Abstract
Background—Podophyllotoxin, a polyphenolic compound with major applications in cancer treatment, is in short supply, as its source is now an endangered plant species. It is therefore essential to find another available plant that produces high levels of podophyllotoxin. Some Juniperus species are known to contain podophyllotoxin, more exactly within their needles, but are still unused in this regard. Objective—The aim is to develop an efficient green ultrasound-assisted extraction protocol for podophyllotoxin from commercially available Juniperus scopulorum varieties. Methodology—To highlight optimal extraction conditions, a single-factor experiment was performed to identify the parameters (extraction duration, frequency, temperature extraction, preliminary grinding, and plant material concentration) influencing extraction. A multifactorial approach using the Box–Behnken design was then applied to determine the exact optimal extraction conditions. Results—The conditions for extracting the maximum amount of podophyllotoxin consist of 51.5 min of extraction time, in ethanol at 69.3%, and at 58.8 °C. This is the highest podophyllotoxin extraction yield ever obtained from Juniperus scopulorum needles. Compared with the reference protocol for the extracting of podophyllotoxin from Juniperus, ultrasonic extraction is an eco-friendly protocol, safe for humans, and the podophyllotoxin extracted by this method has much greater stability, allowing its purification. The analyses also indicate a variation in the podophyllotoxin content of Juniperus scopulorum needles, (from 7.02 to 10.34 mg/g DW) depending on the variety and year of purchase. Conclusion—Extraction in ethanol at 69.3% and at 58.8 °C for 51.5 min allows the extraction of up to 10.34 mg podophyllotoxin/g from freeze-dried Juniperus scopulorum needles.
1. Introduction
Podophyllotoxin is a lignan, a polyphenol, a natural plant metabolite (Figure 1) [1] isolated in the 19th century [2,3,4,5] and presenting remarkable properties: mitotoxic, neurotoxic, insecticidal, antimicrobial, anti-inflammatory, antispasmodic, hypolipidemic, immunosuppressive, antioxidative, analgesic, and cathartic activities [6].
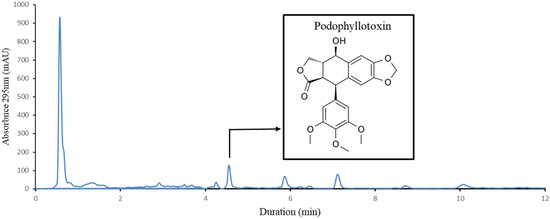
Figure 1.
HPLC chromatogram of J. scopulorum extract and chemical structure of podophyllotoxin.
Furthermore, podophyllotoxin is the unique molecule used as a precursor for the hemisynthesis of some anticancer drugs: etoposide, teniposide, or etopophos with topoisomerase II inhibition ability [7,8,9,10,11,12,13,14,15,16]. These anticancer drugs are involved in the treatment of certain cancers, including lung, breast, testicular, gastric, and blood [17,18,19].
The literature also attributes antiviral activity to podophyllotoxin against Herpes simplex type 1 [20], to some derivatives against HIV [21], and etoposide can fight cytokinin storms in patients infected with the COVID-19 virus [22].
Despite its clinical importance, the supply of podophyllotoxin is currently problematic. Podophyllotoxin is classically extracted from the rhizomes of Podophyllum peltatum or Sinopodophyllum hexandrum Royle [1,23], yielding up to 4% DW podophyllotoxin [24]. However, with slow regeneration and without organized cultivation, the Sinopodophyllum hexandrum Royle overexploitation (due to its podophyllotoxin) has led the plant to being classified as “critically endangered”, in Appendix II of The Convention on International Trading of Endangered Species (CITES). Although complete chemical synthesis of podophyllotoxin is possible, the complex structure makes it economically unviable [15].
Thus, since 2018, there has often been a shortage of etoposide injections, a problem most often solved by switching to etopophos injections [25]. However, without podophyllotoxin available, there is no way to produce etoposide or etopophos, and consequently, cancer patients will no longer be able to receive the treatment they vitally need. Therefore, to continue to ensure the future podophyllotoxin needs, one promising idea is to find other biological materials as plant sources containing a significant amount of podophyllotoxin.
The Juniperus genus appears to be interesting as an alternative natural source of podophyllotoxin. The occurrence of cytotoxic lignan (especially podophyllotoxin) has been described in the needles of Juniperus species since 1953 [26], then confirmed in Juniperus virginiana [27,28,29,30,31,32,33], Juniperus × media [31,32,33,34], Juniperus rigida [35,36,37], Juniperus horizontalis [33,38], Juniperus sabina [31,34,39], and other species including Juniperus scopulorum [31,32,33]. The most interesting species is Juniperus bermudiana, with 22.6 mg podophyllotoxin/g DW [31]. However, this species is endangered and protected by the International Union for Conservation of Nature (“https://www.iucn.org” (accessed on 2 November 2023)). Although Juniperus needles don’t contain as much podophyllotoxin as Sinopodophyllum hexandrum rhizomes, some Juniperus yet contain around 2% DW podophyllotoxin [31], which is the most credible amount found in an alternative natural source. In addition, some Juniperus species are suitable for cultivation, providing a greater amount of plant material than Podophyllum [33]. Juniperus is therefore a potentially creditable source of podophyllotoxin. Among all the Juniperus species, Juniperus scopulorum has not been extensively studied even though it contains podophyllotoxin [31,32,33]. Moreover, it has several cultivated varieties, including Juniperus scopulorum Blue Arrow and Juniperus scopulorum Skyrocket. Thus, this work proposes to explore the credibility of cultivated varieties of these two Juniperus scopulorum as a source of podophyllotoxin.
However, studying the podophyllotoxin content of these plants requires the development and optimization of an efficient extraction protocol. Ultrasonic-assisted extraction appears to be the smartest choice, as this non-conventional technology requires less extraction solvent, a shorter extraction duration, and less thermal degradation while offering higher extraction yield than conventional approaches [40,41]. Additionally, this extraction method is suitable with the use of a green solvent (e.g., ethanol, which also solubilizes phenolic compounds), enabling green extraction. Green extraction is often the preferred extraction approach since it is safer for the environment and human health.
In the ultrasonic extraction approach, thermal, mechanical, and cavitation effects are used to extract the bioactive components from the cells. Indeed, above 20 kHz, ultrasonic radiation extraction of phenolic compounds from solid matrices using liquid solvents creates cavitation bubbles close to the plant cells, which collapse, rupturing the cell wall and cell membrane, and releasing the cell contents. Sonication time, temperature, solvent selection, wave frequency, and ultrasonic wave distribution affect the extraction. The probe and bath are the two main systems currently used, but the ultrasonic bath offers better reproducibility and repeatability and limits the problem of sample contamination and foam production [42,43,44,45,46,47]. Therefore, we herein preferred to use an ultrasonic bath.
The literature supports the value of ultrasound for podophyllotoxin extraction: ultrasonic pretreatment can help to extract podophyllotoxin from Podophyllum peltatum rhizomes without podophyllotoxin degradation [48]. Next, Corbin et al. (2015) [49] developed the most efficient extraction protocol for secoisolariciresinol (a precursor of podophyllotoxin) from flax seeds using ultrasound. However, to date, there is no protocol for extracting podophyllotoxin from Juniperus needles using ultrasound. Therefore, it is interesting to develop this extraction approach.
Considering all this data, green ultrasound-assisted extraction of podophyllotoxin from cultivated varieties of Juniperus scopulorum will be developed in this work. First, single-factor experiments will identify the factors affecting the extraction. Then, a multifactorial approach will be developed to determine optimal extraction conditions. Finally, the optimized protocol will be applied to different Juniperus scopulorum varieties to compare its efficiency with the reference protocol and measure the stability of the extracted podophyllotoxin.
This study aims to determine the interest in J. scopulorum as a new source of podophyllotoxin (for the pharmaceutical industry) and the credibility of ultrasonic-assisted extraction to achieve it.
2. Materials and Methods
2.1. Plant Material
Juniperus scopulorum Blue Arrow and Juniperus scopulorum Skyrocket plants were obtained from Promesse de fleurs (Houplines, France). Some plants were purchased in 2019 and others in 2021. The plants were kept in pots for one month in a phytotronic room at 25 °C under a 12 h photoperiod. Plant irrigation was conducted via the water reservoir of the pots. After one month, needles were collected, stored for 48 h at −80 °C, and then lyophilized for 72 h under secondary vacuum (temperature below 50 °C and pressure below 0.12 mbar) using a CHRIST ALPHA 2-8 LD-plus apparatus coupled with a vacuum pump. The lyophilized needles were used as the starting material for extraction.
2.2. Chemicals
Podophyllotoxin standard of over 98% purity was purchased from Sigma-Aldrich (Saint-Quentin-Fallavier, France). Analytical grade extraction solvents of over 99.9% purity were purchased from Grosseron (Saint-Quentin-Fallavier, France).
2.3. Grinding Approach
Some (lyophilized) samples underwent preliminary mechanical grinding on an IKA A11 mill equipped with a stainless steel beater. The samples were ground twice for 30 s at 28,000 rpm. The powder was then passed over a 60 mesh sieve to obtain particles of 250 μm in size.
2.4. Ultrasound-Assisted Extraction (USAE) Optimization
USAE was conducted using an electronic bath, Elmasonic P30H, with an inner dimension of 240 × 100 × 137 mm, electrical power of 320 W, maximal heating power of 320 W, variable frequencies (0, 37, and 80 kHz), and equipped with a digital timer and a temperature controller. The samples (5/10/20 mg) were placed in a 1.5 mL polypropylene tube and suspended in a corresponding volume of aqueous ethanol. During the ultrasonic treatment, the tubes were maintained immersed in the bath by a float. To conduct the study, various material concentrations were tested (10, 20, and 50 g/L), and several aqueous ethanol concentrations were used (40, 50, 60, 70, 80, 90, and 100% (v/v)). Also, different extraction durations (10, 20, 30, 40, 60, and 80 min), several extraction temperatures (20, 40, 60, and 80 °C), and three ultrasound frequencies (0, 37, and 80 kHz) were tested. Additionally, the impact of a preliminary grinding (made with a mechanical grinder) was evaluated. The extracts were filtered (0.45 μm) before HPLC analysis.
2.5. Reference Solid/Liquid Extraction
The Renouard et al. (2011) [31] protocol, which offers the highest podophyllotoxin extraction yield from Juniperus needles, was used as a reference. In brief, the Juniperus needles are ground in absolute methanol using an Ultraturax (Ultraturrax T25 basic) for 3 min at 19,000 rpm with a mass/volume ratio of 10 mg/mL. The extract is then left to macerate for 5 h under agitation at 25 °C. The solid residue is then centrifuged (15 min—3000 rpm) to retain only the supernatant containing the podophyllotoxin.
2.6. High-Performance Liquid Chromatography (HPLC) Analysis
The podophyllotoxin quantification was carried out on an Agilent liquid chromatographic system including an Agilent 1260 Infinity G5611A Quaternary Pump (with an include degasser), an Agilent 1260 Infinity II G7129A vial sampler, and an Agilent 1260 Infinity II G7117C diode array detector (DAD). The entire HPLC chain was controlled by HPLC 1260 Infinity software. The separation was performed at 35 °C on a KINETEX (Phenomenex) F5 column (100 × 3.0 mm i.d; 2.6 μm), with 5 μL as the injection volume. The mobile phase was composed of HPLC-grade solvents: water (solvent A) and methanol (solvent B). With a flow rate of 0.7 mL/min, a nonlinear gradient was applied for the mobile phase variation as follows: from 0 to 2 min of A–B: 70:30 (v/v) to 38:62 (v/v), from 2 to 9 min of A–B: 38:62 (v/v) to 33:67 (v/v), and from 9 to 12 min of A–B: 33:67 (v/v) to 5:95 (v/v). Then, the column was rinsed with absolute methanol for 10 min. Detection was performed at 295 nm. Podophyllotoxin was identified due to its retention time and UV spectrum in comparison to the reference standard. Quantification was performed using a calibration curve of podophyllotoxin ranging from 125 μg/mL to 1 mg/mL, with a correlation coefficient higher than 0.999, LOD = 35.73 μg/mL, and LOQ = 108.27 μg/mL.
2.7. Experimental Design
Response surface plots and factorial experiment design were used to determine the optimal podophyllotoxin extraction conditions with XLSTAT 2022.2.1 software (Addinsoft, Paris, France). The preliminary experiments allowed the assignment of the three independent variables affecting extraction yield and their range values. The variables were coded at three levels: −1, 0, and 1, and the selected values were X1—ethanol concentration (60, 75, and 90% (v/v)), X2—incubation duration (30, 55, and 80 min), and X3—temperature (40, 60, and 80 °C) (Table 1). Eighteen observations of different combinations were prepared, as presented in Table 2. All the observations were carried out in five independent replicates. The equation calculation was provided by the XLSTAT2022 software (Addinsoft, Paris, France).

Table 1.
Identities, code units, coded levels, and actual experimental values of the three independent variables.

Table 2.
Results of experimental design.
2.8. Statistical Analysis
Each experiment was conducted at least 3 times. A comparative statistical analysis of groups was performed using the Student’s t-test. The statistical tests were significant at p < 0.05. The graphical and statistical treatments were performed using Microsoft Excel 2010 software, except for the experimental design, which was performed using XLSTAT2022 software (Addinsoft, Paris, France).
3. Results
3.1. Preliminary Single Factor Experiments
Single-factor experiments were chosen to evaluate the relative impact of different conditions on the extraction yield of podophyllotoxin from Juniperus scopulorum needles. Previously, we developed an HPLC separation gradient enabling precise identification and quantification of podophyllotoxin (Figure 1). The impact of different parameters identified as important in the literature was tested: extraction duration, ultrasound frequency, ethanol concentration, solvent/material ratio, prior grinding, and extraction temperature. These preliminary single-factor experiments determined the main limiting factors. In the second stage, in order to measure the direct impact and the interaction effect between these parameters, they will be applied in the design of the experiments.
On the other hand, as the objective is to develop a green extraction protocol, an extraction solvent suitable for green chemistry must be chosen. Conventionally, different organic solvents can be used to extract podophyllotoxin from Juniperus needles: methanol, ethanol, and chloroform [31]. Ethanol appears to be the best choice because it is non-toxic, has minimal environmental impact, and can be mixed with water to improve its ability to solubilize podophyllotoxin.
As there is no protocol for podophyllotoxin USAE, we determine the parameter ranges to test based on the secoisolariciresinol USAE study [49]. For secoisolariciresinol, the optimal content is obtained with an extraction time of 60 min and a sample/solvent concentration of 5 mg/mL. Therefore, for the podophyllotoxin USAE, we tested an extraction time of up to 80 min and a sample/solvent concentration from 5 to 20 mg/mL. As the optimal solvent concentration was not exactly determined for secoisolariciresinol USAE, we tested from 40% to 100% ethanol, considering podophyllotoxin solubility. For the other parameters, we tested the largest condition allowed by the apparatus (temperature between 20 and 80 °C, frequencies equal to 0, 37, or 80 KHz).
A preliminary experiment was conducted with several extraction durations (10, 20, 30, 40, 60, and 80 min) using fixed ultrasound frequencies (37 kHz), the aqueous ethanol concentration (80%), the plant material concentration (10 g DW/L), and temperature (40 °C), but without prior grinding. The results presented in Figure 2a highlight that increasing the extraction duration improves the podophyllotoxin extraction yield by up to 40 min and remains at this maximum for at least 80 min.
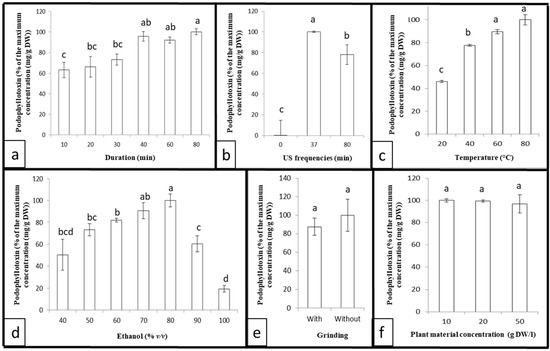
Figure 2.
Podophyllotoxin content extracted from Juniperus scopulorum needles by a function of (a) extraction duration, (b) ultrasound frequency, (c) extraction temperature, (d) ethanol concentration, (e) grinding, and (f) plant material concentration. Values are means ± SD of 5 independent replicates. Different letters represent significant differences between the various extraction conditions (p < 0.05).
The sonication frequency can also influence extraction. Three ultrasound frequencies (0, 37, and 80 kHz) were tested with a fixed extraction duration (40 min), aqueous ethanol concentration (80%), plant material concentration (10 g DW/L), and temperature (40 °C), but without prior grinding. It seems (Figure 2b) that at 0 kHz, there is no significant podophyllotoxin extraction. Then, 37 kHz offers a slightly higher extraction yield compared to 80 kHz, due to the stability of the podophyllotoxin [49,50,51,52], with better repeatability. It can be noted that the apparatus allows only a choice between 0, 37, or 80 kHz.
It has also been established that temperature can have an impact on ultrasonic-assisted extraction. Thus, the effect of temperature was evaluated at 20, 40, 60, and 80 °C using a fixed extraction duration (40 min), plant material concentration (10 g DW/L), ultrasound frequency (37 kHz), aqueous ethanol concentration (80%), plant material concentration (10 g DW/L), and temperature (40 °C), but without prior grinding. The results (Figure 2c) show that increasing the temperature improves the extraction yield of the podophyllotoxin up to 60 °C; then, the maximum reached remains unchanged.
Different concentrations of aqueous ethanol solution were tested (40, 50, 60, 70, 80, 90, and 100% (v/v)) using a fixed extraction duration (40 min), ultrasound frequency (37 kHz), plant material concentration (10 g DW/L), and temperature (40 °C), but without prior grinding. The extraction yield reached a maximum—increasing up to 70% and decreasing after 80% (Figure 2d).
The effect of previous grinding was analyzed using a fixed extraction duration (40 min), ultrasound frequency (37 kHz), aqueous ethanol concentration (80%), plant material concentration (10 g DW/L), and temperature (40 °C). As expected, previous grinding had no statistically significant effect on podophyllotoxin extraction yield (Figure 2e). Sonication caused cavitation bubbles that were generated close to the sample. These cavitation bubbles collapse, breaking down the cell walls and releasing the cell contents: phenolic compounds, etc. [46,48,53,54]. Sonication, therefore, makes grinding unnecessary.
The effect of various concentrations of plant material was also studied with 10, 20, and 50 g DW/L using a fixed extraction duration (40 min), ultrasound frequency (37 kHz), aqueous ethanol concentration (80%), plant material concentration (10 g DW/L), and temperature (40 °C), but without prior grinding. The results (Figure 2f) indicate that the concentration of plant material has no statistically significant impact on the podophyllotoxin extraction yield.
3.2. Development of a Multifactorial Approach
Knowing that interaction between the independent variables is possible, an experimental factorial design was performed and completed by statistical analysis and 3D surface response plots [55]. This method allows the ultrasound-assisted extraction of podophyllotoxin from Juniperus scopulorum needles to be optimized quickly and accurately.
Preliminary experiments have shown that the four variables affecting podophyllotoxin extraction are: extraction duration, ultrasonic frequency, aqueous ethanol concentration, and extraction temperature. Nevertheless, the frequency cannot be exactly adjusted in this ultrasonic bath. The frequency can only be set to 0, 37, or 80 kHz. Therefore, the 37 kHz frequency was chosen as it provides the highest podophyllotoxin extraction yield. Hence, the experimental factorial design will be realized considering only three variables: aqueous ethanol concentration (X1, ranging from 60 to 90% (v/v)), extraction duration (X2, ranging from 30 to 80 min), and extraction temperature (X3, ranging from 40 to 80 °C). The codes used for each independent variable are shown in Table 1. Moreover, based on our preliminary experiment, a concentration of 10 mg DW/L of plant material was chosen, without previous grinding.
Herein, a Box–Behnken design was performed. For the experiments, eighteen different conditions were randomized using independent process variables. Each condition consists of 5 independent replicates, and the podophyllotoxin content was determined by HPLC (Table 2).
The results show that the podophyllotoxin extracted from freeze-dried Juniperus scopulorum needles ranges from 5.81 mg/g DW (Obs10) to 7.14 mg/g DW (Obs15). Using multiple regression analysis, the following second-order polynomial equation was obtained: Y = 6.946 + 0.012 × X1 − 0.034 × X2 − 0.108 × X3 − 0.318 × X12 − 0.371 × X1X2 − 3.33.10−3 × X1X3 − 0.4 × X22 + 0.251 × X2X3 − 0.473 × X32 to represent the podophyllotoxin content (Y) as a function of the different variables (X1, X2, and X3).
The results of the statistical analysis (Table 3) show that the quadratic coefficients X12, X22, and X32, as well as the interaction coefficients X1X2 and X2X3, seem to significantly influence the extraction of podophyllotoxin from freeze-dried Juniperus scopulorum needles. Concomitantly, each linear coefficient (X1, X2 and X3) and the interaction coefficient X1X3 were not significant (p > 0.05). Thus, each variable (incubation duration, temperature, and percentage of ethanol) did not have a direct impact on the podophyllotoxin extraction. However, the interaction between the variables, percentage of ethanol incubation time, and incubation time–temperature induced a significant indirect impact on the extraction yield of podophyllotoxin from the freeze-dried J. scopulorum.

Table 3.
Statistical analysis of regression coefficient.
Table 4 presents the results of the variance analysis (ANOVA) and the fit of the model obtained. Considering the model presenting the predicted podophyllotoxin extraction yield versus the experimentally obtained podophyllotoxin extraction yield, the high F-value (10.82) and the low p-value (1.3 × 10−3) show that the model is highly significant. Then, the coefficient of determination, R2 (0.924), and the adjusted R2 (0.839) values confirm that the model for podophyllotoxin extraction yield from freeze-dried Juniperus scopulorum needles closely matches the predicted model. Also, the low value of the coefficient of variation (CV = 1.79%) demonstrates that the model fits the experimental values.

Table 4.
ANOVA of the predicted model for USAE of podophyllotoxin from freeze-dried Juniperus scopulorum needles.
According to the analysis, the 3D plots (Figure 3) clearly highlight that the extraction of podophyllotoxin from freeze-dried Juniperus scopulorum needles reached a maximum. Increasing the temperature and extraction duration leads to a decrease in podophyllotoxin content (Figure 3C), as suggested by the observed negative linear and quadratic coefficients.
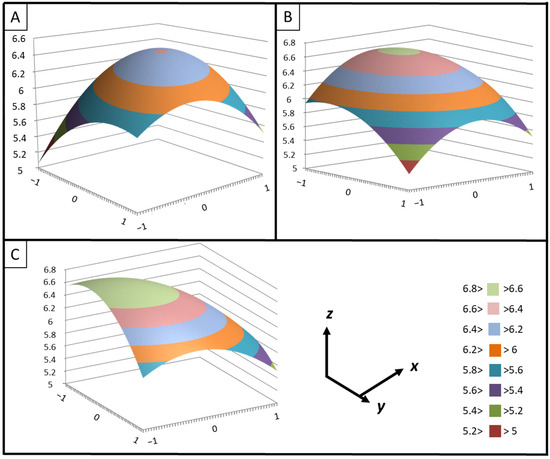
Figure 3.
Predicted surface response plots of the podophyllotoxin extraction yield (y) as a function of (A): ethanol concentration (x) and extraction duration (z), (B): ethanol concentration (x) and extraction temperature (z), and (C): extraction duration (x) and extraction temperature (z).
Considering the adjusted second-order polynomial equation, it appears that the optimum conditions are: 69.3% ethanol concentration, 51.5 min incubation duration, and 58.8 °C temperature. Applying these conditions, a podophyllotoxin content of 7.02 mg/g DW was extracted from the freeze-dried Juniperus scopulorum needles.
3.3. Variation of Podophyllotoxin Content in Different Varieties of Juniperus scopulorum
In this study, the podophyllotoxin content of two varieties of Juniperus scopulorum, Blue Arrow and Skyrocket, was quantified by applying the optimal conditions previously established. Under the same conditions, the effect of the year of purchase of the two varieties on the podophyllotoxin content was evaluated. The results (Figure 4) demonstrate that the variety and year of purchase can significantly affect the podophyllotoxin content. Juniperus scopulorum Blue Arrow, bought in 2021, presented a podophyllotoxin content of about 7.02 mg/g DW, while the same variety bought in 2019 presented a podophyllotoxin content of about 10.26 mg/g DW. Moreover, Juniperus scopulorum Skyrocket, bought in 2019, presented about 10.34 mg of podophyllotoxin/g DW. In brief, this protocol allows up to 10.34 mg podophyllotoxin/g DW to be extracted from freeze-dried Juniperus scopulorum needles.
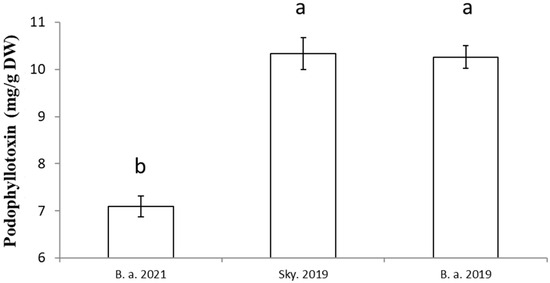
Figure 4.
Comparison of podophyllotoxin content in needles (obtained using optimized extraction protocol) between several Juniperus scopulorum samples and/or varieties. Values are means ± SD of 5 independent replicates. Different letters represent significant differences between the various extraction conditions (p < 0.05).
3.4. Comparison of the Optimized Protocol with the Reference Protocol
The podophyllotoxin extraction protocol offering the best extraction yield from Juniperus needles known today is the protocol of Renouard et al., 2011 [31], a protocol consisting of grinding the needles using an Ultraturax in 100% methanol followed by maceration (5 h). As previously proved, podophyllotoxin content can vary between species; we tested both protocols (the optimized ultrasound-assisted protocol developed here and the Renouard et al., 2011 [31] protocol) on the same sample (Juniperus scopulorum Blue Arrow bought in 2021). The result (Figure 5) shows that no significant difference was detected between these two protocols. Therefore, the ultrasound-assisted extraction protocol developed in this study is equal to the Renouard et al. (2011) [31] protocol and also offers the best extraction yield of podophyllotoxin from Juniperus scopulorum needles.
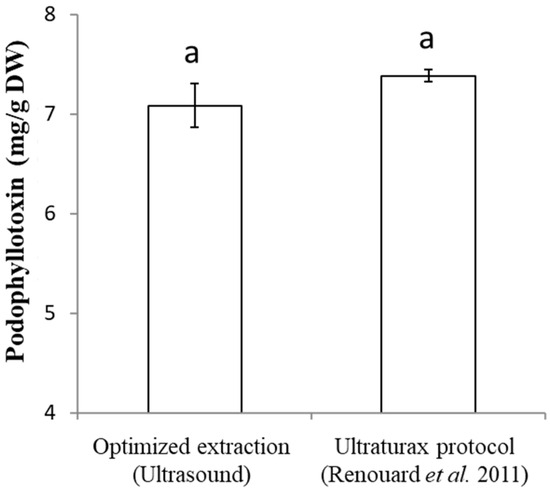
Figure 5.
Comparison of podophyllotoxin content in Juniperus scopulorum needles obtained using the optimized extraction protocol vs. Renouard et al., 2011 protocol [31]. Values are means ± SD of 5 independent replicates. Different letters represent significant differences between the various extraction conditions (p < 0.05).
3.5. The Juniperus Extract Stability
To assess the interest in developing an extraction protocol for a specific/interesting molecule, it is crucial to consider the stability of the extracted molecule. For this purpose, it is necessary to evaluate the duration of the presence of podophyllotoxin in the extract without decreasing its content. The experiments show (Figure 6) that podophyllotoxin stays stable in the extract at 25 °C for up to 6 days. A statistically significant decrease was measured after 10 days. This is an interesting observation compared with the protocol of Renouard et al. (2011) [31], which detected a decrease after 6 h.
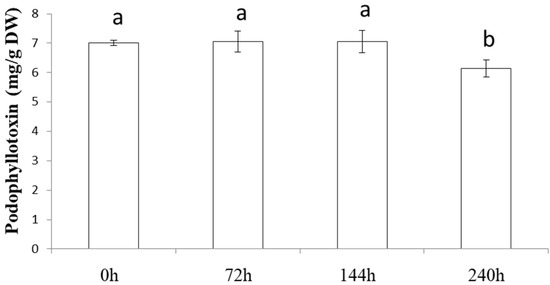
Figure 6.
Stability study of podophyllotoxin content in Juniperus scopulorum needles obtained using the optimized extraction protocol. Values are means ± SD of 3 independent replicates. Different letters represent significant differences between the various extraction conditions (p < 0.05).
4. Discussion
The preliminary experiment highlights the involvement of ultrasound frequency in the podophyllotoxin extraction process (Figure 2b). The literature explains that ultrasound frequency can improve the solubility of podophyllotoxin in the extraction solvent, due to the cavitation effect and the modification of the diffusion coefficient of the targeted molecule. Up to 60 kHz, the frequency does not affect the stability of phenolic compounds after their extraction [50]. However, at 80 KHz, a significant impact is observed. Tchabo et al. (2015) [56] also measured a decrease in total phenolic compound concentration in mulberry above and below 33.82 KHz. It has already been established that the high frequency of ultrasound can lead to the degradation of phenolic compounds and, therefore, also of podophyllotoxin. [51,52]. Additionally, a similar study conducted on secoisolariciresinol, a podophyllotoxin precursor, showed better extraction yield at 30 kHz than at 45 kHz [49], illustrating the negative impact of high frequencies on lignans. Thus, using an ultrasound frequency of 37 kHz allows a better extraction yield of podophyllotoxin compared to 0 and 80 kHz.
The multifactorial approach demonstrates that the interaction between the percentage of ethanol and incubation duration is involved in the extraction of podophyllotoxin from Juniperus scopulorum needles. Indeed, incubation duration has a significant impact: the longer the treatment lasts, the more cavitation bubbles emerge and collapse, and the more the proportion of molecules released from cells increases [45,47]. Concomitantly, in order to release the molecules from the cell, it is essential to solubilize the molecule. De facto, the percentage of ethanol will unduly affect the extraction yield of the podophyllotoxin. Moreover, the percentage of ethanol modifies the viscosity of the solvent, whereas the cavitation threshold depends on the viscosity of the solvent [47,57]. A modification of the cavitation threshold will lead to a modification of the incubation duration, resulting in cell perforation. Then, the collapse of the cavitation bubble is more intense at low vapor pressure, which depends on the percentage of ethanol [58]. Thus, this interaction and its impact on podophyllotoxin extraction yield seem obvious.
The interaction of temperature and incubation duration has a significant favorable impact on the extraction yield of podophyllotoxin from Juniperus scopulorum needles. Temperature decreases solvent viscosity and surface tension, leading to attenuating the effects of sonication [58]. Then, a positive effect of temperature is associated with an increase in cavitation bubbles [59]. As the temperature can increase the number of cavitation bubbles, the extraction duration will define how long these numerous bubbles will act (perforate the cell to extract the podophyllotoxin), explaining the benefit of the interaction between temperature and incubation duration.
In this study, the optimized protocol was established to extract up to 10.34 mg podophyllotoxin/g DW from freeze-dried Juniperus scopulorum needles. Protocols with podophyllotoxin extraction yields of 8.5 mg/g DW [33] and 4.9 mg/g DW [32] have been reported in the literature, but this amount of podophyllotoxin has never been extracted from Juniperus scopulorum needles. It should be noted that this content appears to be consistent with the published content: between 0.2 mg/g DW (Juniperus communis) and 22.6 mg/g DW (Juniperus bermudiana) [31].
However, compared with the extraction protocol that yielded the highest amount of podophyllotoxin from Juniperus needles [31], there is no statistically significant difference. Thus, this USAE protocol is as effective as the protocol of Renouard et al., 2011 [31], which is the reference protocol. Furthermore, this USAE protocol only requires ethanol as the organic extraction solvent, which is an eco-friendly solvent with minor toxicity for humans, unlike the Renouard et al. (2011) [31] protocol, which uses methanol, a neurotoxic solvent. Also, the Renouard et al. (2011) [31] protocol requires 5 h of maceration at 25 °C after Ultraturax grinding to extract the maximum podophyllotoxin amount, while this USAE protocol does not require prior grinding and extraction lasts less than an hour. Therefore, USAE is less time-consuming, and thus more economical. Additionally, using Ultraturax, samples must be processed one by one, while the ultrasonic bath allows treating twenty samples simultaneously. Then, the Renouard et al. (2011) [31] protocol demonstrates a statistically significant podophyllotoxin loss as early as one hour after extraction, which can pose a problem for the pharmaceutical industry, because once extracted, the podophyllotoxin must immediately be purified. However, the protocol herein developed allows the extract to be stored for 6 days without loss of podophyllotoxin, thus giving more latitude/time for purification of the active ingredient. Furthermore, the impact of the variety and/or year of purchase of the plant on podophyllotoxin content is a result that could be expected. Indeed, Peanparkdee Patrawatt and Iwamoto (2019) [60] and Zainol et al. (2020) [61] detected differences in the content of ultrasonically extracted phenolic compounds depending on geographical origin, cultivars, or plant species. In addition, regarding podophyllotoxin content specifically, damage to Podophyllum peltatum leaves increases the podophyllotoxin content [62]. A difference has also been shown in the needles of different Juniperus varieties: for instance, the Juniperus virginiana variety Blue Clouds contains 384.5 mg/100 g DW, whereas the variety Kosteri contains 4.3 mg/100 g DW [32]. Additionally, podophyllotoxin is a phytoalexin, i.e., a molecule produced by the plant to protect itself and/or as a response to stress. Therefore, the content of podophyllotoxin will be modified depending on the plant’s basic protective ability and/or the stresses suffered by the plant [62,63]. In this study, the significant variation in podophyllotoxin levels according to the year of purchase and/or variety shows the impact of the environment and genetics on the production of molecules. To maximize the interest in J. scopulorum as a source of podophyllotoxin, it could be interesting to identify varieties and environmental factors that could stimulate podophyllotoxin production, along the lines of what has been conducted for one of these precursors in flax [64] or for podophyllotoxin in Podophyllum peltatum, considering the way the leaves are harvested and stored [62,65].
In conclusion, it is important to remember that podophyllotoxin is a well-known precursor of anti-cancer drugs, and the current plant source, Sinopodophyllum hexandrum, is now prohibited for the extraction of podophyllotoxin. A new alternative plant source is therefore being sought. Juniperus species are known to contain podophyllotoxin in their needles. Hence, we developed a protocol for the extraction of podophyllotoxin from Juniperus scopulorum needles. This study focused on a green chemistry approach: ultrasound-assisted extraction to avoid environmental damage, and on commercially available Juniperus scopulorum varieties to ensure the availability of the plant source. The protocol developed involved extraction in 69.3% ethanol for 51.5 min at 58.8 °C and allowed up to 10.34 mg/g DW podophyllotoxin to be extracted from freeze-dried Juniperus scopulorum needles, equaling the extraction yield obtained with the reference protocol, although a variation in the podophyllotoxin content was measured according to the variety and year of purchase of the plant. A stable podophyllotoxin content for six days was observed, which constitutes an interesting stability period to ensure the purification of the molecule after its extraction.
Thus, in the area of availability and extraction of podophyllotoxin, this work highlights a newly available and quantitively credible source of this active molecule in J. scopulorum needles. In addition, it offers an efficient, rapid, environmentally friendly ultrasonic extraction protocol using a bath that can act on several samples simultaneously, enabling it to be adapted to an industrial scale.
An interesting prospect would be to identify the genetic and environmental factors stimulating the production of podophyllotoxin by J. scopulorum to increase the amount of podophyllotoxin produced and therefore the gains in credibility of this plant as a source of anticancer agents.
Author Contributions
Conceptualization, S.R.; Methodology, A.R.; Validation, C.V.; Investigation, C.V., A.R. and S.R.; Writing—original draft, S.R.; Writing—review & editing, A.R. and S.R.; Supervision, C.V.; Funding acquisition, C.V. and S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Ligue contre le Cancer de Dordogne France”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
The project received, for its realization, the financial support of the “Ligue contre le Cancer de Dordogne France”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Canel, C.; Moraes, R.M.; Dayan, F.; Ferreira, D. Podophyllotoxin. Phytochemistry 2000, 54, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Kingston, D.G. Anticancer Agents from Natural Products, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2011; p. 97. [Google Scholar]
- Podwyssotzki, V. The active constituents of podophyllotoxin. Pharm. J. Trans. 1881, 12, 217–218. [Google Scholar]
- Podwyssotzki, V. On the active constituents of podophyllin. Am. J. Pharm. 1882, 12, 102–115. [Google Scholar]
- Podwyssotzki, V. Pharmakologische Studien uber Podophyllum peltatum. Arch. Exp. Path. Pharmakol. 1884, 13, 29–52. [Google Scholar] [CrossRef]
- Yu, X.; Che, Z.P.; Xu, H. Recent Advances in the Chemistry and Biology of Podophyllotoxins. Chem. Eur. J. 2016, 10, 1002–1006. [Google Scholar] [CrossRef]
- Stoll, A.; Renz, J.; Wartburg, A.V. The isolation of podophyllotoxin glucoside. J. Am. Chem. Soc. 1954, 76, 3103–3104. [Google Scholar] [CrossRef]
- Stoll, A.; von Wartburg, A.; Angliker, E.; Renz, J. The isolation of 40-Demethylpodophyllotoxin glucoside from rhizomes podophyllum emodi wall. J. Am. Chem. Soc. 1954, 76, 5004–5005. [Google Scholar] [CrossRef]
- Emmenggger, H.; Stahelin, H.; Rutschmann, J.; von Wartburg, A. Chemistry and Pharmacology of podophyllum glucosides and derivatives. I. Arzneim. 1961, 11, 327–333. [Google Scholar]
- Kuhn, M.; von Wartburg, A. Mitosis-inhibiting substances. XXI. Synthesis of epipodophyllotoxin b-D-glucopyranoside. Helv. Chim. Acta 1968, 51, 1631–1641. [Google Scholar] [CrossRef]
- Kuhn, M.; von Wartburg, A. Mitosis-inhibiting substances. Glycosidation process. II. Glycosides of 4”-demethylepipodophyllotoxin. Helv. Chim. Acta 1969, 52, 948–955. [Google Scholar] [CrossRef]
- Keller-Juslen, C.; Kuhn, M.; VonWartburg, A.; Staehelin, H. Synthesis and antimitotic activity of glycosidic lignan derivatives related to podophyllotoxin. J. Med. Chem. 1971, 14, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Joel, S. The clinical pharmacology of etoposide. An update. Cancer Treat. Rev. 1996, 22, 179–221. [Google Scholar] [CrossRef] [PubMed]
- Stahelin, H.F.; von Wartburg, A. The chemical and biological route from podophyllotoxin glucoside to etoposide: Ninth cain memorial award lecture. Cancer Res. 1991, 51, 5–15. [Google Scholar] [PubMed]
- Gordaliza, M.; Garcia, P.A.; Miguel del Corral, J.M. Podophyllotoxin: Distribution, sources, applications, and new cytotoxic derivatives. Toxicon 2004, 44, 441–459. [Google Scholar] [CrossRef]
- Ardalani, H.; Avan, A.; Ghayour-Mobarhan, M. Podophyllotoxin: A novel potential natural anticancer agent. Avicenna J. Phytomed. 2017, 7, 285–294. [Google Scholar] [PubMed]
- Holm, B.; Sehested, M.; Jesen, P.B. Improved targeting of brain tumors using dexrazoxane rescue of topoisomerase II combined with supra-lethal doses of etoposide and teniposide. Clin. Cancer Res. 1998, 4, 1367–1373. [Google Scholar]
- Ekstrom, K.; Hoffman, K.; Linne, T.; Eriksoon, B.; Glimelius, B. Single-dose etoposide in advanced pancreatic and biliary cancer, a phase II study. Oncol. Rep. 1998, 5, 931–934. [Google Scholar] [CrossRef]
- Zi, C.T.; Yang, L.; Kong, Q.H.; Li, H.M.; Yang, X.Z.; Ding, Z.T.; Jiang, Z.H.; Hu, J.M.; Zhou, J. Glucoside derivatives of podophyllotoxin: Synthesis, physicochemical properties, and cytotoxicity. Drug Des. Dev. Ther. 2019, 13, 3683–3692. [Google Scholar] [CrossRef]
- Hammonds, T.R.; Denyer, S.P.; Jackson, D.E.; Irving, W.L. Studies to show that with podophyllotoxine the early replicative stages of herpes virus type 1 depend upon functional cytoplasmic microtubules. J. Med. Microbiol. 1996, 45, 167–172. [Google Scholar] [CrossRef]
- Chen, S.W.; Wang, Y.H.; Jin, Y.; Tian, X.; Zheng, Y.T.; Luo, D.Q.; Tu, Y.Q. Synthesis and anti-HIV-1 activities of novel podophyllotoxin derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 2091–2095. [Google Scholar] [CrossRef]
- Maulin, P.; Eduardo, D.; Daniel, S.; Parag, D.; Ashwin, C.; Michael, B.; Roberto, C.; Gerard, C.J. Etoposide as salvage therapy for cytokine storm due to coronavirus disease 2019. Chest 2021, 159, E7–E11. [Google Scholar]
- Hartwell, J.L.; Schrecker, A.W. Components of Podophyllin. V. The Constitution of Podophyllotoxin. J. Am. Chem. Soc. 1951, 73, 2909–2916. [Google Scholar] [CrossRef]
- Purohit, M.C.; Bahuguna, R.; Maithani, U.C.; Purohit, A.N.; Rawat, M.S.M. Variation in podophylloresin and podophyllotoxin contents in different populations of Podophyllum hexandrum. Curr. Sci. India 1999, 77, 1078–1080. [Google Scholar]
- Li, H.; Cimino, S.K. Clinical impact of the etoposide injection shortage. J. Oncol. Pharm. Pract. 2020, 26, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Hartwell, J.L.; Johnson, J.M.; Fitzgerald, D.B.; Belkin, M. Podophyllotoxin from Juniperus species savinin. J. Am. Chem. Soc. 1953, 75, 235–236. [Google Scholar] [CrossRef]
- Cushman, K.E.; Maqbool, M.; Gerard, P.D.; Bedir, E.; Lata, H.; Moraes, R.M. Variation of podophyllotoxin in leaves of Eastern red cedar (Juniperus virginiana). Planta Med. 2003, 69, 477–478. [Google Scholar]
- Gawde, A.; Cantrell, C.L.; Zheljazkov, V.D. Dual extraction of essential oil and podophyllotoxin from Juniperus virginiana. Ind. Crops Prod. 2009, 30, 276–280. [Google Scholar] [CrossRef]
- Gawde, A.; Zheljazkov, V.D.; Maddox, V.; Cantrell, C.L. Bioprospection of Eastern red cedar from nine physiographic regions in Mississippi. Ind. Crops Prod. 2009, 30, 59–64. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Zheljazkov, V.D.; Osbrink, W.L.; Castro, A.; Maddox, V.; Craker, L.E.; Astatkie, T. Podophyllotoxin and essential oil profile of Juniperus and related species. Ind. Crops Prod. 2013, 43, 668–676. [Google Scholar] [CrossRef]
- Renouard, S.; Lopez, T.; Hendrawati, O.; Dupré, P.; Doussot, J.; Falguieres, A.; Ferroud, C.; Hagège, D.; Lamblin, F.; Lainé, E.; et al. Podophyllotoxin and deoxypodophyllotoxin in Juniperus bermudiana and 12 other Juniperus species: Optimization of extraction, method validation, and quantification. J. Agric. Food Chem. 2011, 59, 8101–8107. [Google Scholar] [CrossRef]
- Och, M.; Och, A.; Cie’sla, L.; Kubrak, T.; Pecio, L.; Stochmal, A.; Kocki, J.; Bogucka-Kocka, A. Study of cytotoxic activity, podophyllotoxin, and deoxypodophyllotoxin content in selected Juniperus species cultivated in Poland. Pharm. Biol. 2015, 53, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.I.; Nedialkov, P.T.; Tashev, A.N.; Olech, M.; Nowak, R.; Ilieva, Y.E.; Kokanova-Nedialkova, Z.K.; Atanasova, T.N.; Angelov, G.; Najdenski, H.M. Junipers of various origins as potential sources of the anticancer drug precursor podophyllotoxin. Molecules 2021, 26, 5179. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Zühlke, S.; Spiteller, M. Chemometric evaluation of the anti-cancer pro-drug podophyllotoxin and potential therapeutic analogues in Juniperus and Podophyllum species. Phytochem. Anal. 2011, 22, 128–143. [Google Scholar] [CrossRef]
- Woo, K.W.; Choi, S.U.; Park, J.C.; Lee, K.R. A new lignan glycoside from Juniperus rigida. Arch. Pharm. Res. 2011, 34, 2043–2049. [Google Scholar] [CrossRef]
- Han, J.W.; Shim, D.W.; Shin, W.Y.; Kim, M.K.; Shim, E.J.; Sun, X.; Koppula, S.; Kim, T.J.; Kang, T.B.; Lee, K.H. Juniperus rigida Sieb. extract inhibits inflammatory responses via attenuation of TRIF-dependent signaling and inflammasome activation. J. Ethnopharmacol. 2016, 190, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, D.; Li, D.; Zhang, S. Quality evaluation of Juniperus rigida Sieb. et Zucc. based on phenolic profiles, bioactivity, and HPLC fingerprint combined with chemometrics. Front. Pharmacol. 2017, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Cantrell, C.L.; Donega, M.A.; Astatkie, T.; Heidel, B. Podophyllotoxin concentration in Junipers in the Big Horn Mountains in Wyoming. HortScience 2012, 47, 1696–1697. [Google Scholar] [CrossRef]
- Xu, S.; Li, X.; Liu, S.; Tian, P.; Li, D. Juniperus sabina L. as a source of podophyllotoxins: Extraction optimization and anticholinesterase activities. Int. J. Mol. Sci. 2022, 23, 10205. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xie, J.; Xu, Y.; Zou, L.; Zhang, L.; Luo, Z. Natural deep eutectic solvent enhanced pulse-ultrasonication assisted extraction as a multistability protective and efficient green strategy to extract anthocyanin from blueberry pomace. LWT 2021, 144, 111220. [Google Scholar] [CrossRef]
- Yusoff, I.; Taher, Z.; Rahmat, Z.; Chua, L. A review of ultrasound-assisted extraction for plant bioactive compounds: Phenolics, flavonoids, thymols, saponins and proteins. Food Res. Int. 2022, 157, 111268. [Google Scholar] [CrossRef]
- Miller, D.L.; Pislaru, S.V.; Greenleaf, J.E. Sonoporation: Mechanical DNA delivery by ultrasonic cavitation. Somat. Cell Mol. Gen. 2002, 27, 115–134. [Google Scholar] [CrossRef]
- Karshafian, R.; Bevan, P.D.; Williams, R.; Samac, S.; Burns, P.N. Sonoporation by ultrasound-activated microbubble contrast agents: Effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med. Biol. 2009, 35, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Ohta, S.; Suzuki, K.; Miyagawa, S.; Ogino, Y.; Villacorte, M.; Wada, Y.; Yamada, G. Sonoporation in developmental biology. In Electroporation and Sonoporation in Developmental Biology; Nakamura, H., Ed.; Springer: Tokyo, Japan, 2009; pp. 317–326. [Google Scholar]
- Gang, D.; Wang, J.; Dudareva, N.; Nam, K.; Simon, J.; Lewinsohn, E.; Pichersky, E. An investigation of the storage and biosynthesis of phenylpropenes in sweet basil. Plant. Physiol. 2001, 125, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef]
- Zhao, S.; Baik, O. Application of ultrasound as pretreatment for extraction of podophyllotoxin from rhizomes of Podophyllum peltatum. Ultrason. Sonochem. 2012, 19, 22–31. [Google Scholar] [CrossRef]
- Corbin, C.; Fidel, T.; Leclerc, E.A.; Barakzoy, E.; Sagot, N.; Falguiéres, A.; Renouard, S.; Blondeau, J.P.; Ferroud, C.; Doussot, J.; et al. Development and validation of an efficient ultrasound assisted extraction of phenolic compounds from flax (Linum usitatissimum L.) seeds. Ultrason. Sonochem. 2015, 26, 176–185. [Google Scholar] [CrossRef]
- Soria, A.C.; Villamiel, M. Effect of ultrasound on the technological properties and bioactivity of food: A review. Trends Food Sci. Technol. 2010, 21, 323–331. [Google Scholar] [CrossRef]
- Lavilla, I.; Bendicho, C. Fundamentals of ultrasound-assisted extraction. In Water Extraction of Bioactive Compounds; González, H.D., González Muñoz, M.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 291–316. [Google Scholar]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011, 18, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Tchabo, W.; Ma, Y.; Engmann, F.N.; Zhang, H. Ultrasound-assisted enzymatic extraction (UAEE) of phytochemical compounds from mulberry (Morus nigra) must and optimization study using response surface methodology. Ind. Crops Prod. 2015, 63, 214–225. [Google Scholar] [CrossRef]
- Mason, T.; Lorimer, J. General principles. In Applied Sonochemistry: Uses of Power Ultrasound in Chemistry and Processing; Mason, T.J., Lorimer, J.P., Eds.; Wiley: Weinheim, Germany, 2002; pp. 25–74. [Google Scholar]
- Santos, H.; Lodeiro, C.; Capelo-Martínez, J. The power of ultrasound. In Ultrasound in Chemistry: Analytical Applications; Capelo-Martínez, J.L., Ed.; Wiley: Weinheim, Germany, 2009; pp. 1–16. [Google Scholar]
- Shirsath, S.; Sonawane, S.; Gogate, P. Intensification of extraction of natural products using ultrasonic irradiations—A review of current status. Chem. Eng. Process. Process Intensif. 2012, 53, 10–23. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Patrawatt, J.; Iwamoto, S. Effects of extraction conditions on phenolic content, anthocyanin content and antioxidant activity of bran extracts from Thai rice cultivars. J. Cereal Sci. 2019, 86, 86–91. [Google Scholar] [CrossRef]
- Zainol, M.; Mohd Subri, I.; Zamri, I.; Mohd Zin, Z.; Fisal, A.; Mamat, H. Antioxidative properties and proximate analysis of spent coffee ground (SCG) extracted using ultrasonic methanol assisted technique as a potential functional food ingredient. Food Res. 2020, 4, 636–644. [Google Scholar] [CrossRef]
- Bedir, E.; Tellez, M.; Lata, H.; Khan, I.; Cushman, K.E.; Moraes, R.M. Post-harvest and scale-up extraction of American mayapple leaves for podophyllotoxin production. Ind. Crops Prod. 2006, 24, 3–7. [Google Scholar] [CrossRef]
- Renouard, S.; Corbin, C.; Drouet, S.; Medvedec, B.; Doussot, J.; Colas, C.; Maunit, B.; Bhambra, A.; Gontier, E.; Jullian, N.; et al. Investigation of Linum flavum (L.) hairy root cultures for the production of anticancer aryltetralin lignans. Int. J. Mol. Sci. 2018, 19, 990. [Google Scholar] [CrossRef]
- Garros, L.; Drouet, S.; Corbin, C.; Decourtil, C.; Fidel, T.; Lebas de Lacour, J.; Leclerc, E.A.; Renouard, S.; Tungmunnithum, D.; Doussot, J.; et al. Insight into the influence of cultivar type, cultivation year, and site on the lignans and related phenolic profiles, and the health-promoting antioxidant potential of flax (Linum usitatissimum L.) seeds. Molecules 2018, 23, 2636. [Google Scholar] [CrossRef]
- Cushman, K.E.; Moraes, R.M.; Gerard, P.D.; Bedir, E.; Silva, B.; Khan, I.A. Frequency and timing of leaf removal affect growth and podophyllotoxin content of Podophyllum peltatum in full sun. Planta Med. 2006, 72, 824–829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).