Effect of Different Local Vibration Durations on Knee Extensors’ Maximal Isometric Strength
Abstract
Featured Application
Abstract
1. Introduction
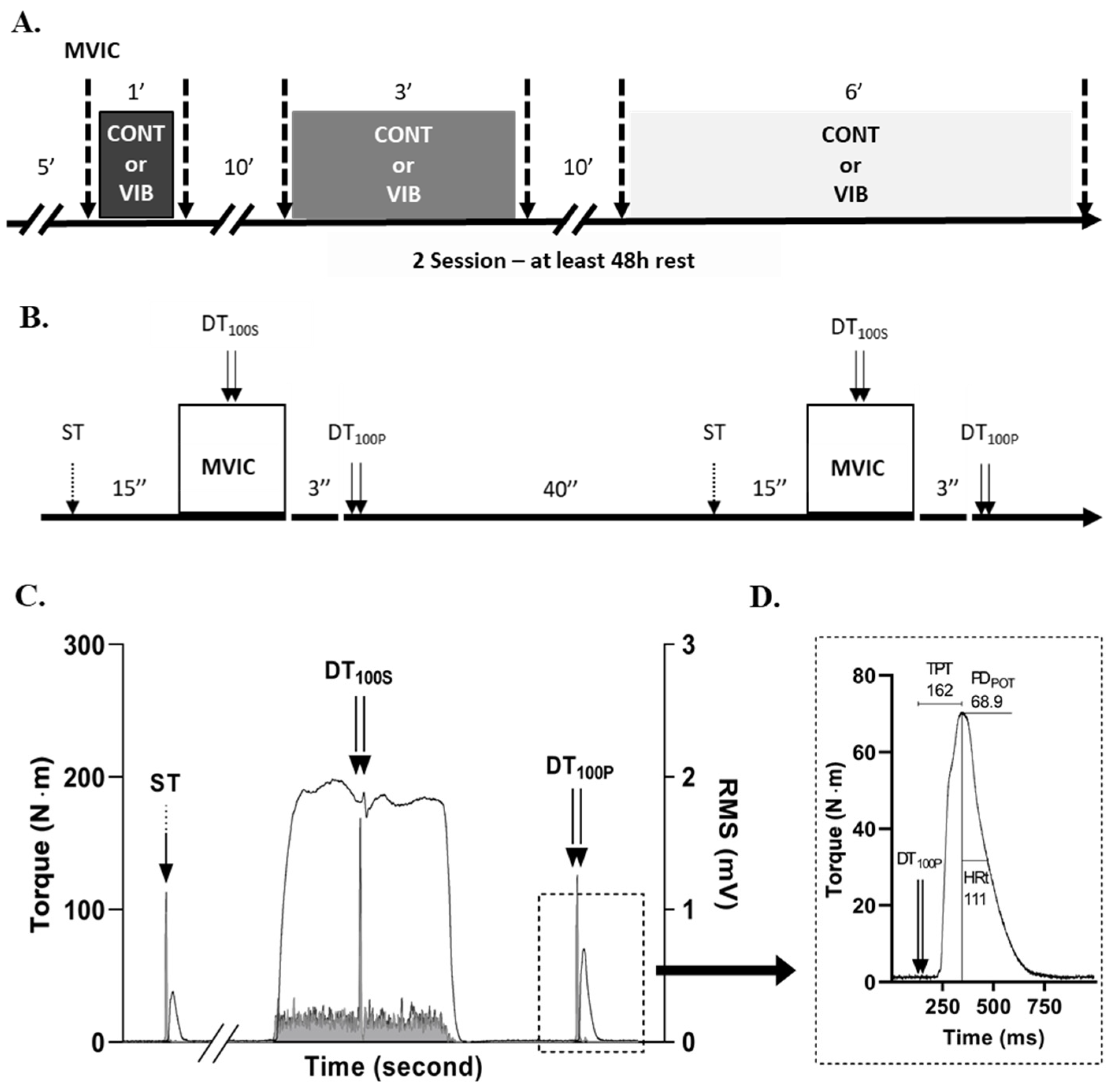
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
2.3. Maximum Isometric Torque and Rate of Torque Development
2.4. Electrical Nerve Stimulation and M-Wave Amplitude
2.5. Electromyographic Activity
2.6. Interpolated Twitch Technic
2.7. Potentiated Doublet Stimulation
2.8. Statistical Analysis
3. Results
3.1. MVIC and Central Fatigue Markers
3.2. Peripheral Fatigue Markers
3.3. Electromyographic Activity during LV
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribot-Ciscar, E.; Rossi-Durand, C.; Roll, J.P. Muscle Spindle Activity Following Muscle Tendon Vibration in Man. Neurosci. Lett. 1998, 258, 147–150. [Google Scholar] [CrossRef]
- Roll, J.P.; Vedel, J.P. Kinaesthetic Role of Muscle Afferents in Man, Studied by Tendon Vibration and Microneurography. Exp. Brain Res. 1982, 47, 177–190. [Google Scholar] [CrossRef]
- Hayward, L.F.; Nielsen, R.P.; Heckman, C.J.; Hutton, R.S. Tendon Vibration-Induced Inhibition of Human and Cat Triceps Surae Group I Reflexes: Evidence of Selective Ib Afferent Fiber Activation. Exp. Neurol. 1986, 94, 333–347. [Google Scholar] [CrossRef]
- Burke, D.; Hagbarth, K.E.; Löfstedt, L.; Wallin, B.G. The Responses of Human Muscle Spindle Endings to Vibration during Isometric Contraction. J. Physiol. 1976, 261, 695–711. [Google Scholar] [CrossRef] [PubMed]
- Roll, J.P.; Vedel, J.P.; Ribot, E. Alteration of Proprioceptive Messages Induced by Tendon Vibration in Man: A Microneurographic Study. Exp. Brain Res. 1989, 76, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.; Hagbarth, K.E.; Löfstedt, L.; Wallin, B.G. The Responses of Human Muscle Spindle Endings to Vibration of Non-Contracting Muscles. J. Physiol. 1976, 261, 673–693. [Google Scholar] [CrossRef]
- Gillies, J.D.; Burke, D.J.; Lance, J.W. Supraspinal Control of Tonic Vibration Reflex. J. Neurophysiol. 1971, 34, 302–309. [Google Scholar] [CrossRef]
- Desmedt, J.E.; Godaux, E. Mechanism of the Vibration Paradox: Excitatory and Inhibitory Effects of Tendon Vibration on Single Soleus Muscle Motor Units in Man. J. Physiol. 1978, 285, 197–207. [Google Scholar] [CrossRef]
- De Gail, P.; Lance, J.W.; Neilson, P.D. Differential Effects on Tonic and Phasic Reflex Mechanisms Produced by Vibration of Muscles in Man. J. Neurol. Neurosurg. Psychiatry 1966, 29, 1–11. [Google Scholar] [CrossRef]
- Herda, T.J.; Ryan, E.D.; Smith, A.E.; Walter, A.A.; Bemben, M.G.; Stout, J.R.; Cramer, J.T. Acute Effects of Passive Stretching vs Vibration on the Neuromuscular Function of the Plantar Flexors. Scand. J. Med. Sci. Sport. 2009, 19, 703–713. [Google Scholar] [CrossRef]
- Ushiyama, J.; Masani, K.; Kouzaki, M.; Kanehisa, H.; Fukunaga, T. Difference in Aftereffects Following Prolonged Achilles Tendon Vibration on Muscle Activity during Maximal Voluntary Contraction among Plantar Flexor Synergists. J. Appl. Physiol. 2005, 98, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- Yoshitake, Y.; Shinohara, M.; Kouzaki, M.; Fukunaga, T. Fluctuations in Plantar Flexion Force Are Reduced after Prolonged Tendon Vibration. J. Appl. Physiol. 2004, 97, 2090–2097. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barrera-Curiel, A.; Colquhoun, R.J.; Hernandez-Sarabia, J.A.; DeFreitas, J.M. The Effects of Vibration-Induced Altered Stretch Reflex Sensitivity on Maximal Motor Unit Firing Properties. J. Neurophysiol. 2019, 121, 2215–2221. [Google Scholar] [CrossRef] [PubMed]
- Souron, R.; Besson, T.; McNeil, C.J.; Lapole, T.; Millet, G.Y. An Acute Exposure to Muscle Vibration Decreases Knee Extensors Force Production and Modulates Associated Central Nervous System Excitability. Front. Hum. Neurosci. 2017, 11, 519. [Google Scholar] [CrossRef]
- Jackson, S.W.; Turner, D.L. Prolonged Muscle Vibration Reduces Maximal Voluntary Knee Extension Performance in Both the Ipsilateral and the Contralateral Limb in Man. Eur. J. Appl. Physiol. 2003, 88, 380–386. [Google Scholar] [CrossRef]
- Kouzaki, M.; Shinohara, M.; Fukunaga, T. Decrease in Maximal Voluntary Contraction by Tonic Vibration Applied to a Single Synergist Muscle in Humans. J. Appl. Physiol. 2000, 89, 1420–1424. [Google Scholar] [CrossRef]
- Konishi, Y.; Kubo, J.; Fukudome, A. Effects of Prolonged Tendon Vibration Stimulation on Eccentric and Concentric Maximal Torque and Emgs of the Knee Extensors. J. Sports Sci. Med. 2009, 8, 548–552. [Google Scholar]
- Saito, A.; Ando, R.; Akima, H. Effects of Prolonged Patellar Tendon Vibration on Force Steadiness in Quadriceps Femoris during Force-Matching Task. Exp. Brain Res. 2016, 234, 209–217. [Google Scholar] [CrossRef]
- Souron, R.; Besson, T.; Millet, G.Y.; Lapole, T. Acute and Chronic Neuromuscular Adaptations to Local Vibration Training. Eur. J. Appl. Physiol. 2017, 117, 1939–1964. [Google Scholar] [CrossRef]
- Fry, A.; Folland, J.P. Prolonged Infrapatellar Tendon Vibration Does Not Influence Quadriceps Maximal or Explosive Isometric Force Production in Man. Eur. J. Appl. Physiol. 2014, 114, 1757–1766. [Google Scholar] [CrossRef]
- Cattagni, T.; Billet, C.; Cornu, C.; Jubeau, M. No Alteration of the Neuromuscular Performance of Plantar-Flexor Muscles After Achilles Tendon Vibration. J. Sport Rehabil. 2016, 26, 1–3. [Google Scholar] [CrossRef][Green Version]
- Ekblom, M.M.N.; Thorstensson, A. Effects of Prolonged Vibration on H-Reflexes, Muscle Activation, and Dynamic Strength. Med. Sci. Sport. Exerc. 2011, 43, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Farabet, A.; Souron, R.; Millet, G.Y.; Lapole, T. Changes in Tibialis Anterior Corticospinal Properties after Acute Prolonged Muscle Vibration. Eur. J. Appl. Physiol. 2016, 116, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Amann, M.; Duchateau, J.; Meeusen, R.; Rice, C.L. Neural Contributions to Muscle Fatigue: From the Brain to the Muscle and Back Again. Med. Sci. Sports Exerc. 2016, 48, 2294–2306. [Google Scholar] [CrossRef] [PubMed]
- Place, N. Quantification of Central Fatigue: A Central Debate. Eur. J. Appl. Physiol. 2021, 121, 2375–2376. [Google Scholar] [CrossRef]
- Place, N.; Millet, G.Y. Quantification of Neuromuscular Fatigue: What Do We Do Wrong and Why? Sports Med. 2020, 50, 439–447. [Google Scholar] [CrossRef]
- Dotan, R.; Woods, S.; Contessa, P. On the Reliability and Validity of Central Fatigue Determination. Eur. J. Appl. Physiol. 2021, 121, 2393–2411. [Google Scholar] [CrossRef] [PubMed]
- Maffiuletti, N.A.; Aagaard, P.; Blazevich, A.J.; Folland, J.; Tillin, N.; Duchateau, J. Rate of Force Development: Physiological and Methodological Considerations. Eur. J. Appl. Physiol. 2016, 116, 1091–1116. [Google Scholar] [CrossRef]
- Kennouche, D.; Varesco, G.; Espeit, L.; Féasson, L.; Souron, R.; Rozand, V.; Millet, G.Y.; Lapole, T. Acute Effects of Quadriceps Muscle versus Tendon Prolonged Local Vibration on Force Production Capacities and Central Nervous System Excitability. Eur. J. Appl. Physiol. 2022, 122, 2451–2461. [Google Scholar] [CrossRef]
- Abbruzzese, M.; Minatel, C.; Reni, L.; Favale, E. Postvibration Depression of the H-Reflex as a Result of a Dual Mechanism: An Experimental Study in Humans. J. Clin. Neurophysiol. 2001, 18, 460–470. [Google Scholar] [CrossRef]
- Bongiovanni, L.G.; Hagbarth, K.E.; Stjernberg, L. Prolonged Muscle Vibration Reducing Motor Output in Maximal Voluntary Contractions in Man. J. Physiol. 1990, 423, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Hultborn, H.; Meunier, S.; Morin, C.; Pierrot-Deseilligny, E. Assessing Changes in Presynaptic Inhibition of I a Fibres: A Study in Man and the Cat. J. Physiol. 1987, 389, 729–756. [Google Scholar] [CrossRef]
- Curtis, D.R.; Eccles, J.C. Synaptic Action during and after Repetitive Stimulation. J. Physiol. 1960, 150, 374–398. [Google Scholar] [CrossRef] [PubMed]
- Souron, R.; Baudry, S.; Millet, G.Y.; Lapole, T. Vibration-Induced Depression in Spinal Loop Excitability Revisited. J. Physiol. 2019, 597, 5179–5193. [Google Scholar] [CrossRef]
- Lapole, T.; Mesquita, R.N.O.; Baudry, S.; Souron, R.; Brownstein, C.G.; Rozand, V. Can Local Vibration Alter the Contribution of Persistent Inward Currents to Human Motoneuron Firing? J. Physiol. 2023, 601, 1467–1482. [Google Scholar] [CrossRef] [PubMed]
- Nito, M.; Yoshimoto, T.; Hashizume, W.; Shindo, M.; Naito, A. Vibration Decreases the Responsiveness of Ia Afferents and Spinal Motoneurons in Humans. J. Neurophysiol. 2021, 126, 1137–1147. [Google Scholar] [CrossRef]
- Ribot-Ciscar, E.; Vedel, J.P.; Roll, J.P. Vibration Sensitivity of Slowly and Rapidly Adapting Cutaneous Mechanoreceptors in the Human Foot and Leg. Neurosci. Lett. 1989, 104, 130–135. [Google Scholar] [CrossRef]
- Rozand, V.; Pageaux, B.; Marcora, S.M.; Papaxanthis, C.; Lepers, R. Does Mental Exertion Alter Maximal Muscle Activation? Front. Hum. Neurosci. 2014, 8, 755. [Google Scholar] [CrossRef]
- Garnier, Y.M.; Lepers, R.; Stapley, P.J.; Papaxanthis, C.; Paizis, C. Changes in Cortico-Spinal Excitability Following Uphill versus Downhill Treadmill Exercise. Behav. Brain Res. 2017, 317, 242–250. [Google Scholar] [CrossRef]
- Garnier, Y.M.; Paizis, C.; Lepers, R. Corticospinal Changes Induced by Fatiguing Eccentric versus Concentric Exercise. Eur. J. Sport Sci. 2019, 19, 166–176. [Google Scholar] [CrossRef]
- Babault, N.; Pousson, M.; Michaut, A.; Van Hoecke, J. Effect of Quadriceps Femoris Muscle Length on Neural Activation during Isometric and Concentric Contractions. J. Appl. Physiol. 2003, 94, 983–990. [Google Scholar] [CrossRef]
- Strojnik, V.; Komi, P.V. Neuromuscular Fatigue after Maximal Stretch-Shortening Cycle Exercise. J. Appl. Physiol. 1998, 84, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Garnier, Y.M.; Lepers, R.; Dubau, Q.; Pageaux, B.; Paizis, C. Neuromuscular and Perceptual Responses to Moderate-Intensity Incline, Level and Decline Treadmill Exercise. Eur. J. Appl. Physiol. 2018, 118, 2039–2053. [Google Scholar] [CrossRef]
- Portney, L.G. Foundations of Clinical Research: Applications to Evidence-Based Practice, 4th ed.; Portney, L.G., Ed.; F. A. Davis Company: Philadelphia, PA, USA, 2020; ISBN 9780803661134. [Google Scholar]
- Bongiovanni, L.G.; Hagbarth, K.E. Tonic Vibration Reflexes Elicited during Fatigue from Maximal Voluntary Contractions in Man. J. Physiol. 1990, 423, 1–14. [Google Scholar] [CrossRef]
- Pfenninger, C.; Grosboillot, N.; Digonet, G.; Lapole, T. Effects of Prolonged Local Vibration Superimposed to Muscle Contraction on Motoneuronal and Cortical Excitability. Front. Physiol. 2023, 14, 1106387. [Google Scholar] [CrossRef]
- Souron, R.; Oriol, M.; Millet, G.Y.; Lapole, T. Intermediate Muscle Length and Tendon Vibration Optimize Corticospinal Excitability During Knee Extensors Local Vibration. Front. Physiol. 2018, 9, 1266. [Google Scholar] [CrossRef]
- Eklund, G.; Hagbarth, K.E. Normal Variability of Tonic Vibration Reflexes in Man. Exp. Neurol. 1966, 16, 80–92. [Google Scholar] [CrossRef]
| 1 Min | 3 Min | 6 Min | p-Values/ES (pη²) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | PRE | POST | C × D | C × T | C × D × T | ||
| MVIC (N∙m) | CONT | 225.1 ± 83.7 | 225.9 ± 77.6 | 220.9 ± 83.8 | 210.4 ± 75.7 | 209.5 ± 73 | 205.8 ± 72 | 0.192/0.119 | 0.045/0.275 | 0.379/0.072 |
| LV | 211.2 ± 81.7 # | 213.2 ± 82.6 | 215.8 ± 75.1 | 212.2 ± 75.1 | 209.3 ± 76.8 | 216.5 ± 76.8 | ||||
| VAL (%) | CONT | 87.2 ± 10.1 | 85.9 ± 10.4 | 86 ± 10.3 | 82.3 ± 9.7 | 82.8 ± 10.4 | 82 ± 10.1 | 0.143/0.139 | 0.113/0.182 | 0.837/0.014 |
| LV | 84.5 ± 10.8 | 84.0 ± 12.2 | 86.3 ± 8.4 | 84.4 ± 8 | 85.3 ± 8.6 | 85.1 ± 8.4 | ||||
| RMSMVIC.M−1 RF (%) | CONT | 6.79 ± 3 | 6.42 ± 2.88 | 6.53 ± 2.72 | 6.31 ± 2.5 | 5.96 ± 2.16 | 6.1 ± 2.49 | 0.714/0.026 | 0.845/0.003 | 0.611/0.037 |
| LV | 6.61 ± 2.57 | 5.88 ± 1.96 | 6.15 ± 2.44 | 5.92 ± 2.07 | 6.57 ± 2.88 | 6.39 ± 2.87 | ||||
| RMSMVIC.M−1 VL (%) | CONT | 4.94 ± 3.03 | 5.09 ± 3.34 | 5.52 ± 4.09 | 4.85 ± 3.25 | 4.69 ± 3.2 | 4.75 ± 3.17 | 0.737/0.014 | 0.533/0.031 | 0.695/0.028 |
| LV | 5.68 ± 3.96 | 5.14 ± 3.28 | 4.98 ± 3.15 | 4.47 ± 2.57 | 4.9 ± 2.67 | 5.11 ± 2.99 | ||||
| T50 (N∙m) | CONT | 50.1 ± 24.7 | 50.4 ± 24.6 | 44.4 ± 24.9 | 43.5 ± 24.8 | 42.3 ± 22.7 | 47.4 ± 23.9 | 0.147/0.137 | 0.522/0.032 | 0.659/0.032 |
| LV | 43.6 ± 23.1 | 42 ± 22.7 | 44.4 ± 19 | 44.2 ± 21.6 | 45.8 ± 25.3 | 47.7 ± 23.3 | ||||
| PDPOT (N⋅m) | CONT | 82.2 ± 23.2 | 84.4 ± 24 | 80.6 ± 24.6 | 80 ± 23.3 | 80.82 ± 23.28 | 77.7 ± 21.9 | 0.321/0.084 | 0.229/0.109 | 0.147/0.137 |
| LV | 80.5 ± 24.6 | 81.4 ± 24.4 | 82.7 ± 25.9 | 82.8 ± 25.8 | 79.14 ± 23.48 | 79.6 ± 24.6 | ||||
| TPT (ms) | CONT | 152.1 ± 16.8 | 149.3 ± 20.5 | 149.8 ± 15.2 | 150.8 ± 18.7 | 143.32 ± 14 | 145 ± 21.7 | 0.015/0.319 | 0.982/0.000 | 0.256/0.100 |
| LV | 144.5 ± 16.9 | 143.9 ± 16.9 | 139.7 ± 13.8 $ | 142.1 ± 17.4 $ | 148.9 ± 18.8 $ | 151.9 ± 23.2 $ | ||||
| HRt (ms) | CONT | 81.8 ± 18.9 | 82.2 ± 18.1 | 89.7 ± 36.8 | 84 ± 30 | 91.54 ± 32.42 | 95.3 ± 40.3 | 0.024/0.250 | 0.280/0.089 | 0.231/0.109 |
| LV | 83 ± 20 | 96.2 ± 43.9 | 97.5 ± 36.8 | 91.7 ± 29.6 | 86.18 ± 33.64 | 88.6 ± 41.5 | ||||
| PP RF (mV) | CONT | 2.34 ± 0.71 | 2.4 ± 0.75 | 2.24 ± 0.88 | 2.23 ± 0.86 | 2.14 ± 0.55 | 2.14 ± 0.71 | 0.619/0.036 | 0.299/0.083 | 0.882/0.010 |
| LV | 2.12 ± 0.72 | 2.24 ± 0.84 | 2.24 ± 0.6 | 2.25 ± 0.61 | 2.18 ± 0.75 | 2.26 ± 0.86 | ||||
| PP VL (mV) | CONT | 4.98 ± 1.74 | 4.98 ± 1.78 | 4.71 ± 2.62 | 4.73 ± 2.7 | 4.38 ± 1.79 | 4.32 ± 1.71 | 0.432/0.053 | 0.399/0.055 | 0.946/0.004 |
| LV | 4.28 ± 2.6 | 4.39 ± 2.66 | 4.59 ± 1.89 | 4.69 ± 1.83 | 4.83 ± 2.7 | 4.79 ± 2.78 | ||||
| 1 Min | 3 Min | 6 Min | p-Values/ES (pη²/K’s W Coef.) | Grouped | p-Values/ES | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CONT | LV | CONT | LV | CONT | LV | T | C | C × D | CONT | LV | ||
| MVIC (%) | 1.9 ± 7.1 | 1.2 ± 4.4 | −3.6 ± 7.9 | −1.9 ± 5.4 | −1.2 ± 12.2 | 4.1 ± 8.8 | 0.113/0.127 | N/A | N/A | −1 ± 3.9 | 1.2 ± 3.7 | 0.108/0.461 |
| VAL (%) | −1.4 ± 3.4 | −0.7 ± 6 | −4.2 ± 6.3 | −2.1 ± 3.6 | −0.9 ± 6.2 | −0.2 ± 4.2 | N/A | 0.125/0.172 | 0.824/0.015 | −2.2 ± 2.6 * | −1 ± 2.3 | 0.125/0.439 |
| RMSMVIC.M−1 RF (%) | 0.7 ± 18.7 | −3.5 ± 18.8 | −9.9 ± 15 | −6.8 ± 14 | −0.6 ± 14.4 | −1.3 ± 19.6 | N/A | 0.845/0.003 | 0.611/0.037 | −3.2 ± 11.4 | −3.8 ± 10.6 | 0.845/−0.344 |
| RMSMVIC.M−1 VL (%) | 8.6 ± 27.8 | 0.4 ± 26.9 | −8.8 ± 15.8 | −9.8 ± 18.5 | 8.8 ± 35.8 | 8.3 ± 24.8 | N/A | 0.533/0.031 | 0.369/0.695 | 2.9 ± 16.2 | −0.4 ± 10.1 | 0.533/−0.053 |
| T50 (%) | 3.3 ± 25.8 | −2.4 ± 25.2 | 23.1 ± 39.8 | 3.6 ± 35.3 | −1.6 ± 20 | 12.8 ± 29.9 | N/A | 0.702/0.012 | 0.673/0.030 | 8 ± 32.2 | 4.9 ± 29 | 0.566/−0.171 |
| PDPOT (%) | 2.7 ± 3.9 | 1.2 ± 6.1 | −0.2 ± 4.7 | 0.1 ± 5.6 | −3.1 ± 9.7 | 0.6 ± 7 | 0.813/0.032 | N/A | N/A | −0.2 ± 3.8 | 0.6 ± 3.8 | 0.300/0.248 |
| TPT (%) | −1.1 ± 3.2 | −0.6 ± 2.1 | 2.9 ± 12.2 | 1.4 ± 8.6 | 2.1 ± 12.8 | 3.2 ± 12.2 | 0.616/0.051 | N/A | N/A | 1.3 ± 6.1 | 1.3 ± 5 | 0.730/0.064 |
| HRt (%) | 0.8 ± 6.6 | 16.2 ± 49.1 | −4.1 ± 11.7 | −3.5 ± 10.6 | 3.1 ± 8.5 | 1.6 ± 6.5 | 0.332/0.082 | N/A | N/A | −0.1 ± 5.9 | 4.8 ± 18.1 | 0.510/0.302 |
| PP RF (%) | 2.8 ± 6.1 | 5.1 ± 9.6 | 0.1 ± 5.5 | 0.5 ± 5.3 | −1.1 ± 25.1 | 2.5 ± 11.1 | 0.348/0.080 | N/A | N/A | 0.6 ± 9.4 | 2.7 ± 4.8 | 0.433/0.271 |
| PP VL (%) | 0.2 ± 5.3 | 2.6 ± 8 | 0.6 ± 8.3 | 4.1 ± 8.6 | 0.4 ± 19.8 | −2.4 ± 5.5 | 0.316/0.084 | N/A | N/A | 0.4 ± 7.8 | 1.4 ± 4.3 | 0.465/0.201 |
| 1 Min | 3 Min | 6 Min | ||||
|---|---|---|---|---|---|---|
| CONT | LV | CONT | LV | CONT | LV | |
| RMSCOND.M−1 RF(%) | 0.023 ± 0.013 | 0.028 ± 0.019 | 0.024 ± 0.02 | 0.029 ± 0.024 | 0.042 ± 0.042 | 0.036 ± 0.037 |
| RMSCOND.M−1 VL (%) | 0.056 ± 0.037 | 0.051 ± 0.025 | 0.056 ± 0.031 | 0.061 ± 0.058 | 0.084 ± 0.107 | 0.084 ± 0.081 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amiez, N.; Zografou, S.; Timbert, T.; Martin, A.; Methenitis, S.; Babault, N.; Paizis, C. Effect of Different Local Vibration Durations on Knee Extensors’ Maximal Isometric Strength. Appl. Sci. 2023, 13, 11376. https://doi.org/10.3390/app132011376
Amiez N, Zografou S, Timbert T, Martin A, Methenitis S, Babault N, Paizis C. Effect of Different Local Vibration Durations on Knee Extensors’ Maximal Isometric Strength. Applied Sciences. 2023; 13(20):11376. https://doi.org/10.3390/app132011376
Chicago/Turabian StyleAmiez, Nicolas, Stella Zografou, Tom Timbert, Alain Martin, Spyridon Methenitis, Nicolas Babault, and Christos Paizis. 2023. "Effect of Different Local Vibration Durations on Knee Extensors’ Maximal Isometric Strength" Applied Sciences 13, no. 20: 11376. https://doi.org/10.3390/app132011376
APA StyleAmiez, N., Zografou, S., Timbert, T., Martin, A., Methenitis, S., Babault, N., & Paizis, C. (2023). Effect of Different Local Vibration Durations on Knee Extensors’ Maximal Isometric Strength. Applied Sciences, 13(20), 11376. https://doi.org/10.3390/app132011376









