Economic Analysis of the Production Process of Probiotics Based on the Biological and Physiological Parameters of the Cells
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Cultures
2.2. Stresses
2.3. Fluid Bed Drying and Coating
2.4. Simulated Gastrointestinal Conditions
2.5. Intestinal Epithelial Cell Culture
2.6. Adhesion Assay
2.7. Imaging Flow Cytometry
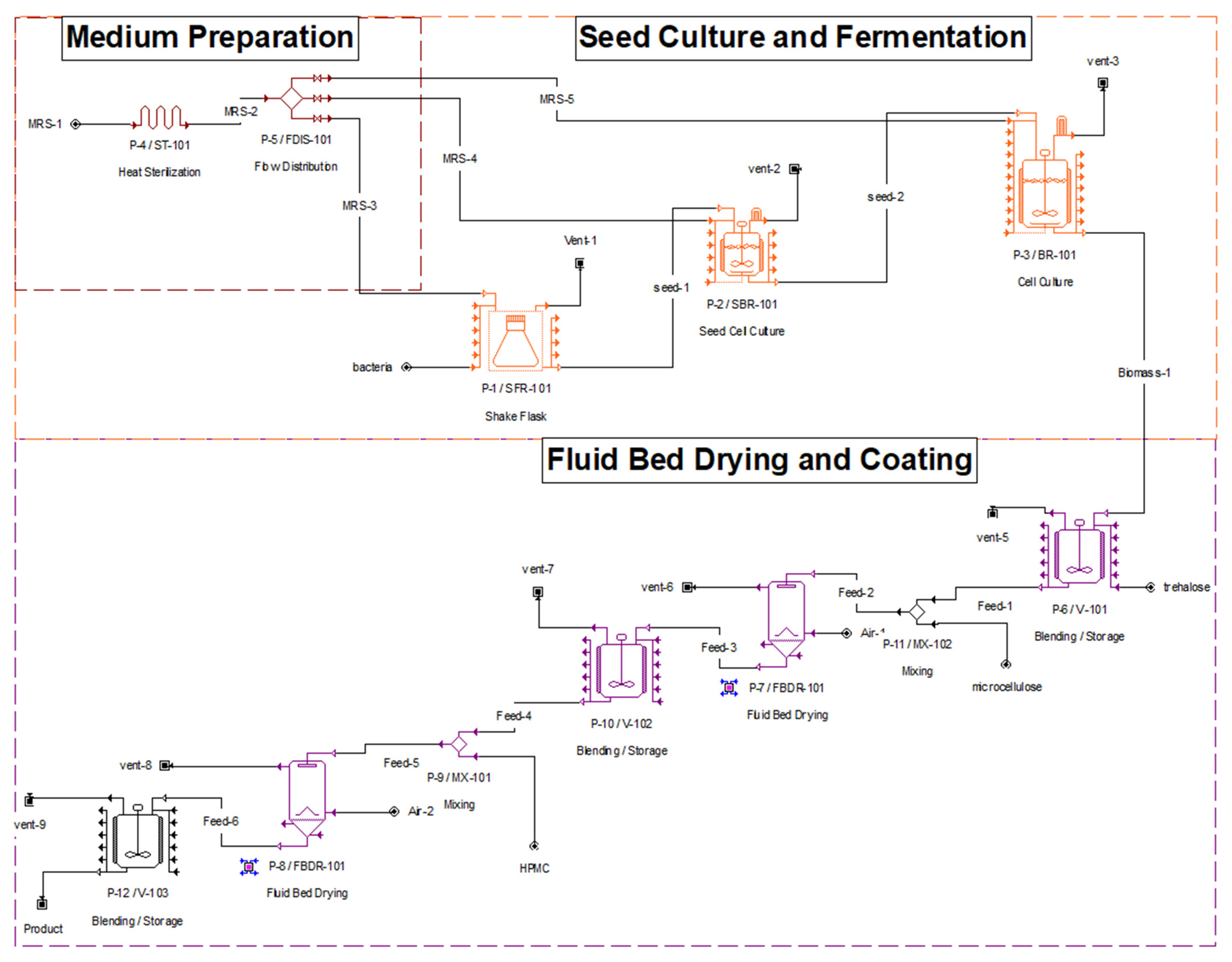
2.8. Process Simulation in SuperPro Designer
2.9. Sensitivity Analysis
2.10. Statistical Analysis
3. Results and Discussion
3.1. In Vitro Simulated Digestion
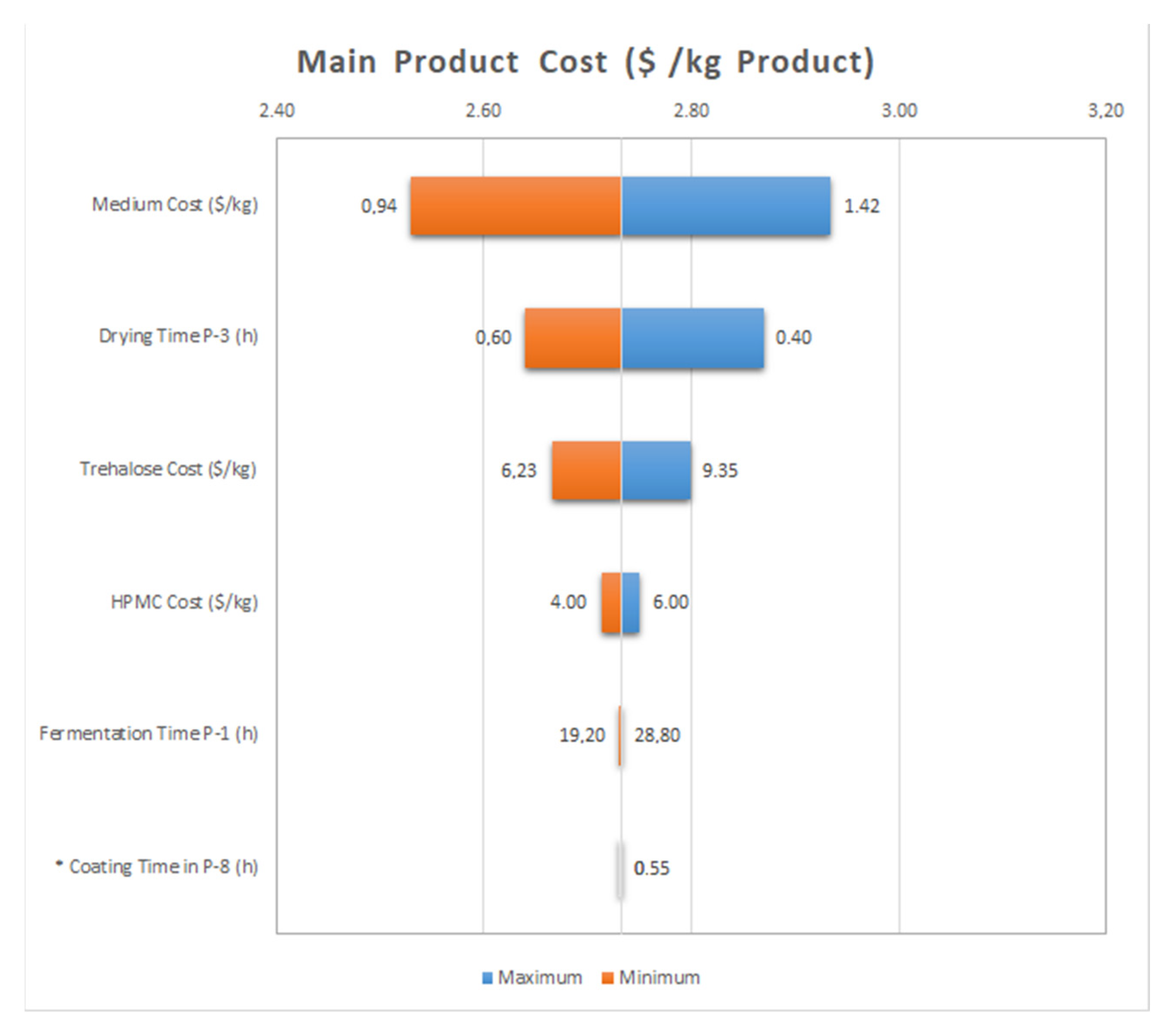
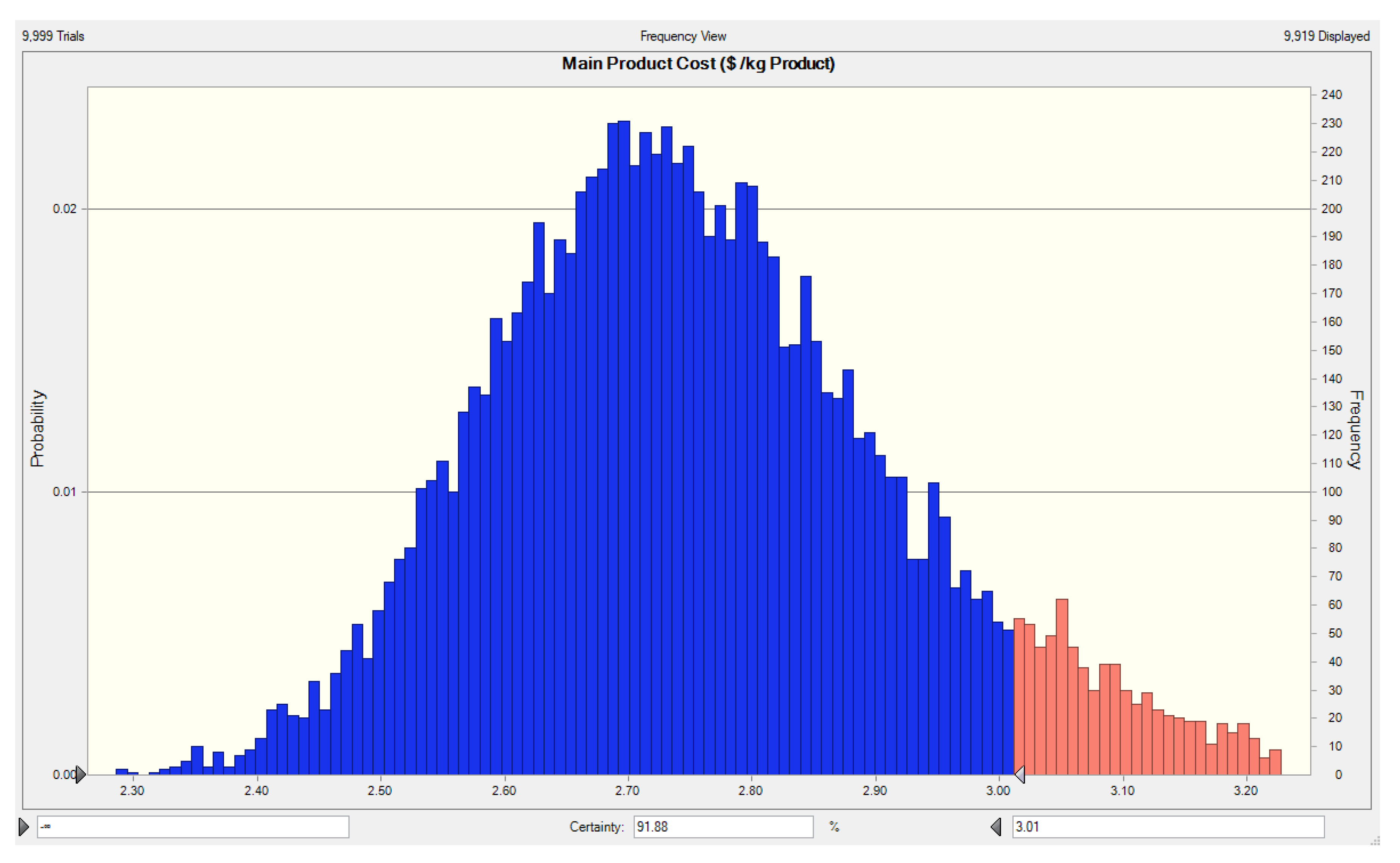
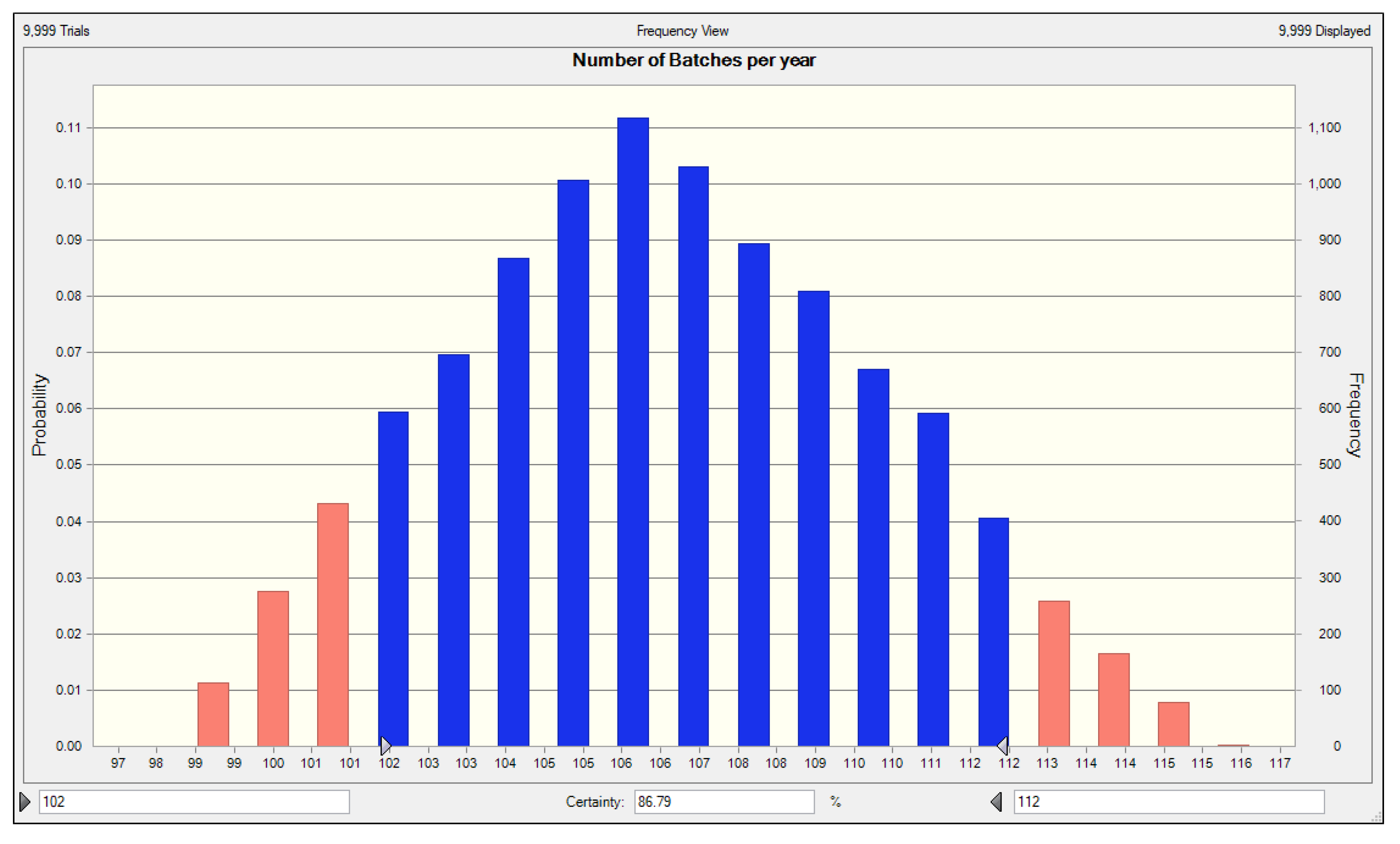
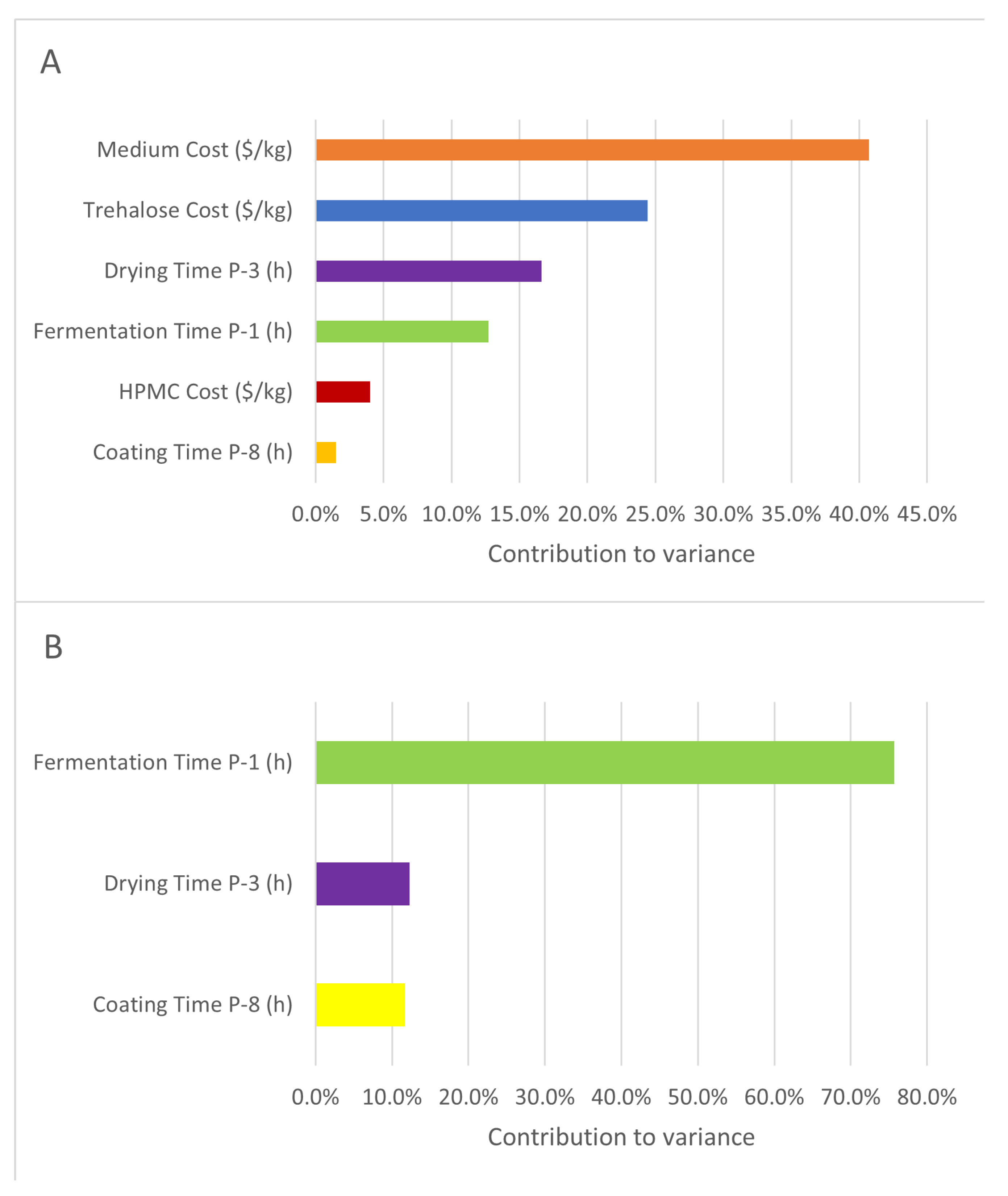
3.2. Process Design and Economic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Report of a Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic Acid Bacteria, Córdoba, Argentina, 1–4 October 2001; Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food, London, Ontario, Canada, 30 April–1 May 2002; Joint FAO WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food (Ed.) FAO Food and Nutrition Paper; Food and Agriculture Organization of the United Nations [u.a]: Rome, Italy, 2006; ISBN 978-92-5-105513-7. [Google Scholar]
- Pereira, J.V.; Gamage, H.K.A.H.; Cain, A.K.; Hayes, E.; Paulsen, I.T.; Tetu, S.G. High-Throughput Viability Testing of Microbial Communities in a Probiotic Product Using Flow Cytometry. Appl. Microbiol. 2023, 3, 1068–1082. [Google Scholar] [CrossRef]
- George Kerry, R.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of Probiotics for Human Health: A Review. J. Food Drug Anal. 2018, 26, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Broeckx, G.; Vandenheuvel, D.; Claes, I.J.J.; Lebeer, S.; Kiekens, F. Drying Techniques of Probiotic Bacteria as an Important Step towards the Development of Novel Pharmabiotics. Int. J. Pharm. 2016, 505, 303–318. [Google Scholar] [CrossRef]
- Fu, N.; Chen, X.D. Towards a Maximal Cell Survival in Convective Thermal Drying Processes. Food Res. Int. 2011, 44, 1127–1149. [Google Scholar] [CrossRef]
- Vass, P.; Domokos, A.; Pantea, E.; Szilágyi, B.; Molnár, M.; Záhonyi, P.; Nagy, B.; Nagy, Z.K. Processing of Thermosensitive Biological API from Suspension Using an Integrated Continuous Granulation—Drying—Milling Line into Powder Ready for Tableting. Dry. Technol. 2023, 41, 492–502. [Google Scholar] [CrossRef]
- Assari, M.R.; Basirat Tabrizi, H.; Najafpour, E. Energy and Exergy Analysis of Fluidized Bed Dryer Based on Two-Fluid Modeling. Int. J. Therm. Sci. 2013, 64, 213–219. [Google Scholar] [CrossRef]
- Mohideen Batcha, M.F.; Amirnordin, S.H.; Md Yudin, A.S. 4—Fluidized Bed Dryers. In Drying Technology in Food Processing; Jafari, S.M., Malekjani, N., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 67–122. ISBN 978-0-12-819895-7. [Google Scholar]
- Hathi, Z.; Mettu, S.; Priya, A.; Athukoralalage, S.; Lam, T.N.; Choudhury, N.R.; Dutta, N.K.; El-Omar, E.M.; Gong, L.; Mohan, G.; et al. Methodological Advances and Challenges in Probiotic Bacteria Production: Ongoing Strategies and Future Perspectives. Biochem. Eng. J. 2021, 176, 108199. [Google Scholar] [CrossRef]
- Han, S.; Lu, Y.; Xie, J.; Fei, Y.; Zheng, G.; Wang, Z.; Liu, J.; Lv, L.; Ling, Z.; Berglund, B.; et al. Probiotic Gastrointestinal Transit and Colonization After Oral Administration: A Long Journey. Front. Cell. Infect. Microbiol. 2021, 11, 609722. [Google Scholar] [CrossRef]
- Qi, Y.; Huang, L.; Zeng, Y.; Li, W.; Zhou, D.; Xie, J.; Xie, J.; Tu, Q.; Deng, D.; Yin, J. Pediococcus Pentosaceus: Screening and Application as Probiotics in Food Processing. Front. Microbiol. 2021, 12, 762467. [Google Scholar] [CrossRef]
- Song, A.-X.; Mao, Y.-H.; Siu, K.-C.; Tai, W.C.S.; Wu, J.-Y. Protective Effects of Exopolysaccharide of a Medicinal Fungus on Probiotic Bacteria during Cold Storage and Simulated Gastrointestinal Conditions. Int. J. Biol. Macromol. 2019, 133, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, Z.; Zhang, M.; Meng, L.; Ming, Z.; Liu, J. Bioinspired Oral Delivery of Gut Microbiota by Self-Coating with Biofilms. Sci. Adv. 2020, 6, eabb1952. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Han, S.; Zhang, S.; Wang, K.; Lv, L.; McClements, D.J.; Xiao, H.; Berglund, B.; Yao, M.; Li, L. The Role of Probiotic Exopolysaccharides in Adhesion to Mucin in Different Gastrointestinal Conditions. Curr. Res. Food Sci. 2022, 5, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.-N.; Wu, H.; Wang, X.-J.; He, L.-J.; Guo, C.-F. The Mechanism of Antimicrobial Activity of Conjugated Bile Acids against Lactic Acid Bacilli. Microorganisms 2023, 11, 1823. [Google Scholar] [CrossRef]
- Mukherjee, S.; Hooper, L.V. Antimicrobial Defense of the Intestine. Immunity 2015, 42, 28–39. [Google Scholar] [CrossRef]
- Long, S.L.; Gahan, C.G.M.; Joyce, S.A. Interactions between Gut Bacteria and Bile in Health and Disease. Mol. Asp. Med. 2017, 56, 54–65. [Google Scholar] [CrossRef]
- Zielińska, D.; Rzepkowska, A.; Radawska, A.; Zieliński, K. In Vitro Screening of Selected Probiotic Properties of Lactobacillus Strains Isolated from Traditional Fermented Cabbage and Cucumber. Curr. Microbiol. 2015, 70, 183–194. [Google Scholar] [CrossRef]
- Safeer Abbas, M.; Afzaal, M.; Saeed, F.; Asghar, A.; Jianfeng, L.; Ahmad, A.; Ullah, Q.; Elahi, S.; Ateeq, H.; Shah, Y.A.; et al. Probiotic Viability as Affected by Encapsulation Materials: Recent Updates and Perspectives. Int. J. Food Prop. 2023, 26, 1324–1350. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Wang, Q.; Zhang, Z.; Nie, W.; Shang, L. Armored Probiotics for Oral Delivery. Smart Med. 2023. [Google Scholar] [CrossRef]
- Buss, M.T.; Ramesh, P.; English, M.A.; Lee-Gosselin, A.; Shapiro, M.G. Spatial Control of Probiotic Bacteria in the Gastrointestinal Tract Assisted by Magnetic Particles. Adv. Mater. 2021, 33, 2007473. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.S.; Kwon, M.; Son, N.; Kim, S.-K.; Son, M.-Y. Multilayer Coating with Red Ginseng Dietary Fiber Improves Intestinal Adhesion and Proliferation of Probiotics in Human Intestinal Epithelial Models. J. Microbiol. Biotechnol. 2023, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rajam, R.; Subramanian, P. Encapsulation of Probiotics: Past, Present and Future. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 46. [Google Scholar] [CrossRef]
- Kiepś, J.; Juzwa, W.; Dembczyński, R. Imaging Flow Cytometry Demonstrates Physiological and Morphological Diversity within Treated Probiotic Bacteria Groups. Int. J. Mol. Sci. 2023, 24, 6841. [Google Scholar] [CrossRef]
- Anandharaj, M.; Rani, R.P.; Swain, M.R. Production of High-Quality Probiotics by Fermentation. In Microbial Functional Foods and Nutraceuticals; Gupta, V.K., Treichel, H., Shapaval, V., Antonio De Oliveira, L., Tuohy, M.G., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 235–266. ISBN 978-1-119-04901-2. [Google Scholar]
- Fiore, W.; Arioli, S.; Guglielmetti, S. The Neglected Microbial Components of Commercial Probiotic Formulations. Microorganisms 2020, 8, 1177. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Kiepś, J.; Juzwa, W.; Olejnik, A.; Sip, A.; Tomaszewska-Gras, J.; Dembczyński, R. The Effects of Cellular Membrane Damage on the Long-Term Storage and Adhesion of Probiotic Bacteria in Caco-2 Cell Line. Nutrients 2023, 15, 3484. [Google Scholar] [CrossRef] [PubMed]
- Bucka-Kolendo, J.; Sokołowska, B. Lactic Acid Bacteria Stress Response to Preservation Processes in the Beverage and Juice Industry. Acta Biochim. Pol. 2017, 64, 459–464. [Google Scholar] [CrossRef]
- da Cruz Rodrigues, V.C.; da Silva, L.G.S.; Simabuco, F.M.; Venema, K.; Antunes, A.E.C. Survival, Metabolic Status and Cellular Morphology of Probiotics in Dairy Products and Dietary Supplement after Simulated Digestion. J. Funct. Foods 2019, 55, 126–134. [Google Scholar] [CrossRef]
- Wang, A.; Lin, J.; Zhong, Q. Enteric Rice Protein-Shellac Composite Coating to Enhance the Viability of Probiotic Lactobacillus Salivarius NRRL B-30514. Food Hydrocoll. 2021, 113, 106469. [Google Scholar] [CrossRef]
- Huang, S.; Vignolles, M.-L.; Chen, X.D.; Le Loir, Y.; Jan, G.; Schuck, P.; Jeantet, R. Spray Drying of Probiotics and Other Food-Grade Bacteria: A Review. Trends Food Sci. Technol. 2017, 63, 1–17. [Google Scholar] [CrossRef]
- Fu, N.; Huang, S.; Xiao, J.; Chen, X.D. Producing Powders Containing Active Dry Probiotics With the Aid of Spray Drying. Adv. Food Nutr. Res. 2018, 85, 211–262. [Google Scholar] [CrossRef] [PubMed]
- Haffner, F.B.; Diab, R.; Pasc, A.; Haffner, F.B.; Diab, R.; Pasc, A. Encapsulation of Probiotics: Insights into Academic and Industrial Approaches. Aimsmates 2016, 3, 114–136. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Ayrapetyan, M.; Williams, T.; Oliver, J.D. Relationship between the Viable but Nonculturable State and Antibiotic Persister Cells. J. Bacteriol. 2018, 200, e00249-18. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s), and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
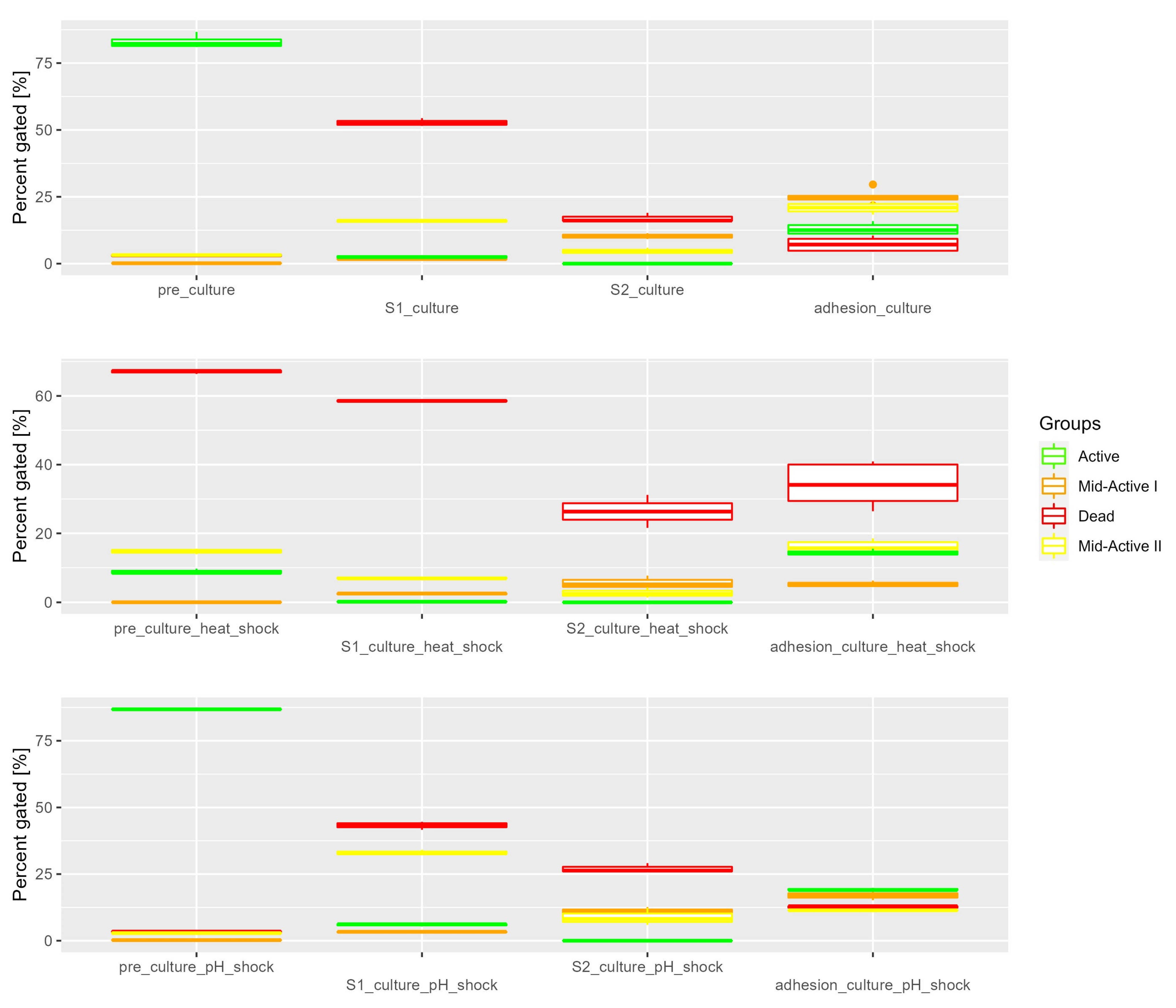
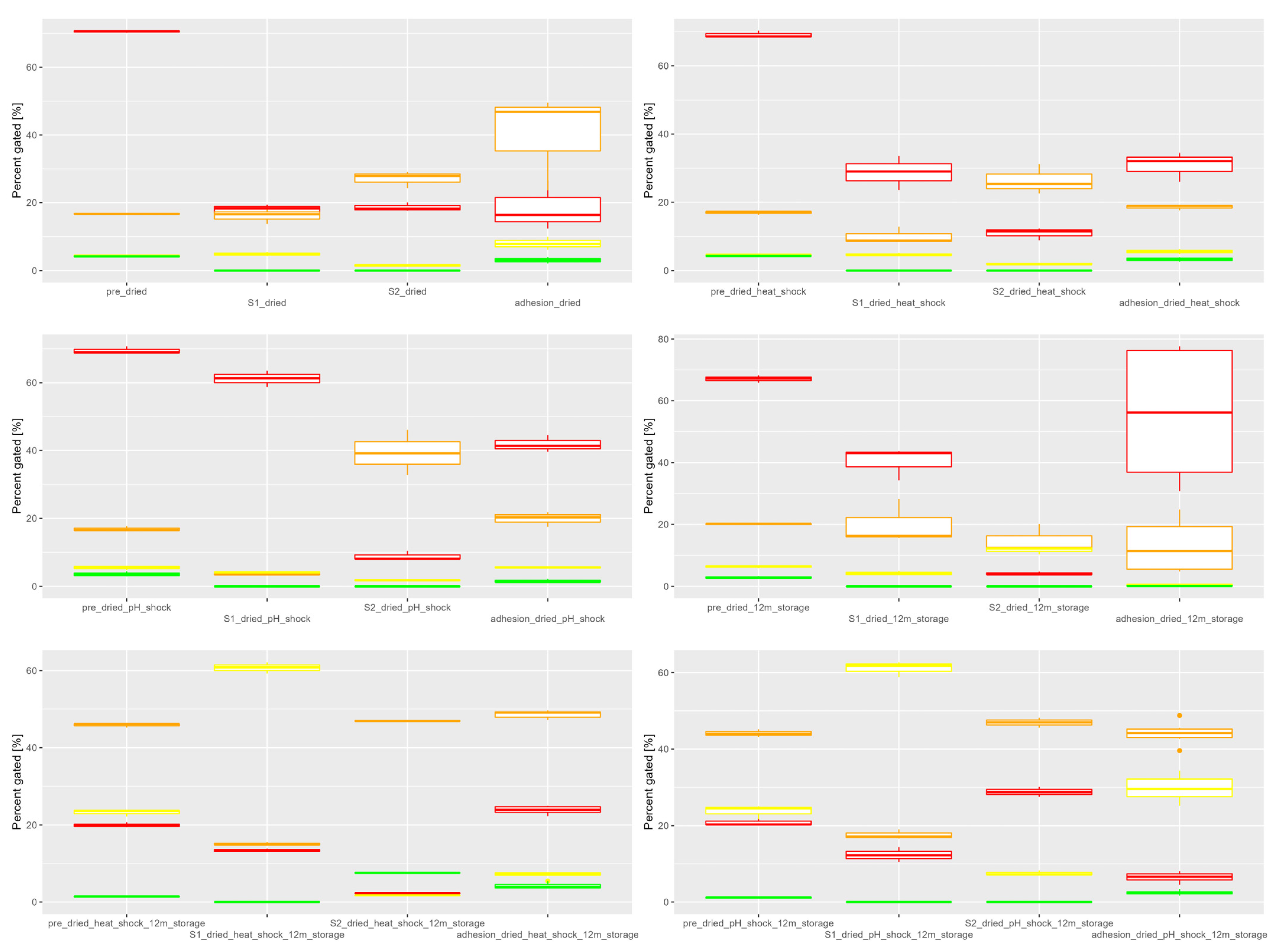
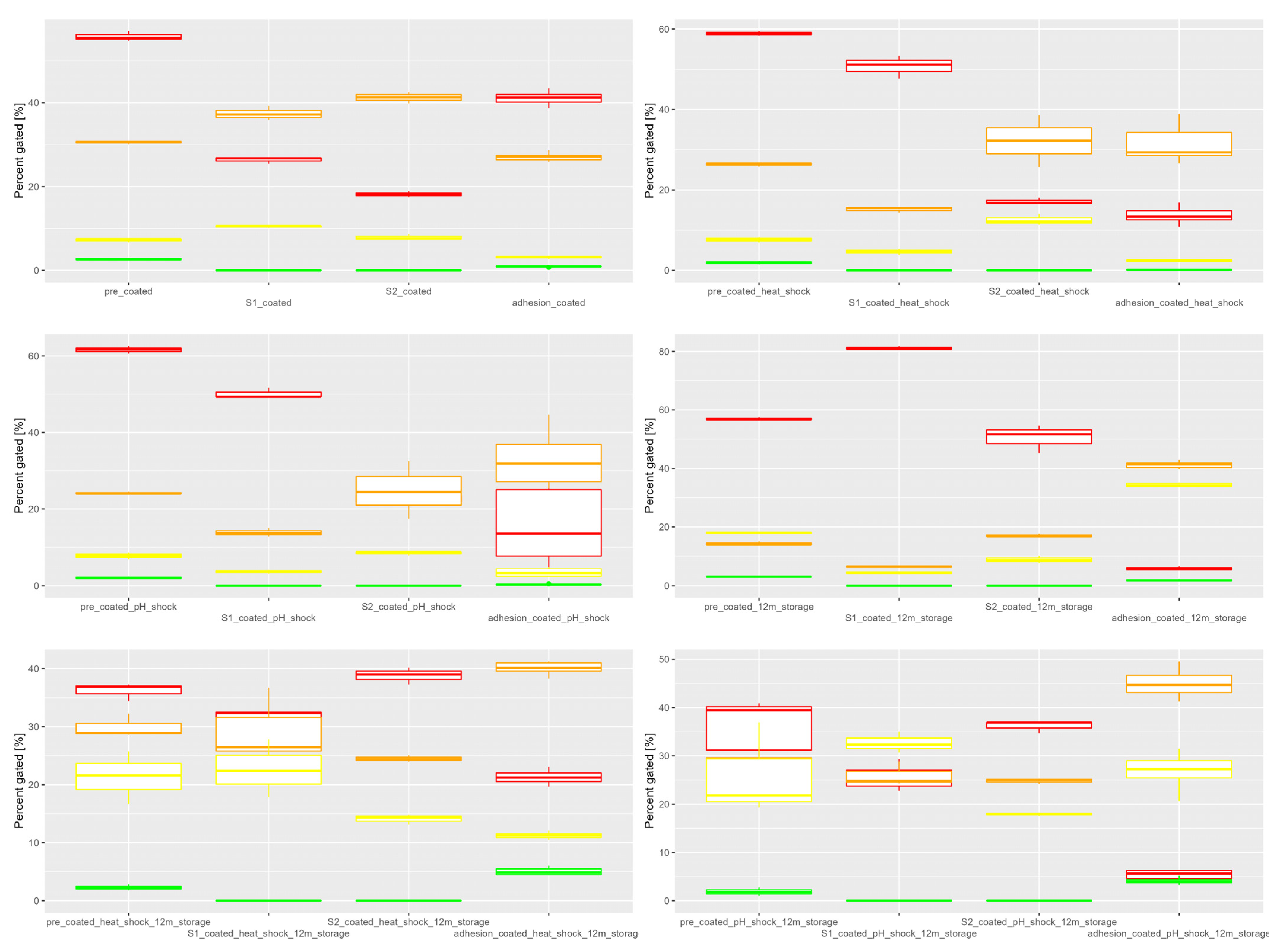
| Constituent | SGF | SIF |
|---|---|---|
| pepsin | 2000 U/mL | - |
| pancreatin | - | 100 U/mL (based on trypsin) |
| bile | - | 10 mM |
| CaCl2 | 0.075 mM | 0.3 mM |
| pH | 3 | 7 |
| Parameter | Unit | |
|---|---|---|
| DFC | $ | 1,584,000 |
| TPDC | $ | 861,000 |
| TPIC | $ | 516,000 |
| CFC | $ | 207,000 |
| Operating cost | $ | 100,000 |
| Batch size | kg | 326.91 |
| Cost basis annual | kg/year | 34,979 |
| Gross margin | % | 71.98 |
| Return on investment (ROI) | % | 11.51 |
| Payback time (PBT) | year | 4.33 |
| Net present value (NPV at 7%) | $ | 909,000 |
| Revenues (per year) | $ | 350,000 |
| Equipment | Size | Purchase cost (PC) ($) |
|---|---|---|
| Fluid bed dryer | 415.97 L | 107,000 |
| Bioreactor | 296.11 L | 150,000 |
| Seed bioreactor | 18.34 L | 29,950 |
| Heat sterilizer | 66.17 L/h rated by throughput | 30,000 |
| Blending tank | 308.29 L | 11,400 |
| Shake flask rack | 2 L | 1000 |
| Variant | Unit Production Cost ($/kg) | Production Cost ($/1 kg VB Cells Pre-Digestion) | Production Cost ($/1 kg VB Cells after Digestion) |
|---|---|---|---|
| Dried A | 2.59 | 30.58 cde ± 1.05 | 173.83 a ± 32.59 |
| Dried, 12 months B | 2.59 | 28.09 cde ± 2.45 | 22.19 cde ± 2.11 |
| Dried heat shock A | 2.59 | 29.40 cde ± 0.13 | 134.20 b ± 12.05 |
| Dried heat shock 12 months B | 2.59 | 10.52 e ± 0.36 | 27.91 cde ± 0.98 |
| Dried pH shock A | 2.63 | 29.35 cde ± 1.99 | 144.51 b ± 10.82 |
| Dried pH shock 12 months B | 2.63 | 10.58 e ± 0.37 | 35.11 cd ± 2.87 |
| Coated A | 3.01 | 30.31 cde ± 1.73 | 38.18 c ± 3.36 |
| Coated 12 months B | 3.01 | 14.36 cde ± 0.27 | 34.13 cde ± 4.06 |
| Coated heat shock A | 3.01 | 31.45 cde ± 2.77 | 24.03 cde ± 2.48 |
| Coated heat shock 12 months B | 3.01 | 12.73 de ± 1.87 | 21.38 cde ± 1.41 |
| Coated pH shock A | 3.05 | 31.00 cde ± 1.77 | 36.22 cd ± 2.44 |
| Coated pH shock 12 months B | 3.05 | 10.95 e ± 1.24 | 17.02 de ± 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiepś, J.; Olejnik, A.; Juzwa, W.; Dembczyński, R. Economic Analysis of the Production Process of Probiotics Based on the Biological and Physiological Parameters of the Cells. Appl. Sci. 2023, 13, 11541. https://doi.org/10.3390/app132011541
Kiepś J, Olejnik A, Juzwa W, Dembczyński R. Economic Analysis of the Production Process of Probiotics Based on the Biological and Physiological Parameters of the Cells. Applied Sciences. 2023; 13(20):11541. https://doi.org/10.3390/app132011541
Chicago/Turabian StyleKiepś, Jakub, Anna Olejnik, Wojciech Juzwa, and Radosław Dembczyński. 2023. "Economic Analysis of the Production Process of Probiotics Based on the Biological and Physiological Parameters of the Cells" Applied Sciences 13, no. 20: 11541. https://doi.org/10.3390/app132011541
APA StyleKiepś, J., Olejnik, A., Juzwa, W., & Dembczyński, R. (2023). Economic Analysis of the Production Process of Probiotics Based on the Biological and Physiological Parameters of the Cells. Applied Sciences, 13(20), 11541. https://doi.org/10.3390/app132011541







