Evaluation of Dentin Tubule Occlusion Using Pre-Treatment with No-Ozone Cold Plasma: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tooth Preparation
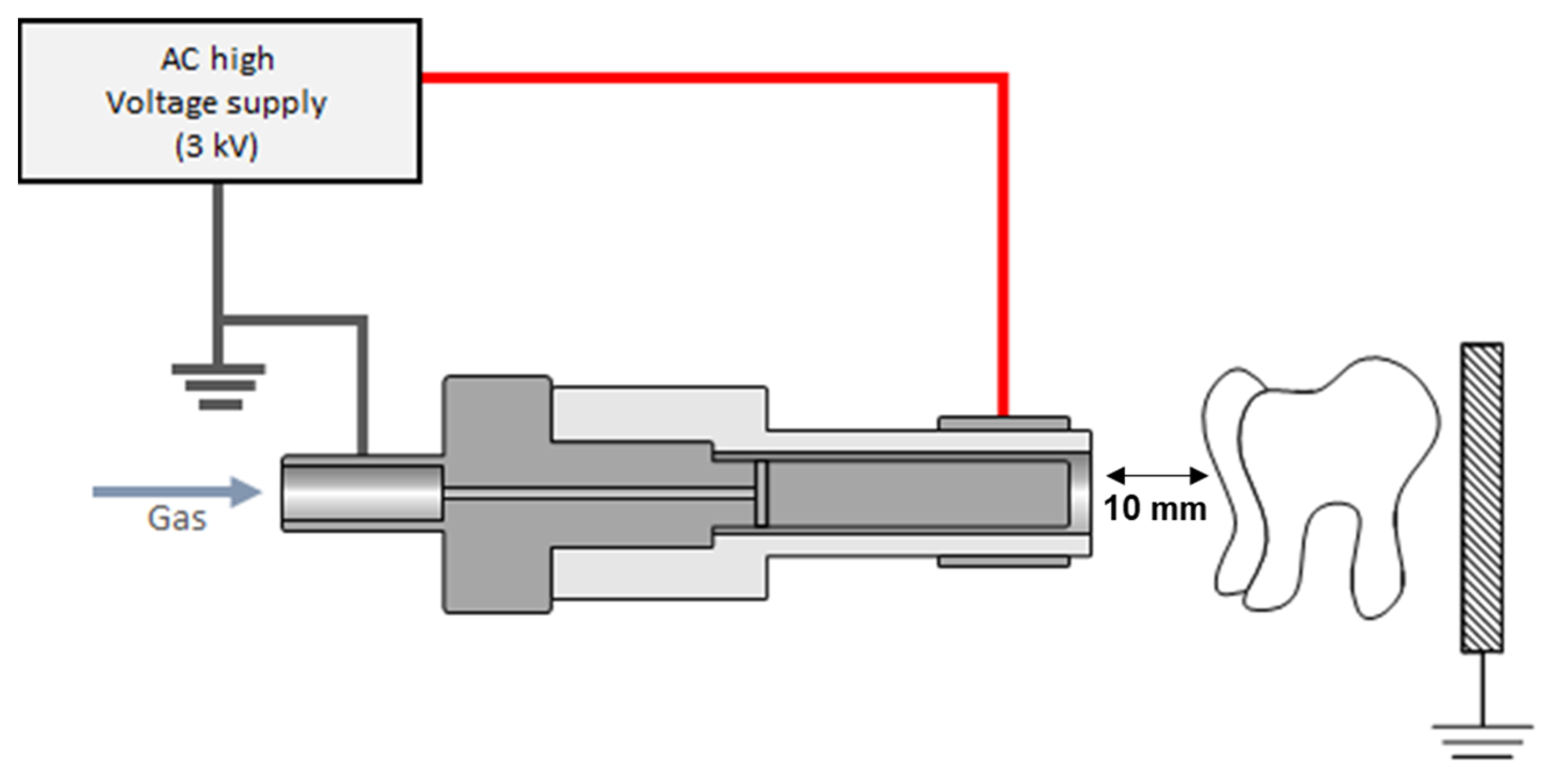
2.3. Plasma Device
2.4. Electron Probe Microanalyzer Analysis
2.5. Scanning Electron Microscopy and Energy Dispersive X-ray Spectrometry Analysis
2.6. Statistical Analysis
3. Results
3.1. F− Level Measurement Using EPMA Analysis
3.2. Effect of Two Types of Meshes on the Improvement of APF Application via NCP Pre-Treatment
3.3. Dentinal Tubule Occlusion: SEM Analysis
3.4. Evaluation of the Dentin Re-Mineralization Effect by NCP Pre-Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mason, S.; Burnett, G.R.; Patel, N.; Patil, A.; Maclure, R. Impact of toothpaste on oral health-related quality of life in people with dentine hypersensitivity. BMC Oral Health 2019, 19, 226. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Tenenbaum, H.C.; Wilder, R.S.; Quock, R.; Hewlett, E.R.; Ren, Y.F. Pathogenesis, diagnosis and management of dentin hypersensitivity: An evidence-based overview for dental practitioners. BMC Oral Health 2020, 20, 220. [Google Scholar] [CrossRef] [PubMed]
- Elovikova, T.M.; Ermishina, E.Y.; Uvarova, L.V.; Koshcheev, A.S. The increased sensitivity of dentin: The mechanisms of remineralization using toothpaste with tin fluoride. Stomatologiia 2019, 95, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Abuzinadah, S.H.; Alhaddad, A.J. A randomized clinical trial of dentin hypersensitivity reduction over one month after a single topical application of comparable materials. Sci. Rep. 2021, 11, 2793. [Google Scholar] [CrossRef] [PubMed]
- Aminoshariae, A.; Kulild, J.C. Current concepts of dentinal hypersensitivity. J. Endod. 2021, 47, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Guerra, F.; Corridore, D.; Cocco, F.; Arrica, M.; Rinaldo, F.; Mazur, M.; Sanavia, C.; Nardi, G.M.; Campus, G. Oral health sentinel-based surveillance: A pilot study on dentinal hypersensitivity pain. Clin. Ter. 2017, 168, e333–e337. [Google Scholar] [CrossRef]
- Longridge, N.N.; Youngson, C.C. Dental Pain: Dentine Sensitivity, Hypersensitivity and Cracked Tooth Syndrome. Prim. Dent. J. 2019, 8, 44–51. [Google Scholar] [CrossRef]
- Bertani, G.; Di Tinco, R.; Bertoni, L.; Orlandi, G.; Pisciotta, A.; Rosa, R.; Rigamonti, L.; Signore, M.; Bertacchini, J.; Sena, P.; et al. Flow-dependent shear stress affects the biological properties of pericyte-like cells isolated from human dental pulp. Stem Cell Res. Ther. 2023, 14, 31. [Google Scholar] [CrossRef]
- Suge, T.; Ishikawa, K.; Matsuo, T.; Ebisu, S. Duration of dentin tubule occlusion by the calcium phosphate precipitation method: An in vivo study in beagle dogs. Dent. Mater. J. 2021, 40, 1020–1026. [Google Scholar] [CrossRef]
- Mukherjee, M.; Kalita, T.; Barua, P.; Barman, A.; Thonai, S.; Mahanta, P.S.; Medhi, H. Efficacy of Smear Layer Removal of Human Teeth Root Canals Using Herbal and Chemical Irrigants: An In Vitro Study. Cureus 2023, 15, e40467. [Google Scholar] [CrossRef]
- Charoenlarp, P.; Wanachantararak, S.; Vongsavan, N.; Matthews, B. Pain and the rate of dentinal fluid flow produced by hydrostatic pressure stimulation of exposed dentine in man. Arch. Oral Biol. 2007, 52, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Banfield, N.; Addy, M. Dentine hypersensitivity: Development and evaluation ofamodel in situ to study tubulepatency. J. Clin. Periodontol. 2004, 31, 325–335. [Google Scholar] [CrossRef] [PubMed]
- George, A.A.; Muruppel, A.M.; Lal, S. A Comparative Evaluation of the Effectiveness of Three Different Modalities in Occluding Dentinal Tubules: An In Vitro Study. J. Contemp. Dent. Pract. 2019, 20, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.O.C.; Cruz, A.A.C.F.; Santos, D.O.; Douglas-DE-Oliveira, D.W.; Flecha, O.D.; Gonçalves, P.F. Sensitivity and specificity of assessment scales of dentin hypersensitivity—An accuracy study. Braz. Oral Res. 2020, 34, e043. [Google Scholar] [CrossRef]
- Bamise, C.T.; Esan, T.A. Mechanisms and treatment approaches of dentine hypersensitivity: A literature review. Oral Health Prev. Dent. 2011, 9, 353–367. [Google Scholar]
- Kijsamanmith, K.; Wallanon, P.; Pitchayasatit, C.; Kittiratanaviwat, P. The effect of fluoride iontophoresis on seal ability of self-etch adhesive in human dentin in vitro. BMC Oral Health 2022, 22, 109. [Google Scholar] [CrossRef]
- Paik, Y.; Kim, M.J.; Kim, H.; Kang, S.W.; Choi, Y.K.; Kim, Y.I. The Effect of Biomimetic Remineralization of Calcium Phosphate Ion Clusters-Treated Enamel Surfaces on Bracket Shear Bond Strength. Int. J. Nanomed. 2023, 18, 4365–4379. [Google Scholar] [CrossRef]
- Ramli, R.; Ghani, N.; Taib, H.; Mat-Baharin, N.H. Successful management of dentin hypersensitivity: A narrative review. Dent. Med. Probl. 2022, 59, 451–460. [Google Scholar] [CrossRef]
- Yuan, P.; Shen, X.; Liu, J.; Hou, Y.; Zhu, M.; Huang, J.; Xu, P. Effects of dentifrice containing hydroxyapatite on dentinal tu-bule occlusion and aqueous hexavalent chromium cations sorption: A preliminary study. PLoS ONE 2012, 7, e45283. [Google Scholar] [CrossRef]
- Jafari, B.; Katoozian, H.R.; Tahani, M.; Ashjaee, N. A comparative study of bone remodeling around hydroxyapatite-coated and novel radial functionally graded dental implants using finite element simulation. Med. Eng. Phys. 2022, 102, 103775. [Google Scholar] [CrossRef]
- Braun, A.; Cichocka, A.; Semaan, E.; Krause, F.; Jepsen, S.; Frentzen, M. Root surfaces after ultrasonic instrumentation with a polishing fluid. Quintessence Int. 2007, 38, e490–e496. [Google Scholar] [PubMed]
- Tschoppe, P.; Zandim, D.L.; Martus, P.; Kielbassa, A.M. Enamel and dentine remineralization by nano-hydroxyapatite toothpastes. J. Dent. 2011, 39, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Gamagedara, T.P.; Ziana, H.F. Effects of hydroxyapatite nanoparticles on liver enzymes and blood components. J. Clin. Investig. Stud. 2018, 1, 1–5. [Google Scholar]
- Nam, S.-H.; Choi, B.B.R.; Kim, G.-C. The Whitening Effect and Histological Safety of Nonthermal Atmospheric Plasma Inducing Tooth Bleaching. Int. J. Environ. Res. Public Health 2021, 18, 4714. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lee, H.Y.; Lee, H.J.; Kim, J.B.; Kim, S.; Joo, J.Y.; Kim, G.C. Retention Improvement in Fluoride Application with Cold Atmospheric Plasma. J. Dent. Res. 2018, 97, 179–183. [Google Scholar] [CrossRef]
- Park, S.R.; Lee, H.W.; Hong, J.W.; Lee, H.J.; Kim, J.Y.; Choi, B.B.; Kim, G.C.; Jeon, Y.C. Enhancement of the killing effect of low-temperature plasma on Streptococcus mutans by combined treatment with gold nanoparticles. J. Nanobiotechnol. 2014, 8, 29. [Google Scholar] [CrossRef]
- Choi, B.B.R.; Choi, J.H.; Hong, J.W.; Song, K.W.; Lee, H.J.; Kim, U.K.; Kim, G.C. Selective Killing of Melanoma Cells With Non-Thermal Atmospheric Pressure Plasma and p-FAK Antibody Conjugated Gold Nanoparticles. Int. J. Med. Sci. 2017, 14, 1101–1109. [Google Scholar] [CrossRef]
- Eggers, B.; Wagenheim, A.M.; Jung, S.; Kleinheinz, J.; Nokhbehsaim, M.; Kramer, F.J.; Sielker, S. Effect of Cold Atmospheric Plasma (CAP) on Osteogenic Differentiation Potential of Human Osteoblasts. Int. J. Mol. Sci. 2022, 23, 2503. [Google Scholar] [CrossRef]
- Choi, B.-B.; Choi, J.-H.; Kang, T.-H.; Lee, S.-J.; Kim, G.-C. Enhancement of Osteoblast Differentiation Using No-Ozone Cold Plasma on Human Periodontal Ligament Cells. Biomedicines 2021, 9, 1542. [Google Scholar] [CrossRef]
- Park, N.S.; Yun, S.E.; Lee, H.Y.; Lee, H.J.; Choi, J.H.; Kim, G.C. No-ozone cold plasma can kill oral pathogenic microbes in H2O2-dependent and independent manner. Sci. Rep. 2022, 12, 7597. [Google Scholar] [CrossRef]
- Minetti, E.; Palermo, A.; Malcangi, G.; Inchingolo, A.D.; Mancini, A.; Dipalma, G.; Inchingolo, F.; Patano, A.; Inchingolo, A.M. Dentin, Dentin Graft, and Bone Graft: Microscopic and Spectroscopic Analysis. J. Funct. Biomater. 2023, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Favaro, Z.L.; Soares, P.V.; Cunha-Cruz, J. Prevalence of dentin hypersensitivity: Systematic review and meta-analysis. J. Dent. 2019, 81, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Davari, A.; Ataei, E.; Assarzadeh, H. Dentin hypersensitivity: Etiology, diagnosis and treatment; a literature review. J. Dent. 2013, 14, 136–145. [Google Scholar]
- West, N.X.; Seong, J.; Davies, M. Management of dentine hypersensitivity: Efficacy of professionally and self-administered agents. J. Clin. Periodontol. 2015, 42, S256–S302. [Google Scholar] [CrossRef]
- Lee, H.Y.; Choi, J.H.; Hong, J.W.; Kim, G.C. Comparative study of the Ar and He atmospheric pressure plasmas on E-cadherin protein regulation for plasma plasma mediated transdermal drug delivery. J. Phys. D Appl. Phys. 2018, 51, 11. [Google Scholar] [CrossRef]
- Abu, H.A.; Martinho, F.C.; Sellan, P.L.B.; Pampuri, C.R.; Torres, C.R.G.; Pucci, C.R. Effect of Remineralization Pretreatments on Human Dentin Permeability and Bond Strength. Int. J. Dent. 2023, 2023, 2182651. [Google Scholar] [CrossRef]
- Berg, C.; Unosson, E.; Engqvist, H.; Xia, W. Comparative Study of Technologies for Tubule Occlusion and Treatment of Dentin Hypersensitivity. J. Funct. Biomater. 2021, 12, 27. [Google Scholar] [CrossRef]
- Yuan, P.; Liu, S.; Lv, Y.; Liu, W.; Ma, W.; Xu, P. Effect of a dentifrice containing different particle sizes of hydroxyapatite on dentin tubule occlusion and aqueous Cr (VI) sorption. Int. J. Nanomed. 2019, 14, 5243–5256. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, B.-B.; Park, S.-A.; Choi, J.-H.; Park, S.-R.; Kim, G.-C. Evaluation of Dentin Tubule Occlusion Using Pre-Treatment with No-Ozone Cold Plasma: An In Vitro Study. Appl. Sci. 2023, 13, 11728. https://doi.org/10.3390/app132111728
Choi B-B, Park S-A, Choi J-H, Park S-R, Kim G-C. Evaluation of Dentin Tubule Occlusion Using Pre-Treatment with No-Ozone Cold Plasma: An In Vitro Study. Applied Sciences. 2023; 13(21):11728. https://doi.org/10.3390/app132111728
Chicago/Turabian StyleChoi, Byul-Bora, Seung-Ah Park, Jeong-Hae Choi, Sang-Rye Park, and Gyoo-Cheon Kim. 2023. "Evaluation of Dentin Tubule Occlusion Using Pre-Treatment with No-Ozone Cold Plasma: An In Vitro Study" Applied Sciences 13, no. 21: 11728. https://doi.org/10.3390/app132111728
APA StyleChoi, B.-B., Park, S.-A., Choi, J.-H., Park, S.-R., & Kim, G.-C. (2023). Evaluation of Dentin Tubule Occlusion Using Pre-Treatment with No-Ozone Cold Plasma: An In Vitro Study. Applied Sciences, 13(21), 11728. https://doi.org/10.3390/app132111728





