meta-Analysis. Front. Plant Sci. 2022, 11, 1927. [Google Scholar] [CrossRef]
- Cosme, M.; Fernández, I.; Van der Heijden, M.G.; Pieterse, C.M. Non-mycorrhizal plants: The exceptions that prove the rule. Trends Plant Sci. 2018, 23, 577–587. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, Y.N.; Wu, Q.S. Quantitative estimation of water uptake by mycorrhizal extraradical hyphae in citrus under drought stress. Sci. Hortic. 2018, 229, 132–136. [Google Scholar] [CrossRef]
- Li, J.; Meng, B.; Chai, H.; Yang, X.; Song, W.; Li, S.; Lu, A.; Zhang, T.; Sun, W. Arbuscular Mycorrhizal Fungi Alleviate Drought Stress in C3 (Leymus chinensis) and C4 (Hemarthria altissima) Grasses via Altering Antioxidant Enzyme Activities and Photosynthesis. Front. Plant Sci. 2019, 10, 499. [Google Scholar] [CrossRef]
- Boutasknit, A.; Baslam, M.; Ait-El-Mokhtar, M.; Anli, M.; Ben-Laouane, R.; Douira, A.; El Modafar, C.; Mitsui, T.; Wahbi, S.; Meddich, A. Arbuscular Mycorrhizal Fungi Mediate Drought Tolerance and Recovery in Two Contrasting Carob (Ceratonia siliqua L.) Ecotypes by Regulating Stomatal, Water Relations, and (In)Organic Adjustments. Plants 2020, 9, 80. [Google Scholar] [CrossRef]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef]
- Hardie, K. The effect of removal the extraradical hyphae on water uptake by vesicular-arbuscular mycorrhyzal plant. New Phytol. 1985, 101, 677–684. [Google Scholar] [CrossRef]
- Birhane, E.; Sterck, F.J.; Fetene, M.; Bongers, F.; Kuyper, T.W. Arbuscular mycorrhizal fungi enhance photosynthesis, water use efficiency, and growth of frankincense seedlings under pulsed water availability conditions. Oecologia 2012, 169, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Augé, R.M.; Toler, H.D.; Saxton, A.M. Arbuscular mycorrhizal symbiosis alters stomatal conductance of host plants more under drought than under amply watered conditions: A meta-analysis. Mycorrhiza 2015, 25, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Watts-Williams, S.J.; Cavagnaro, T.R.; Tyerman, S.D. Variable effects of arbuscular mycorrhizal fungal inoculation on physiological and molecular measures of root and stomatal conductance of diverse Medicago truncatula accessions. Plant Cell Environ. 2018, 42, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Saboor, A.; ArifAli, M.; Danish, S.; Ahmed, N.; Fahad, S.; Datta, R.; Ansari, M.J.; Nasif, O.; Rahman, M.R.; Glick, B.R. Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-defcient soils. Sci. Rep. 2021, 11, 18468. [Google Scholar] [CrossRef] [PubMed]
- Beltrano, J.; Ruscitti, M.; Arango, M.C.; Ronco, M. Effects of arbuscular mycorrhiza inoculation on plant growth, biological and physiological parameters and mineral nutrition in pepper grown under different salinity and p levels. J. Soil Sci. Plant Nutr. 2013, 13, 123–141. [Google Scholar] [CrossRef]
- Yan, Q.; Li, S.; Xiao, X.; Chen, J.; Liu, J.; Lin, C.; Guan, R.; Wang, D. Arbuscular mycorrhizal fungi improve the growth and drought tolerance of Cinnamomum migao by enhancing physio-biochemical responses. Ecol. Evol. 2022, 12, e9091. [Google Scholar] [CrossRef]
- Zhu, X.C.; Song, F.B.; Liu, S.Q.; Liu, T.D.; Zou, X. Arbuscular mycorrhizae improves photosynthesis and water status of Zea mays L. under drought stress. Plant Soil Environ. 2012, 58, 186–191. [Google Scholar] [CrossRef]
- Zhu, X.; Cao, Q.; Sun, L.; Yang, X.; Yang, W.; Zhang, H. Stomatal Conductance and Morphology of Arbuscular Mycorrhizal Wheat Plants Response to Elevated CO2 and NaCl Stress. Front. Plant Sci. 2018, 19, 1363. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.N.; Qin, Q.Y.; Ma, W.Y.; Zhou, L.J.; Wu, Q.S.; Xu, Y.J.; Kuča, K.; Hashem, A.; Al-Arjani, A.B.F.; Almutairi, K.F.; et al. Metabolomics reveals arbuscular mycorrhizal fungi-mediated tolerance of walnut to soil drought. BMC Plant Biol. 2023, 23, 118. [Google Scholar] [CrossRef]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef]
- Sangwan, S.; Prasanna, R. Mycorrhizae Helper Bacteria: Unlocking Their Potential as Bioenhancers of Plant-Arbuscular Mycorrhizal Fungal Associations. Microb Ecol. 2022, 84, 1–10. [Google Scholar] [CrossRef]
- Abbaspour, H.; Saeidi-SarAfshari, S.; Abdel-Wahhab, M. Tolerance of mycorrhiza infected pistachio (Pistacia vera L.) seedling to drought stress under glasshouse conditions. J. Plant Physiol. 2012, 169, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Amiri, R.; Nikbakht, A.; Etemadi, N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015, 197, 373–380. [Google Scholar] [CrossRef]
- Ahmad, P.; Ahanger, M.A.; Egamberdieva, D.; Alam, P.; Alyemeni, M.N.; Ashraf, M. Modification of osmolytes and antioxidant enzymes by epibrassinolide in chickpea seedlings under mercury (Hg) toxicity. J. Plant Growth Regul. 2018, 37, 309–322. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and artifacts. Plant Physiol. 2018, 180, 1246–1250. [Google Scholar] [CrossRef]
- Bárzana, G.R.; Aroca, R.; Ruiz-Lozano, J.M. Localized and non-localized effects of arbuscular mycorrhizal symbiosis on accumulation of osmolytes and aquaporins and on antioxidant systems in maize plants subjected to total or partial Fernández-Lizarazo and Moreno-Fonseca: Mechanisms for tolerance to water-deficit stress in plants inoculated with arbuscular mycorrhizal fungi. A review root drying. Plant Cell Environ. 2015, 38, 1613–1627. [Google Scholar]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Wu, Q.S.; Cao, M.Q.; Zou, Y.N.; Wu, C.; He, X.H. Mycorrhizal colonization represents functional equilibrium on root morphology and carbon distribution of trifoliate orange grown in a split-root system. Sci. Hortic. 2016, 199, 95–102. [Google Scholar]
- Yooyongwech, S.; Samphumphuang, R.; Tisarum, C.; Theerawitaya, C.; Cha-Um, S. Arbuscular mycorrhizal fungi (AMF) improved water deficit tolerance in two different sweet potato genotypes involves osmotic adjustments via soluble sugar and free proline. Sci. Hortic. 2016, 198, 107–117. [Google Scholar] [CrossRef]
- Wu, H.H.; Zou, Y.N.; Rahman Ni, Q.D.; Wu, Q.S. Mycorrhizas alter sucrose and proline metabolism in trifoliate orange exposed to drought stress. Sci. Rep. 2017, 7, 42389. [Google Scholar] [CrossRef]
- Bozkurt, S.; Mansuroğlu, G.S.; Kara, M.; Önder, S. Responses of lettuce to irrigation levels and nitrogen forms. Afr. J. Agric. Res. 2009, 4, 1171–1177. [Google Scholar]
- Şenyiğit, U.; Kaplan, D. Impact of different irrigation water levels on yield and some quality parameters of lettuce (Lactuca sativa L. Var. Longifolia cv.) under unheated greenhouse condition. Infrastruct. Ecol. Rural Areas 2013, 2, 97–107. [Google Scholar]
- Acar, B.; Paksoy, M.; Türkmen, Ö.; Seymen, M. Irrigation and nitrogen level affect lettuce yield in greenhouse condition. IJIWM 2015, 2, 001–004. [Google Scholar]
- Acar, B. Water-yield relationships of lettuce plants for different irrigation strategies. Int. Sci. J. Mech. Agric. Conserv. Resour. 2020, 5, 177–180. [Google Scholar]
- Sesveren, S. Response of Lactuva Sativa Var. Crispa to deficit irrigation and leonardite treatments. All Life 2022, 15, 105–117. [Google Scholar] [CrossRef]
- Cantrell, I.C.; Linderman, R.G. Preinoculation of lettuce and onion with VA mycorrhizal fungi reduces deleterious effects of soil salinity. Plant Soil 2001, 233, 269–281. [Google Scholar] [CrossRef]
- Azcón, R.; Ambrosano, E.; Charest, C. Nutrient acquisition in mycorrhizal lettuce plants under different phosphorus and nitrogen concentration. Plant Sci. 2003, 165, 1137–1145. [Google Scholar] [CrossRef]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Arbuscular Mycorrhizal Fungi (AMF) Improved Growth and Nutritional Quality of Greenhouse-Grown Lettuce. J. Agric. Food Chem. 2011, 59, 5504–5515. [Google Scholar] [CrossRef]
- Santander, C.; Sanhueza, M.; Olave, J.; Aroca, R.; Cumming, J.; Cornejo, P. Arbuscular Mycorrhizal Colonization Promotes the Tolerance to Salt Stress in Lettuce Plants through an Efficient Modification of Ionic Balance. J Soil Sci. Plant Nutr. 2019, 19, 321–331. [Google Scholar] [CrossRef]
- Epelde, L.; Urra, J.; Anza, M.; Gamboa, J.; Garbisu, C. Inoculation of arbuscular mycorrhizal fungi increases lettuce yield without altering natural soil communities. Arch. Agron. Soil Sci. 2020, 68, 413–430. [Google Scholar] [CrossRef]
- Rasouli, F.; Amini, T.; Asadi, M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Ercisli, S.; Skrovankova, S.; Mlcek, J. Growth and Antioxidant Responses of Lettuce (Lactuca sativa L.) to Arbuscular Mycorrhiza Inoculation and Seaweed Extract Foliar Application. Agronomy 2022, 12, 401. [Google Scholar] [CrossRef]
- Lowery, B.; Hickey, W.J.; Arshad, M.A.; Rattan, L. Soil water parameters and soil quality. Methods Assess. Soil Qual. 1996, 4, 143–155. [Google Scholar]
- Kumar, A.; Sharma, S.; Mishra, S. Influence of Arbuscular Mycorrhizal (AM) Fungi and Salinity on Seedling Growth, Solute Accumulation, and Mycorrhizal Dependency of Jatropha curcas L. J. Plant Growth Regul. 2010, 29, 297–306. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Pituch, K.A.; Stevens, J.P. Applied Multivariate Statistics for the Social Sciences, 6th ed.; Riegert, D., Ed.; Taylor and Francis: New York, NY, USA, 2016. [Google Scholar]
- Gutiérrez, D.A. Yield and Nitrogen Use Efficiency for Lettuce (Lactuca sativa) Grown with Airjection and Non-Aerated Irrigation. Master’s Thesis, Jordan College of Agricultural Sciences and Technology California State University, Fresno, CA, USA, 2020. [Google Scholar]
- Lee, J.S.; Chandra, D.; Son, J. Growth, Physicochemical, Nutritional, and Postharvest Qualities of Leaf Lettuce (Lactuca sativa L.) as Affected by Cultivar and Amount of Applied Nutrient Solution. Horticulturae 2022, 8, 436. [Google Scholar] [CrossRef]
- Kirova, E.B.; Geneva, M.P.; Kostadinov, K.; Filipov, S. Improving Yield and Quality-Related Physiological Characteristics of Lettuce by Integrated Inorganic and Organic Fertilizers Management. Agric. Conspec. Sci. 2021, 87, 127–134. [Google Scholar]
- Gholamhoseini, M.; Ghalavand, A.; Dolatabadian, A.; Jamshidi, E.; Khodaei-Joghan, A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013, 117, 106–114. [Google Scholar] [CrossRef]
- Bengough, A.G.; McKenzie, B.M.; Hallett, P.D.; Valentine, T.A. Root elongation, water stress, and mechanical impedance: A review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 2011, 62, 59–68. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Kumari, J.; Rengel, Z. Root length and root lipid composition contribute to drought tolerance of winter and spring wheat. Plant Soil 2019, 439, 57–73. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, L.; Yang, R.; Han, Y.; Hao, J.; Liu, C.; Fan, S. Effects of exogenous putrescine on the ultrastructure of and calcium ion flow rate in lettuce leaf epidermal cells under drought stress. Hortic. Environ. Biotechnol. 2019, 60, 479–490. [Google Scholar] [CrossRef]
- Nurliana, S.; Fachriza, S.; Hemelda, N.M.; Yuniati, R. Chitosan application for maintaining the growth of lettuce (Lactuca sativa) under drought condition. Earth Environ. Sci. 2021, 980, 012013. [Google Scholar] [CrossRef]
- Al-Karaki, G.; Mcmichael, B.; Zak, J. Field response of wheat to arbuscular mycorrhizal fungi and drought stress. Mychorrhiza 2004, 14, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.D.; Zhang, D.J.; Hu, X.C.; Wu, Q.S.; Jiang, C.J.; Xia, T.J.; Gao, X.B.; Kuča, K. Mycorrhiza-induced changes in root growth and nutrient absorption of tea plants. Plant Soil Environ. 2018, 64, 283–289. [Google Scholar] [CrossRef]
- Meddich, A.; Jaiti, F.; Bourzik, W.; El Asli, A.; Hafidi, M. Use of mycorrhizal fungi as a strategy for improving the drought tolerance in date palm (Phoenix dactylifera). Sci. Hortic. 2015, 192, 468–474. [Google Scholar] [CrossRef]
- Badvi, H.; Alemzade Ansari, N.; Mahmoodi Sorestani, M.; Eskandari, F. Effects of drought stress and mycorrhizal fungi on some morphophysiological characteristics of lettuce (Lactuca sativa L.). Plant Prod. 2015, 38, 27–39. [Google Scholar]
- Ruiz-Lozano, J.M.; Aroca, R.; Zamarreño, A.M.; Molina, S.; Andreo-Jiménez, B.; Porcel, R.; García-Mina, J.M.; Ruyter-Spira, C.; López-Ráez, J.A. Arbuscular mycorrhizal symbiosis induces strigolactone biosynthesis under drought and improves drought tolerance in lettuce and tomato. Plant Cell Environ. 2016, 39, 441–452. [Google Scholar] [CrossRef]
- Gholinezhad, E.; Darvishzadeh, R.; Moghaddam, S.S.; Popović-Djordjević, J. Effect of mycorrhizal inoculation in reducing water stress in sesame (Sesamum indicum L.): The assessment of agrobiochemical traits and enzymatic antioxidant activity. Agric. Water Manag. 2020, 238, 106234. [Google Scholar] [CrossRef]
- Hazzoumi, Z.; Moustakime, Y.; Elharchli, E.; Joutei, K.A. Effect of arbuscular mycorrhizal fungi (AMF) and water stress on growth, phenolic compounds, glandular hairs, and yield of essential oil in basil (Ocimum gratissimum L.). Chem. Biol. Technol. Agric. 2015, 2, 10. [Google Scholar] [CrossRef]
- Abdi, N.; Van Biljon, A.; Steyn, C.; Labuschagne, M. Zn Fertilizer and Mycorrhizal Inoculation Effect on Bread Wheat Cultivar Grown under Water Deficit. Life 2023, 13, 1078. [Google Scholar] [CrossRef]
- Sium, A.; Shawon, A.; Swapan Kumar, R.; Sun Hee, W.; Kailas Dashrath, S.; Abdullah Mohammad, S. Effect of salinity on the morphological, physiological and biochemical properties of lettuce (Lactuca sativa L.) in Bangladesh. Open Agric. 2019, 4, 361–373. [Google Scholar]
- Ain-Lhout, F.; Zunzunegui, M.; Diaz Barradas, M.C.; Tirado, R.; Clavijo, A.; Garcia Novo, F. Comparison of proline accumulation in two mediterranean shrubs subjected to natural and experimental water deficit. Plant Soil 2001, 230, 175–183. [Google Scholar] [CrossRef]
- Zou, Y.N.; Wu, Q.S.; Huang, Y.M.; Ni, Q.D.; He, X.N. Mycorrhizal-Mediated Lower Proline Accumulation in Poncirus trifoliata under Water Deficit Derives from the Integration of Inhibition of Proline Synthesis with Increase of Proline Degradation. PlosOne 2013, 8, e80568. [Google Scholar] [CrossRef] [PubMed]
- Basahi, J.M.; Ismail, M.; Hassan, I.A. Effects of Enhanced UV-B Radiation and Drought Stress on Photosynthetic Performance of Lettuce (Lactuca sativa L. Romaine) Plants. Annu. Res. Rev. Biol. 2014, 4, 1739–1756. [Google Scholar] [CrossRef]
- Duangpan, S.; Sujitto, S.; Eksomtramage, T. Genotypic Variation in Proline Accumulation during Sequential Drought and Rewatering in Response to Drought Preconditioning. Int. J. Agric. Technol. 2017, 13, 927–940. [Google Scholar]
- Santos, R.C.; Godoy, J.I.; Fávero, A.P. Melhoramento do Amendoim. Santos, R.C. O Agronegócio do Amendoim no Brasil. Camp. Gd. Embrapa Algodão 2005, 4, 124–192. [Google Scholar]
- Yavuz, N.; Seymen, M.; Kal, Ü. Impacts of water stress and harvest time on physio-biochemical characteristics of lettuce. Int. J. Agric. Nat. Sci. 2021, 14, 61–77. [Google Scholar]
- Mombeini, M.; Alamzadeh Ansar, N.; Abdossi, V.H.; Naseri, A. Reducing destructive effects of drought stress on cucumber through seed priming with silicic acid, pyridoxine, and ascorbic acid along with foliar spraying with silicic acid. Agric. Conspec. Sci. 2021, 86, 35–49. [Google Scholar]
- Kiran, S. Effects of Vermicompost on Some Morphological, Physiological and Biochemical Parameters of Lettuce (Lactuca sativa var. crispa) under Drought Stress. Not. Bot. Horti Agrobo. 2019, 47, 352–358. [Google Scholar] [CrossRef][Green Version]
- Han, Y.; Fan, S.; Zhang, Q.; Wang, Y. Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings. Agric. Sci. 2013, 4, 112–115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
