Abstract
Background: This pilot prospective study analysed the clinical use of a new bioceramic premixed CaSi-containing sealer in association with a warm carrier-based technique. Methodology: Healthy patients (n = 38) requiring 40 root canal treatments were enrolled. Periapical X-rays were taken preoperatively, after root canal filling and after 1, 6, and 12 months. Two evaluators assessed the Periapical Index (PAI) and the sealer extrusion. The healing rate and survival rate were also evaluated. Barnard test was used to assess the relationship of each potential prognostic factor with periapical index (PAI) at 12-month follow-up. The significance level was set at 0.05. Results: Root canal treatments (n = 38) were analysed at the end-line (12 months). The total drop-out was 5% (two patients; two teeth). A total of 31 teeth (82%) (PAI 1-2) showed complete healing, while 7 (18%) are still healing. Cumulative survival was 100%. Apical extrusion of the sealers was observed in 18 cases (47%). Of these extrusions, nine (50%) resulted radiographically undetectable after 12 months. Conclusions: The study supports the use of premixed CaSi-based bioceramic sealers in association with carrier-based techniques. Periapical extrusion of the sealer and its radiographic modification or disappearance are possible events reported in the first 12 months.
1. Introduction
Warm carrier-based techniques associated with different sealers have been shown to be effective and offer clinical advantages due to their ease of use, short learning curve, and reliable technical and clinical outcomes [1]. Warm procedures have traditionally been associated with the use of epoxy resin-based sealers, which are still considered the “gold standard” and revealing success rates from 81% to 96% after 3–5 years [2,3,4]. Unfortunately, epoxy-resin sealers are highly hydrophobic and require a root canal with no moisture in order to achieve a stable seal with no voids [5].
In the last few years, calcium silicate-based materials have shown increasing popularity in endodontic treatment. The introduction of calcium silicate-based materials as endodontic sealers is particularly attractive for their chemical and physical properties. These materials are able to set in the presence of moisture and blood, such as in wide apexes [6,7]. Calcium silicate-based sealers are biocompatible and osteoconductive towards circulating and periapical MSC populations, as observed in recent histological and in vitro studies [8]. The use of these sealers was proposed in combination with the cold single cone technique [9,10], thanks to their specific and innovative properties such as the ability to expand into the root canal, the capacity to produce new apatite formation and to seal the discrepancies between guttapercha and dentinal walls [11,12,13,14,15]. Reconsidering the application of these sealers with a warm technique, such as the carrier-based technique, could be an interesting new perspective in an attempt to simplify the technique.
A new category of premixed CaSi-based or containing sealers has been recently developed and characterised by different compositions [16,17,18]. AH Plus Bioceramic sealer (Dentsply, Konstanz, Germany) is a novel premixed ready-to-be-used sealer with hydraulic properties and adequate flowability. The chemical and physical properties have been investigated in some recent studies, which have reported the ability of the sealer to release calcium and alkalise the environment [19,20,21] and the ability to induce the formation of small apatite deposits when immersed in simulated body fluids [19]. These properties showed the sealer suitability for clinical use.
Despite their promising characteristics and considering the recent introduction on the market, a limited number of short-term clinical investigations have been conducted [9,22,23,24]. Further research is needed to fully understand their performance, clinical outcomes, and occurrence of post-operative pain after filling.
The aim of this clinical pilot prospective cohort study was to evaluate the 12-month outcome, survival, and periapical healing rate of endodontically affected teeth filled with AH Plus Bioceramic sealer in association with warm carrier-based technique. Post-operative pain after root canal filling was assessed after 1 day, 1 week, and 1 month.
2. Materials and Methods
2.1. Study Design and Sample
The study was designed in December 2021 as a pilot prospective clinical study. This design was chosen to provide preliminary data on the effectiveness of the new treatment in order to design a larger randomised clinical trial that was planned for January 2023. No major modifications were made to the study design after its initial conception. The patients were treated in the Endodontic Clinical Section—Dental Clinic, University of Bologna, by a pool of postgraduate master operators (n = 8) in accordance with standardized protocols and under the strict supervision of the experienced tutors of the master. All the operators, before the study started, were adequately instructed and trained in sealer application and obturation technique. The study was approved by the ethical committee (597-2022-SPER-AUSLBO).
The study adhered to the principles of the Declaration of Helsinki, as modified in 2013 [25]. The clinical staff provided written and verbal information to patients before enrolment.
All patients provided a signed informed consent to accept the treatment plan and to follow the hygiene program. The study was designed in compliance with the STROBE checklist [26] and the guidelines published by Dodson in 2007 [27].
2.2. Study Population
Table 1a,b provide the inclusion and exclusion criteria for the clinical study.

Table 1.
(a) Inclusion Criteria. (b) Exclusion criteria.
2.3. Primary Root Canal Treatment
Nerve block anaesthesia (1.7 mL, mepivacaine chloridrate, Scandonest 3%, Septodont, St.-Maur-des-Fosses, France) and local anaesthesia (1.8 mL mepivacaine chloridrate, Scandonest 2% with 1:100,000 adrenaline, Septodont, St.-Maur-des-Fosses, France) were performed. The total duration of each endodontic session was 60 to 90 min. Dental dam isolation was positioned on the affected tooth. A straight-line access was performed with a diamond bur mounted on high-speed water-cooled handpieces (Cefla, Imola, Italy). A preoperative working length was estimated using periapical radiographs. The crown-down technique was used. Gates-Glidden burs #2 and #3 were utilised when necessary, only in the coronal third. NiTi instruments were used to shape the canals in the coronal, medium, and apical third (Rotate, VDW, Munchen, Germany). An electronic apex locator (Root ZX, Morita, Osaka, Japan) with K-file #10 was used to determine the working length during the entire clinical procedure. Intra-oral periapical X-rays were performed to confirm the working length during the root canal instrumentation. Each root canal was shaped in the apical third with an apical diameter of #25.04 at least.
Irrigation was performed after the use of each instrument with a total of 5 mL of 5% NaOCl solution (Niclor 5, OGNA, Muggiò, Italy).
2.4. Secondary Root Canal Treatment
An initial pathway was created with Gates-Glidden burs #3 and #4 (Dentsply Maillefer, Ballaigues, Switzerland) to approximately 3–4 mm depth in the gutta-percha. Reciprocating NiTi instruments (Reciproc Blue, VDW, Munchen, Germany) were then used with Silver Reciproc Endomotor in the “Reciproc All” setting. After each step, the material entrapped among the instrument threads was removed using a sterile sponge. The working length was established after the removal of root canal remnants using periapical X-ray and an electronic apex locator. An apical enlargement was performed with Reciproc Blue #40 and #50 when needed. Irrigation was performed using a total amount of 5.0 mL of 5% NaOCl. When necessary, a dental surgery microscope (OMS3200 Dental Microscope, Zumax Medical Co., Suzhou, China) was used to detect the access to root canal orifices and to identify the presence of remnants.
2.5. Root Canal Filling Procedures
A premixed CaSi-containing bioceramic sealer (Ah Plus Bioceramic, Dentsply, Konstanz, Germany) was used in association with a warm carrier-based technique (Thermafil, Dentsply, Konstanz, Germany). AH Plus Bioceramic is mostly composed of zirconium dioxide (50–70%) as a radiopacifier and tricalcium silicate (10–15%) as a bioactive component. Dimethyl sulfoxide and traces of lithium carbonate and thickening agents are also reported by the manufacturer.
The sealer was applied with a sterile K-file inserted into the canal to reach the WL—3 mm and gently moved around the root canal walls. The carrier was heated using a dedicated obturation oven (Thermaprep obturation, Dentsply, Konstanz, Germany) and slowly inserted into the canal at WL—0.5 mm. The excess of the carrier was cut with a round bur. An X-ray was performed to verify the quality of the root canal obturation. Finally, a small cotton pellet and a temporary restoration (Coltosol, Coltene, Altstaetten, Switzerland) were positioned in the access cavity and maintained until definitive restoration. In case of severe pain, a medical prescription to take NSAID medications (such as ibuprofen or ketoprofen) was prepared by the university staff. In this case, the event was recorded, and the patient was excluded from the study.
2.6. Tooth Restoration
Teeth were definitely restored within 2 weeks under rubber dam isolation. Temporary restoration was removed using ultrasonic tips, and a crown was restored under rubber dam isolation. Self-etching dentinal bonding agent primer and bonding (Clearfil SE BOND, Kuraray, Osaka, Japan) were applied, photo-cured (Elipar, 3M ESPE, St. Paul, MN, USA) for 30 s and layered by flowable (G_Aenial Flow, GC Corporation, Tokyo, Japan) and composite (G-Aenial, GC Corporation, Tokyo, Japan) resins applied incrementally with 1.5 mm layers.
2.7. Radiological Evaluation
X-rays were taken after the root canal filling using a parallel technique. The following parameters were used: the target–film distance was approx. 30 cm, 0.41 s exposure at 70 Kw and 8 mA. The radiographs were developed in a standard developer unit at 20 °C (Euronda s.p.a., Vicenza, Italy), 12 s developing time, and 25 s fixing time according to the manufacturer instructions.
Intra-oral periapical X-rays and clinical criteria were used to classify the final outcome, with each patient monitored at 1, 6, and 12 months of follow-up. The root canal obturation was considered “adequate” when the filling material was detected at 0–1.0 mm from the radiological apex. Overfilling, short filling, and sealer extrusion were recorded. X-rays were digitalised using a slide scanner with a mean resolution of 1000 dpi and a magnification factor of 20×.
Periapical Index (PAI) [28] was used to score the preoperative diagnosis and endpoint evaluations, which were evaluated in a double-blind manner by two operators (university researchers trained in this analysis) who did not perform the root canal treatment. PAI calibration was performed using well-defined instructions and periapical radiographs with different periapical lesion scores. To ensure their reliability, the evaluators independently assessed the X-rays. In the event of any discrepancies between their assessments, these were extensively discussed until a mutual consensus was achieved. Sealer extrusion was recorded and measured on each periapical X-ray using open-source software (Image J, Bethesda, MD, USA).
2.8. Post-Operative Pain Assessment
Post-operative pain was assessed as Patient Reported Outcome (PRO) using a 10 cm Visual Analogical Scale, divided into 0–100 steps, with 0 indicating no pain and 100 indicating the most intense pain [29]. Post-operative pain was evaluated after root canal filling (T0), after 1 day (T1), after 7 days (T7), after 1 month (T28), and after 12 months (T365).
When a tooth presented a PAI 1 or 2, the tooth was considered “radiographically healed”.
When a tooth presented an improvement in PAI, the tooth was considered as “radiographically healing”.
2.9. Statistical Methods
Variables were summarised as counts and percentages. Barnard CSM (Convexity, Symmetry, and Minimization) test was used to assess the relationship of each potential prognostic factor with PAI at 12-month follow-up (1–2 [healed] vs. ≥3 [still healing]). Barnard test is an exact unconditional test recommended for association in 2 × 2 tables due to its power and preservation of test size [30,31]. Operationally, it starts with the most extreme table and sequentially adds more extreme ones based on the smallest p-value calculated by iteratively maximising the probability of a 2 × 2 table. Effect sizes were expressed as differences in percentages with 95% confidence intervals (CIs) derived by matching Barnard CSM p-values.
The 95% CIs for healing and survival rates were obtained with the Bayesian-derived Jeffreys method [32]. The significance level was set at 0.05, and all tests were two-sided. Data analysis was performed with the “Exact” R package [33].
3. Results
Demographic Information
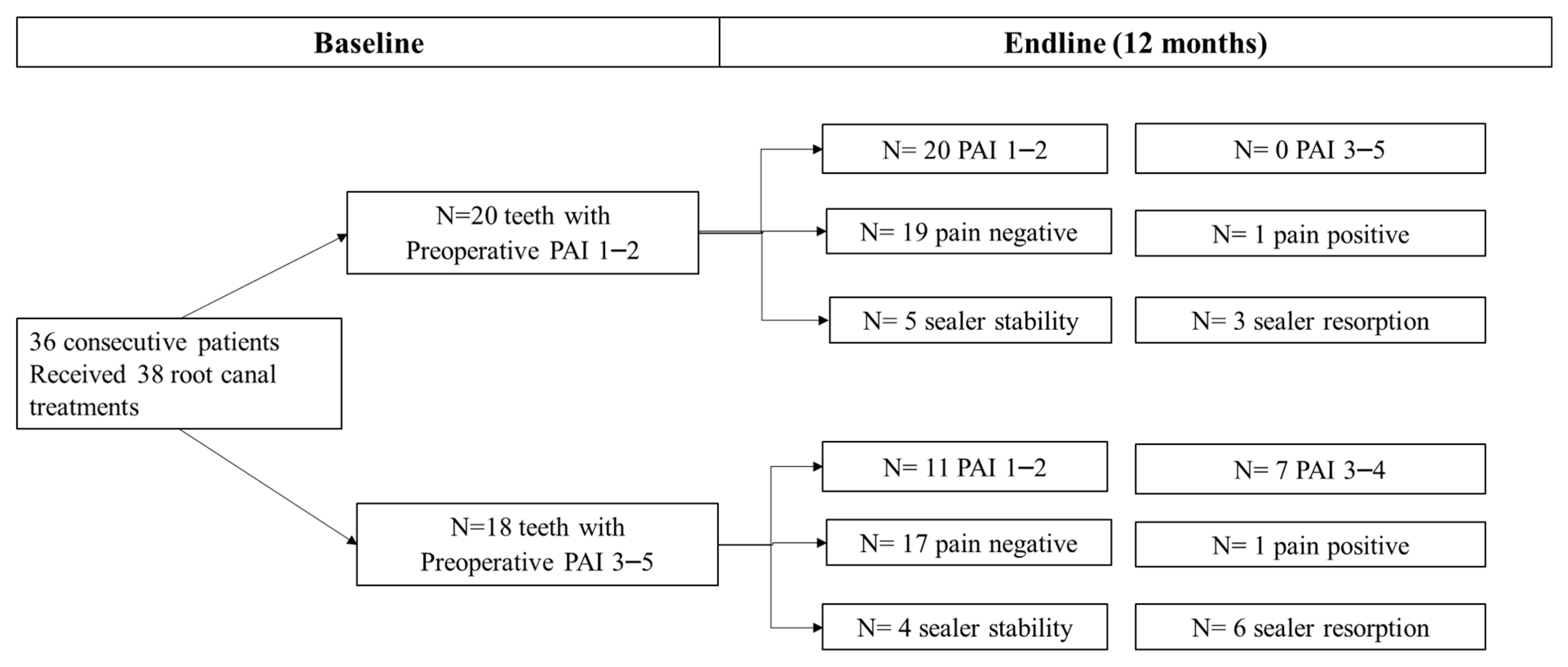
A total of 38 patients requiring 40 root canal treatments were accepted to be included in the study (Figure 1). Two patients contributing with two root canal treatments (5%) were unable to complete the follow-up and were excluded. A total of 38 teeth were analysed at the end-line. Information on patient and tooth-related parameters is reported in Table 2, while obturation-related parameters are reported in Table 3a,b.
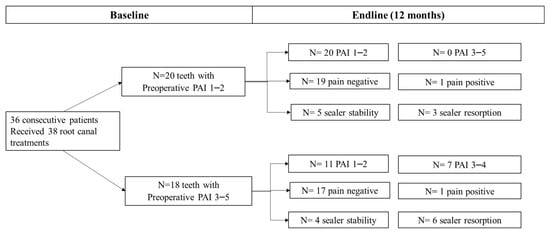
Figure 1.
Endline PAI, frequency of post-operative Pain and sealer stability between teeth with preoperative PAI 1–2 versus teeth with a preoperative PAI 3–5. No differences in post-operative pain distribution were observed at 12 months between the two groups. Two root canal treatments still presented a slight tenderness to percussion at 1-year follow-up. Teeth with preoperative PAI > 2 had a higher percentage of healing lesions and a higher percentage of sealer resorption.

Table 2.
Patient and tooth-related characteristics of the study at baseline.

Table 3.
(a) Patient-related characteristics of the study sample at 12-month follow-up (1–2 [healed] vs. ≥3 [still healing]). (b) Obturation-related parameters of the study sample at 12-month follow-up (1–2 [healed] vs. ≥3 [still healing]).
The pilot cohort included a high number of teeth with a periapical lesion (PAI ≥ 3) (47%), pulp necrosis (21%), and re-exacerbated periapical lesion (26%). The majority of root canal obturation length was considered adequate (79%), while 10% resulted in overfilled and 13% were underfilled. Sealer periapical extrusion was observed in a high percentage of cases (47%). Most of the radiographic extrusion resulted in smaller than 5 mm.
The cumulative percentage of healed teeth at 12 months was 82% (95% CI 67–91%). Seven out of thirty-eight teeth presented a periapical radiolucency after 12 months (18%). Cumulative survival rate was 100% (95%CI = 94–100%).
Patient-related characteristics (age, sex, tooth location, and type) did not influence the healing percentage. Interestingly, at the tooth level, the type of treatment (root canal treatment vs. retreatment) did not influence the healing percentage (p-value = 0.472). Initial PAI was significantly related to periapical healing; that is, teeth with a preoperative PAI > 2 had lower healing rates at 12 months (p-value = 0.001) (Table 3a).
As shown in Table 3b, obturation length, sealer extrusion and extrusion size did not influence the outcome (all p-values > 0.05). Interestingly, healed teeth exhibited a lower periapical sealer resorption as compared with healing teeth (43% vs. 75%), but the difference was not statistically significant due to small sample sizes (p-value = 0.333).
Table 4 and Table 5 report the post-operative pain intensity according to VAS during the follow-up. No pain was observed in 84% of the cases 1 day after treatment. This percentage increased, reaching 95% at 1 year after root canal filling. No severe pain after filling was recorded in any case. A total of two root canal treatments still presented slight tenderness to percussion at 1-year follow-up. The percentage of healing was significantly influenced by post-operative pain detected at one day (p-value = 0.029), one week (p-value = 0.007), one month (p-value = 0.007), and 12 months (p-value = 0.011) after obturation; that is, patients with no pain had higher healing percentages (Table 4).

Table 4.
Pain-related parameters of the study sample, overall and by periapical index (PAI) at 12-month follow-up (1–2 [healed] vs. ≥ 3 [still healing]).

Table 5.
Post-operative pain intensity according to VAS at 1 day, 7 days, 28 days and 1 year after root canal obturation.
Figure 1 depicts endline PAI, frequency of post-operative pain and sealer stability between teeth with no preoperative periapical lesion (PAI 1–2) versus teeth with a preoperative periapical lesion (PAI 3–5). No differences in post-operative pain distribution were observed at 12 months between the two groups. Differently, teeth with preoperative PAI > 2 had a higher percentage of healing lesions and a higher percentage of sealer resorption. When comparing the extrusion frequencies in upper or lower jaws, we found a higher percentage of extrusions in maxillary locations, namely 12/18 teeth, when compared to the mandibular sites (6/18 teeth). Sealer resorption occurred in 6/9 maxillary teeth and 3/9 mandibular teeth.
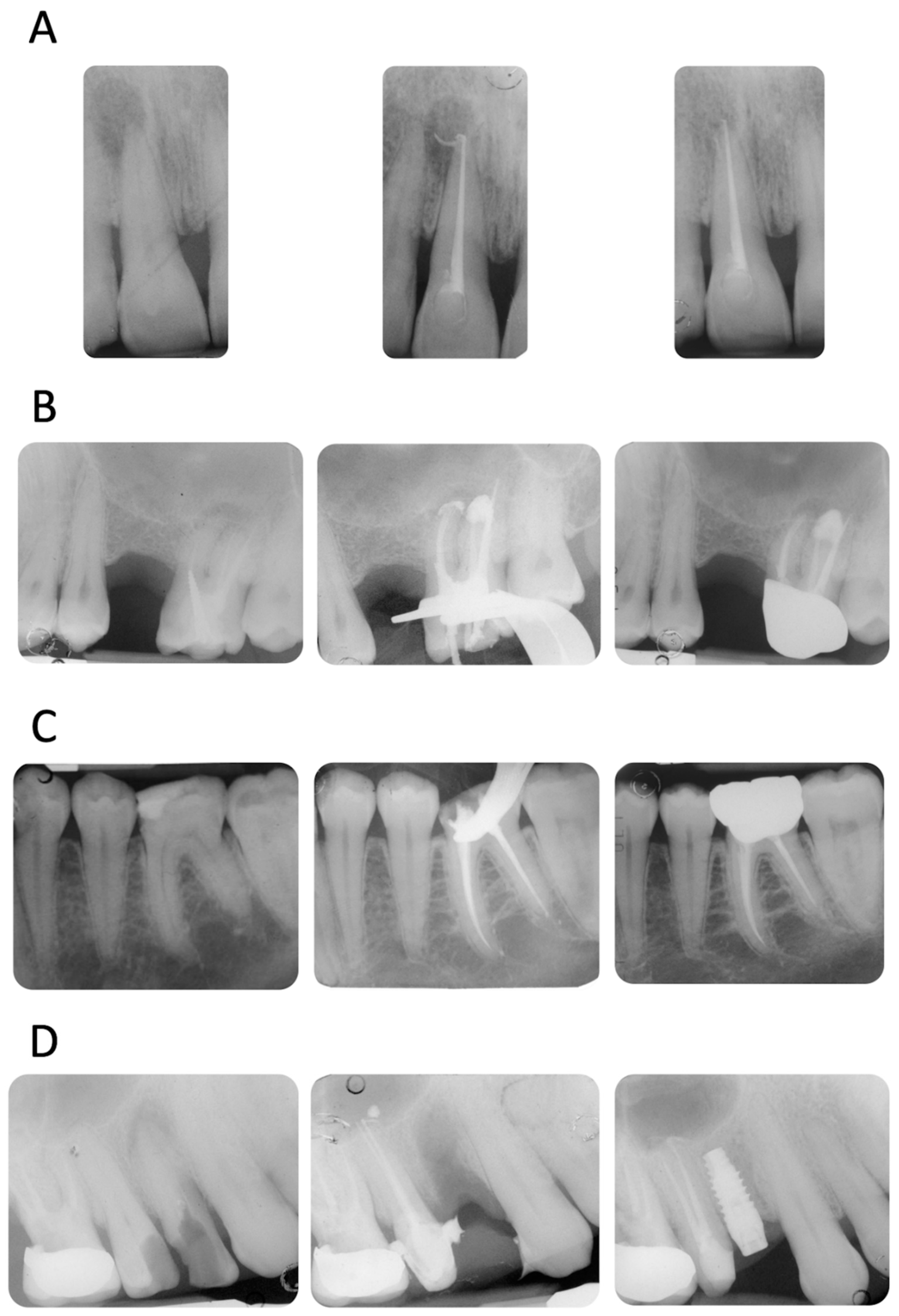
Four representative cases included in the study are reported in Figure 2A–D.
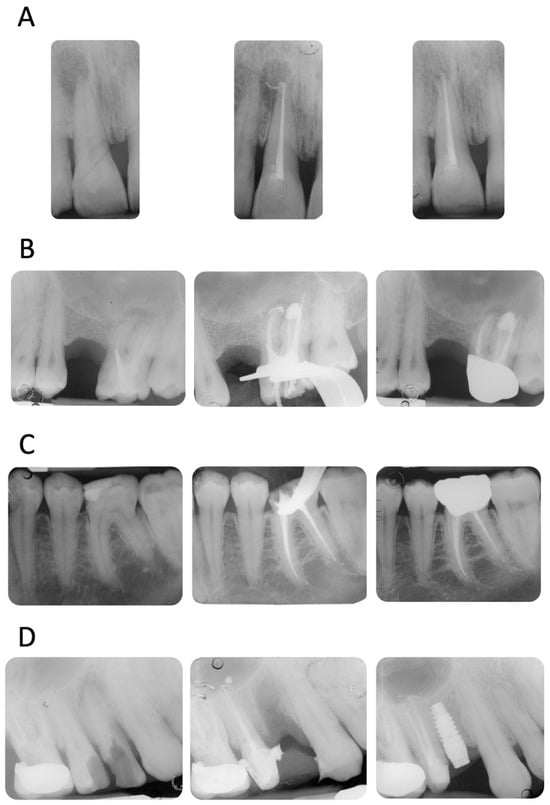
Figure 2.
(A) Upper incisor with a periapical lesion (PAI = 5). The periapical X-ray at 12 months showed resolution of the periapical pathology (PAI = 2). Note the disappearance of sealer extrusion. (B) Upper molar with previous failed root canal treatment (PAI = 4). The mesio-buccal root revealed a periapical lesion, a metal post, and an incomplete filling of the apical third. It is interesting to observe the sealer morphology after root canal obturation and after 12 months of follow-up. The extrusion around the mesio-buccal root seems to have disappeared, while the one in the palatal root is still present. No post-operative pain was reported (VAS = 0 throughout the entire treatment and follow-up). (C) Lower first molar with a deep carious lesion. Slight periapical extrusion was observed in the mesial and distal canals. Uneventful healing was observed at the 12-month follow-up. (D) Upper second premolar with a deep carious lesion and no periapical radiolucencies (PAI = 2). Slight modification of the sealer extrusion was observed at the 12-month recall.
4. Discussion
This pilot study analysed the outcome of root canal treatments filled with a recently introduced premixed CaSi-containing bioceramic sealer and warm carrier-based technique. The cumulative percentage of healed teeth was approx. 81.6%, with no extractions. The cumulative survival rate was 100%. These data are in line with previously published studies on carrier-based techniques used with an epoxy resin sealer [2,3,4]. A total of 8 teeth still presented a radiographically detectable periapical radiolucency at 12 months, which was stable or improved compared to the preoperative periapical lesion. Approximately half of the endodontically treated teeth had a diagnosis of necrotic pulp (21%) or a re-exacerbated periapical lesion (26%) at baseline. This condition critically affected the 12-month healing outcome, as demonstrated by the statistical analysis in Table 3a,b. Other obturation-related parameters were analysed to assess the potential effect of the sealer and technique on the healing rate of the root canal performed.
Interestingly, a high percentage of periapical sealer extrusion (47% of the total) was observed. The analysis of periapical X-rays demonstrated some modification of their morphology or partially complete disappearance over time. The extrusion, at least in the present study, is mainly composed only of sealers. Recent studies confirmed that warm-carrier-based systems may induce a great percentage of extrusion, ranging from approx. 25% to 58% of the cases [23,29]. A conventional gold-standard sealer (AH Plus), mainly composed of epoxy-resin components, offered a high percentage of radiographic extrusion (30%) [23], likely attributable to the higher flow when heating is applied [34]. In the present study, teeth that showed partial or complete resorption (and radiographic disappearance) of apical extrusion were associated with lower percentages of healing at 12 months but were not statistically significant (p > 0.05). Longer follow-up (4 years, according to the European Society of Endodontology) may reveal a more significant association with the effect of sealer resorption on healing outcome [35].
Only a small number of studies evaluated the presence and the role of periapical extrusion on healing and their effect on clinical results [36,37]. Previous studies found the non-significant effect of sealer extrusions on periapical healing (such as in the case of epoxy resin-based sealers and calcium hydroxide-based sealers) [36,37] as also indicated by a recent meta-analysis [38]. The sealer composition, the ability to release bioactive ions, and the bioactivity properties could influence the periapical bone healing and formation of new bone tissue detectable by radiographic inspection [39]. The biological consequences of extrusion are probably modest if the sealer is mainly composed of biocompatible components [40] that induce fast bone regeneration during their degradation and release of bioactive ions [21,23,39].
The non-complete healing observed in this study may be explained by the fact that biological phases of periapical bone remodelling need time to remove the sealer radiopacifiers and to complete the bone regeneration. Moreover, the potential bioactivity (apatite nucleation ability) of the sealer could induce the formation of hard tissue in the periapical lesion, which may appear less radio opaque than healthy periapical bone. This aspect needs to be elucidated at longer follow-up to assess if the complete resolution of the periapical healing proceeds or remains stable.
In previous times, the use of mineral trioxide aggregate (MTA) and other CaSi-based cement were tested as an approach to achieve effective sealing of wide apexes [7] while simultaneously facilitating the development of a durable and biologically active barrier in proximity to the periapical bone and into the internal surface of the canal. The formation of a biocompatible, osteoconductive cement barrier can stabilise the sealing, potentially stimulating the generation of new bone tissue [41,42]. Hence, CaSi-based cements create the so-called “biomimetic remineralisation” of demineralised dentin [43,44]. Tay et al. first proposed the use of CaSi Portland-derived cements to induce the solid interfibrillar remineralisation of root demineralised dentin [43,44].
The percentage of tri Calcium Silicate component in the total sealer composition is probably lower than in the traditional powder/liquid cement. It is important to remark that the CaSi component is the bioactive ingredient of the material [43,44].
The present study analysed post-operative pain at the different end points. Post-operative pain was assessed as a patient-related outcome. A recent meta-analysis reported that root canal filling procedures are some of the most associated factors that affect post-operative pain [45]. During the obturation steps, the endodontic sealer establishes direct contact with periapical tissues. Consequently, the physical and chemical properties of the sealer could influence the magnitude of post-operative endodontic pain.
Interestingly, the persistence of (mild) pain in the first month after root canal filling was significantly associated with slower periapical healing. Two teeth presented only slight tenderness after occlusal load but no periapical recrudescence at 12 months follow-up. A previous study on a bioceramic CaSi-based sealer showed a similar trend, with an overall reduction in pain intensity during the first 1–2 weeks [29]. In another study, the authors reported that unintentional apical extrusion of calcium silicate-based root canal sealers leads to post-operative pain results that are comparable to resin-based sealers [46]. Pontoriero et al. compared four different types of bioceramic sealer and reported that the presence of post-operative pain was not affected by the extrusion of the sealer [24]. Other conditions may affect the persistence of pain after endodontic therapy, and the presence of specific microbiota (and an elevated number of pathogens) may alter the healing steps [47].
5. Conclusions
This pilot preliminary study opens new questions for the next clinical studies on bioceramic sealers. When an extrusion of sealer occurs in a periapical area with bone deficit, how will the periapical area heal? Will it form mineralised tissue, fibrous tissue, or an inert deposit of biocompatible sealer?
In theory, the extrusion of a bioactive sealer such as AH Plus Bioceramic could potentially enhance the formation of new mineralised tissue at the apex area and promote the creation of new bone and bone-like tissue at periapical levels. However, the question remains whether extrusion should be considered an index of perfect healing or merely a modest defect of therapy. It is also unclear whether extruded AH Plus Bioceramic used with the carrier-based technique will induce dentin remineralisation of the root canal. Further studies are needed to investigate the optimal amount of sealer extrusion for promoting healing and apical bone regeneration. In conclusion, it is important to note that this is an early study; a more detailed analysis of non-linear relationships across variables will be performed in the upcoming randomised clinical study. Despite the need for further research, this study supports the routine clinical use of flowable premixed sealers in combination with the warm carrier-based technique.
Author Contributions
Conceptualization, C.P. and M.G.G.; methodology, C.P.; software, A.S. and F.Z.; validation, A.S.; formal analysis, A.S., F.Z. and J.L.; investigation, C.P., A.S. and F.Z.; resources, C.P.; data curation, A.S., F.Z. and J.L.; writing—original draft preparation, C.P. and A.S.; writing—review and editing, C.P., M.G.G. and A.S.; visualisation, A.S. and F.Z.; supervision, C.P.; project administration, C.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was approved by the ethical committee (597-2022-SPER-AUSLBO).
Informed Consent Statement
Informed consent was obtained from all subjects.
Data Availability Statement
Data available upon reasonable request.
Conflicts of Interest
The authors deny any conflict of interest.
References
- Mirfendereski, M.; Roth, K.; Bing, F.; Dubrowski, A.; Carnahan, H.; Azarpazhooh, A.; Basrani, B.; Torneck, C.D.; Friedman, S. Technique acquisition in the use of two thermoplasticized root filling methods by inexperienced dental students: A micro-CT analysis. J. Endod. 2009, 35, 1512–1517. [Google Scholar] [CrossRef] [PubMed]
- Pirani, C.; Zamparini, F.; Peters, O.A.; Iacono, F.; Gatto, M.R.; Generali, L.; Gandolfi, M.G.; Prati, C. The fate of root canals obturated with Thermafil: 10-year data for patients treated in a master program. Clin. Oral Investig. 2019, 23, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.; Gatti, R.; Glickman, G.N.; Opperman, L.A. Comparative analysis of carrier-based obturation and lateral compaction: A retrospective clinical outcomes study. Int. J. Dent. 2012, 2012, 954675. [Google Scholar] [CrossRef][Green Version]
- Demirci, G.K.; Caliskan, M.K. A prospective randomized comparative study of cold lateral condensation versus Core/Gutta-percha in teeth with periapical lesions. J. Endod. 2016, 42, 206–210. [Google Scholar] [CrossRef]
- Zhou, H.M.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.F.; Haapasalo, M. Physical properties of 5 root canal sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef]
- Prati, C.; Siboni, F.; Polimeni, A.; Bossu, M.; Gandolfi, M.G. Use of calcium-containing endodontic sealers as apical barrier in fluid-contaminated wide-open apices. J. Appl. Biomater. Funct. Mater. 2014, 12, 263–270. [Google Scholar] [CrossRef]
- Pace, R.; Giuliani, V.; Nieri, M.; Di Nasso, L.; Pagavino, G. Mineral trioxide aggregate as apical plug in teeth with necrotic pulp and immature apices: A 10-year case series. J. Endod. 2014, 40, 1250–1254. [Google Scholar] [CrossRef]
- Sanz, J.L.; López-García, S.; Rodríguez-Lozano, F.J.; Melo, M.; Lozano, A.; Llena, C.; Forner, L. Cytocompatibility and bioactive potential of AH Plus Bioceramic Sealer: An in vitro study. Int. Endod. J. 2022, 55, 1066–1080. [Google Scholar] [CrossRef]
- Chybowski, E.A.; Glickman, G.N.; Patel, Y.; Fleury, A.; Solomon, E.; He, J. Clinical Outcome of Non-Surgical Root Canal Treatment Using a Single-cone Technique with Endosequence Bioceramic Sealer: A Retrospective Analysis. J. Endod. 2018, 44, 941–945. [Google Scholar] [CrossRef]
- Angerame, D.; De Biasi, M.; Pecci, R.; Bedini, R. Filling ability of three variants of the single-cone technique with bioceramic sealer: A micro-computed tomography study. J. Mater. Sci. Mater. Med. 2020, 31, 91. [Google Scholar] [CrossRef]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Perut, F.; Ciapetti, G.; Mongiorgi, R.; Prati, C. New Portland cement-based materials for endodontics mixed with articaine solution: A study of cellular response. J. Endod. 2008, 34, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Siboni, F.; Botero, T.; Bossù, M.; Riccitiello, F.; Prati, C. Calcium silicate and calcium hydroxide materials for pulp capping: Biointeractivity, porosity, solubility and bioactivity of current formulations. J. Appl. Biomater. Funct. Mater. 2015, 13, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Van Landuyt, K.; Taddei, P.; Modena, E.; Van Meerbeek, B.; Prati, C. Environmental scanning electron microscopy connected with energy dispersive x-ray analysis and Raman techniques to study ProRoot mineral trioxide aggregate and calcium silicate cements in wet conditions and in real time. J. Endod. 2010, 36, 851–857. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Siboni, F.; Primus, C.M.; Prati, C. Ion release, porosity, solubility, and bioactivity of MTA Plus tricalcium silicate. J. Endod. 2014, 40, 1632–1637. [Google Scholar] [CrossRef]
- Primus, C.M.; Tay, F.R.; Niu, L.N. Bioactive tri/dicalcium silicate cements for treatment of pulpal and periapical tissues. Acta Biomater. 2019, 96, 35–54. [Google Scholar] [CrossRef]
- Primus, C.; Gutmann, J.L.; Tay, F.R.; Fuks, A.B. Calcium silicate and calcium aluminate cements for dentistry reviewed. J. Am. Ceram. Soc. 2022, 105, 1841–1863. [Google Scholar] [CrossRef]
- Camilleri, J.; Atmeh, A.; Li, X.; Meschi, N. Present status and future directions: Hydraulic materials for endodontic use. Int. Endod. J. 2022, 55, 710–777. [Google Scholar] [CrossRef]
- Zamparini, F.; Prati, C.; Taddei, P.; Spinelli, A.; Di Foggia, M.; Gandolfi, M.G. Chemical-Physical Properties and Bioactivity of New Premixed Calcium Silicate-Bioceramic Root Canal Sealers. Int. J. Mol. Sci. 2022, 23, 13914. [Google Scholar] [CrossRef]
- Souza, L.C.; Neves, G.S.T.; Kirkpatrick, T.; Letra, A.; Silva, R. Physicochemical and Biological Properties of AH Plus Bioceramic. J. Endod. 2023, 49, 69–76. [Google Scholar] [CrossRef]
- Donnermeyer, D.; Schemkämper, P.; Bürklein, S.; Schäfer, E. Short and Long-Term Solubility, Alkalizing Effect, and Thermal Persistence of Premixed Calcium Silicate-Based Sealers: AH Plus Bioceramic Sealer vs. Total Fill BC Sealer. Materials 2022, 15, 7320. [Google Scholar] [CrossRef] [PubMed]
- Zavattini, A.; Knight, A.; Foschi, F.; Mannocci, F. Outcome of root canal treatments using a new calcium silicate root canal sealer: A non-randomized clinical trial. J. Clin. Med. 2020, 9, 782. [Google Scholar] [CrossRef] [PubMed]
- Zamparini, F.; Spinelli, A.; Cardinali, F.; Ausiello, P.; Gandolfi, M.G.; Prati, C. The Use of Premixed Calcium Silicate Bioceramic Sealer with Warm Carrier-Based Technique: A 2-Year Study for Patients Treated in a Master Program. J. Funct. Biomater. 2023, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Pontoriero, D.I.K.; Ferrari Cagidiaco, E.; Maccagnola, V.; Manfredini, D.; Ferrari, M. Outcomes of Endodontic-Treated Teeth Obturated with Bioceramic Sealers in Combination with Warm Gutta-Percha Obturation Techniques: A Prospective Clinical Study. J. Clin. Med. 2023, 12, 2867. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Med. Assoc. 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M. STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Epidemiology 2007, 18, 805–835. [Google Scholar] [CrossRef]
- Dodson, T.B. A guide for preparing a patient-oriented research manuscript. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2007, 104, 307–315. [Google Scholar] [CrossRef]
- Ørstavik, D.; Kerekes, K.; Eriksen, H.M. The periapical index: A scoring system for radiographic assessment of apical periodontitis. Endod. Dent. Traumatol. 1986, 2, 20–34. [Google Scholar] [CrossRef]
- Fonseca, B.; Coelho, M.S.; Bueno, C.E.D.S.; Fontana, C.E.; Martin, A.S.; Rocha, D.G.P. Assessment of Extrusion and Postoperative Pain of a Bioceramic and Resin-Based Root Canal Sealer. Eur. J. Dent. 2019, 13, 343–348. [Google Scholar] [CrossRef]
- Barnard, G.A. A new test for 2 × 2 tables. Nature 1945, 156, 177. [Google Scholar] [CrossRef]
- Lydersen, S.; Fagerland, M.W.; Laake, P. Recommended tests for association in 2 × 2 tables. Stat. Med. 2009, 28, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.D.; Cai, T.T.; Das Gupta, A. Interval estimation for a binomial proportion. Stat. Sci. 2001, 16, 101–133. [Google Scholar] [CrossRef]
- Calhoun, P. Exact: Unconditional Exact Test. R Package Version 3.2, 2022. Available online: https://CRAN.R-project.org/package=Exact (accessed on 18 October 2023).
- Donnermeyer, D.; Schäfer, E.; Bürklein, S. Real-time Intracanal Temperature Measurement during Different Obturation Techniques. J. Endod. 2018, 44, 1832–1836. [Google Scholar] [CrossRef]
- European Society of Endodontology. Quality guidelines for endodontic treatment: Consensus report of the European Society of Endodontology. Int. Endod. J. 2006, 39, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Rôças, I.N.; Alves, F.R.; Loghin, S.; Siqueira, J.F., Jr. Apically Extruded Sealers: Fate and Influence on Treatment Outcome. J. Endod. 2016, 42, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.F.B.; Scheeren, B.; van der Waal, S.V. The Effect of Unintentional AH-Plus Sealer Extrusion on Resolution of Apical Periodontitis After Root Canal Treatment and Retreatment-A Retrospective Case-control Study. J. Endod. 2023, 49, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Aminoshariae, A.; Kulild, J.C. The impact of sealer extrusion on endodontic outcome: A systematic review with meta-analysis. Aust. Endod. J. 2020, 46, 123–129. [Google Scholar] [CrossRef]
- Gandolfi, M.G.; Iezzi, G.; Piattelli, A.; Prati, C.; Scarano, A. Osteoinductive potential and bone-bonding ability of ProRoot MTA, MTA Plus and Biodentine in rabbit intramedullary model: Microchemical characterization and histological analysis. Dent. Mater. 2017, 33, 221–238. [Google Scholar] [CrossRef]
- Geurtsen, W.; Leyhausen, G. Biological aspects of root canal filling materials--histocompatibility, cytotoxicity, and mutagenicity. Clin. Oral. Investig. 1997, 1, 5–11. [Google Scholar] [CrossRef]
- Von Arx, T. Mineral Trioxide Aggregate (MTA) a success story in apical surgery. Swiss. Dent. J. 2016, 126, 573–595. [Google Scholar]
- Giacomino, C.M.; Wealleans, J.A.; Kuhn, N.; Diogenes, A. Comparative Biocompatibility and Osteogenic Potential of Two Bioceramic Sealers. J. Endod. 2019, 45, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H.; Rueggeberg, F.A.; Loushine, R.J.; Weller, R.N. Calcium phosphate phase transformation produced by the interaction of the portland cement component of white mineral trioxide aggregate with a phosphate-containing fluid. J. Endod. 2007, 33, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H. Guided tissue remineralisation of partially demineralised human dentine. Biomaterials 2008, 29, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Mekhdieva, E.; Del Fabbro, M.; Alovisi, M.; Comba, A.; Scotti, N.; Tumedei, M.; Carossa, M.; Berutti, E.; Pasqualini, D. Postoperative Pain following Root Canal Filling with Bioceramic vs. Traditional Filling Techniques: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 4509. [Google Scholar] [CrossRef] [PubMed]
- Drumond, J.P.S.C.; Maeda, W.; Nascimento, W.M.; Campos, D.L.; Prado, M.C.; de-Jesus-Soares, A.; Frozoni, M. Comparison of Postobturation Pain Experience after Apical Extrusion of Calcium Silicate- and Resin-Based Root Canal Sealers. J. Endod. 2021, 47, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Buonavoglia, A.; Zamparini, F.; Lanave, G.; Pellegrini, F.; Diakoudi, G.; Spinelli, A.; Lucente, M.S.; Camero, M.; Vasinioti, V.I.; Gandolfi, M.G.; et al. Endodontic Microbial Communities in Apical Periodontitis. J. Endod. 2023, 49, 178–189. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).