The Use of Effective Microorganisms as a Sustainable Alternative to Improve the Quality of Potatoes in Food Processing
Abstract
1. Introduction
2. Material and Methods
2.1. Characteristics of Potato Cultivars
2.2. Characteristics of the EM Farming Preparation
2.3. Field Studies
2.4. Collection and Taking Samples for Qualitative Research
2.5. Determination of Content and Yield of Dry Matter and Starch
2.6. Chip Quality Determination
2.7. Methodology of Soil Sample Evaluation
2.8. Soil Conditions
2.9. Meteorological Conditions
2.10. Statistical Calculations
3. Results
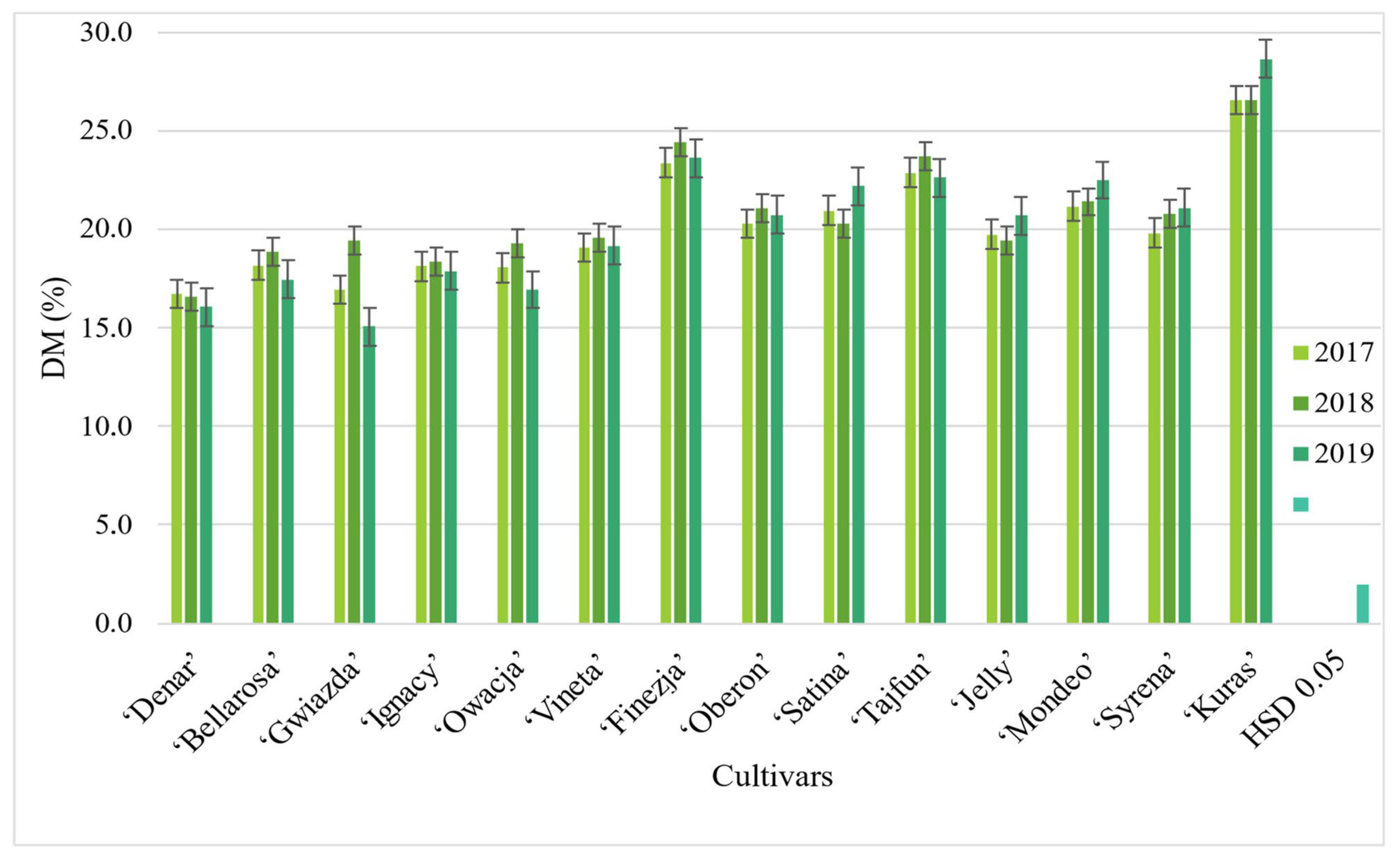
3.1. Content and Yield of Dry Matter
3.2. Starch Content and Yield
3.3. Quality of Chips
3.4. Variability of Features and Their Contribution to the Total Variance
3.5. Interaction of Features
4. Discussion
4.1. Influence of Cultivation Technology on Yield of Dry Matter and Starch and Quality of Chips
4.2. Influence of Biotic and Abiotic Factors on the Quality of Chips
4.3. Phenotypic Variability of Potato and Its Products
4.4. Relationship between Qualitative Features and Yield of Dry Matter and Starch
5. Orientation towards the Future
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janas, R. Możliwości wykorzystania Efektywnych Mikroorganizmów w ekologicznych systemach produkcji roślin uprawnych. Problemy Inżynierii Rolniczej 2009, 3, 111–119. (In Polish) [Google Scholar]
- Schneider, Z. Biochemiczne podstawy funkcjonowania EM-FarmingTM. Naturalne probiotyczne mikroorganizmy. Wyd. Stowarzyszenie Ekosystem. Licheń 2009, 2009, 14–16. (In Polish) [Google Scholar]
- Kołodziejczyk, M. Effectiveness of nitrogen fertilization and application of microbial preparations in potato cultivation. TJAF 2014, 38, 299–310. [Google Scholar] [CrossRef]
- Kołodziejczyk, M. Effect of nitrogen fertilization and microbial preparations on potato yielding. Plant Soil Environ. 2014, 60, 379–386. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M. Wpływ użyźniacza glebowego UGmax na plon ziemniaka i jego strukturę. Biuletyn IHAR 2013, 267, 107–112. (In Polish) [Google Scholar] [CrossRef]
- Szewczuk, C.; Sugier, D.; Baran, S.; Bielińska, E.J.; Gruszczyk, M. Wpływ preparatów użyźniających i zróżnicowanych dawek nawozów na wybrane właściwości chemiczne gleb oraz plon i cechy jakościowe bulw ziemniaka. Ann. UMCS 2016, E-71, 65–79. (In Polish) [Google Scholar] [CrossRef]
- Paśmionka, I.; Kotarba, C. Możliwości wykorzystania efektywnych mikroorganizmów w ochronie środowiska. Kosmos. Problemy Nauk Biologicznych 2015, 64, 173–184. (In Polish) [Google Scholar]
- Vaitkevičienė, N.; Jariene, E.; Danilcenko, H.; Sawicka, B. Effect of biodynamic preparations on the content of some mineral elements and starch in tubers of three colored potato cultivars. J. Elem. 2021, 21, 927–9935. [Google Scholar] [CrossRef]
- Vaitkevičienė, N. The Effect of Biodynamic Preparations on the Accumulation of Biologically Active Compounds in the Tubers of Different Genotypes of Ware Potatoes. Doctoral Dissertation, Aleksandras Stulginskis University, Kaunas, Lithuania, 2016; 121p. [Google Scholar]
- Martyniuk, S.; Księżak, J. Ocena wpływu pseudomikrobiologicznych biopreparatów stosowanych w uprawie roślin. Pol. J. Argon. 2011, 6, 27–33. (In Polish) [Google Scholar]
- Kaczmarek, Z.; Owczarzak, W.; Mrugalska, L.; Grzelak, M. The influence of effective microorganisms for some of physical and water properties on arable-humus horizons of mineral soils. J. Res. Appl. Agric. Eng. 2007, 52, 73–77. [Google Scholar]
- Szembowski, B. Experiences of the farm in Trankwice with the EM-Farming TM biotechnology. In Natural Probiotic Microorganisms; Publishing House of the Ecosystem Association; Basilica of Our Lady of Licheń: Wielkopolskie, Poland, 2009; pp. 56–58. (In Polish) [Google Scholar]
- Wu, F.; Wang, W.; Ma, Y.; Liu, Y.; Ma, X.; An, L.; Feng, H. Prospect of beneficial microorganisms applied in potato cultivation for sustainable agriculture. Afr. J. Microbiol. Res. 2013, 7, 2150–2158. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B. The effect of application of biopreparations and fungicides on the yield and selected parameters of seed value of seed potatoes. Acta Agrophys. 2018, 25, 239–255. [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B. Wpływ stosowania fungicydów, preparatów mikrobiologicznych i wyciągów z ziół na kształtowanie plonu ziemniaka. Fragm. Agronom. 2018, 35, 81–105. (In Polish) [Google Scholar] [CrossRef]
- Pszczółkowski, P.; Sawicka, B.; Danilčenko, H.; Jariene, E. The role of microbiological preparations in improving the quality of potato tubers. In Proceedings of the International Scientific Conference ‘New Trends in Food Safety and Quality’, Akademija, Lithuania, 5–7 December 2017; Aleksandras Stulginskis University: Akademija, Lithuania, 2017; pp. 22–23. ISBN 978-609-449-120-7. [Google Scholar]
- Sawicka, B.; Pszczółkowski, P.; Noaema, A.H.; Krochmal-Marczak, B.; Kiełtyka-Dadasiewicz, A. Effective microorganisms in agriculture and food processing. In Contemporary Research on the State of the Environment and the Medicinal Use of Plants; Chwil, M., Michał, M., Wyd, S., Eds.; University of Life Sciences in Lublin: Lublin, Poland, 2019; pp. 45–65. ISBN 978-83-7259-310-8. (In Polish). [Google Scholar] [CrossRef]
- Sawicka, B.; Pszczółkowski, P.; Kiełtyka-Dadasiewicz, A.; Ćwintal, M.; Krochmal-Marczak, B. Effect of effective microorganisms on the quality of potatoes in food processing. Appl. Sci. 2021, 11, 1415. [Google Scholar] [CrossRef]
- Huk, W. Znaczenie i zastosowanie mikroorganizmów w uprawie roślin. In Naturalne Probiotyczne Mikroorganizmy; Basilica of Our Lady of Licheń: Wielkopolskie, Poland, 2009; pp. 61–63. (In Polish) [Google Scholar]
- Higa, T. Effective microorganisms—Their role in Kyusei Nature Farming and sustainable agriculture. In Proceedings of the Third International Conference on Kyusei Nature Farming; USDA: Washington, DC, USA, 1996. [Google Scholar]
- Higa, T. Rewolucja w ochronie naszej planety. In Fundacja Rozwój, SGGW, Wydawnictwo; Greenland-Technologia EM: Warszawa, Poland, 2003. (In Polish) [Google Scholar]
- Higa, T.; Parr, J.F. Beneficial and effective microorganisms for a sustainable agriculture and environment. Int. Nat. Farming Res. Cent. Atami. Jaoan D 1994, 16, 3. [Google Scholar]
- Gałązka, A.; Kocoń, A. Evaluation of the effectiveness of preparations with beneficial microorganisms on the enzymatic activity of soil. Stud. Rep. IUNG-PIB 2015, 45, 143–154. [Google Scholar] [CrossRef]
- Wadas, W.; Dziugieł, T. Changes in Assimilation Area and Chlorophyll Content of Very Early Potato (Solanum tuberosum L.) Cultivars as Influenced by Biostimulants. Agronomy 2020, 10, 387. [Google Scholar] [CrossRef]
- Lancaster, S.H.; Haney, R.L.; Senseman, S.A.; Hons, F.M.; Handler, J.M. Soil microbial activity is affected by Roundup Weather Max and pesticides applied to cotton (Gossypium hirsutum). J. Agric. Food Chem. 2006, 54, 7221–7226. [Google Scholar] [CrossRef]
- Jarienė, E.; Vaitkevičienė, N.; Danilčenko, H.; Tajner-Czopek, A.; Rytel, A.; Kucharska, A.; Sokół-Łętowska, A.; Gertchen, M.; Jeznach, M. Effect of biodynamic preparations on the phenolic antioxidants in potatoes with colored-flesh. Biol. Agric. Hortic. 2017, 35, 132–142. [Google Scholar] [CrossRef]
- Egbuna, C.; Sawicka, B.; Tijjanzi, H.; Toskë, L.; Jonathan, K.; Ifemeje, C.; Skiba, D.; Lukong, B.C. Biopesticides, Safety Issues and Market Trends. In Natural Remedies for Pest, Disease and Weed Control. PART I. Green Approach to Pest and Disease Control; EGBUNA-9780128193044 Chapter: FM-CTR-01; Egbuna, C., Sawicka, B., Eds.; Elsevier Inc.; Academic Press: New York, NY, USA, 2020; pp. 43–54. ISBN 978-0-12-819304-4. [Google Scholar] [CrossRef]
- Van Vliet, P.C.J.; Bloem, J.; de Goede, R.G.M. Microbial diversity, nitrogen loss and grass production after addition of Effective Micro-organisms® (EM) to slurry manure. Appl. Soil Ecol. 2006, 32, 188–198. [Google Scholar] [CrossRef]
- WRB. World Reference Base for Soil Resources 2014 International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports 106; Food and Agriculture Organization of The United Nations: Rome, Italy, 2014. [Google Scholar]
- Roztropowicz, S.; Czerko, Z.; Głuska, A.; Goliszewski, W.; Gruczek, T.; Lis, B.; Lutomirska, B.; Nowacki, W.; Rykaczewska, K.; Sowa-Niedziałkowska, G.; et al. Methodic of Observation, Measurements and Sample Take in Agricultural Experiments with Potato; Plant Breeding Acclimatization Institute: Jadwisin, Poland, 1999; p. 50. (In Polish) [Google Scholar]
- Grudzińska, M.; Zgórska, K. Effect of sugar content in potato tubers on colour of chips. Żywność Nauka Technol. Jakość 2008, 5, 107–115. [Google Scholar]
- PN-EN ISO 10520; Skrobia Naturalna [Norma] Oznaczanie Zawartości Skrobi: Metoda Polarymetryczna Ewersa. Polski Komitet Normalizacyjny: Warszawa, Poland, 2002; ISBN 8323695156. (In Polish)
- Mozolewski, W. Research on Relations between the Quality of Potato Cultivars and the Quality of PC and FF; Spread. Monogr.; Uniwersytet Warmińsko-Mazurski: Olsztyn, Poland, 2005; p. 77. (In Polish) [Google Scholar]
- EN ISO 8586:2014-03; Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Sensory Evaluation Experts. ISO: Geneva, Switzerland, 2014. Available online: https://sklep.pkn.pl/pn-en-iso-8586-2014-03e.html (accessed on 20 November 2020).
- PN-A-74780; Processed Potatoes. They Have Potato Snacks. Polish Accreditation Committee: Warsaw, Poland, 1996.
- Ryzak, M.; Bartmiński, P.; Bieganowski, A. Methods of determination of granulometric distribution of mineral soils. Acta Agrophysica Theses Monogr. 2009, 175, 97. [Google Scholar]
- KQ/PB-34 version 04 from 07.10.2011. Zakres Akredytacji Laboratorium Badawczego/Scope of Accreditation for Testing Laboratory Nr/No AB 1186. 2011. Available online: https://www.nil.gov.pl/app/uploads/2021/09/AB-774-zakres19.pdf (accessed on 30 December 2020). (In Polish)
- ISO 10390:2005; Soil Quality—Determination of pH. ISO: Geneva, Switzerland, 2005; This Standard has been Revised by ISO 10390:2021. Available online: https://www.iso.org/standard/40879.html (accessed on 9 June 2023).
- Polish Standard PN-R-04023: 1996; Chemical and Agricultural Analysis of Soil. Determination of Available Phosphorus Content in Mineral Soils. Polish Committee for Standardization: Warsaw, Poland, 1996. (In Polish)
- Polish Standard PN-R-04022: 1996 + AZ1: 2002; Chemical and Agricultural Analysis of soil. Determination of Available Potassium Content in Mineral Soils. Polish Committee for Standardization: Warsaw, Poland, 2002.
- Polish Standard PN-R-04020 1994 + AZ1: 2004; Chemical and Agricultural Analysis of Soil. Polish Committee for Standardization: Warsaw, Poland, 2004. (In Polish)
- Polish Standard PN-R-04017: 1992; Content of Copper Soluble in HCl in Mineral Soil. Polish Committee for Standardization: Warsaw, Poland, 1992. (In Polish)
- Polish Standard PN-R-04019: 1993; Content of Manganese Soluble in HCl in Mineral Soil. Polish Committee for Standardization: Warsaw, Poland, 1993. (In Polish)
- Polish Standard PN-R-04021: 1994; The Content of Iron Soluble in HCl in Mineral Soil. Polish Committee for Standardization: Warsaw, Poland, 1994. (In Polish)
- Nawrocki, S.; Nawozowe, Z.; Część, I. Liczby Graniczne do Wyceny Zawartości w Glebach Makro-i Mikroelementów; Wyd. IUNG: Puławy, Poland, 1985; p. 38ss. (In Polish) [Google Scholar]
- Skowera, B. Changes of hydrothermal conditions in the Polish area (1971−2010). Fragm. Agron. 2014, 31, 74–87. [Google Scholar]
- SAS Institute Inc. SAS/STAT®9.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Koronacki, J. Statistics, for Students of Technical and Natural Sciences; Scientific and Technical Publishing House: Warsaw, Poland, 2009; p. 491. (In Polish)
- Boligłowa, E. Ochrona ziemniaka przed chorobami i szkodnikami przy użyciu Efektywnych Mikroorganizmów (EM) z udziałem ziół. In Wybrane Zagadnienia Ekologiczne We Współczesnym Rolnictwie; Red, Z.Z., Ed.; PIMR: Poznań, Poland, 2005; pp. 165–170. (In Polish) [Google Scholar]
- Boligłowa, E.; Gleń, K. Assessment of Effective Microorganism activity (EM) in winter wheat protection against fungal diseases. Ecol. Chem. Eng. A 2008, 15, 23–27. [Google Scholar]
- Martyniuk, S. Production of microbiological preparations on the example of symbiotic bacteria in legumes. J. Res. Appl. Agric. Eng. 2010, 55, 20–23. (In Polish) [Google Scholar]
- Małuszyńska, E.; Szydłowska, A.; Martyniak, D.; Dziamba, S.; Dziamba, J. Wpływ preparatów zawierających efektywne mikroorganizmy na zdolność kiełkowania nasion z upraw ekologicznych. Biuletyn IHAR 2012, 263, 33–42. (In Polish) [Google Scholar] [CrossRef]
- Polivanova, O.B.; Tiurin, K.N.; Sivolapova, A.B.; Goryunova, S.V.; Zhevora, S.V. Influence of Increased Radiation Background on Antioxidative Responses of Helianthus tuberosus L. Antioxidants 2023, 12, 956. [Google Scholar] [CrossRef]
- Lisińska, G. Technological value and consumption quality of Polish potato cultivars. Zesz. Probl. Post. Sci. Agric. 2006, 511, 81–94. (In Polish) [Google Scholar]
- Krzysztofik, B.; Sułkowski, K. Zmiany składu chemicznego bulw ziemniaka podczas przechowywania i ich wpływ na wybrane właściwości chipsów. Inżynieria Rolnicza 2013, 4, 161–169. (In Polish) [Google Scholar]
- Zychnowska, M.; Krygier, K.; Iwańczuk, M. Analiza zawartości i jakości tłuszczu w polskich smażonych chipsach ziemniaczanych. Bromatolo. Chem. Toksykol. 2015, 48, 622–629. (In Polish) [Google Scholar]
- Gonçalves, E.M.; Pereira, N.; Silva, M.; Alvarenga, N.; Ramos, A.C.; Alegria, C.; Abreu, M. Influence of Air-Drying Conditions on Quality, Bioactive Composition and Sensorial Attributes of Sweet Potato Chips. Foods 2023, 12, 1198. [Google Scholar] [CrossRef]
- Kita, A.; Figiel, A. Effects of thermal treatment parameters on selected properties of potato chips. Acta Agrophys. 2009, 14, 609–617. [Google Scholar]
- Khayatnezhad, M.; Shahriari, R.; Gholamin, R.; Jamaati-e-Somarin, S.; Zabihi-e-Mahmoodabad, R. Correlation and path analysis between yield and yield components in potato (Solanum tuberosum L.). Middle-East J. Sci. Res. 2011, 7, 17–21. [Google Scholar]
- Dighton, J.; Tugay, T.; Zhdanova, N. Fungi and Ionizing Radiation from Radionuclides: Fungi and Ionizing Radiation. FEMS Microbiol. Lett. 2008, 281, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Kalita, M.C.; Thakur, D. Broad Spectrum Antimicrobial Activity of Forest-Derived Soil Actinomycete, Nocardia sp. PB-52. Front. Microbiol. 2016, 18, 347. [Google Scholar] [CrossRef]
- Chęciński, G.; Czernaś, K. Pożyteczne mikroorganizmy “ProBio-Emy” i ich praktyczne zastosowanie. Gaz Woda i Technika Sanitarna 2012, 2, 58–61. (In Polish) [Google Scholar]
- Anonymous 2023. Common Agricultural Policy for 2023–2027 28 Cap Strategic Plans at a Glance 2023. Available online: https://agriculture.ec.europa.eu/system/files/2022-12/csp-at-a-glance-eu-countries_en.pdf (accessed on 9 June 2023).
| Cultivars | Maturity Group | Color of Skin | Color of the Flesh | Shape of the Tubers | Depth of the Tuber Eyes at 9° Scale * |
|---|---|---|---|---|---|
| ‘Denar’ | Very early | Yellow | Light yellow | Round oval | 7.0 |
| ‘Bellarosa’ | Early | Red | Yellow | Round oval | 7.0 |
| ‘Gwiazda’ | Early | Yellow | Yellow | Round oval | 7 |
| ‘Ignacy’ | Early | Yellow | Light yellow | Round oval | 6.5 |
| ‘Owacja’ | Early | Yellow | Light yellow | Round oval | 7.0 |
| ‘Vineta’ | Early | Yellow | Yellow | Round | 7.0 |
| ‘Finezja’ | Medium-early | Yellow | Light yellow | Round oval | 7.0 |
| ‘Oberon’ | Medium-early | Red | Light yellow | Oval | 7.0 |
| ‘Satina’ | Medium-early | Yellow | Yellow | Round oval | 7.5 |
| ‘Tajfun’ | Medium-early | Yellow | Yellow | Oval | 7.0 |
| ‘Jelly’ | Medium-late | Yellow | Yellow | Oval | 7.5 |
| ‘Mondeo’ | Medium-late | Yellow | Creamy | Round oval | 7.0 |
| ‘Syrena’ | Medium-late | Yellow | Yellow | Oval | 7.0 |
| ‘Kuras’ | Late | Yellow | Creamy | Round | 6.0 |
| Year | Content of Assimilable Macronutrients (mg kg−1 Soil) | Content of Humus [%] | pH KCl | Content of Micronutrients (mg kg−1 Soil) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P2O5 | K2O | Mg | Cu | Mn | Zn | Fe | B | |||
| 2017 | 207 | 121 | 75 | 0.99 | 6.10 | 5.72 | 311 | 41.3 | 3822 | 6.23 |
| 2018 | 193 | 111 | 70 | 1.04 | 5.92 | 4.80 | 319 | 50.5 | 3726 | 5.74 |
| 2019 | 221 | 114 | 68 | 1.00 | 5.77 | 7.98 | 265 | 43.2 | 3676 | 6.00 |
| Average | 207 | 115 | 71 | 1.01 | 5.93 | 6.17 | 298 | 45.0 | 3741 | 5.99 |
| Month | Rainfall (mm) | Air Temperature (°C) | Hydrothermal Coefficient of Sielianinov * | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2017 | 2018 | 2019 | 2017 | 2018 | 2019 | |
| April | 43.0 | 61.8 | 47.1 | 11.1 | 8.8 | 10.0 | 1.3 | 2.3 | 1.6 |
| May | 141.4 | 120.3 | 47.9 | 14.7 | 12.8 | 15.3 | 3.1 | 3.0 | 1.0 |
| June | 85.2 | 46.7 | 87.3 | 15.9 | 16.7 | 19.1 | 1.8 | 0.9 | 1.5 |
| July | 69.7 | 45.2 | 114.1 | 21.1 | 19.4 | 20.5 | 1.1 | 0.8 | 1.8 |
| August | 95.8 | 6.1 | 41.0 | 19.2 | 21.4 | 19.5 | 1.6 | 0.1 | 0.7 |
| September | 19.6 | 130.2 | 11.8 | 14.6 | 15.5 | 15.5 | 0.4 | 2.8 | 0.3 |
| Total | 454.7 | 410.3 | 349.2 | - | - | - | - | - | - |
| Experimental Factors | Content of DM (%) | Yield of DM (t.ha−1) | |
|---|---|---|---|
| Pre-treatment treatments | Control object | 20.2 a | 7.57 b |
| Exposition I * | 20.4 a | 9.53 a | |
| Exposition II ** | 20.6 a | 9.28 a | |
| HSDp0.05 | ns *** | 0.42 | |
| Cultivars | ‘Denar’ | 16.3 d | 7.98 b |
| ‘Bellarosa’ | 18.0 c | 6.88 c | |
| ‘Gwiazda’ | 16.5 d | 7.35 b | |
| ‘Ignacy’ | 18.0 c | 8.79 b | |
| ‘Owacja’ | 17.7c | 6.56 c | |
| ‘Vineta’ | 19.3 c | 7.86 b | |
| ‘Finezja’ | 23.9 b | 9.56 b | |
| ‘Oberon’ | 20.8 b | 8.26 b | |
| ‘Satina’ | 21.5 b | 10.33 a | |
| ‘Tajfun’ | 23.0 b | 9.88 b | |
| ’Jelly’ | 20.2 b | 8.31 b | |
| ’Mondeo’ | 22.1 b | 10.49a | |
| ’Syrena’ | 21.0 b | 9.42 ab | |
| ’Kuras’ | 28.0 a | 11.41 a | |
| HSDp0.05 | 3.8 | 1.97 | |
| Years | 2017 | 20.1 a | 9.10 b |
| 2018 | 20.7 a | 7.35 c | |
| 2019 | 20.3 a | 9.89 a | |
| HSDp0.05 | ns *** | 0.42 | |
| Mean | 20.4 | 8.79 | |
| Experimental Factors | Starch Content (%) | Yield of Starch (t·ha−1) | |
|---|---|---|---|
| Pre-treatment treatments | Control object | 15.2 a | 5.73 b |
| Exposition I * | 15.4 a | 7.15 a | |
| Exposition II ** | 15.4 a | 6.96 a | |
| HSDp0.05 | ns *** | 0.33 | |
| Cultivars | ‘Denar’ | 12.4 c | 6.05 b |
| ‘Bellarosa’ | 13.8 c | 5.23 c | |
| ‘Gwiazda’ | 13.3 c | 5.71 b | |
| ‘Ignacy’ | 13.6 c | 6.63 b | |
| ‘Owacja’ | 13.8 c | 5.02 c | |
| ‘Vineta’ | 14.5 bc | 5.92 b | |
| ‘Finezja’ | 18.0 a | 7.20 a | |
| ‘Oberon’ | 15.6 b | 6.21 b | |
| ‘Satina’ | 15.7 b | 7.66 a | |
| ‘Tajfun’ | 17.4 ab | 7.46 a | |
| ’Jelly’ | 14.8 bc | 6.17 b | |
| ’Mondeo’ | 16.2 b | 7.82 a | |
| ’Syrena’ | 15.5 b | 7.05 a | |
| ’Kuras’ | 20.3 a | 8.48 a | |
| HSDp0.05 | 2.9 | 1.54 | |
| Years | 2017 | 15.1 b | 6.84 b |
| 2018 | 15.7 a | 5.57 c | |
| 2019 | 15.2 b | 7.43 a | |
| HSDp0.05 | 0.6 | 0.33 | |
| Mean | 15.4 | 6.61 | |
| Experiment Factors | Color (Scale 9°) | Organoleptic Assessment (Scale 5°) | Visual Assessment (5° Scale) | Moisture (%) | Wet Places (%) | Fat Content (%) | |
|---|---|---|---|---|---|---|---|
| Pre-planting treatments | Control object | 7.90 a | 4.20 a | 4.00 a | 2.10 b | 1.90 c | 25.90 a |
| Exposition I | 7.90 a | 3.60 b | 3.70 b | 2.20 a | 3.60 a | 21.70 b | |
| Exposition II | 7.90 a | 3.60 b | 3.70 b | 1.90 c | 2.30 b | 21.91 b | |
| HSDp0.05 | ns * | 0.19 | 0.20 | 0.10 | 0.13 | 1.16 | |
| Cultivars | ‘Denar’ | 7.80 a | 3.67 bc | 3.78 b | 2.06 bc | 3.44 d | 27.44 a |
| ‘Bellarosa’ | 7.76 a | 2.89 d | 3.00 c | 2.58 a | 6.56 a | 24.89 a | |
| ‘Gwiazda’ | 7.63 a | 3.72 b | 3.39 c | 2.64 a | 5.00 c | 24.01 a | |
| ‘Ignacy’ | 8.08 a | 3.17 c | 3.94 b | 2.39 ab | 2.56 e | 23.55 b | |
| ‘Owacja’ | 6.93 bc | 3.89 b | 3.94 b | 1.89 c | 1.67 f | 24.65 a | |
| ‘Vineta’ | 8.26 a | 3.89 b | 4.11 b | 2.11 b | 1.67 f | 21.92 b | |
| ‘Finezja’ | 8.09 a | 3.83 b | 4.00 b | 2.44 a | 1.06 g | 21.24 b | |
| ‘Oberon’ | 7.68 a | 3.83 b | 3.67 bc | 1.67 c | 1.33 g | 23.16 b | |
| ‘Satina’ | 8.54 a | 4.50 a | 4.22 ab | 1.50 d | 0.00 i | 21.05 b | |
| ‘Tajfun’ | 7.53 b | 3.33 c | 3.17 c | 2.17 b | 3.33 d | 21.20 b | |
| ‘Jelly’ | 8.71 a | 4.89 a | 4.89 a | 1.60 d | 0.56 h | 21.05 b | |
| ‘Mondeo’ | 7.94 a | 4.06 b | 3.83 b | 2.17 b | 3.22 d | 23.67 b | |
| ‘Syrena’ | 8.11 a | 4.00 b | 3.72 b | 1.67 d | 0.56 h | 23.02 b | |
| ‘Kuras’ | 7.58 b | 3.56 c | 3.50 c | 2.11 b | 5.44 b | 23.50 b | |
| HSDp0.05 | 1.13 | 0.60 | 0.63 | 0.34 | 0.43 | 4.02 | |
| Years | 2017 | 8.06 a | 4.05 a | 4.01 a | 2.24 a | 2.85 a | 23.56 a |
| 2018 | 7.67 a | 3.51 c | 3.61 b | 1.88 b | 2.02 b | 22.07 b | |
| 2019 | 7.96 a | 3.84 b | 3.78 b | 2.17 a | 2.93 a | 23.89 a | |
| HSDp0.05 | ns * | 0.19 | 0.20 | 0.10 | 0.13 | 1.16 | |
| Mean | 7.90 | 3.80 | 3.80 | 2.07 | 2.60 | 23.17 | |
| Specification | Significance of Effect | Percentage Contribution of the Variance of Total Variance | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Year | Treatment | Cultivar | Year x Treatment | Year x Cultivar | Treatment x Cultivar | T x Y x C | Year | Treatment | Cultivar | Year x Treatment | Year x Cultivar | Treatment x Cultivar | T x Y x C | |
| Content of DM (%) | ns ** | ns | ** | ns | ns | ns | ns | 6.9 | 7.7 | 37.7 | 13.0 | 18.1 | 17.8 | 3.2 |
| Content of starch (%) | ** | ns | ** | ns | ns | ns | ns | 23.3 | 6.2 | 18.6 | 25.3 | 18.5 | 9.2 | 2.1 |
| Yield of DM (t ha−1) | ** | ** | ** | * | ns | ns | ns | 19.5 | 20.5 | 24.4 | 15.9 | 6.8 | 7.9 | 6.2 |
| Yield of starch | ** | ** | ** | * | * | ns | ns | 16.3 | 16.4 | 32.8 | 30.6 | 15.6 | 6.8 | 0.9 |
| Color (scale 9°) | ns | ns | ** | ns | ns | ns | ns | 16.5 | 14.5 | 40.3 | 9.1 | 8.0 | 6.5 | 4.9 |
| Organoleptic ass. (scale 5°) | ** | ** | ** | ns | ns | ns | ns | 25.7 | 18.8 | 29.4 | 12.9 | 5.3 | 7.0 | 1.6 |
| Visual assessment (5° scale) | ** | ** | ** | ns | ns | ns | ns | 16.9 | 17.4 | 32.2 | 18.7 | 8.1 | 4.0 | 2.1 |
| Moisture (%) | ** | ** | ** | ns | ns | ns | ns | 33.7 | 14.6 | 23.4 | 12.8 | 7.9 | 7.1 | 2.4 |
| Wet places (%) | ** | * | ** | * | ns | ns | ns | 35.4 | 11.9 | 29.9 | 13.6 | 7.4 | 6.9 | 1.7 |
| Fat content (%) | ** | * | ** | ns | ns | ns | ns | 23.1 | 12.1 | 39.2 | 13.1 | 5.8 | 6.1 | 1.1 |
| Specification | x1 | x2 | x3 | x4 | x5 | x6 | x7 | x8 | x9 | x10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 20.38 | 8.79 | 15.36 | 6.61 | 7.90 | 3.80 | 3.80 | 2.07 | 2.60 | 23.17 |
| Median | 20.13 | 8.74 | 15.10 | 6.57 | 7.87 | 3.83 | 3.81 | 2.11 | 2.11 | 23.33 |
| Standard deviation | 3.00 | 2.54 | 2.18 | 1.86 | 0.43 | 0.49 | 0.45 | 0.25 | 0.93 | 1.75 |
| Kurtosis | 0.48 | −0.46 | 0.44 | −0.50 | 0.13 | 0.24 | 0.54 | −1.12 | −0.74 | 0.22 |
| Skewness | 0.68 | 0.26 | 0.69 | 0.25 | −0.16 | 0.32 | 0.44 | −0.04 | 0.58 | 0.69 |
| Range | 14.53 | 11.55 | 10.90 | 8.60 | 1.78 | 2.00 | 1.89 | 1.14 | 6.56 | 6.39 |
| Minimum | 14.53 | 3.78 | 10.90 | 2.90 | 6.93 | 2.89 | 3.00 | 1.50 | 0.00 | 21.05 |
| Maximum | 29.07 | 15.33 | 21.80 | 11.50 | 8.71 | 4.89 | 4.89 | 2.64 | 6.56 | 27.44 |
| Coefficient of variation (%) | 14.70 | 28.85 | 14.22 | 28.12 | 5.50 | 12.85 | 11.93 | 12.07 | 35.78 | 7.55 |
| Specification | x1 | x2 | x3 | x4 | x5 | x6 | x7 | x8 | x9 | x10 |
|---|---|---|---|---|---|---|---|---|---|---|
| x1 | 1.00 | |||||||||
| x2 | 0.50 ** | 1.00 | ||||||||
| x3 | 0.97 ** | 0.44 ** | 1.00 | |||||||
| x4 | 0.48 ** | 0.99 ** | 0.44 ** | 1.00 | ||||||
| x5 | 0.03 | 0.16 | 0.00 | 0.14 | 1.00 | |||||
| x6 | 0.08 | 0.10 | 0.05 | 0.08 | 0.55 ** | 1.00 | ||||
| x7 | −0.06 | 0.03 | −0.09 | 0.01 | 0.64 ** | 0.83 ** | 1.00 | |||
| x8 | −0.12 | −0.12 | −0.08 | −0.09 | −0.32 * | −0.72 ** | −0.57 ** | 1.00 | ||
| x9 | −0.02 | −0.06 | 0.00 | −0.05 | −0.48 ** | −0.72 ** | −0.75 ** | 0.73 ** | 1.00 | |
| x10 | −0.49 ** | −0.19 | −0.49 ** | −0.18 * | −0.48 ** | −0.42 ** | −0.28 * | 0.26 * | 0.42 ** | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pszczółkowski, P.; Sawicka, B.; Barbaś, P.; Skiba, D.; Krochmal-Marczak, B. The Use of Effective Microorganisms as a Sustainable Alternative to Improve the Quality of Potatoes in Food Processing. Appl. Sci. 2023, 13, 7062. https://doi.org/10.3390/app13127062
Pszczółkowski P, Sawicka B, Barbaś P, Skiba D, Krochmal-Marczak B. The Use of Effective Microorganisms as a Sustainable Alternative to Improve the Quality of Potatoes in Food Processing. Applied Sciences. 2023; 13(12):7062. https://doi.org/10.3390/app13127062
Chicago/Turabian StylePszczółkowski, Piotr, Barbara Sawicka, Piotr Barbaś, Dominika Skiba, and Barbara Krochmal-Marczak. 2023. "The Use of Effective Microorganisms as a Sustainable Alternative to Improve the Quality of Potatoes in Food Processing" Applied Sciences 13, no. 12: 7062. https://doi.org/10.3390/app13127062
APA StylePszczółkowski, P., Sawicka, B., Barbaś, P., Skiba, D., & Krochmal-Marczak, B. (2023). The Use of Effective Microorganisms as a Sustainable Alternative to Improve the Quality of Potatoes in Food Processing. Applied Sciences, 13(12), 7062. https://doi.org/10.3390/app13127062










