Roasting Extract of Handroanthus impetiginosus Enhances Its Anticancer Activity in A549 Lung Cancer Cells and Improves Its Antioxidant and Anti-Inflammatory Effects in Normal Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Roasted Taheebo Extract
2.3. Antioxidant Screening
2.3.1. Total Phenolic Content (TPC) Determination
2.3.2. Total Flavonoid Content (TFC) Determination
2.3.3. DPPH-Free Radical Scavenging Activity Assessment
2.3.4. Procedure for Reducing Power Activity (RPA)
2.4. Cell Culture
2.5. In Vitro Cytotoxicity of Taheebo
2.6. Measurement of Antioxidant Enzyme Activity
2.6.1. Determination of GPx Activity
2.6.2. Determination of SOD Activity
2.6.3. Determination of CAT Activity
2.6.4. Determination of GST Activity
2.6.5. Reactive Oxygen Species (ROS) Generation
2.7. Nitrite Levels (NO) Determination
2.8. Wound-Healing Assay
2.9. Reverse Transcription-Polymerase Chain Reaction (RT-PCR and qRT-PCR)
3. Results
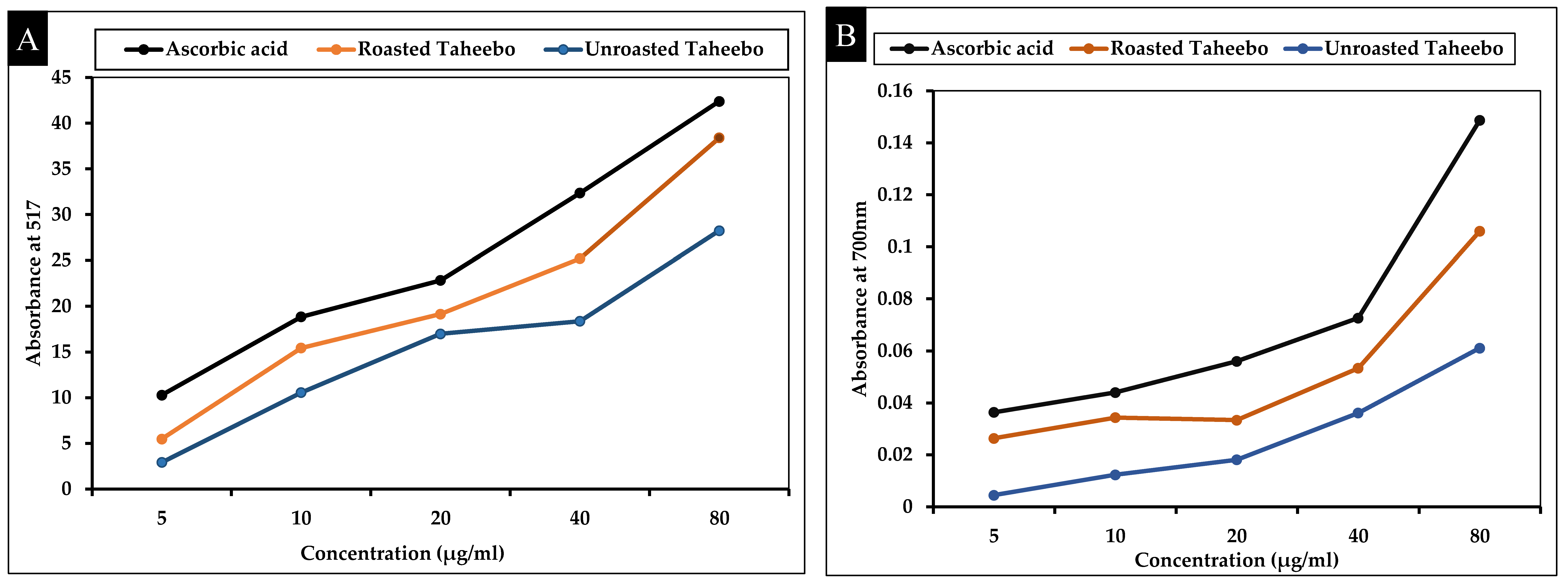
3.1. Antioxidant Activities of Taheebo and Roasted Taheebo
TPC, TFC, and Antioxidant Effects
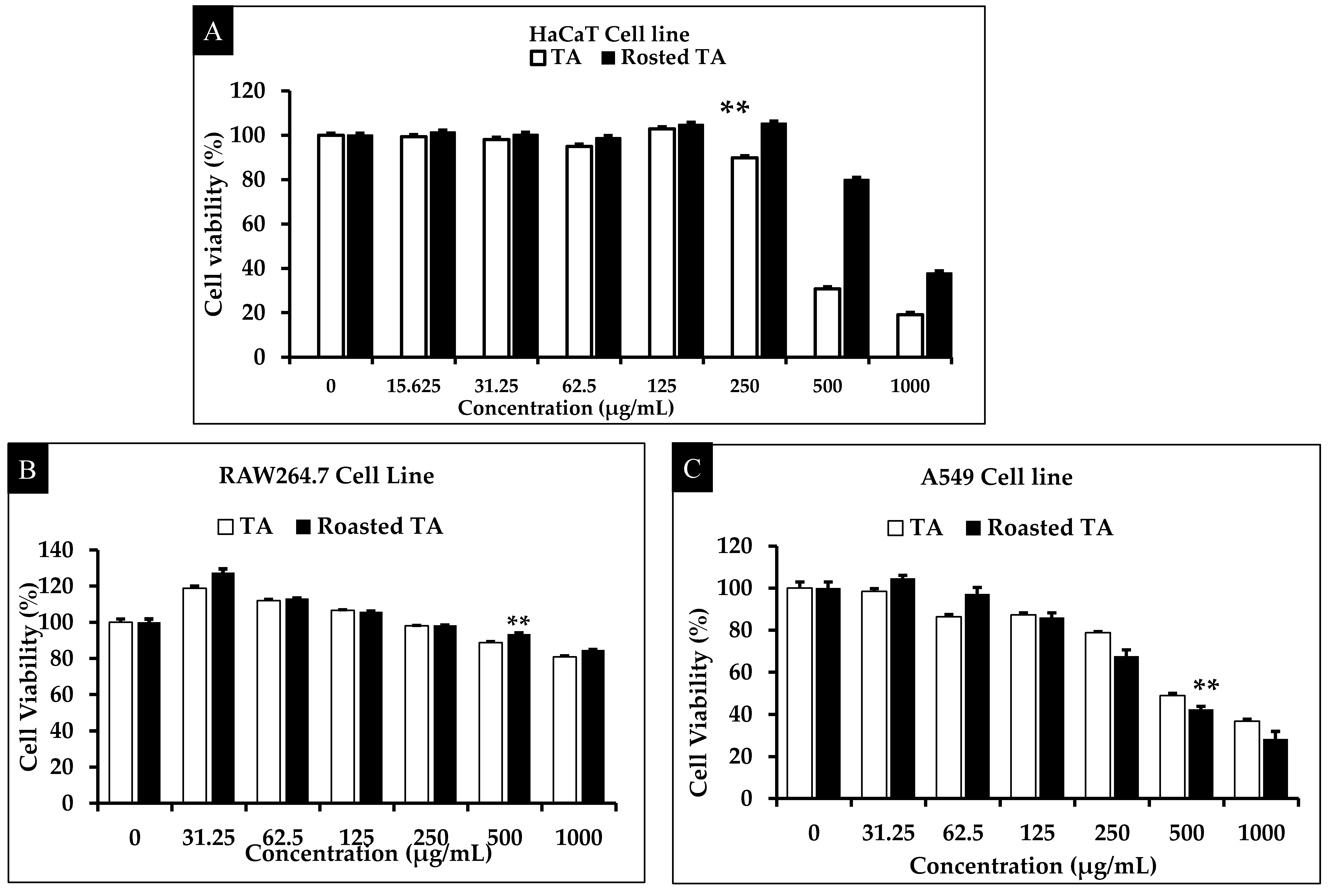
3.2. Evaluation of Cytotoxicity
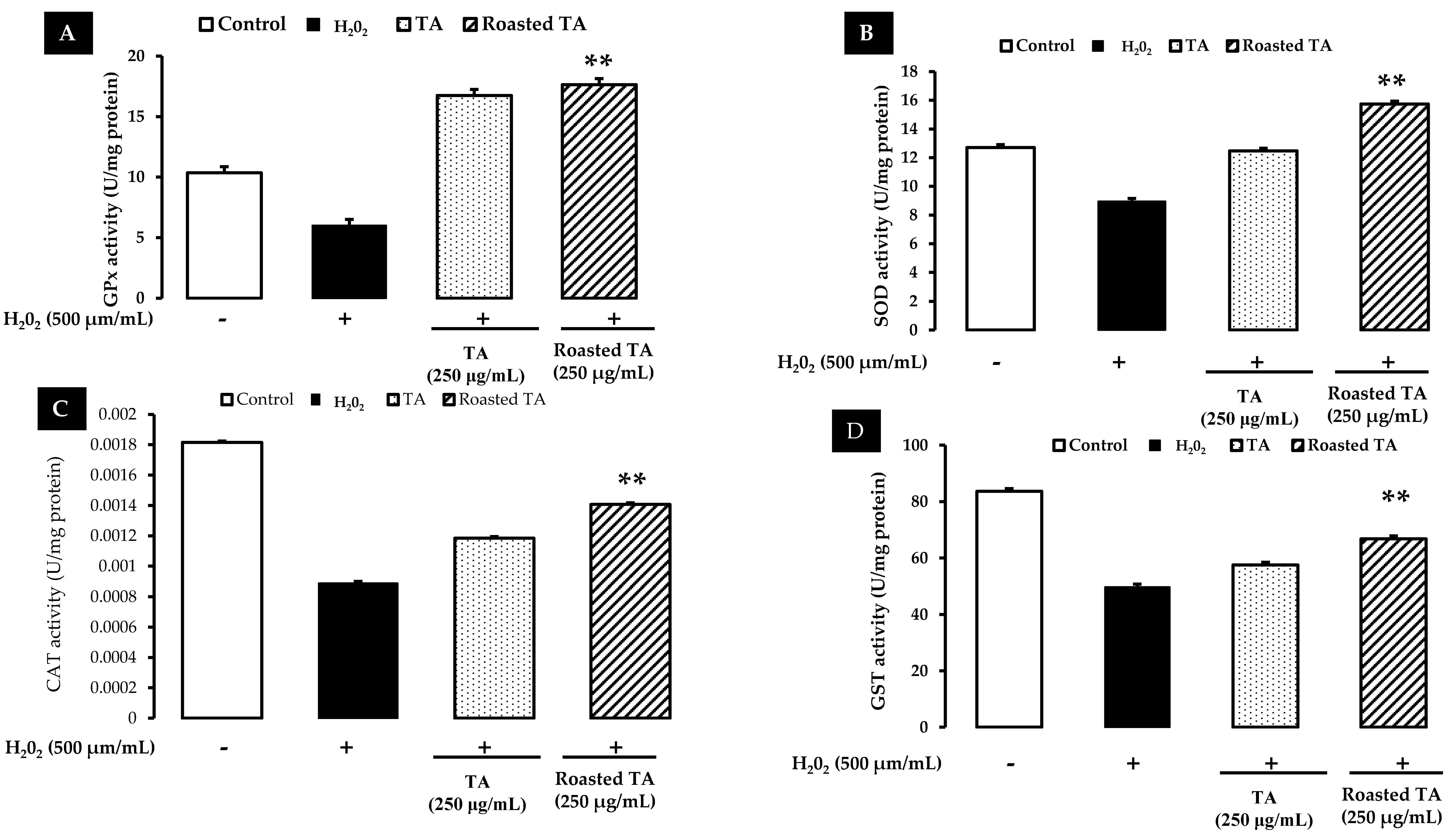
3.3. Enzymatic Activity of Antioxidants
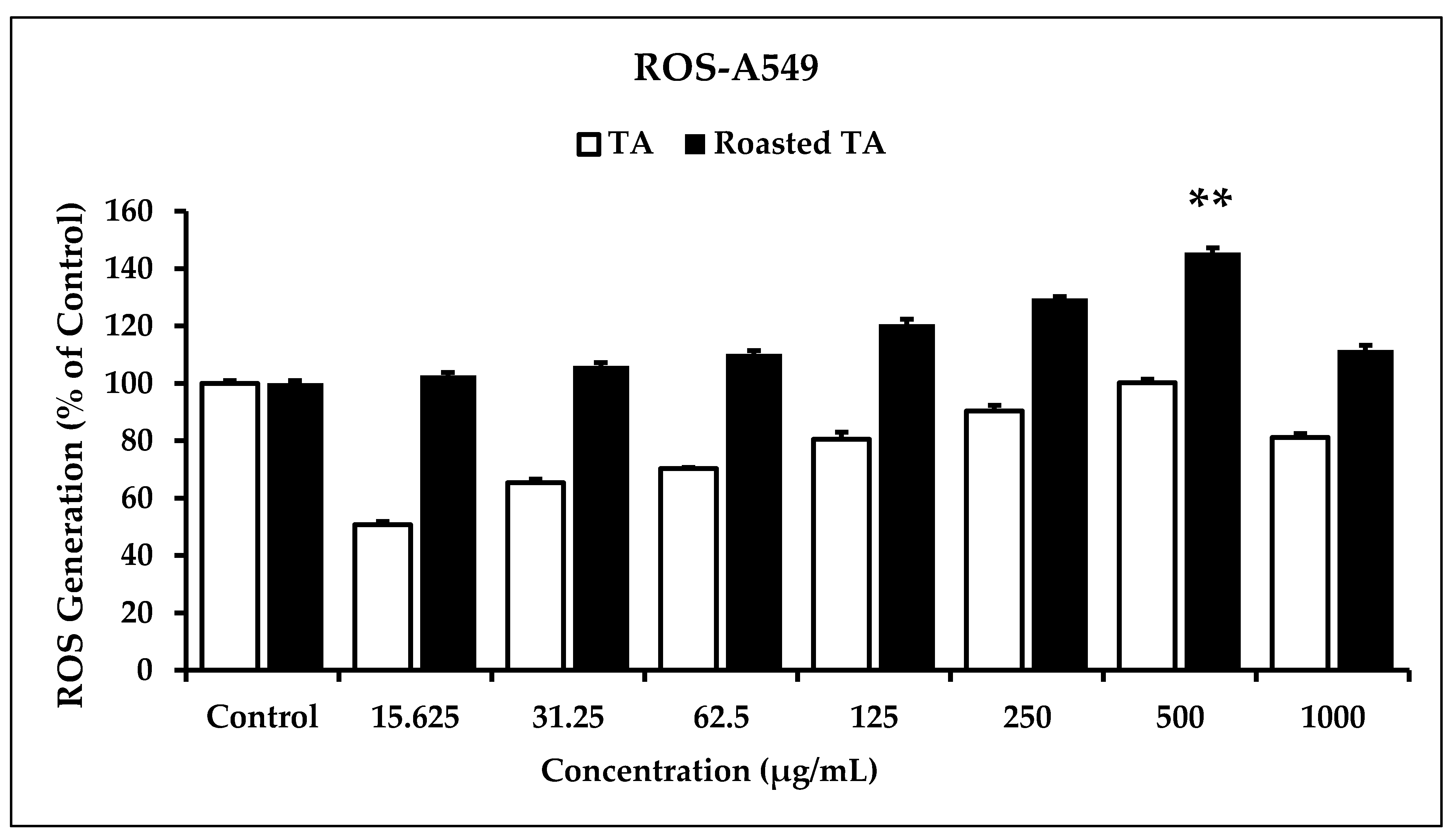
3.4. In Vitro ROS Induced by Roasted Taheebo on Cancer Cells
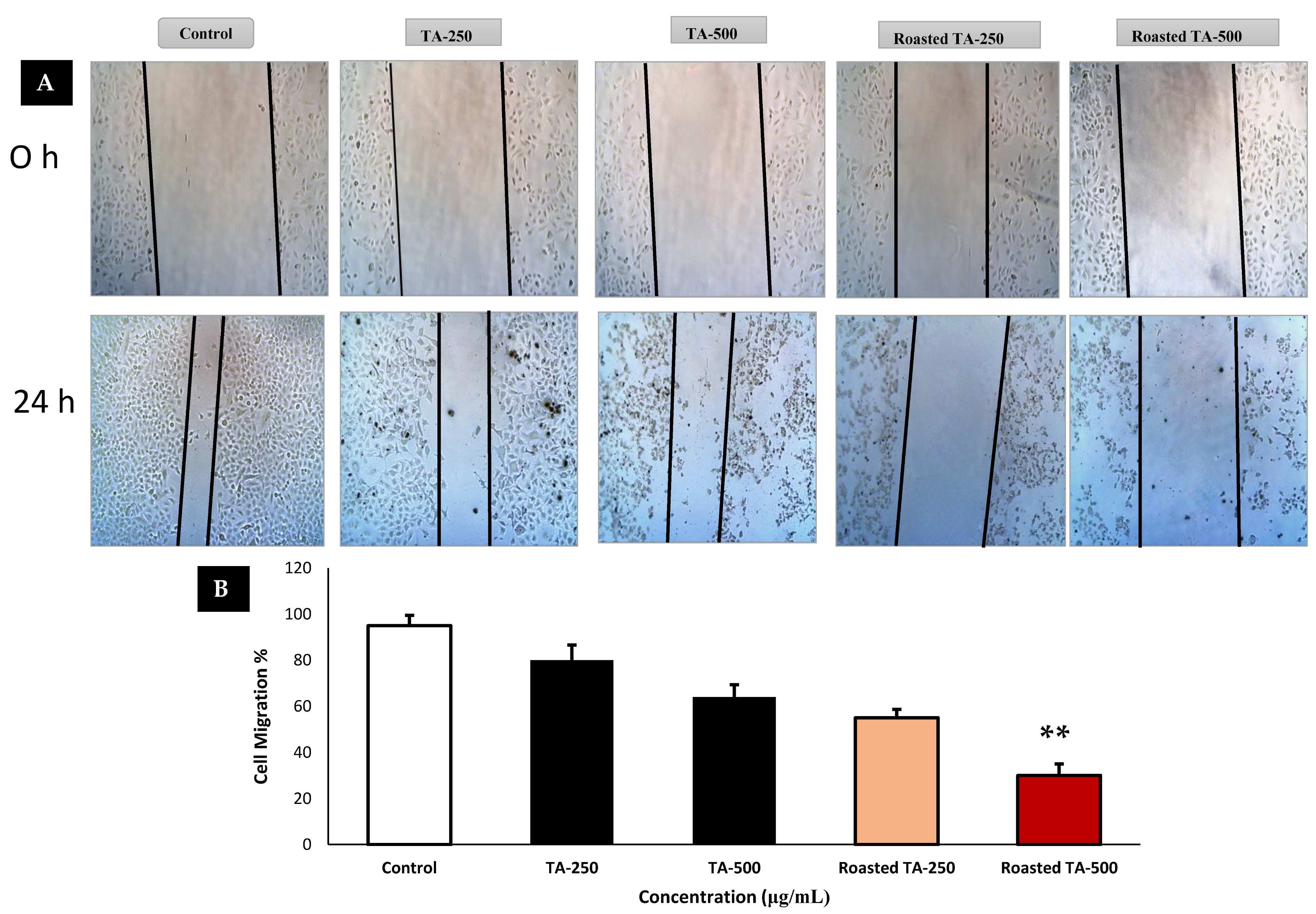
3.5. Cancer Cells Migration Is Inhibited by Taheebo
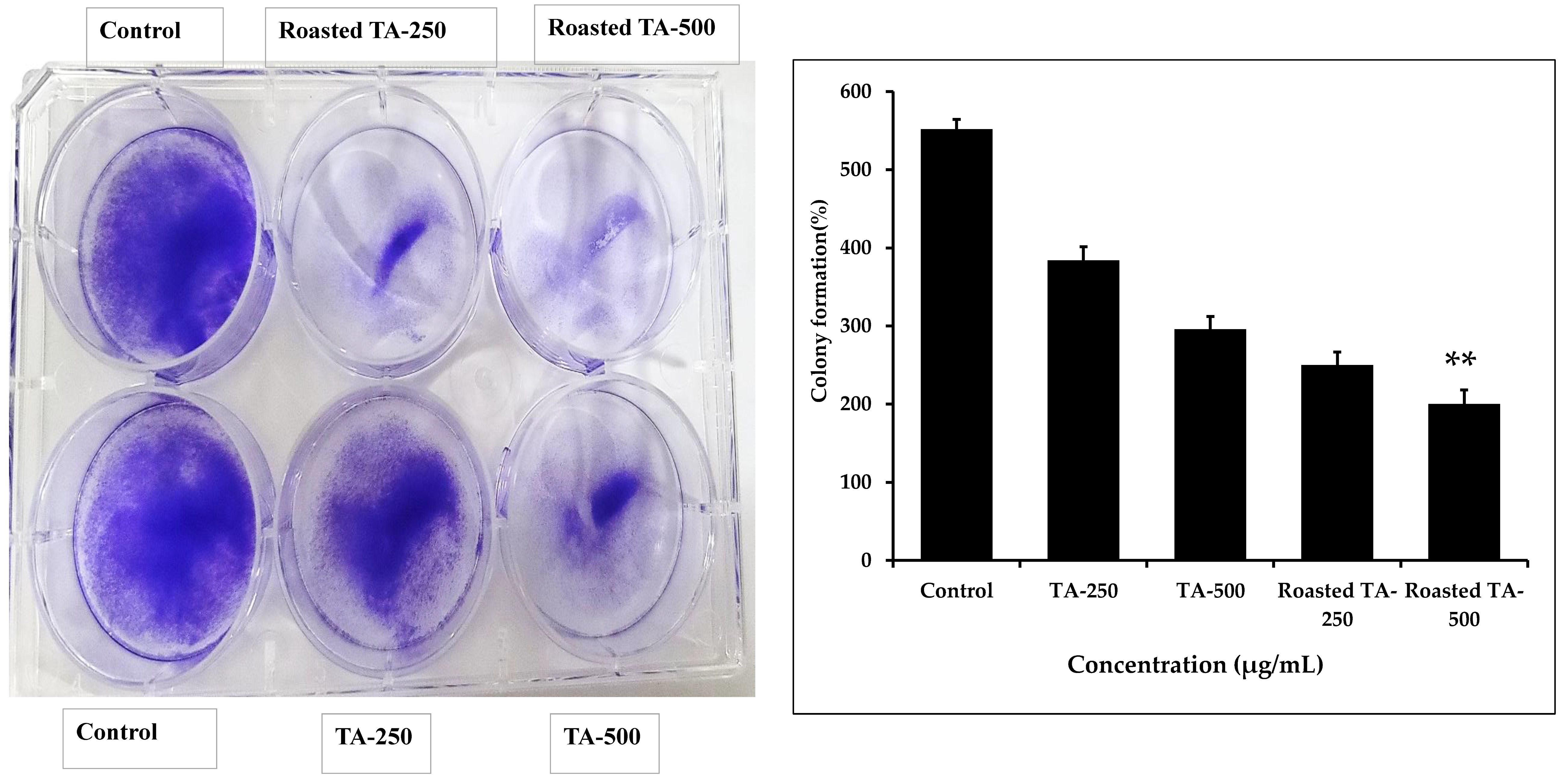
3.6. Inhibition of Colony Formation in Cancer Cells
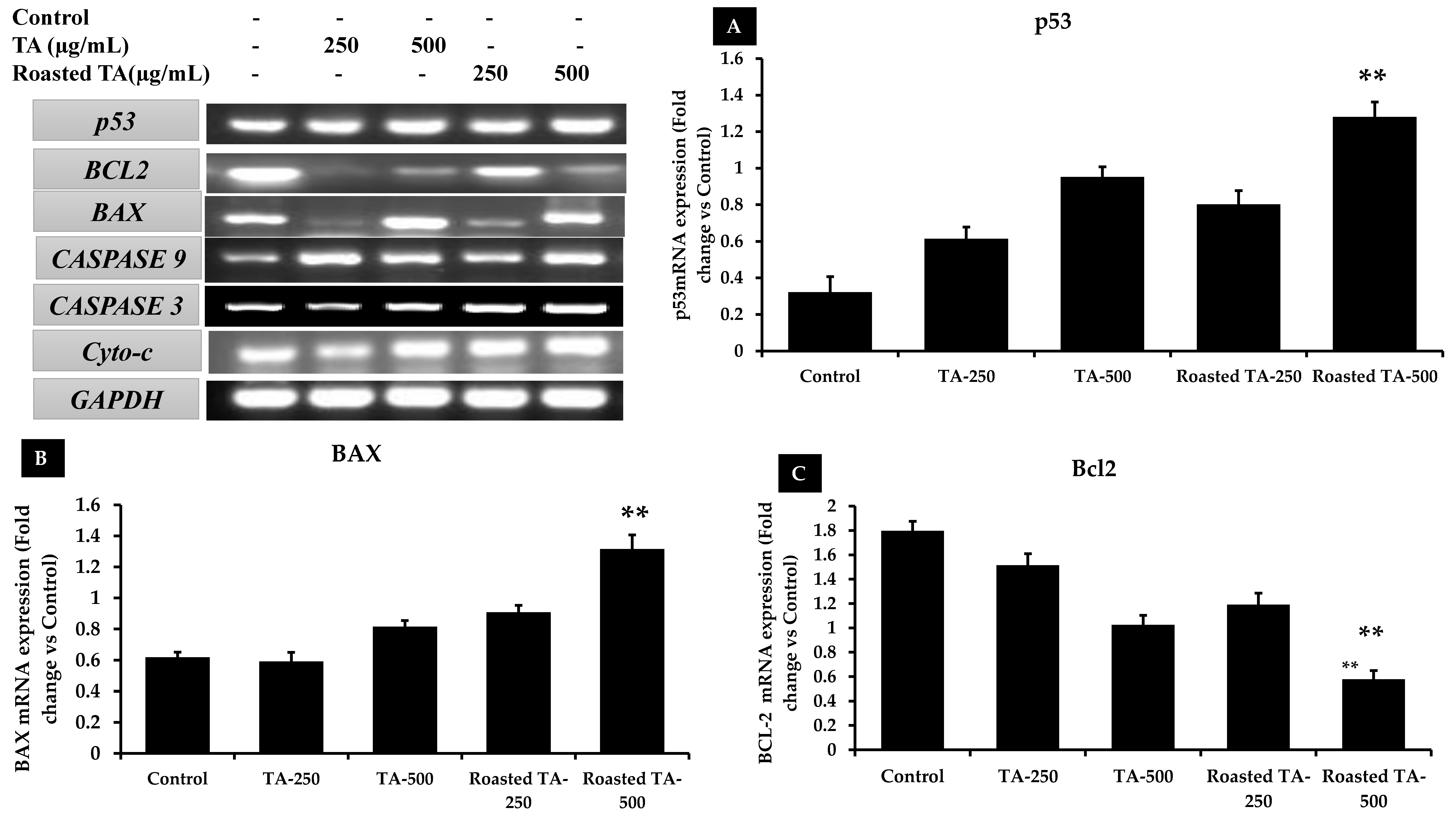
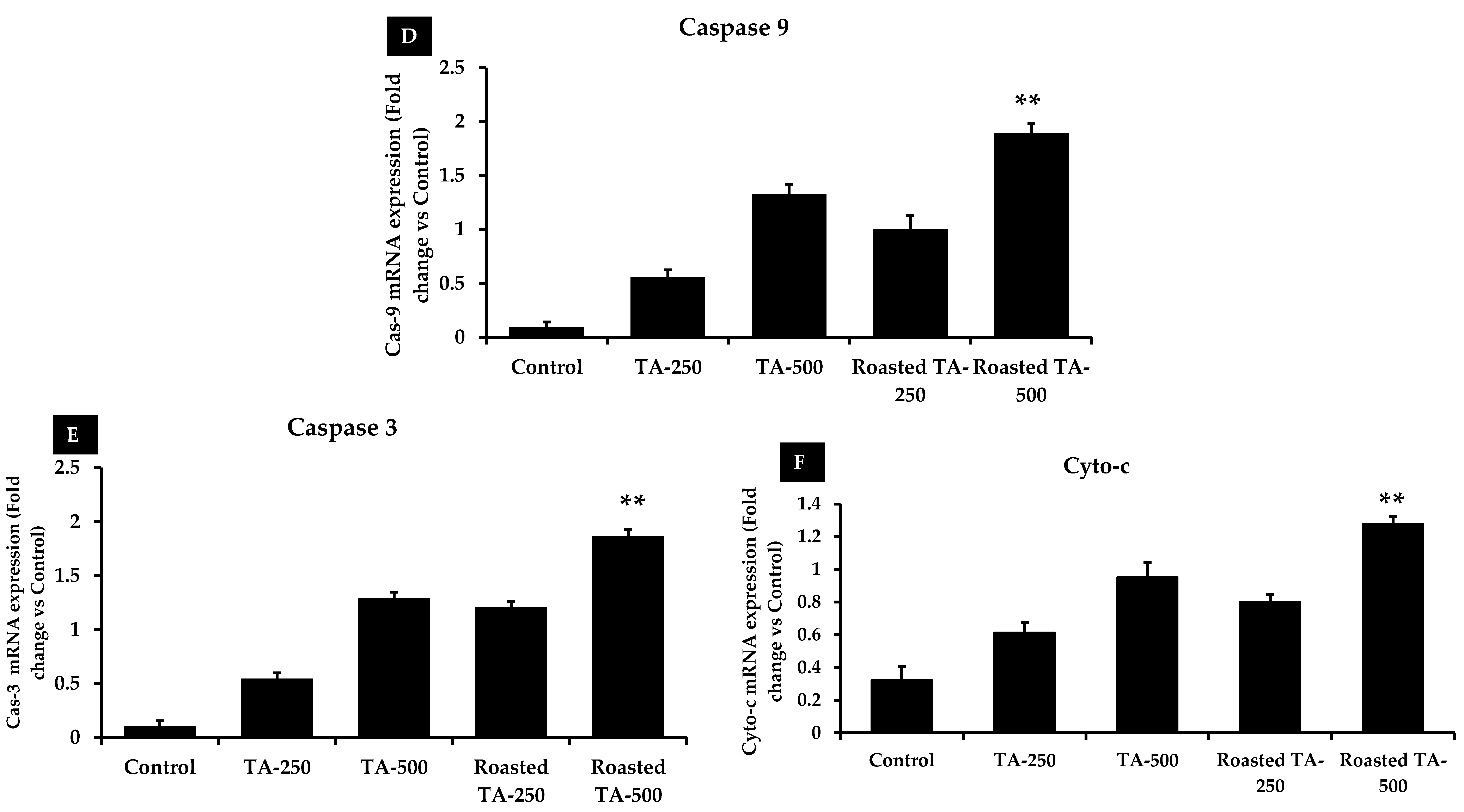
3.7. Taheebo Induces Apoptosis by Regulating Apoptotic Gene Expression
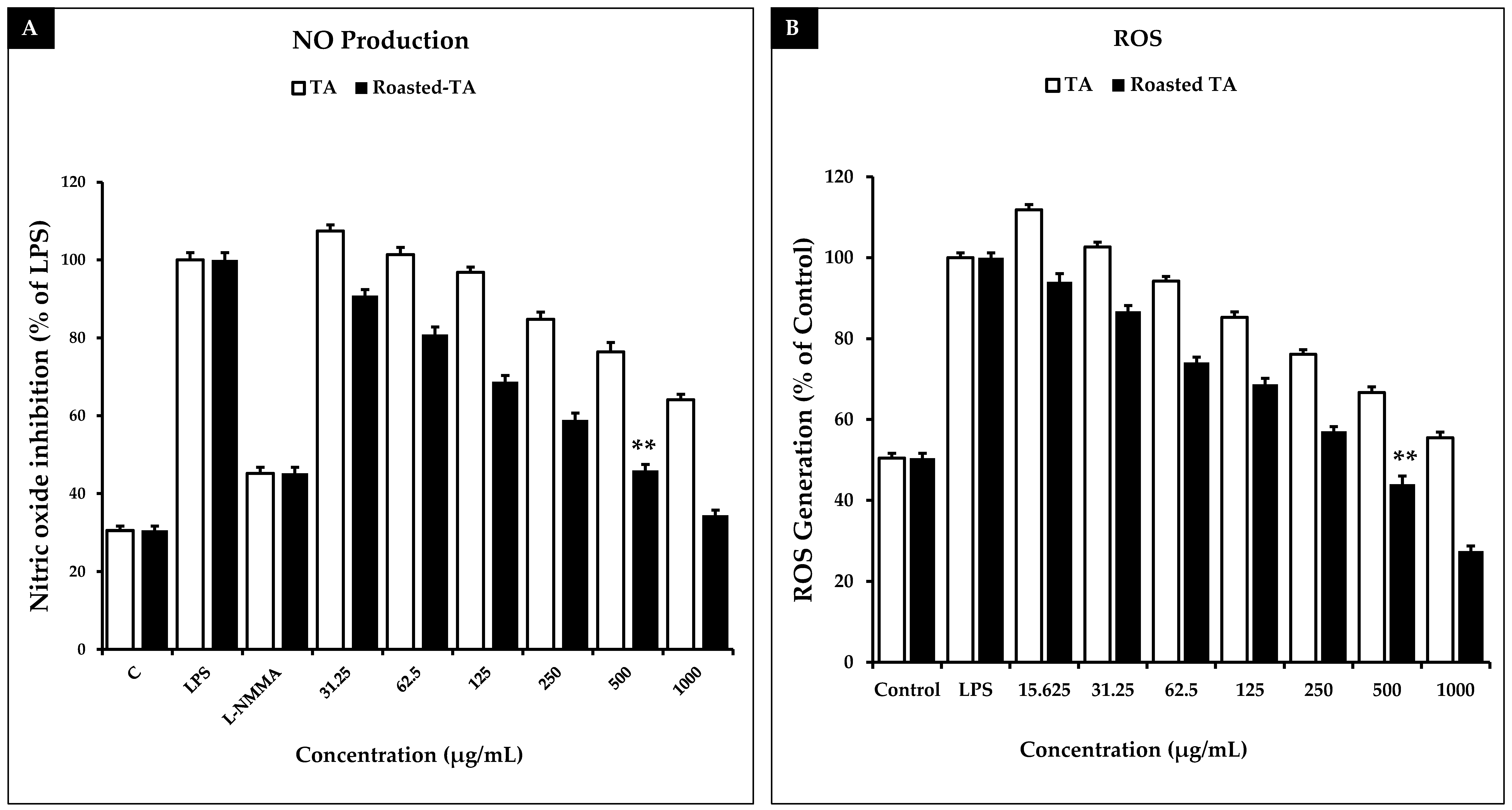
3.8. Taheebo Extract Increased NO Production and Inhibited ROS Generation Induced by LPS
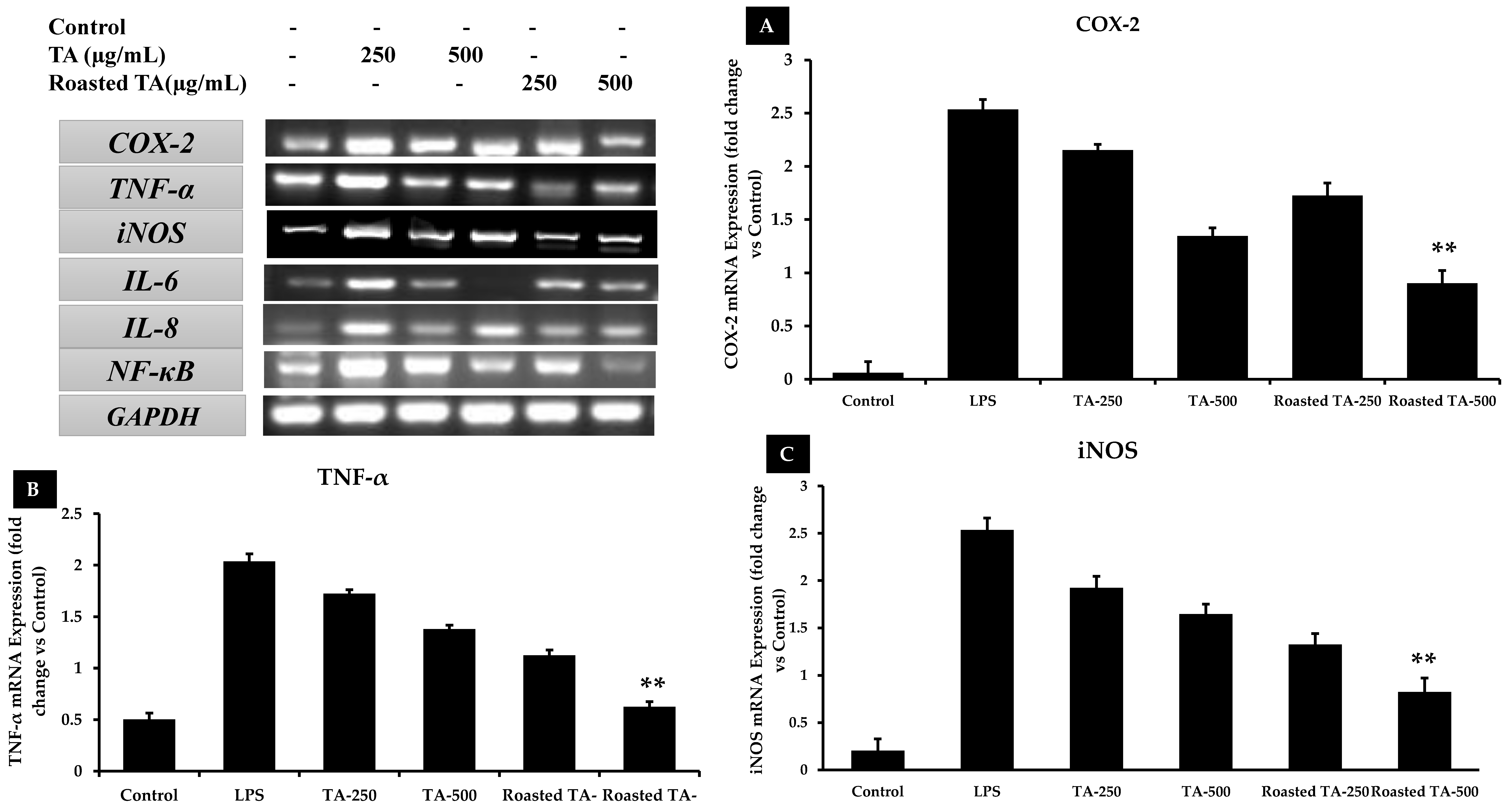
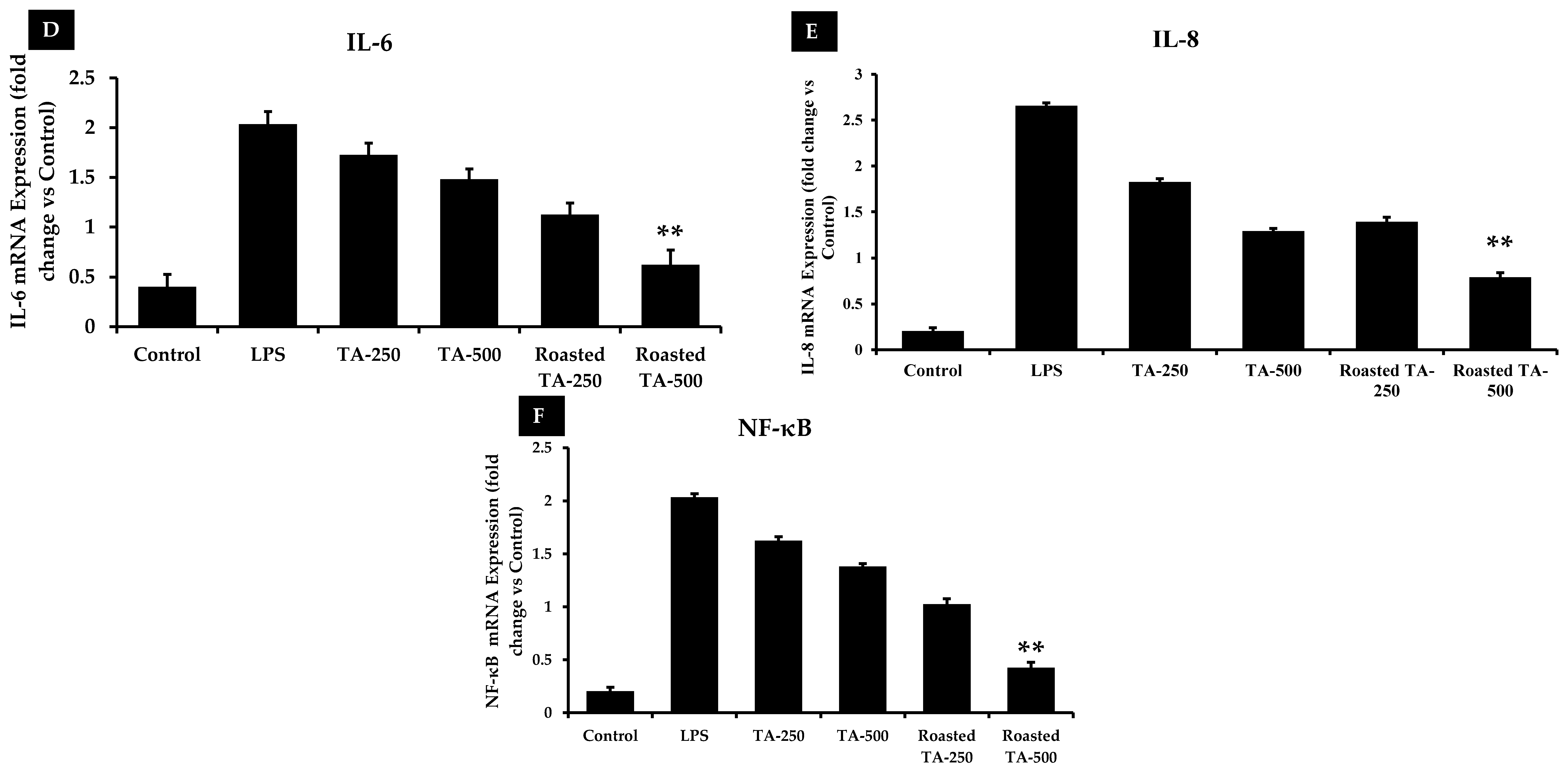
3.9. Taheebo Extract Inhibited the Increased Levels of Inflammation-Related Cytokines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ngo, L.T.; Okogun, J.I.; Folk, W.R. 21st Century natural product research and drug development and traditional medicines. Nat. Prod. Rep. 2013, 30, 584–592. [Google Scholar] [CrossRef]
- Akter, R.; Yang, D.U.; Ahn, J.C.; Awais, M.; Nahar, J.; Ramadhania, Z.M.; Kim, J.Y.; Lee, G.J.; Kwak, G.-Y.; Lee, D.W.; et al. Comparison of In Vitro Estrogenic Activity of Polygoni multiflori Radix and Cynanchi wilfordii Radix via the Enhancement of ERα/β Expression in MCF7 Cells. Molecules 2023, 28, 2199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hunto, S.T.; Yang, Y.; Lee, J.; Cho, J.Y. Tabebuia impetiginosa: A comprehensive review on traditional uses, phytochemistry, and immunopharmacological properties. Molecules 2020, 25, 4294. [Google Scholar] [CrossRef]
- Sobreiro, M.B.; Vieira, L.D.; Nunes, R.; Novaes, E.; Coissac, E.; Silva-Junior, O.B.; Grattapaglia, D.; Collevatti, R.G. Chloroplast genome assembly of Handroanthus impetiginosus: Comparative analysis and molecular evolution in Bignoniaceae. Planta 2020, 252, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jausoro, V.; Llorente, B.E.; Apóstolo, N.M. Structural differences between hyperhydric and normal in vitro shoots of Handroanthus impetiginosus (Mart. ex DC) Mattos (Bignoniaceae). Plant Cell Tissue Organ Cult. (PCTOC) 2010, 101, 183–191. [Google Scholar] [CrossRef]
- Edwards, S.E.; da Costa Rocha, I.; Williamson, E.M.; Heinrich, M. Lapacho Handroanthus impetiginosus (Mart. ex DC.) Mattos. In Phytopharmacy: An Evidence-Based Guide to Herbal Medicinal Products; Wiley: Hoboken, NJ, USA, 2015; p. 234. [Google Scholar]
- Jones, K. Pau d’Arco: Immune Power from the Rain Forest; Inner Traditions/Bear & Co: Rochester, VT, USA, 1995. [Google Scholar]
- Park, B.-S.; Lee, K.-G.; Shibamoto, T.; Lee, S.-E.; Takeoka, G.R. Antioxidant Activity and Characterization of Volatile Constituents of Taheebo (Tabebuia impetiginosa Martius ex DC). J. Agric. Food Chem. 2002, 51, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Malange, K.F.; dos Santos, G.G.; Kato, N.N.; Toffoli-Kadri, M.C.; Carollo, C.A.; Silva, D.B.; Portugal, L.C.; Alves, F.M.; Rita, P.H.S.; Parada, C.A.; et al. Tabebuia aurea decreases hyperalgesia and neuronal injury induced by snake venom. J. Ethnopharmacol. 2018, 233, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Suo, M.; Isao, H.; Kato, H.; Takano, F.; Ohta, T. Anti-inflammatory constituents from Tabebuia avellanedae. Fitoterapia 2012, 83, 1484–1488. [Google Scholar] [CrossRef]
- Pires, T.C.S.P.; Dias, M.I.; Calhelha, R.C.; Carvalho, A.M.; Queiroz, M.-J.R.P.; Barros, L.; Ferreira, I.C.F.R. Bioactive Properties of Tabebuia impetiginosa-Based Phytopreparations and Phytoformulations: A Comparison between Extracts and Dietary Supplements. Molecules 2015, 20, 22863–22871. [Google Scholar] [CrossRef]
- Byeon, S.E.; Chung, J.Y.; Lee, Y.G.; Kim, B.H.; Kim, K.H.; Cho, J.Y. In vitro and in vivo anti-inflammatory effects of taheebo, a water extract from the inner bark of Tabebuia avellanedae. J. Ethnopharmacol. 2008, 119, 145–152. [Google Scholar] [CrossRef]
- Castellanos, J.R.G.; Prieto, J.M.; Heinrich, M. Red Lapacho (Tabebuia impetiginosa)—A global ethnopharmacological commodity? J. Ethnopharmacol. 2009, 121, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mowrey, D. Ancient Herb. Modern Medicine; Mountainwest Institute of Herbal Sciences: Salt Lake City, UT, USA, 2001. [Google Scholar]
- Jin, Y.; Jeong, K.M.; Lee, J.; Zhao, J.; Choi, S.-Y.; Baek, K.-S. Development and Validation of an Analytical Method Readily Applicable for Quality Control of Tabebuia impetiginosa (Taheebo) Ethanolic Extract. J. AOAC Int. 2018, 101, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.J.L.; Johanes, I.; Pinheiro, A.A.V. Anticancer potential and toxicity of the genus Handroanthus Mattos (Bignoniaceae): A systematic review. Toxicon 2022, 217, 131–142. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.C.V.; Carvalho, A.; Rahme, S.W.; Araújo, A.d.R.B.; Malard, P.F.; Brunel, H.S.S. Evaluation of cell viability and nitric oxide release after treatment of human hepatocellular carcinoma cells with a homeopathic compound of Graviola (Anonna muricata) and Purple Ipe (Handroanthus impetiginosus). Med. Res. Arch. 2023, 11, 4091. [Google Scholar] [CrossRef]
- Lee, M.H.; Choi, H.M.; Hahm, D.-H.; Her, E.; Yang, H.-I.; Yoo, M.C.; Kim, K.S. Analgesic and anti-inflammatory effects in animal models of an ethanolic extract of Taheebo, the inner bark of Tabebuia avellanedae. Mol. Med. Rep. 2012, 6, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-E.; Park, B.-S.; Yum, J.H. Antioxidative activity of taheebo (Tabebuia impetiginosa Martius ex DC.) extracts on the H2O2-induced NIH3T3 cells. J. Med. Plants Res. 2012, 6, 5258–5265. [Google Scholar]
- Iwamoto, K.; Fukuda, Y.; Tokikura, C.; Noda, M.; Yamamoto, A.; Yamamoto, M.; Yamashita, M.; Zaima, N.; Iida, A.; Moriyama, T. The anti-obesity effect of Taheebo (Tabebuia avellanedae Lorentz ex Griseb) extract in ovariectomized mice and the identification of a potential anti-obesity compound. Biochem. Biophys. Res. Commun. 2016, 478, 1136–1140. [Google Scholar] [CrossRef]
- Mukherjee, B.; Telang, N.; Wong, G.Y.C. Growth inhibition of estrogen receptor positive human breast cancer cells by Taheebo from the inner bark of Tabebuia avellandae tree. Int. J. Mol. Med. 1998, 24, 253–260. [Google Scholar] [CrossRef]
- Böhler, T.; Nolting, J.; Gurragchaa, P.; Lupescu, A.; Neumayer, H.-H.; Budde, K.; Kamar, N.; Klupp, J. Tabebuia avellanedae extracts inhibit IL-2-independent T-lymphocyte activation and proliferation. Transpl. Immunol. 2008, 18, 319–323. [Google Scholar] [CrossRef]
- Tahara, T.; Watanabe, A.; Yutani, M.; Yamano, Y.; Sagara, M.; Nagai, S.; Saito, K.; Yamashita, M.; Ihara, M.; Iida, A. STAT3 inhibitory activity of naphthoquinones isolated from Tabebuia avellanedae. Bioorganic Med. Chem. 2020, 28, 115347. [Google Scholar] [CrossRef]
- Freitas, A.E.; Budni, J.; Lobato, K.R.; Binfaré, R.W.; Machado, D.G.; Jacinto, J.; Veronezi, P.O.; Pizzolatti, M.G.; Rodrigues, A.L.S. Antidepressant-like action of the ethanolic extract from Tabebuia avellanedae in mice: Evidence for the involvement of the monoaminergic system. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Lee, J.H. Effects of oven-drying, roasting, and explosive puffing process on isoflavone distributions in soybeans. Food Chem. 2009, 112, 316–320. [Google Scholar] [CrossRef]
- Kim, W.; Kim, S.-Y.; Kim, D.-O.; Kim, B.-Y.; Baik, M.-Y. Puffing, a novel coffee bean processing technique for the enhancement of extract yield and antioxidant capacity. Food Chem. 2018, 240, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.-S.; Shin, J.-S.; Kim, W.; Baik, M.-Y.J.F. Increases in Ginsenoside Rg3, Compound K, and Antioxidant Activity of Cultivated Wild Panax Ginseng (CWPG) by Puffing. Foods 2022, 11, 2936. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kumar, A.; Kumar, V.; Kumar, S.; Saini, R.K.; Nayi, P.; Gehlot, R. Recent advancements and applications of explosion puffing. Food Chem. 2023, 403, 134452. [Google Scholar] [CrossRef] [PubMed]
- Ramadhania, Z.M.; Yang, D.U.; Moektiwardojo, M.; Han, Y.; Park, J.K.; Rupa, E.J.; Yang, D.C.; Lee, S.J.; Kang, S.C. Enhanced Anti-Skin Aging Effects of Fermented Black Ginseng (Panax ginseng C.A. Meyer) by Aspergillus niger KHNT-1. Appl. Sci. 2022, 13, 550. [Google Scholar] [CrossRef]
- Nahar, J.; Boopathi, V.; Rupa, E.J.; Awais, M.; Valappil, A.K.; Morshed, M.N.; Murugesan, M.; Akter, R.; Yang, D.U.; Mathiyalagan, R. Protective Effects of Aquilaria agallocha and Aquilaria malaccensis Edible Plant Extracts against Lung Cancer, Inflammation, and Oxidative Stress—In Silico and In Vitro Study. Appl. Sci. 2023, 13, 6321. [Google Scholar] [CrossRef]
- Akter, R.; Kwak, G.-Y.; Ahn, J.C.; Mathiyalagan, R.; Ramadhania, Z.M.; Yang, D.C.; Kang, S.C. Protective Effect and Potential Antioxidant Role of Kakadu Plum Extracts on Alcohol-Induced Oxidative Damage in HepG2 Cells. Appl. Sci. 2021, 12, 236. [Google Scholar] [CrossRef]
- Bak, M.-J.; Jun, M.; Jeong, W.-S. Antioxidant and Hepatoprotective Effects of the Red Ginseng Essential Oil in H2O2-Treated HepG2 Cells and CCl4-Treated Mice. Int. J. Mol. Sci. 2012, 13, 2314–2330. [Google Scholar] [CrossRef]
- Piao, M.J.; Yoo, E.S.; Koh, Y.S.; Kang, H.K.; Kim, J.; Kim, Y.J.; Kang, H.H.; Hyun, J.W. Antioxidant Effects of the Ethanol Extract from Flower of Camellia japonica via Scavenging of Reactive Oxygen Species and Induction of Antioxidant Enzymes. Int. J. Mol. Sci. 2011, 12, 2618–2630. [Google Scholar] [CrossRef]
- Sikdar, S.; Lallemand, B.; Dubois, J. Induction of Phase II Enzymes Glutathione-S-Transferase and NADPH: Quinone Oxydoreductase 1 with Novel Sulforaphane Derivatives in Human Keratinocytes: Evaluation of the Intracellular GSH Level. Pharmacol. Pharm. 2014, 05, 937–943. [Google Scholar] [CrossRef]
- Nagano, T.; Tachihara, M.; Nishimura, Y. Mechanism of Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitors and a Potential Treatment Strategy. Cells 2018, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Morshed, N.; Ahn, J.C.; Mathiyalagan, R.; Rupa, E.J.; Akter, R.; Karim, R.; Jung, D.H.; Yang, D.U.; Yang, D.C.; Jung, S.K. Antioxidant Activity of Panax ginseng to Regulate ROS in Various Chronic Diseases. Appl. Sci. 2023, 13, 2893. [Google Scholar] [CrossRef]
- Saeed, A.; Marwat, M.S.; Shah, A.H.; Naz, R.; Abidin, S.Z.-U.; Akbar, S.; Khan, R.; Navid, M.T.; Saeed, A. Assessment of total phenolic and flavonoid contents of selected fruits and vegetables. Indian J. Tradit. Knowl. (IJTK) 2019, 18, 686–693. [Google Scholar]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Lue, B.-M.; Nielsen, N.S.; Jacobsen, C.; Hellgren, L.; Guo, Z.; Xu, X. Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human low density lipoprotein assays. Food Chem. 2010, 123, 221–230. [Google Scholar] [CrossRef]
- Mushtaq, S.; Abbasi, B.H.; Uzair, B.; Abbasi, R. Natural products as reservoirs of novel therapeutic agents. EXCLI J. 2018, 17, 420–451. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef]
- Su, X.-L.; Wang, J.-W.; Che, H.; Wang, C.-F.; Jiang, H.; Lei, X.; Zhao, W.; Kuang, H.-X.; Wang, Q.-H. Clinical application and mechanism of traditional Chinese medicine in treatment of lung cancer. Chin. Med. J. 2020, 133, 2987–2997. [Google Scholar] [CrossRef]
- Ghuman, S.; Ncube, B.; Finnie, J.; McGaw, L.; Njoya, E.M.; Coopoosamy, R.; Van Staden, J. Antioxidant, anti-inflammatory and wound healing properties of medicinal plant extracts used to treat wounds and dermatological disorders. S. Afr. J. Bot. 2019, 126, 232–240. [Google Scholar] [CrossRef]
- Angusti, A.; Manfredini, S. The Antioxidants and Pro-Antioxidants Network: An Overview. Curr. Pharm. Des. 2004, 10, 1677–1694. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Zaidieh, T.; Smith, J.R.; Ball, K.E.; An, Q. ROS as a novel indicator to predict anticancer drug efficacy. BMC Cancer 2019, 19, 1224. [Google Scholar] [CrossRef]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef]
- Rao, P.C.; Begum, S.; Sahai, M.; Sriram, D.S. Coptisine-induced cell cycle arrest at G2/M phase and reactive oxygen species–dependent mitochondria-mediated apoptosis in non-small-cell lung cancer A549 cells. Tumor Biol. 2017, 39, 1010428317694565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Zamzami, N.; Marchetti, P.; Castedo, M.; Decaudin, D.; Macho, A.; Hirsch, T.; A Susin, S.; Petit, P.X.; Mignotte, B.; Kroemer, G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 1995, 182, 367–377. [Google Scholar] [CrossRef]
- Guo, R.; Chen, X.; Nguyen, T.; Chai, J.; Gao, Y.; Wu, J.; Li, J.; Abdel-Rahman, M.A.; Chen, X.; Xu, X. The Strong Anti-Tumor Effect of Smp24 in Lung Adenocarcinoma A549 Cells Depends on Its Induction of Mitochondrial Dysfunctions and ROS Accumulation. Toxins 2022, 14, 590. [Google Scholar] [CrossRef]
- Hu, T.-Y.; Ju, J.-M.; Mo, L.-H.; Ma, L.; Hu, W.-H.; You, R.-R.; Chen, X.-Q.; Chen, Y.-Y.; Liu, Z.-Q.; Qiu, S.-Q. Anti-inflammation action of xanthones from Swertia chirayita by regulating COX-2/NF-κB/MAPKs/Akt signaling pathways in RAW 264.7 macrophage cells. Phytomedicine 2019, 55, 214–221. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Bao, Y.; Wang, X.; Zhai, J.; Zhan, X.; Zhang, H. Characterization and anti-inflammation of a polysaccharide produced by Chaetomium globosum CGMCC 6882 on LPS-induced RAW 264.7 cells. Carbohydr. Polym. 2021, 251, 117129. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Yarrow, J.C.; E Perlman, Z.E.; Westwood, N.J.; Mitchison, T.J. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol. 2004, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, C.M. Bcl-2 family members and disease. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2004, 1644, 169–177. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Abrahamse, H. Increased oxidative stress induced by rubus bioactive compounds induce apoptotic cell death in human breast cancer cells. Oxidative Med. Cell. Longev. 2019, 2019, 6797921. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.M.; Yu, Z.X.; Ferrans, V.J.; Lowenstein, R.A.; Finkel, T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 11848–11852. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; St. Clair, D.K. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef]
- Omoyeni, O.A.; Hussein, A.; Meyer, M.; Green, I.; Iwuoha, E. Pleiocarpa pycnantha leaves and its triterpenes induce apoptotic cell death in Caco-2 cells in vitro. BMC Complement. Altern. Med. 2015, 15, 224. [Google Scholar] [CrossRef]
- Kang, M.H.; Reynolds, C.P. Bcl-2 Inhibitors: Targeting Mitochondrial Apoptotic Pathways in Cancer Therapy. Clin. Cancer Res. 2009, 15, 1126–1132. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.H.; Viacava Follis, A.; Kriwacki, R.W.; Moldoveanu, T. Discoveries and controversies in BCL-2 protein-mediated apoptosis. FEBS J. 2016, 283, 2690–2700. [Google Scholar] [CrossRef] [PubMed]
- Julien, O.; Wells, J.A. Differentiation. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zihan, X.; Yizhe, W.; Yanyang, L.; Zhixi, L.; Xi, Y. Trilobatin induces apoptosis and attenuates stemness phenotype of acquired gefitinib resistant lung cancer cells via suppression of NF-κB pathway. Nutr. Cancer 2022, 74, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Yu, X.; Yu, T.; Zheng, X.; Chu, Q. Radix tetrastigma inhibits the non-small cell lung cancer via Bax/Bcl-2/Caspase-9/Caspase-3 pathway. Nutr. Cancer 2022, 74, 320–332. [Google Scholar] [PubMed]
- Lin, W.-W.; Karin, M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J. Clin. Investig. 2007, 117, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Cunha, I.W.; Lopes, A.; Falzoni, R.; Soares, F.A. Sarcomas Often Express Constitutive Nitric Oxide Synthases (NOS) But Infrequently Inducible NOS. Appl. Immunohistochem. Mol. Morphol. 2006, 14, 404–410. [Google Scholar] [CrossRef]
- Gupta, S.C.; Sundaram, C.; Reuter, S.; Aggarwal, B.B. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2010, 1799, 775–787. [Google Scholar] [CrossRef]
- Knott, A.B.; Bossy-Wetzel, E. Nitric oxide in health and disease of the nervous system. Antioxid. Redox Signal. 2009, 11, 541–553. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Riley, D.P.; Caputi, A.P.; Salvemini, D. Antioxidant therapy: A new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 2001, 53, 135–159. [Google Scholar] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-J.; Kwon, Y.-G.; Chung, H.-T.; Lee, S.-K.; Simmons, R.L.; Billiar, T.R.; Kim, Y.-M. Antioxidant enzymes suppress nitric oxide production through the inhibition of NF-κB activation: Role of H2O2 and nitric oxide in inducible nitric oxide synthase expression in macrophages. Nitric Oxide 2001, 5, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Naeem, M.; Noh, J.-K.; Lee, E.H.; Yoo, J.-W. Dexamethasone phosphate-loaded folate-conjugated polymeric nanoparticles for selective delivery to activated macrophages and suppression of inflammatory responses. Macromol. Res. 2015, 23, 485–492. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Patel, G.; Xue, Q.; Njateng, G.S.S.; Cai, S.; Cheng, G.; Kai, G. In vitro and in vivo anti-inflammatory effects of different extracts from Epigynum auritum through down-regulation of NF-κB and MAPK signaling pathways. J. Ethnopharmacol. 2020, 261, 113105. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hu, G.; Wang, C.; Xu, H.; Chen, X.; Qian, A. Cepharanthine, an Alkaloid from Stephania cepharantha Hayata, Inhibits the Inflammatory Response in the RAW264.7 Cell and Mouse Models. Inflammation 2013, 37, 235–246. [Google Scholar] [CrossRef]
| Gene | Primer Sequences (5′-3′) |
|---|---|
| p53 | F: TCT TGGGCC TGT GTT ATC TCC R: CGC CCA TGC AGG AAC TGT TA |
| Bcl2 | F: GAA GGG CAG CCG TTA GGAAA R: GCG CCC AAT ACG ACC AAA TC |
| BAX | F: GGT TGC CCT CTT CTA CTT T R: AGC CAC CCT GGT CTT G |
| CASPASE 3 | F: GAA GGA ACA CGC CAG GAA AC R: GCA AAG TGA AAT GTA GCA CCA A |
| CASPASE 9 | F: GCC CGA GTT TGA GAG GAA AA R: CAC AGC CAG ACC AGG AC |
| Cyto-c | F: CAGAAGGAAGTTAGGCC R: CGTCGCAGTGGATGATGTG |
| COX-2 | F: CCT GAG CAT CTA CGG TTT GC R: ACT GCT CAT CAC CCC ATT CA |
| TNF-α | F: GCCAGAATGCTGCAGGACTT R: GGCCTAAGGTCCACTTGTGTCA |
| iNOS | F: CCT GAG CAT CTA CGG TTT GC R: ACT GCT CAT CAC CCC ATT CA |
| IL-6 | F: AGGGTTGCCAGATGCAATAC R: AAACCAAGGCACAGTGGAAC |
| IL-8 | F: CCGGAGAGGAGACTTCACAG R: GGAAATTGGGGTAGGAAGGA |
| NF-κB | F: GCAAAGGGAACATTCCGATAT R: GCGACATCACATGGAAATCTA |
| GAPDH | F: CAA GGT CAT CCA TGA CAA CTT TG R: GTC CAC CAC CCT GTT GCT GTA G |
| Samples | Total Phenolic Contents (TPC) (µg AAE/g Extract) | Total Flavonoid Contents (TFC) (µg AAE/g Extract) | DPPH Scavenging (µg AAE/g Extract) | Reducing Power (µg AAE/g Extract) |
|---|---|---|---|---|
| Roasted Taheebo | 65,263.64 ± 0.029 | 1526.82 ± 0.001 | 989.62 ± 0.015 | 15.59 ± 0.006 |
| Unroasted taheebo | 54,877.27 ± 0.069 | 1185.36 ± 0.001 | 767.73 ± 0.025 | 11.66 ± 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahar, J.; Morshed, M.N.; Rupa, E.J.; Lee, J.H.; Kariyarath Valappil, A.; Awais, M.; Hun, K.J.; Sook, L.J.; Al-Amin, M.; Ahn, J.C.; et al. Roasting Extract of Handroanthus impetiginosus Enhances Its Anticancer Activity in A549 Lung Cancer Cells and Improves Its Antioxidant and Anti-Inflammatory Effects in Normal Cells. Appl. Sci. 2023, 13, 13171. https://doi.org/10.3390/app132413171
Nahar J, Morshed MN, Rupa EJ, Lee JH, Kariyarath Valappil A, Awais M, Hun KJ, Sook LJ, Al-Amin M, Ahn JC, et al. Roasting Extract of Handroanthus impetiginosus Enhances Its Anticancer Activity in A549 Lung Cancer Cells and Improves Its Antioxidant and Anti-Inflammatory Effects in Normal Cells. Applied Sciences. 2023; 13(24):13171. https://doi.org/10.3390/app132413171
Chicago/Turabian StyleNahar, Jinnatun, Md Niaj Morshed, Esrat Jahan Rupa, Jung Hyeok Lee, Anjali Kariyarath Valappil, Muhammad Awais, Ko Jeong Hun, Lee Ji Sook, Md. Al-Amin, Jong Chan Ahn, and et al. 2023. "Roasting Extract of Handroanthus impetiginosus Enhances Its Anticancer Activity in A549 Lung Cancer Cells and Improves Its Antioxidant and Anti-Inflammatory Effects in Normal Cells" Applied Sciences 13, no. 24: 13171. https://doi.org/10.3390/app132413171
APA StyleNahar, J., Morshed, M. N., Rupa, E. J., Lee, J. H., Kariyarath Valappil, A., Awais, M., Hun, K. J., Sook, L. J., Al-Amin, M., Ahn, J. C., Yang, D. C., & Jung, S.-K. (2023). Roasting Extract of Handroanthus impetiginosus Enhances Its Anticancer Activity in A549 Lung Cancer Cells and Improves Its Antioxidant and Anti-Inflammatory Effects in Normal Cells. Applied Sciences, 13(24), 13171. https://doi.org/10.3390/app132413171








