Chemical Composition, Anti-α-Glucosidase Activity, and Molecular Modelling Studies of Cleistocalyx operculatus Essential Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Extraction of Essential Oil from Leaves of C. operculatus
2.3. Gas Chromatography–Mass Spectrometry (GC/MS) Analysis of Essential Oil of C. operculatus
2.4. Evaluation of α-Glucosidase Enzyme Inhibition of Essential Oil of C. operculatus
2.5. Molecular Docking Simulation
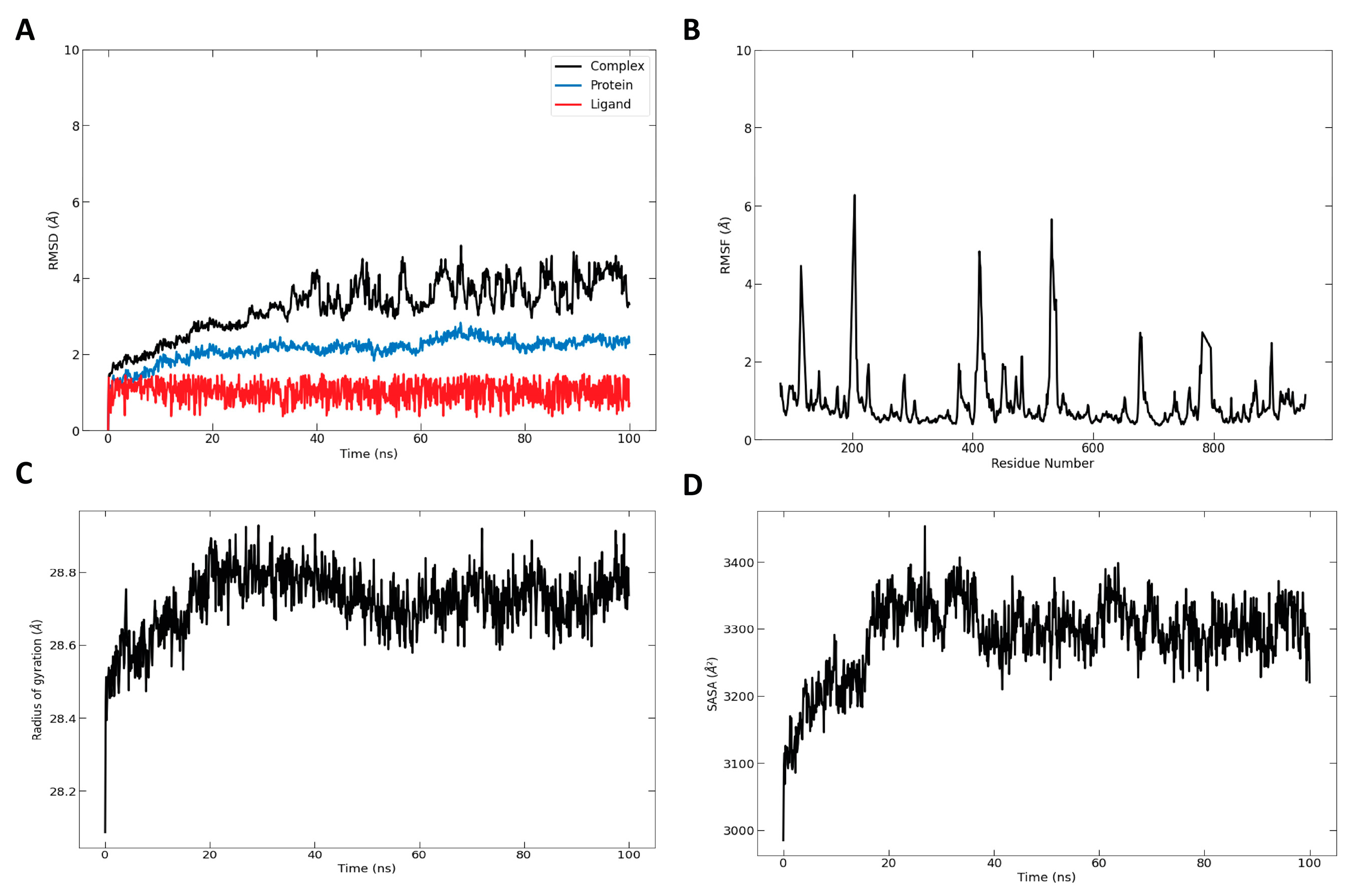
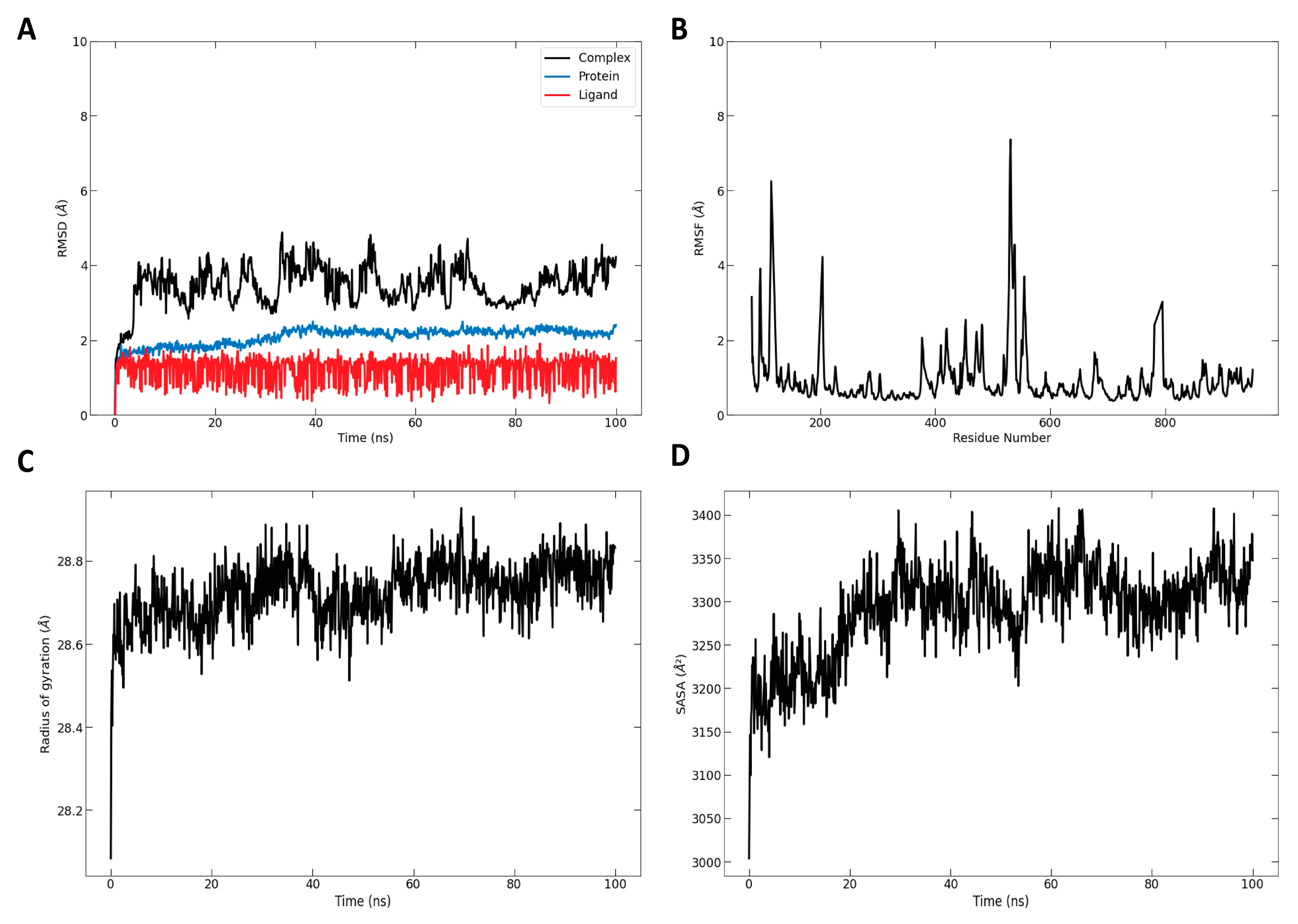
2.6. Molecular Dynamic Simulation
3. Result and Discussion
3.1. Percentage Yield and Chemical Composition of C. operculatus Essential Oil
3.2. In Vitro α-Glucosidase Inhibitory Activity of C. operculatus Essential Oil
3.3. Modeling the Structures of α-Glucosidase Complexes with Main Essential Oil Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casirola, D.M.; Ferraris, R.P. alpha-Glucosidase inhibitors prevent diet-induced increases in intestinal sugar transport in diabetic mice. Metabolism 2006, 55, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Wu, K.; Ji, R.; Zhao, Y.; Lu, J.; Yu, Z.; Xu, X.; Huang, J. Structure and function Insight of the α-Glucosidase QsGH13 from Qipengyuania seohaensis sp. SW-135. Front. Microbiol. 2022, 13, 849585. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Moelands, S.V.; Lucassen, P.L.; Akkermans, R.P.; De Grauw, W.J.; Van de Laar, F.A. Alpha-glucosidase inhibitors for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2018, 12, CD005061. [Google Scholar] [CrossRef]
- Nguyen, T.N.T.; Le, T.D.; Nguyen, P.L.; Nguyen, D.H.; Nguyen, H.V.T.; Nguyen, T.K.; Tran, M.H.; Le, T.H.V. α-Glucosidase inhibitory activity and quantitative contribution of phenolic compounds from Vietnamese Aquilaria crassna leaves. Nat. Prod. Commun. 2022, 17, 1–10. [Google Scholar] [CrossRef]
- Martin, A.E.; Montgomery, P.A. Acarbose: An alpha-glucosidase inhibitor. Am. J. Health Syst. Pharm. 1996, 53, 2277–2290. [Google Scholar] [CrossRef]
- Pham, G.N.; Nguyen, T.T.T.; Nguyen-Ngoc, H. Ethnopharmacology, phytochemistry, and pharmacology of Syzygium nervosum. Evid. Based. Complement. Alternat. Med. 2020, 2020, 8263670. [Google Scholar] [CrossRef]
- Dung, N.T.; Kim, J.M.; Kang, S.C. Chemical composition, antimicrobial and antioxidant activities of the essential oil and the ethanol extract of Cleistocalyx operculatus (Roxb.) Merr and Perry buds. Food Chem. Toxicol. 2008, 46, 3632–3639. [Google Scholar] [CrossRef]
- Dung, N.T.; Bajpai, V.K.; Yoon, J.I.; Kang, S.C. Anti-inflammatory effects of essential oil isolated from the buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Food Chem. Toxicol. 2009, 47, 449–453. [Google Scholar] [CrossRef]
- Ngo, T.C.; Dao, D.Q.; Thong, N.M.; Nam, P.C. Insight into the antioxidant properties of non-phenolic terpenoids contained in essential oils extracted from the buds of Cleistocalyx operculatus: A DFT study. RSC Adv. 2016, 6, 30824–30834. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Pokharel, S.K.; Setzer, W.N. Leaf essential oil composition, antimicrobial and cytotoxic activities of Cleistocalyx operculatus from Hetauda, Nepal. Am. J. Essent. Oils. Nat. Prod. 2015, 3, 34–37. [Google Scholar]
- Joulain, D.; Koenig, W. The Atlas of Spectral Data of Sesquiterpene Hydrocarbons; E. B. Verlag: Hamburg, Germany, 1998. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5 online ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- Dat, T.T.H.; Oanh, P.T.T.; Cuong, L.C.V.; Anh, L.T.; Minh, L.T.H.; Ha, H.; Lam, L.T.; Cuong, P.V.; Anh, H.L.T. Pharmacological properties, volatile organic compounds, and genome sequences of bacterial endophytes from the mangrove plant Rhizophora apiculata Blume. Antibiotics 2021, 10, 1491. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, W.; Littlejohn, T.G. Swiss-PDB Viewer (Deep View). Brief. Bioinform. 2001, 2, 195–197. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Dung, N.X.; Van Luu, H.; Khoi, T.T.; Leclercq, P.A. GC and GC/MS analysis of the leaf oil of Cleistocalyx operculatus Roxb. Merr. et Perry (Syn. Eugenia operculata Roxb.; Syzygicum mervosum DC.). J. Essent. Oil Res. 1994, 6, 661–662. [Google Scholar] [CrossRef]
- Thuy, B.T.P.; Hieu, L.T.; My, T.T.A.; Hai, N.T.T.; Loan, H.T.P.; Thuy, N.T.T.; Triet, N.T.; Van Anh, T.T.; Dieu, N.T.X.; Quy, P.T.; et al. Screening for Streptococcus pyogenes antibacterial and Candida albicans antifungal bioactivities of organic compounds in natural essential oils of Piper betle L.; Cleistocalyx operculatus L. and Ageratum conyzoides L. Chem. Pap. 2021, 75, 1507–1519. [Google Scholar] [CrossRef]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef]
- Mai, T.T.; Thu, N.N.; Tien, P.G.; Van Chuyen, N. Alpha-glucosidase inhibitory and antioxidant activities of Vietnamese edible plants and their relationships with polyphenol contents. J. Nutr. Sci. Vitaminol. 2007, 53, 267–276. [Google Scholar] [CrossRef]
- Chukiatsiri, S.; Wongsrangsap, N.; Ratanabunyong, S.; Choowongkomon, K. In vitro evaluation of antidiabetic potential of Cleistocalyx nervosum var. paniala fruit extract. Plants 2022, 12, 112. [Google Scholar] [CrossRef] [PubMed]
- Mai, T.T.; Chuyen, N.V. Anti-hyperglycemic activity of an aqueous extract from flower buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Biosci. Biotechnol. Biochem. 2007, 71, 69–76. [Google Scholar] [CrossRef]
- Mai, T.T.; Yamaguchi, K.; Yamanaka, M.; Lam, N.T.; Otsuka, Y.; Chuyen, N.V. Protective and anticataract effects of the aqueous extract of Cleistocalyx operculatus flower buds on beta-cells of streptozotocin-diabetic rats. J. Agric. Food Chem. 2010, 58, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Tran, G.B.; Le, N.T.; Dam, S.M. Potential use of essential oil isolated from Cleistocalyx operculatus leaves as a topical dermatological agent for treatment of burn wound. Dermatol. Res. Pract. 2018, 2018, 2730169. [Google Scholar] [CrossRef]
- Hu, Y.C.; Luo, Y.D.; Li, L.; Joshi, M.K.; Lu, Y.H. In vitro investigation of 2′,4′-dihydroxy-6′-methoxy-3′,5′-dimethylchalcone for glycemic control. J. Agric. Food Chem. 2012, 60, 10683–10688. [Google Scholar] [CrossRef]
- Tahir, H.U.; Sarfraz, R.A.; Ashraf, A.; Adil, S. Chemical composition and antidiabetic activity of essential oils obtained from two spices (Syzygium aromaticum and Cuminum cyminum). Int. J. Food Prop. 2016, 19, 2156–2164. [Google Scholar] [CrossRef]


| No | Compounds | RT (min) | SI (%) | RIa | RIb | Content (%) |
|---|---|---|---|---|---|---|
| 1 | α-Pinene | 6.70 | 97 | 932 | 932 | 5.6 |
| 2 | β-Pinene | 8.11 | 96 | 975 | 974 | 0.4 |
| 3 | β-Myrcene | 8.58 | 96 | 990 | 988 | 1.1 |
| 4 | Limonene | 10.02 | 90 | 1028 | 1024 | 2.7 |
| 5 | (Z)-β-ocimene | 10.38 | 97 | 1036 | 1032 | 30.4 |
| 6 | (E)-β-ocimene | 10.92 | 97 | 1050 | 1044 | 14.0 |
| 7 | Terpinolene | 12.41 | 94 | 1087 | 1086 | 1.1 |
| 8 | Linalool | 12.98 | 94 | 1100 | 1095 | 0.3 |
| 9 | Allo-ocimene | 14.44 | 96 | 1134 | 1128 | 31.6 |
| 10 | Veratrole | 14.63 | 82 | 1138 | 1141 | 0.2 |
| 11 | Neo-allo-ocimene | 14.81 | 97 | 1142 | 1140 | 1.4 |
| 12 | cis-Chrysanthenyl acetate | 20.03 | 84 | 1260 | 1261 | 0.1 |
| 13 | α-Copaene | 25.09 | 88 | 1377 | 1374 | 0.1 |
| 14 | Geranyl acetate | 25.43 | 94 | 1385 | 1379 | 0.2 |
| 15 | β-caryophyllene | 26.96 | 97 | 1421 | 1422 | 4.6 |
| 16 | β-Copaene | 27.33 | 89 | 1430 | 1430 | 0.1 |
| 17 | γ-Elemene | 27.52 | 89 | 1435 | 1434 | 0.1 |
| 18 | Aromadendrene | 27.73 | 93 | 1440 | 1439 | 0.2 |
| 19 | α-Humulene | 28.34 | 97 | 1455 | 1452 | 1.0 |
| 20 | γ-Muurolene | 29.28 | 92 | 1478 | 1478 | 0.5 |
| 21 | Germacrene-D | 29.47 | 92 | 1482 | 1480 | 0.3 |
| 22 | β-Selinene | 29.68 | 93 | 1487 | 1489 | 0.2 |
| 23 | Viridiflorene | 30.04 | 92 | 1496 | 1496 | 0.4 |
| 24 | α-Bulnesene | 30.54 | 84 | 1509 | 1509 | 0.1 |
| 25 | δ-Amorphene | 30.80 | 90 | 1515 | 1511 | 0.2 |
| 26 | δ-Cadinene | 31.18 | 94 | 1525 | 1522 | 0.4 |
| 27 | (E)-Nerolidol | 32.74 | 95 | 1565 | 1561 | 0.3 |
| 28 | Caryophyllene oxide | 33.51 | 91 | 1584 | 1582 | 0.4 |
| 29 | Eremoligenol | 35.14 | 87 | 1627 | 1629 | 0.2 |
| 30 | α-Cadinol | 36.23 | 81 | 1657 | 1652 | 0.1 |
| Monoterpene hydrocarbons | 88.3 | |||||
| Sesquiterpene hydrocarbons | 8.3 | |||||
| Oxygenated sesquiterpenes | 0.6 | |||||
| Oxygenated monoterpenes | 0.9 | |||||
| Non-terpenes | 0.2 | |||||
| Total | 98.3 | |||||
| Compound | Docking Score (kcal/mol) | Residues Interaction |
|---|---|---|
| allo-ocimene | −5.358 | Trp376, Phe649, His674, Trp613, Trp516, and Ile441 |
| (Z)-β-ocimene | −5.330 | Phe649, Trp376, Leu405, His674, Ile441, Trp481, and Trp516 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, L.T.T.; Nguyen, T.K.; Pham, T.V.; Ha, T.P.; Tran, P.T.D.; Tam, V.T.T.; Dat, T.T.H.; Thai, P.H.; Cuong, L.C.V. Chemical Composition, Anti-α-Glucosidase Activity, and Molecular Modelling Studies of Cleistocalyx operculatus Essential Oil. Appl. Sci. 2023, 13, 11224. https://doi.org/10.3390/app132011224
Tran LTT, Nguyen TK, Pham TV, Ha TP, Tran PTD, Tam VTT, Dat TTH, Thai PH, Cuong LCV. Chemical Composition, Anti-α-Glucosidase Activity, and Molecular Modelling Studies of Cleistocalyx operculatus Essential Oil. Applied Sciences. 2023; 13(20):11224. https://doi.org/10.3390/app132011224
Chicago/Turabian StyleTran, Linh Thuy Thi, Tan Khanh Nguyen, Ty Viet Pham, Tran Phuong Ha, Phan Thi Diem Tran, Vu Thi Thanh Tam, Ton That Huu Dat, Pham Hong Thai, and Le Canh Viet Cuong. 2023. "Chemical Composition, Anti-α-Glucosidase Activity, and Molecular Modelling Studies of Cleistocalyx operculatus Essential Oil" Applied Sciences 13, no. 20: 11224. https://doi.org/10.3390/app132011224
APA StyleTran, L. T. T., Nguyen, T. K., Pham, T. V., Ha, T. P., Tran, P. T. D., Tam, V. T. T., Dat, T. T. H., Thai, P. H., & Cuong, L. C. V. (2023). Chemical Composition, Anti-α-Glucosidase Activity, and Molecular Modelling Studies of Cleistocalyx operculatus Essential Oil. Applied Sciences, 13(20), 11224. https://doi.org/10.3390/app132011224






