Phytochemical Profile, Antilipase, Hemoglobin Antiglycation, Antihyperglycemic, and Anti-Inflammatory Activities of Solanum elaeagnifolium Cav.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materiel
2.2. Preparation of Plant Extracts
2.3. HPLC-DAD Analysis
2.4. In Vitro Activities
2.4.1. Lipase Inhibitory Activity
2.4.2. Antiglycation Activity
2.5. Pharmacological Activities
2.5.1. Animals
2.5.2. Oral Glucose Tolerance Test (OGTT)
2.5.3. Inhibition of Carbohydrate Hydrolase Enzymes, In Vivo
Pancreatic α-Amylase
Pancreatic α-Glucosidase
2.5.4. Anti-Inflammatory Potential
2.6. Statistical Investigation
3. Results and Discussion
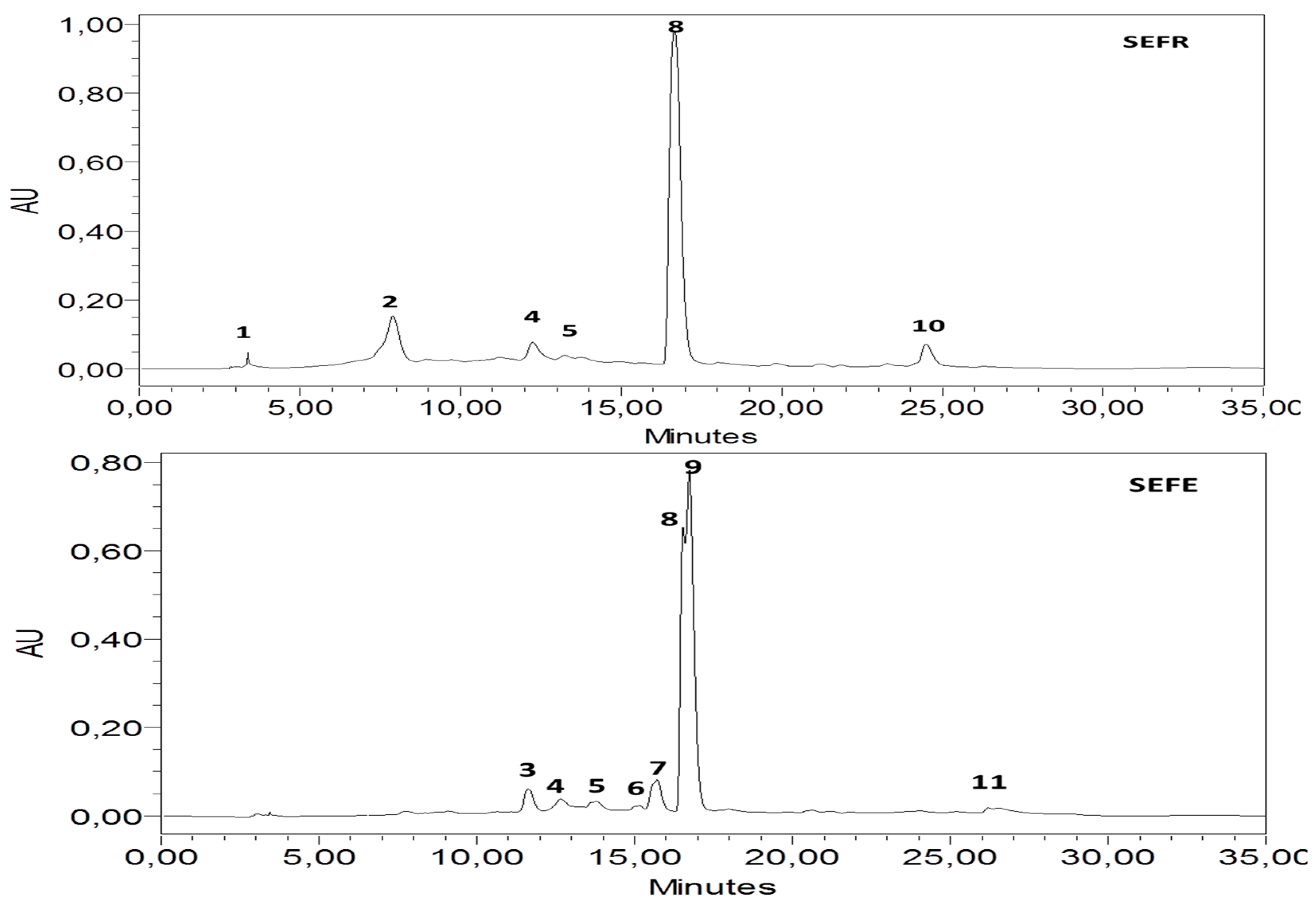
3.1. HPLC-DAD Analysis
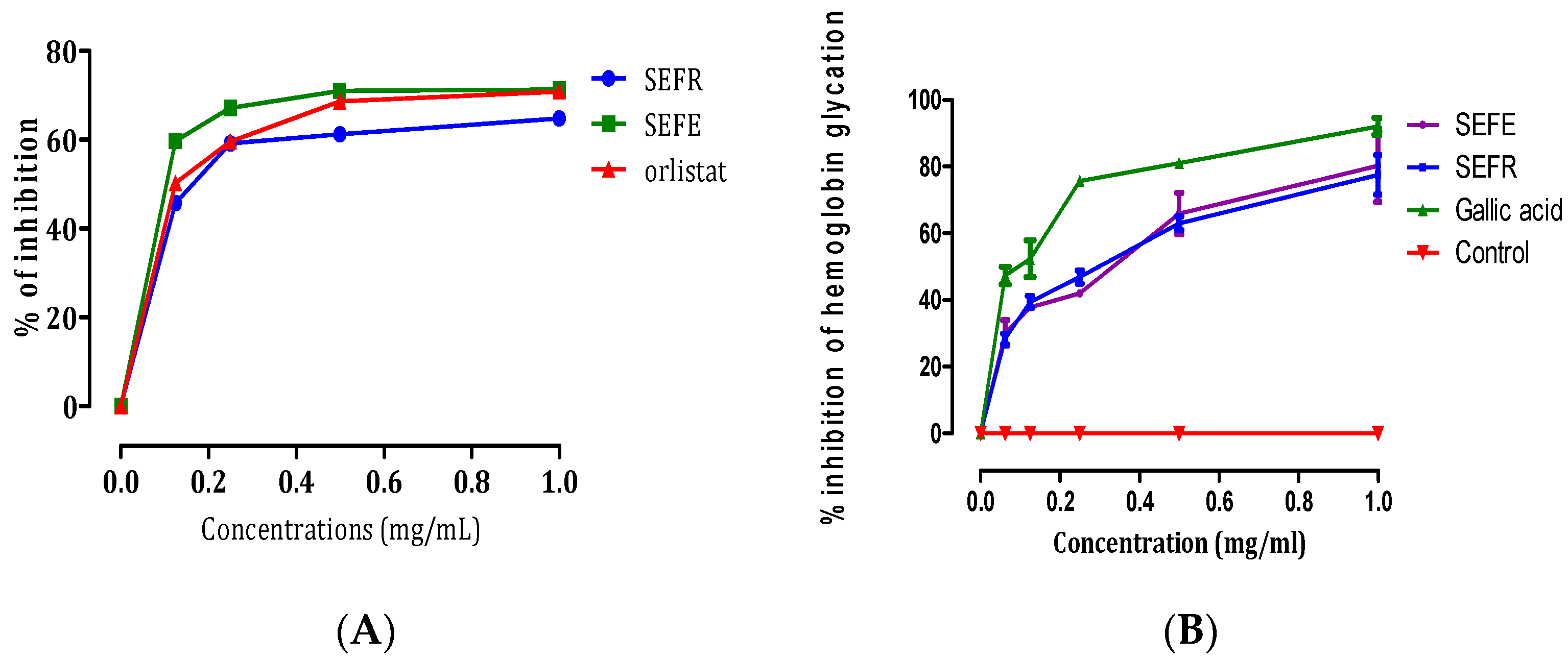
3.2. Lipase Inhibitory and Hemoglobin Antiglycation Activities
3.3. Pharmacological Activities
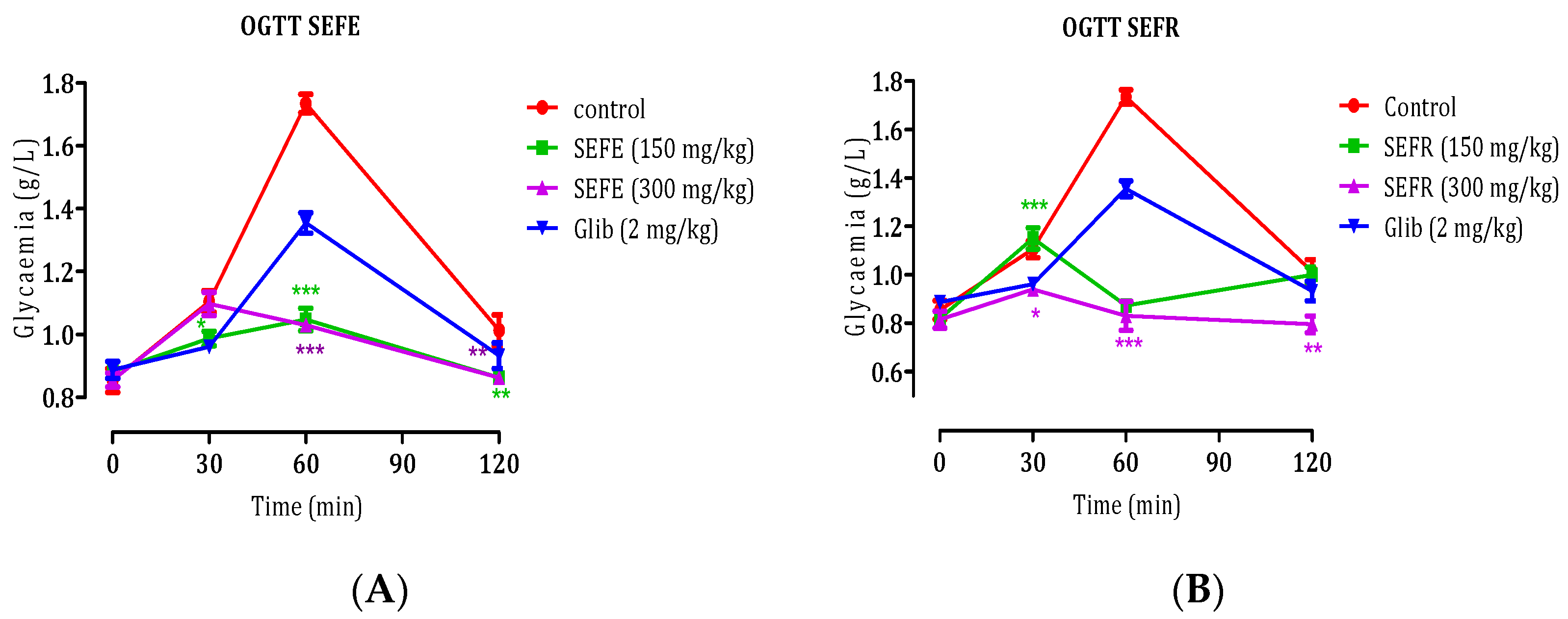
3.3.1. Oral Glucose Tolerance Test (OGTT)
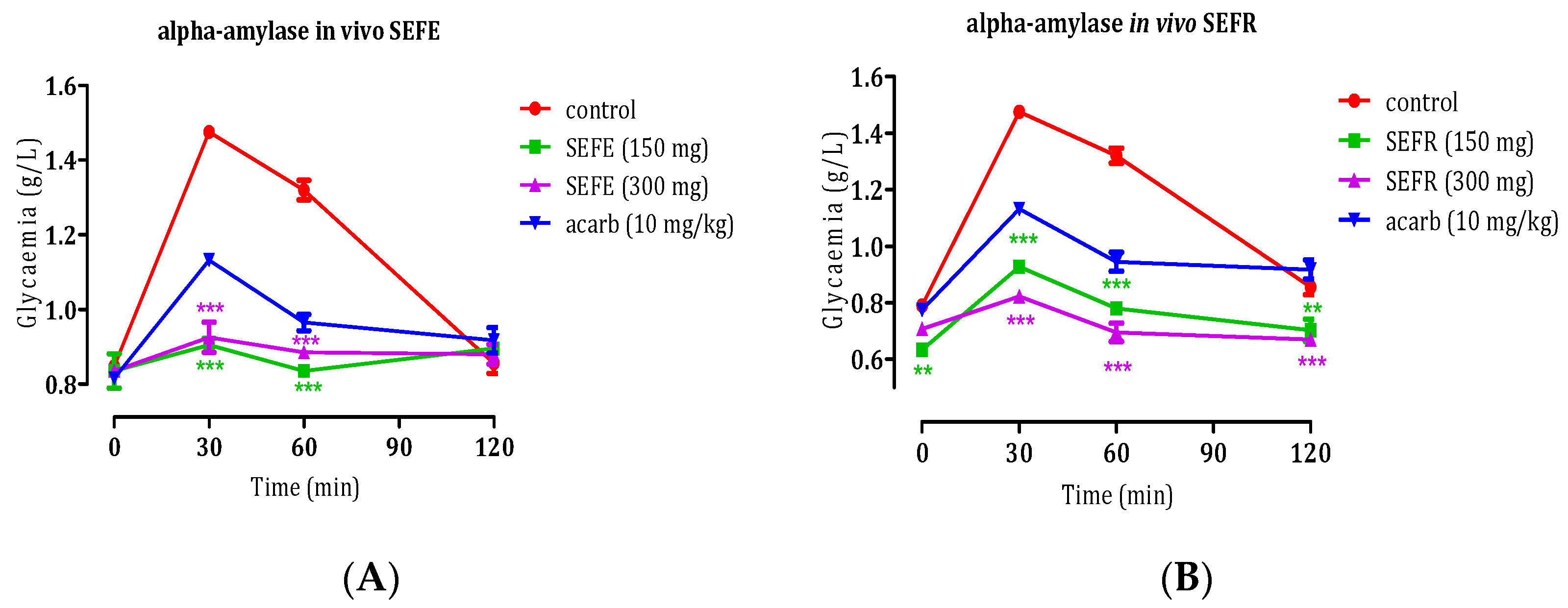
3.3.2. Inhibition of Carbohydrate Hydrolase Enzymes, In Vivo
In Vivo Anti-α-Amylase Activity of S. elaeagnifolium Leaves and Fruits
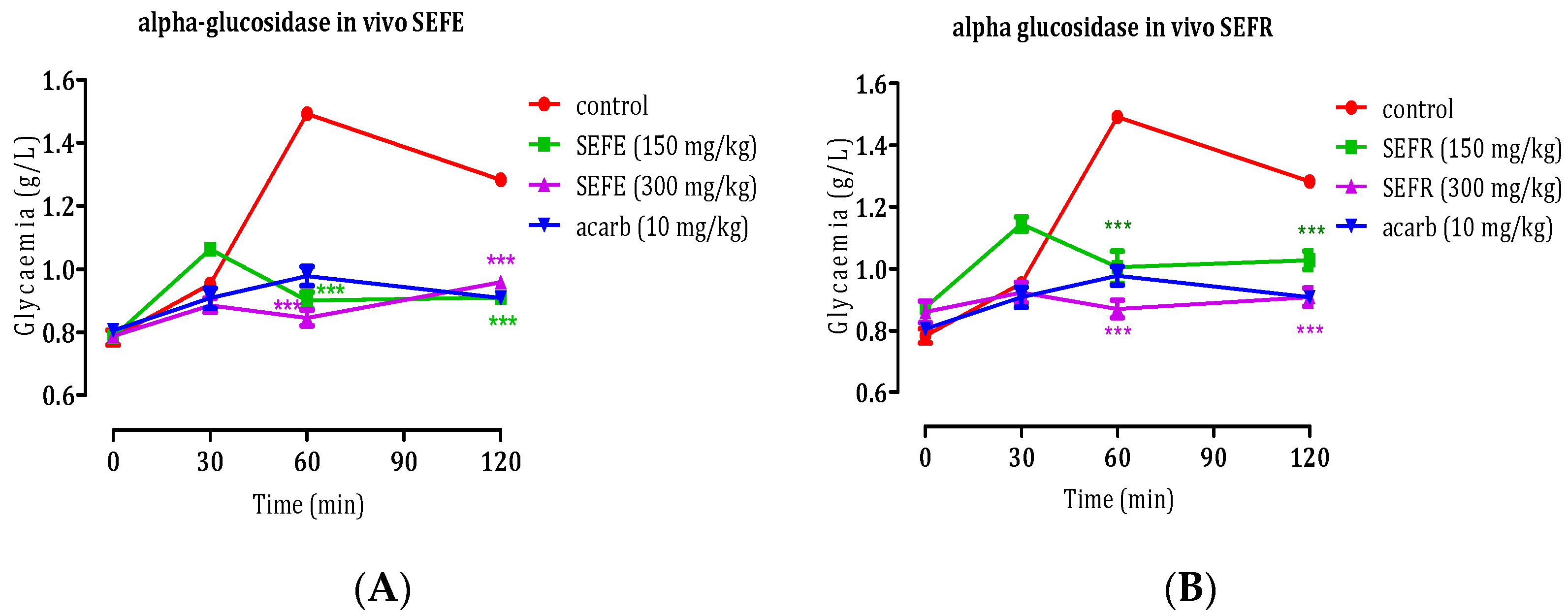
In Vivo Anti-α-Glycosidase Activity of S. elaeagnifolium Leaves and Fruits
3.3.3. Anti-Inflammatory Potential
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiong, W.-T.; Gu, L.; Wang, C.; Sun, H.-X.; Liu, X. Anti-Hyperglycemic and Hypolipidemic Effects of Cistanche Tubulosa in Type 2 Diabetic Db/Db Mice. J. Ethnopharmacol. 2013, 150, 935–945. [Google Scholar] [CrossRef] [PubMed]
- El Ghouizi, A.; Ousaaid, D.; Laaroussi, H.; Bakour, M.; Aboulghazi, A.; Soutien, R.; Hano, C.; Badiaa, L. Ficus carica (Linn.) Leaf and Bud Extracts and Their Combination Attenuates Type-1 Diabetes and Its Complications via the Inhibition of Oxidative Stress. Foods 2023, 12, 759. [Google Scholar] [CrossRef] [PubMed]
- Hbika, A.; Daoudi, N.E.; Bouyanzer, A.; Bouhrim, M.; Mohti, H.; Loukili, E.H.; Mechchate, H.; Al-salahi, R.; Nasr, F.; Bnouham, M.; et al. Artemisia absinthium L. Aqueous and Ethyl Acetate Extracts: Antioxidant Effect and Potential Activity In Vitro and In Vivo against Pancreatic α-Amylase and Intestinal α-Glucosidase. Pharmaceutics 2022, 14, 481. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R.; Medzhitov, R. Origin and Physiological Roles of Inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Umamageswari, M.; Karthikeyan, T.; Maniyar, Y.A. Antidiabetic Activity of Aqueous Extract of Solanum nigrum linn berries in Alloxan Induced Diabetic Wistar Albino Rats. J. Clin. Diagn. Res. 2017, 11, FC16–FC19. [Google Scholar] [CrossRef]
- Dasgupta, N.; Muthukumar, S.P.; Murthy, P.S. Solanum nigrum Leaf: Natural Food Against Diabetes and Its Bioactive Compounds. Res. J. Med. Plants 2016, 10, 181–193. [Google Scholar] [CrossRef]
- Nderitu, K.W.; Mecha, E.; Nyachieo, A. Solanum nigrum Show Anti-Obesity Effects on High-Fat Diet-Fed Sprague Dawley Rats in a Randomized Study. Sci. Afr. 2023, 20, e01713. [Google Scholar] [CrossRef]
- Aabideen, Z.U.; Mumtaz, M.W.; Akhtar, M.T.; Raza, M.A.; Mukhtar, H.; Irfan, A.; Raza, S.A.; Nadeem, M.; Ling, Y.S. Anti-Obesity Effect and UHPLC-QTOF-MS/MS Based Metabolite Profiling of Solanum nigrum Leaf Extract. Asian Pac. J. Trop. Biomed. 2022, 12, 164–174. [Google Scholar] [CrossRef]
- Peng, C.-H.; Cheng, J.-J.; Yu, M.-H.; Dai-Jung, C.; Huang, C.-N.; Wang, C.-J. Solanum nigrum Polyphenols Reduce Body Weight and Body Fat by Affecting Adipocyte and Lipid Metabolism. Food Funct. 2019, 11, 483–492. [Google Scholar] [CrossRef]
- Yeom, Y.-E.; Kim, M.A.; Kim, J.; Lee, C.-M. Anti-Inflammatory Effects of the Extract of Solanum nigrum L. on an Acute Ear Edema Mouse Model. Mater. Technol. 2019, 34, 851–857. [Google Scholar] [CrossRef]
- Campisi, A.; Acquaviva, R.; Raciti, G.; Duro, A.; Rizzo, M.; Santagati, N.A. Antioxidant Activities of Solanum nigrum L. Leaf Extracts Determined in In Vitro Cellular Models. Foods 2019, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, S.; Alarcón, M.; Palomo, I. Aqueous Extract of Tomato (Solanum lycopersicum L.) and Ferulic Acid Reduce the Expression of TNF-α and IL-1β in LPS-Activated Macrophages. Molecules 2015, 20, 15319–15329. [Google Scholar] [CrossRef] [PubMed]
- Houda, M.; Derbré, S.; Jedy, A.; Tlili, N.; Legault, J.; Richomme, P.; Limam, F.; Saidani-Tounsi, M. Combined Anti-AGEs and Antioxidant Activities of Different Solvent Extracts of Solanum elaeagnifolium Cav. (Solanaceae) Fruits during Ripening and Related to Their Phytochemical Compositions. EXCLI J. 2014, 13, 1029–1042. [Google Scholar] [CrossRef]
- Bouslamti, M.; Loukili, E.H.; Elrherabi, A.; Moussaoui, A.; Chebaibi, M.; Bencheikh, N.; Nafidi, H.-A.; Bin Jardan, Y.; Bourhia, M.; Bnouham, M.; et al. Phenolic Profile, Inhibition of α-Amylase and α-Glucosidase Enzymes, and Antioxidant Properties of Solanum elaeagnifolium Cav. (Solanaceae): In Vitro and In Silico Investigations. Processes 2023, 11, 1384. [Google Scholar] [CrossRef]
- Bouslamti, M.; Chelouati, T.; El Moussaoui, A.; El Barnossi, A.; Badiaa, L.; Benjelloun, A. Solanum elaeagnifolium Var. Obtusifolium (Dunal) Dunal: Antioxidant, Antibacterial, and Antifungal Activities of Polyphenol-Rich Extracts Chemically Characterized by Use of In Vitro and In Silico Approaches. Molecules 2022, 27, 8688. [Google Scholar] [CrossRef]
- Balah, M.A.; Hassany, W.M.; Kopici, A.A. Allelopathy of Invasive Weed Solanum elaeagnifolium Cav.: An Investigation in Germination, Growth and Soil Properties. J. Plant Prot. Res. 2022, 62, 58–70. [Google Scholar] [CrossRef]
- Bouslamti, M.; El Barnossi, A.; Kara, M.; Alotaibi, B.; Kamaly, O.; Assouguem, A.; Badiaa, L.; Benjelloun, A. Total Polyphenols Content, Antioxidant and Antimicrobial Activities of Leaves of Solanum elaeagnifolium Cav. from Morocco. Molecules 2022, 27, 4322. [Google Scholar] [CrossRef]
- Badawy, A.; Zayed, R.; Ahmed, S.; Hassanean, H. Phytochemical and Pharmacological Studies of Solanum elaeagnifolium Growing in Egypt. J. Nat. Prod. 2013, 6, 156–167. [Google Scholar]
- Radwan, M.; Badawy, A.; Zayed, R.; Hassanin, H.; Elsohly, M.; Ahmed, S. Cytotoxic Flavone Glycosides from Solanum elaeagnifolium. Med. Chem. Res. 2014, 24, 1326–1330. [Google Scholar] [CrossRef]
- Feki, H.; Koubaa, I.; Damak, M. Secondary Metabolites and Antioxidant Activity of Seed Extracts from Solanum elaeagnifolium Cav. Mediterr. J. Chem. 2014, 2, 639–647. [Google Scholar] [CrossRef]
- Njeh, F.; Feki, H.; Koubaa, I.; Hamed, N.; Mohamed, D.; Ayadi, A.; Hammami, H.; Jarraya, R. Molluscicidal Activity of Solanum elaeagnifolium Seeds against Galba Truncatula Intermediate Host of Fasciola Hepatica: Identification of β-Solamarine. Pharm. Biol. 2015, 54, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Hawas, U.W.; Soliman, G.M.; Abou El-Kassem, L.T.; Farrag, A.R.H.; Mahmoud, K.; León, F. A New Flavonoid C-Glycoside from Solanum elaeagnifolium with Hepatoprotective and Curative Activities against Paracetamol-Induced Liver Injury in Mice. Z. Naturforschung C J. Biosci. 2013, 68, 19–28. [Google Scholar]
- Al-hamaideh, K.; Dmour, I.; El-Elimat, T.; Afifi, F. UPLC-MS Profile and Anti-Proliferative Activity of the Berries of an Aggressive Wild-Growing Weed: Solanum elaeagnifolium Cav. (Solanaceae). Trop. J. Nat. Prod. Res. 2020, 4, 1131–1138. [Google Scholar] [CrossRef]
- Loukili, E.H.; Bouchal, B.; Bouhrim, M.; Abrigach, F.; Genva, M.; Kahina, Z.; Bnouham, M.; Bellaoui, M.; Hammouti, B.; Addi, M.; et al. Chemical Composition, Antibacterial, Antifungal and Antidiabetic Activities of Ethanolic Extracts of Opuntia Dillenii Fruits Collected from Morocco. J. Food Qual. 2022, 2022, 9471239. [Google Scholar] [CrossRef]
- Ouahabi, S.; Loukili, E.H.; Daoudi, N.E.; Chebaibi, M.; Ramdani, M.; Rahhou, I.; Bnouham, M.; Fauconnier, M.-L.; Hammouti, B.; Rhazi, L.; et al. Study of the Phytochemical Composition, Antioxidant Properties, and In Vitro Anti-Diabetic Efficacy of Gracilaria Bursa-Pastoris Extracts. Mar. Drugs 2023, 21, 372. [Google Scholar] [CrossRef]
- Benrahou, K.; Naceiri Mrabti, H.; Bouyahya, A.; Daoudi, N.E.; Bnouham, M.; Mezzour, H.; Mahmud, S.; Alshahrani, M.; Obaidullah, A.; Cherrah, Y.; et al. Inhibition of α-Amylase, α-Glucosidase, Lipase, Intestinal Glucose Absorption and Antidiabetic Properties by Extracts of Erodium Guttatum. Evid. Based Complement. Alternat. Med. 2022, 2022, 5868682. [Google Scholar] [CrossRef]
- Elrherabi, A.; Bouhrim, M.; Rhizlan, A.; Berraaouan, A.; Ziyyat, A.; Mekhfi, H.; Legssyer, A.; Bnouham, M. Phenolic Content, Antioxidant, Hemidiaphragm Glucose Consumption, and Hemoglobin Glycosylation Inhibitory Activities of Lavandula stoechas L. Aqueous Extract. J. Nat. Remedies 2023, 23, 653–660. [Google Scholar] [CrossRef]
- Council of the European Union. Council Directive 86/609/EEC of 24 November 1986 on the Approximation of Laws, Regulations and Administrative Provisions of the Member States Regarding the Protection of Animals Used for Experimental and Other Scientific Purposes, CELEX1. Available online: https://op.europa.eu/en/publication-detail/-/publication/cc3a8ccb-5a30-4b6e-8da8-b13348caeb0c/language-en (accessed on 25 July 2023).
- Daoudi, N.E.; Bouhrim, M.; Ouassou, H.; Abdelkhaleq, L.; Mekhfi, H.; Ziyyat, A.; Aziz, M.; Bnouham, M. Inhibitory Effect of Roasted/Unroasted Argania Spinosa Seeds Oil on α-Glucosidase, α-Amylase and Intestinal Glucose Absorption Activities. S. Afr. J. Bot. 2020, 135, 413–420. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Mechchate, H.; Bourhia, M.; Es-safi, I.; Salamatullah, A.; Alkaltham, M.; Alyahya, H.; Bousta, D.; Bari, A. Glycemic Control Potential of Chemically Characterized Extract from Withania frutescens L. Roots in Severe Diabetes-Induced Mice. Appl. Sci. 2021, 11, 3998. [Google Scholar] [CrossRef]
- Remok, F.; Saidi, S.; Gourich, A.; Zibouh, K.; Maouloua, M.; Makhoukhi, F.; el Menyiy, N.; Touijer, H.; Bouhrim, M.; Sahpaz, S.; et al. Phenolic Content, Antioxidant, Antibacterial, Antihyperglycemic, and α-Amylase Inhibitory Activities of Aqueous Extract of Salvia Lavandulifolia Vahl. Pharmaceuticals 2023, 16, 395. [Google Scholar] [CrossRef] [PubMed]
- Mssillou, I.; Agour, A.; Slighoua, M.; Chebaibi, M.; Amrati, F.E.-Z.; Alshawwa, S.; Kamaly, O.; El Moussaoui, A.; Badiaa, L.; Derwich, E. Ointment-Based Combination of Dittrichia viscosa L. and Marrubium vulgare L. Accelerate Burn Wound Healing. Pharmaceuticals 2022, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The Correlation between Active Oxygens Scavenging and Antioxidative Effects of Flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Yao, L.; Gu, W.; Zhao, S.; Shen, Z.; Lin, Z.; Liu, W.; Yan, T. Pharmacological Activity of Quercetin: An Updated Review. Evid. Based Complement. Alternat. Med. 2022, 2022, e3997190. [Google Scholar] [CrossRef]
- Hou, T.-H.; Chung, J.-P.; Chen, S.-S.; Chang, T. Antioxidation and Antiglycation of 95% Ethanolic Extracts Prepared from the Leaves of Black Nightshade (Solanum nigrum). Food Sci. Biotechnol. 2013, 22, 839–844. [Google Scholar] [CrossRef]
- Singh, M.P.; Pathak, K. Animal Models for Biological Screening of Anti-Diabetic Drugs: An Overview. Euro. J. Exp. Biol. 2015, 5, 37–48. [Google Scholar]
- Shai, L.J.; Masoko, P.; Mokgotho, M.P.; Magano, S.R.; Mogale, A.M.; Boaduo, N.; Eloff, J.N. Yeast Alpha Glucosidase Inhibitory and Antioxidant Activities of Six Medicinal Plants Collected in Phalaborwa, South Africa. S. Afr. J. Bot. 2010, 76, 465–470. [Google Scholar] [CrossRef]
- Elrherabi, A.; Bouhrim, M.; Rhizlan, A.; Berraaouan, A.; Ziyyat, A.; Mekhfi, H.; Legssyer, A.; Bnouham, M. Antihyperglycemic Potential of the Lavandula stoechas Aqueous Extract via Inhibition of Digestive Enzymes and Reduction of Intestinal Glucose Absorption. J. Ayurveda Integr. Med. 2023, 14, 100795. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-Amylase and α-Glucosidase: Potential Linkage for Whole Cereal Foods on Prevention of Hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Dona, A.; Pagès, G.; Kuchel, P. Digestion of Starch: In Vivo and in Vitro Kinetic Models Used to Characterise Oligosaccharide or Glucose Release. Carbohydr. Polym. 2010, 80, 599–617. [Google Scholar] [CrossRef]
- Tomimatsu, T.; Horie, T. Enhanced Glucose Absorption in the Rat Small Intestine Following Repeated Doses of 5-Fluorouracil. Chem. Biol. Interact. 2005, 155, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Benayad, O.; Bouhrim, M.; Tiji, S.; Kharchoufa, L.; Addi, M.; Drouet, S.; Hano, C.; Lorenzo, J.M.; Bendaha, H.; Bnouham, M.; et al. Phytochemical Profile, α-Glucosidase, and α-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules 2021, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Ndebia, E.; Kamgang, R.; Nkeh-ChungagAnye, B. Analgesic and Anti-Inflammatory Properties of Aqueous Extract from Leaves of Solanum torvum (Solanaceae). Afr. J. Tradit. Complement. Altern. Med. AJTCAM Afr. Netw. Ethnomedicines 2006, 4, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Piana, M.; Camponogara, C.; Boligon, A.; Oliveira, S. Solanum Paranense Extracts and Solanine Present Anti-Inflammatory Activity in an Acute Skin Inflammation Model in Mice. Evid. Based Complement. Alternat. Med. 2017, 2017, 4295680. [Google Scholar] [CrossRef]
- Rahman, N.; Haris, M.; Mahmood, R.; ur Rahman, H. Antioxidant and Anti-Inflammatory Potentials of Solanum pubescens Willd an Ethnomedicinal Plant of South Western Andhra Pradesh, India. J. Res. Pharm. 2019, 23, 187–197. [Google Scholar] [CrossRef]
- da Costa, G.A.F.; Morais, M.G.; Saldanha, A.A.; Assis Silva, I.C.; Aleixo, Á.A.; Ferreira, J.M.S.; Soares, A.C.; Duarte-Almeida, J.M.; Lima, L.A.R.d.S. Antioxidant, Antibacterial, Cytotoxic, and Anti-Inflammatory Potential of the Leaves of Solanum Lycocarpum A. St. Hil. (Solanaceae). Evid. Based Complement. Alternat. Med. 2015, 2015, e315987. [Google Scholar] [CrossRef]
- Güran, M.; Şanlıtürk, G.; Kerküklü, N.; Altundag, E.; Yalçin, A.S. Combined Effects of Quercetin and Curcumin on Anti-Inflammatory and Antimicrobial Parameters in Vitro. Eur. J. Pharmacol. 2019, 859, 172486. [Google Scholar] [CrossRef]
| Plant Species | Family | Voucher N° | Used Organ | Region | Co-Ordinates | Date |
|---|---|---|---|---|---|---|
| Solanum elaeagnifolium | Solanaceae | E17/1405 | Leaves (SEFE) | Fez | 34°04′04.2 N, 5°01′26.4 W | December 2021 |
| Fruits (SEFR) | April 2022 |
| Peak | Compounds | SEFR | SEFE | ||
|---|---|---|---|---|---|
| % Area | mg/100 g | % Area | mg/100 g | ||
| 1 | Catechin | 0.49 | 0.49 ± 0.21 | Nd | Nd |
| 2 | Vanillin | 12.13 | 46.72 ± 0.69 | Nd | Nd |
| 3 | Naringin | Nd | Nd | 4.16 | 12.00 ± 0.01 |
| 4 | Quercetin 3-O-β-D-glucoside | 4.36 | 16.72 ± 0.55 | 1.59 | 1.84 ± 0.05 |
| 5 | Rutin | 3.22 | 98.17 ± 0.651 | 1.34 | 3.83 ± 0.23 |
| 6 | Cinnamic acid | Nd | Nd | 0.81 | 2.17 ± 0.44 |
| 7 | p-coumaric acid | Nd | Nd | 6.56 | 75.85 ± 0.13 |
| 8 | Quercetin | 75.84 | 429.02 ± 0.97 | 26.29 | 114.15 ± 0.34 |
| 9 | Trans-3-hydroxycinnamic acid | Nd | Nd | 55.95 | 243.51 ± 0.46 |
| 10 | Kaempferol | 3.96 | 12.09 ± 0.08 | Nd | Nd |
| 11 | Flavone | Nd | Nd | 3.30 | 9.28 ± 0.25 |
| SEFE | SEFR | Orlistat | Gallic Acid | |
|---|---|---|---|---|
| Lipase inhibitory IC50 (mg/mL) | 0.167 ± 0.0006 | 0.106 ± 0.0008 | 0.128 ± 0.003 | - |
| Antiglycation IC50 (mg/mL) | 3.990 ± 0.236 | 3.997 ± 0.140 | - | 3.008 ± 0.03 |
| Diameter (cm) and % of Inhibition | ||||||
|---|---|---|---|---|---|---|
| Treatment | Dose (mg/kg) | 0 h | 3 h | 4 h | 5 h | 6 h |
| Control (NaCl 0.9%) | - | 2.43 ± 0.12 | 3.03 ± 0.03 *** | 3.00 ± 0.00 *** | 2.96 ± 0.03 *** | 2.90 ± 0.05 ** |
| Indomethacine | 10 | 2.30 ± 0.03 | 2.54 ± 0.03 ** (60%) | 2.45 ± 0.03 * (74%) | 2.41 ± 0.02 (80%) | 2.34 ± 0.03 (92%) |
| SEFR Extract | 200 | 2.36 ± 0.06 | 2.86 ± 0.06 *** (17%) | 2.76 ± 0.06 ** (30%) | 2.63 ± 0.03 * (50%) | 2.53 ± 0.03 (64%) |
| 400 | 2.56 ± 0.06 | 2.96 ± 0.03 *** (34%) | 2.83 ± 0.03 * (53%) | 2.70 ± 0.00 (74%) | 2.63 ± 0.03 (86%) | |
| SEFE Extract | 200 | 2.60 ± 0.15 | 3.20 ± 0.00 *** (0%) | 3.06 ± 0.03 ** (20%) | 3.00 ± 0.05 * (25%) | 2.93 ± 0.03 * (30%) |
| 400 | 2.36 ± 0.08 | 2.83 ± 0.06 ** (22%) | 2.73 ± 0.08 * (36%) | 2.60 ± 0.05 (55%) | 2.56 ± 0.03 (58%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouslamti, M.; Elrherabi, A.; Loukili, E.H.; Noman, O.M.; Mothana, R.A.; Ibrahim, M.N.; Abdnim, R.; Slighoua, M.; Bouhrim, M.; Bnouham, M.; et al. Phytochemical Profile, Antilipase, Hemoglobin Antiglycation, Antihyperglycemic, and Anti-Inflammatory Activities of Solanum elaeagnifolium Cav. Appl. Sci. 2023, 13, 11519. https://doi.org/10.3390/app132011519
Bouslamti M, Elrherabi A, Loukili EH, Noman OM, Mothana RA, Ibrahim MN, Abdnim R, Slighoua M, Bouhrim M, Bnouham M, et al. Phytochemical Profile, Antilipase, Hemoglobin Antiglycation, Antihyperglycemic, and Anti-Inflammatory Activities of Solanum elaeagnifolium Cav. Applied Sciences. 2023; 13(20):11519. https://doi.org/10.3390/app132011519
Chicago/Turabian StyleBouslamti, Mohammed, Amal Elrherabi, El Hassania Loukili, Omar M. Noman, Ramzi A. Mothana, Mansour N. Ibrahim, Rhizlan Abdnim, Meryem Slighoua, Mohamed Bouhrim, Mohamed Bnouham, and et al. 2023. "Phytochemical Profile, Antilipase, Hemoglobin Antiglycation, Antihyperglycemic, and Anti-Inflammatory Activities of Solanum elaeagnifolium Cav." Applied Sciences 13, no. 20: 11519. https://doi.org/10.3390/app132011519
APA StyleBouslamti, M., Elrherabi, A., Loukili, E. H., Noman, O. M., Mothana, R. A., Ibrahim, M. N., Abdnim, R., Slighoua, M., Bouhrim, M., Bnouham, M., Lyoussi, B., & Benjelloun, A. S. (2023). Phytochemical Profile, Antilipase, Hemoglobin Antiglycation, Antihyperglycemic, and Anti-Inflammatory Activities of Solanum elaeagnifolium Cav. Applied Sciences, 13(20), 11519. https://doi.org/10.3390/app132011519






