Abstract
Heritage objects with wooden supports can degrade in inappropriate storage conditions or when microclimate factors contribute to the development of biological attacks. Another issue regarding the deterioration of artifacts is the lack of a full understanding of material properties and their behavior during restoration treatments. In this paper, we note the strengthening treatments of artifacts with severely damaged wood and the various treatments against bio-pests. The influence of solvent on dimensional changes was observed for water, acetone, and white spirit. Acetone was found to cause the greatest swelling and deformation of the treated panels. The present work highlights the importance of choosing not only the correct types of solvents for the solubilization of synthetic resins, as well as those used in conservation-restoration treatments, but also the effects they have on polychrome wood panels that have been degraded by xylophagous insects.
1. Introduction
The degradation of cultural heritage objects with wooden supports is a result of the passage of historical time and the fact that wood is a hygroscopic, vulnerable material that responds negatively to environmental conditions in inappropriate storage or exhibition spaces and is prone to physical, chemical, and especially biological deterioration.
Cultural heritage, as defined in Romanian legislation, represents the totality of assets that reflect cultural values and require protection through scientific, legal, and technical measures for conservation and restoration [1]. Romania is part of numerous international organizations (e.g., UNESCO), which work to protect and promote cultural heritage worldwide.
The conservation and restoration of works of art with wooden supports are distinct disciplines that are focused on maintaining the integrity of the original materials and their cultural value. Conservators/restorers must deeply understand the materials used and apply appropriate methods and techniques to conserve works of art [2,3]. Solving practical problems involves research to extend scientific discoveries with applicability in the field of heritage conservation; heritage conservation specialists “need scientific examinations of collections” [4].
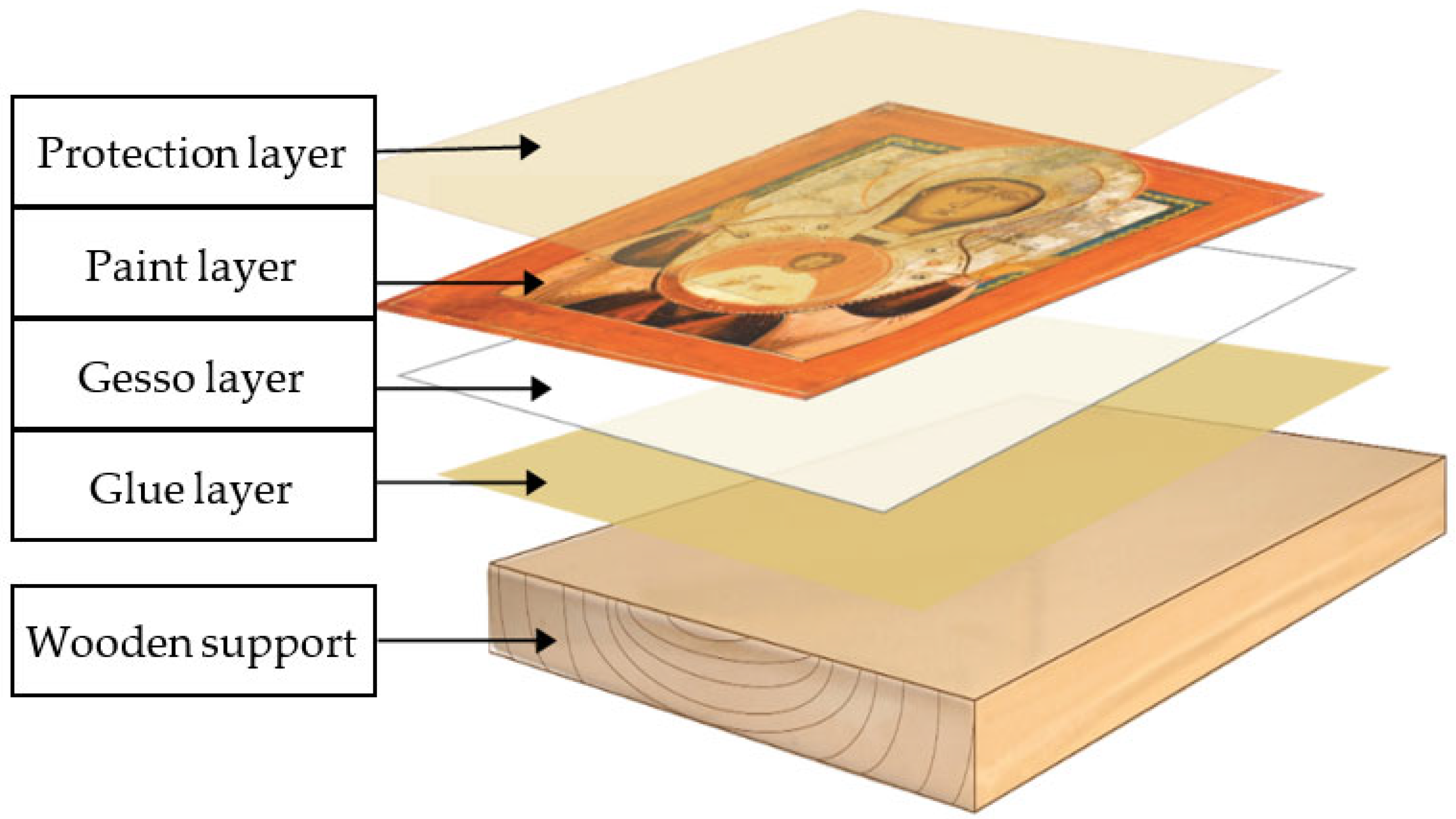
It is important that a cultural heritage object is understood and interpreted both as a whole and as a composite element. It is made of different adjacent or overlapping materials (Figure 1), which have different behaviors, and can be composed as follows: the wooden support, successive layers of glue in different concentrations, the preparation layer (the primer), over which the pictorial layer (the artistic creation) is placed, and the protective varnish. Thus, the wooden support and the complex preparations (the primer) have strengthening, balancing, equalizing, and finishing roles, over which structure the artistic creation is developed (with an aesthetic and/or thematic role). This composite ensemble, in its historical course, accumulates and transmits education, culture, and civilization.
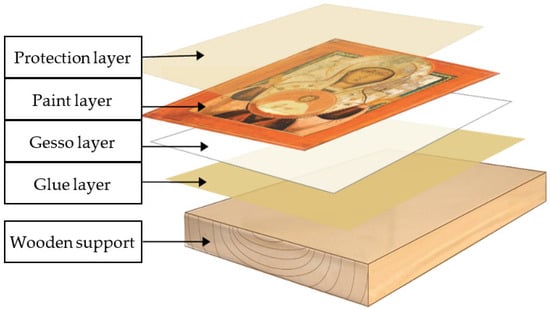
Figure 1.
The successive arrangement of the layers that form the art object.
Both the wooden support and the primer layer, together with the painting film, have elastic properties but exhibit different degrees of elasticity. The cracking of the primer layer (gesso) is often responsible for cracks appearing in paintings. The anisotropy of the wood support, manifesting in the dimensional expansion induced by humidity, inevitably causes deformations of the panels [5]. The relationship between these deformations and the risk of physical damage to the paint layers must be established via modeling and experimental research [5].
We reiterate the importance of knowing and having access to the control mechanisms of dimensional stability, indicating not only the ways in which cultural assets are (or can be) affected but also the processes that are applied to improve dimensional stability. “Stability is defined as: values at which a piece of wood contracts or swells in response to a change in its moisture content” [6].
Wood swelling, as an external dimensional change, is influenced by density and tree species [7,8] and occurs in water vapor, liquid water, or organic solvents. Severe changes in its microclimate, compared to the considered equilibrium humidity parameters of RH 55 ± 5% and a temperature of 22 ± 2 °C, can cause swelling or the dimensional contraction of a panel, producing internal cracks, creating swelling forces in the wood, and initiating splits and fractures in the support or between the support and the painting’s layers [9]. Dimensional changes are different in the three sectioning directions (anisotropy), the most significant being produced in the tangential direction [10,11], with values of between 6 and 12% in the tangential direction, 3–6% in the radial direction, and 0.1–0.3% longitudinally [12,13,14], all these changes occurring in the range of the FSP (fiber saturation point). Rowell (2005) [15] states that for wood to swell, “water or another swelling agent must penetrate the cell wall”, and that moisture does not “concentrate in one place, but spreads throughout the cell wall structure”. In a fiber saturation point (FPS) ranging up to 30%, where water localization in wood is initiated at the cellular level [13], volumetric swelling or contraction occurs, which is also known as “wood movement” [16]. The importance of these changes—as seen through the lens of degradation—can be understood as a bonding problem or the result of deformations and internal stresses [17]. Once the wood swells, internal stresses are produced, called swelling pressures [18,19], which cause deformations. In this context, the moisture content in the wood cells determines a torsional behavior [12]. The time taken to reach the maximum swelling pressure is shorter for thin wooden elements [20].
In addition to dimensional instability, high microclimate conditions favor the development of bio-pests. When wood is wet, it is susceptible to biodeterioration by fungi that decompose it [21], or by xylophagous insects, either independently or symbiotically. The moisture content (MC) of wood affects its performance due to the moisture’s influence on its physical and mechanical properties [22,23]. The mechanical properties, the dimensional changes, the tendency to develop a certain drying defect, and the necessary conditions for the installation and development of biological deterioration are all influenced by wood’s moisture content [24].
During restoration interventions on art objects, it is crucial to consider the behavior of their materials, as they can influence the support and the painting layers. An improvement in the performance of wood should be achieved without additional non-beneficial effects [25]. Solvents are frequently used, either for dissolving polymers, for strengthening purposes, or for antiseptic treatments. The controlled impregnation of wood with monomers can improve its resistance to damage [26]. Evaluation of the impregnation degree [27] must be a ratio between the maximum level of treatment and its efficiency and can be evaluated by weight gain (WPG) [26] and hardness determinations [27]. The consolidation treatments applied to art objects are closely related to the degree of damage: the support can be degraded and exhibit a slightly reduced surface and volume, but situations can frequently be encountered when the surface and volume, in particular, are severely damaged, resulting in significant losses and a reduction in wood density of between 20% and 50% compared to the healthy constituent/reference wood. Such situations will require multiple and complex treatments [28].
Solvents can temporarily swell the wood, which returns to its original dimensions as the solvent evaporates [29]. Due to the hygroscopic nature of wood, it is susceptible to swelling when in contact with liquids [30], depending on the type of liquid being absorbed. Polar liquids with high binding capacity can cause greater swelling, while non-polar or weakly polar organic solvents lead to lesser swelling [31]. The choice of solvents and the composition of the wood strongly influence its swelling. For example, toluene causes minimal swelling, while acetone can lead to swelling that is 3–4.7 times greater than that with toluene [32,33].
The swelling process of wood is complex and is affected by the moisture content and pre-treatment of the wood. Swelling in ethanol–water mixtures can significantly affect the wood structure by partially dissolving lignin [34]. In addition, solvents of low polarity can effectively reduce the stresses in stressed wood, as indicated by the longitudinal shrinkage pattern [31].
A non-exhaustive analysis of the specialized literature shows that most studies focus on new and healthy wood, which does not support polychromy or artistic painting.
These studies examine the sensitivity of wood to the absorption of liquids, such as water [15,32,35,36] or organic solvents [15,33], and focus on wood swelling as a result of these processes.
Different from the specialized literature, our study, and its novelty, refer not only to the dimensional changes produced by swelling but especially to deformations: curvatures and torsions that appear during partial immersion when the wood is in contact with selected solvents. Our study also brings to the forefront and evaluates the behavior of historical wood that has been severely damaged by wood-boring insects, as is found in cultural heritage objects, where a polychrome layer, composed of protein-primer-pigment glue, is present on one of the surfaces (the tangential surface).
Unlike other research, where the samples are conditioned in the oven before the experiments are carried out, in the present case, the samples have a special character where the experiments simulate the real treatments conducted during restoration. Art objects cannot be placed into ovens and conditioned according to the requisite standards, so the dimensional changes can exhibit different and increased variations. In this regard, Mantanis (1995) argues that the presence of water in many organic solvents significantly influences the swelling of wood.
The purpose of the research is to observe the effect of solvents used in conservation and restoration treatments (treatment with liquid solutions—solvents for consolidation or treatment with biocides) on the historical wood (Tilia cordata) found in cultural heritage assets that have been severely and progressively damaged by xylophagous insects. All this is necessary to extend and save the life of heritage objects.
2. Materials and Methods
For this study, lime wood (Tilia cordata), which had been damaged by xylophagous insects and recovered from historical ensembles, and which required replacement due to static considerations, was used. In choosing the wooden material, it was taken into account that polychromy, without aesthetic importance, is present on one of the faces, composed of primer (gesso), a film of a unitary red shade of paint, and a protein glue binder.
The recovered wood was previously thermally and hygroscopically balanced in a controlled environment for 6 months at 20 ÷ 25 °C and RH of 55 ÷ 60%.
Initially, the linden planks had varied sizes and humidity of 8.4 ÷ 9.6%. The historical wood was not processed beforehand with a machine and it was not necessary to prepare it in the same way as a new standardized wood plank.
It was decided to cut and form 15 equal samples, with dimensions of L = 90 × l = 130 × h = 25 ÷ 27 mm; 9 of these were sectioned manually in the longitudinal direction, thus obtaining 18 samples with dimensions of L = 90 × l = 130 × h = 12 mm (±2 mm)—of which 9 samples had a surface with polychromy (P) and 9 samples were without polychromy (FP), respectively, on the back of the panel. The remaining 6 samples retained a thickness of 27 mm.
In the dimensional formation of the samples, it was decided to keep a ratio of 1:5 in relation to the painted panels, which was taken as a reference, and a minimum number of 10–12 annual rings was taken into account for the samples with a thickness of 27 mm. For the sections of 10–12 mm in thickness, there should be a minimum of 5–7 rings (Figure 2).

Figure 2.
Identification of annual rings in the two types of specimens.
Three common liquids were selected for the art conservation treatments: water, acetone, and white spirit D40. These substances are generally used for the transfer of active biocide substances against bio-pests, such as fungi and xylophagous insects, to the wooden support. They are also used to solubilize reinforcing resins.
It was necessary to observe the changes not only on samples with a thickness of 27 mm but also on those with a thickness of 10–12 mm.
The 6 samples with a thickness of 27 mm were immersed, in groups of 2, in each type of selected solvent. The sectioned specimens (12 mm) were also immersed in liquids, with 6 for each solvent, respectively, employing 3 specimens with polychromy and 3 without polychromy.
Partial immersion in the solvents was employed to the level of half the thickness of the samples (Figure 3). This is because, in current practice, art objects with polychromy cannot be totally immersed in solvents or treatment liquids.

Figure 3.
Representation of the technique for immersing the samples.
The samples with a thickness of 12 mm were placed two-by-two in the containers, taking into account the fact that absorption was carried out from the sectioned (cut) face. In the case of the 27-mm-thick specimens, they were placed with the polychrome face outward.
For the samples with a thickness of 27 mm, a quantity of 300 mL of solvent per container was used, and for the samples with a thickness of 12 mm, 150 mL of solvent was used per container. In order to be able to determine the absorption of the solvent, both the mass and the volume of liquid used/set of samples were taken into account Table 1.

Table 1.
Solvents used for the experiment and their parameters.
The damaged volume of the samples was calculated, and a quantity of solvent was taken into account, which, in terms of volume, should not exceed approx. 10% of the total volume of the immersed sample. The immersion time was also taken into account and limited to 15 min—dictated by simulating a treatment stage and in agreement with Rowell (2005), who mentions that swelling does not occur constantly and uniformly; the most important swelling occurs in the first 15 min after immersion, namely, 5.3%; at 30 min, the swelling ratio is 7%, while at 60 min, it is 8.1%.
Apparatus. The preparation and cutting of the samples were performed with a Sheppach 250 electric circular saw, and the sectioning in half, along the longitudinal direction, was performed with a manual saw.
Wood moisture was measured with a digital hygrometer with pins—a digital moisture meter with ±4% deviation and 0.1 accuracy. To measure the mass, the analytical scale Kern EHA 500-1 was used, with a precision of 0.01 g, and the dimensions were measured using the digital caliper.
The variations in deformation measurements were determined with an HBM Machines dial gauge, and the depth of curvature was measured with the Parkside PTM 2 A1 digital apparatus. The devices can be seen in Figure 4.

Figure 4.
The measuring devices used to determine deformation.
To determine the density, which can be defined as the ratio between mass and volume, the ISO 3131-1975 [37] standard was used for the samples with moisture content at the time of testing. Also based on the same standard, the conventional humidity was determined as a ratio between the mass in the dry state and the volume of the sample with a high moisture content—after extraction from (partial) immersion. Thus, Equations (1a) and (1b) were used:
where
- ρw = moisture density at the time of testing;
- mw = mass of the sample at the set humidity at the time of testing, in g;
- vw = volume of the sample at the set humidity at the time of testing, in cm3.
Here,
- ρy = conventional density;
- mi = mass of the dry/conditioned specimen, initially, in g;
- vu = the volume of the sample at the set humidity, at the moment of extraction from the immersion in cm3.
In order to determine the moisture content, the principle found in the EN 13183-1/2002 [38] standard was used; however, due to the fact that historical wooden elements recovered from cultural assets cannot be fully subjected to the rigors of the standards, the calculation formulas and application principles for the field of cultural property restoration were adopted. Since the historical wood pieces cannot be brought to 0% humidity, especially in real conditions when there is polychromy, it was decided to condition the recovered samples in a controlled environment. The recorded humidity had values of between 8.4 and 9.6%—considered the initial humidity (in relation to the standard, u0). Thus, to obtain the moisture content after partial immersion, Formula (2) will be used:
where
- MC = moisture content, as a percentage;
- mu = wet mass of the sample extracted from immersion, in g;
- mi = initial mass, in g.
Linear swelling was calculated according to the ISO 4859-1982 [39] standard, using Formula (3):
where
- αTG = swelling on the tangential section (TG), expressed as a percentage;
- lmax = size of the wet samples when extracted from immersion solvents, in mm;
- lmin = is the size of the dry samples, before immersion, in mm.
The amount of absorbed solvent (Abs.), measured in grams, was calculated with Formula (4a), while Formula (4b) was used to determine the absorbed volume:
where
- MABS = amount absorbed, expressed in g;
- mu = wet mass, after extraction from the solvent, in g;
- mi = initial mass, before treatment, in g.
Here,
- VABS = absorbed volume, expressed in cm3;
- MABS = amount absorbed, expressed in g;
- ρsolvent = solvent density, expressed in g.
Dimensional variation was determined with Formula (5). The readings were obtained with the dial gauge and the digital device. Measurement points were taken on the surface of the specimen, at the same points, before and after immersion.
Here,
- Δ = variation, in mm;
- lu = value of the measured point on the measuring axis after immersion, in mm;
- li = value of the measured point on the initial measurement axis (before immersion), in mm.
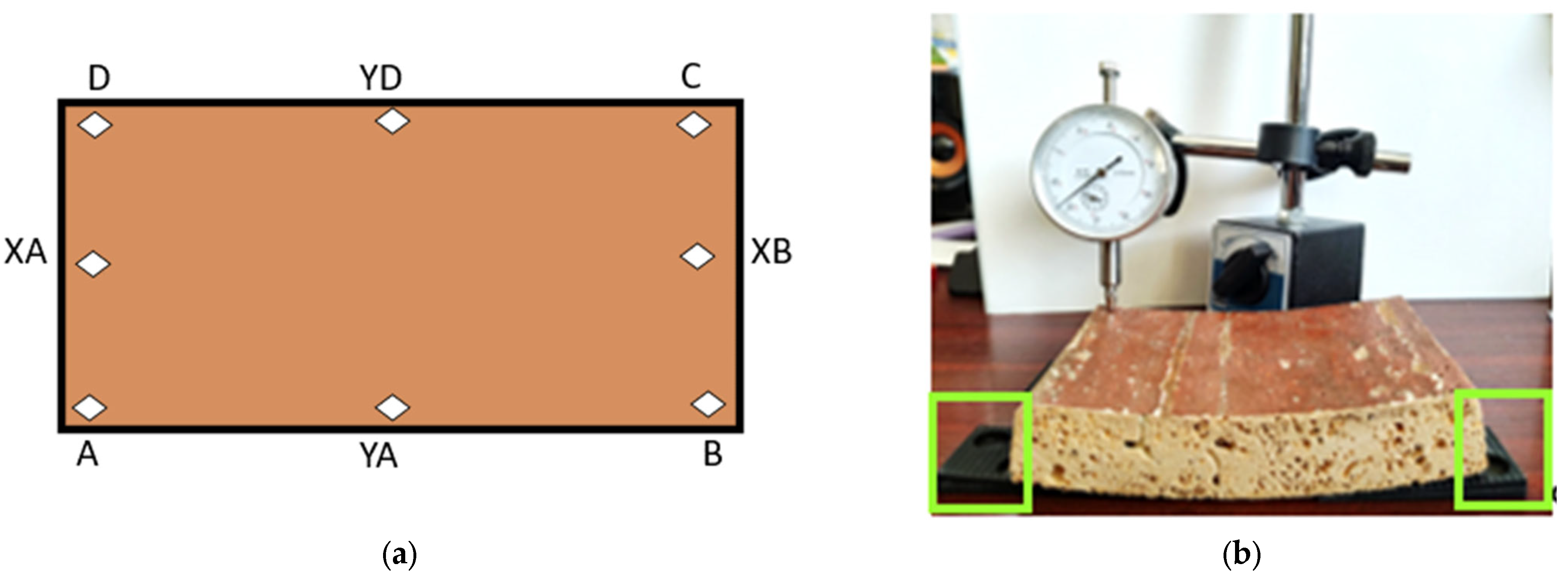
Conventional measurement points on the horizontal axes, D-YD-C, A-YA-B, and XA-XB, were considered, in accordance with Figure 5a. To counteract and balance both the initial deformations and those resulting from extraction from the liquid, compensation wedges with a length of 60 mm and an inclination of from 1 mm to 70 mm were used, as shown in Figure 5b.
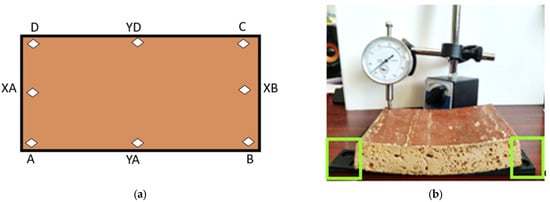
Figure 5.
(a) Marking the measurement points on the samples and the coordinates on the axes; (b) measuring the deflection with the dial gauge, highlighting the compensation wedges.
3. Results
3.1. Fluid Absorption and Swelling Dimensional Changes
The absorption of moisture implicitly leads to the volume increase of the wood.
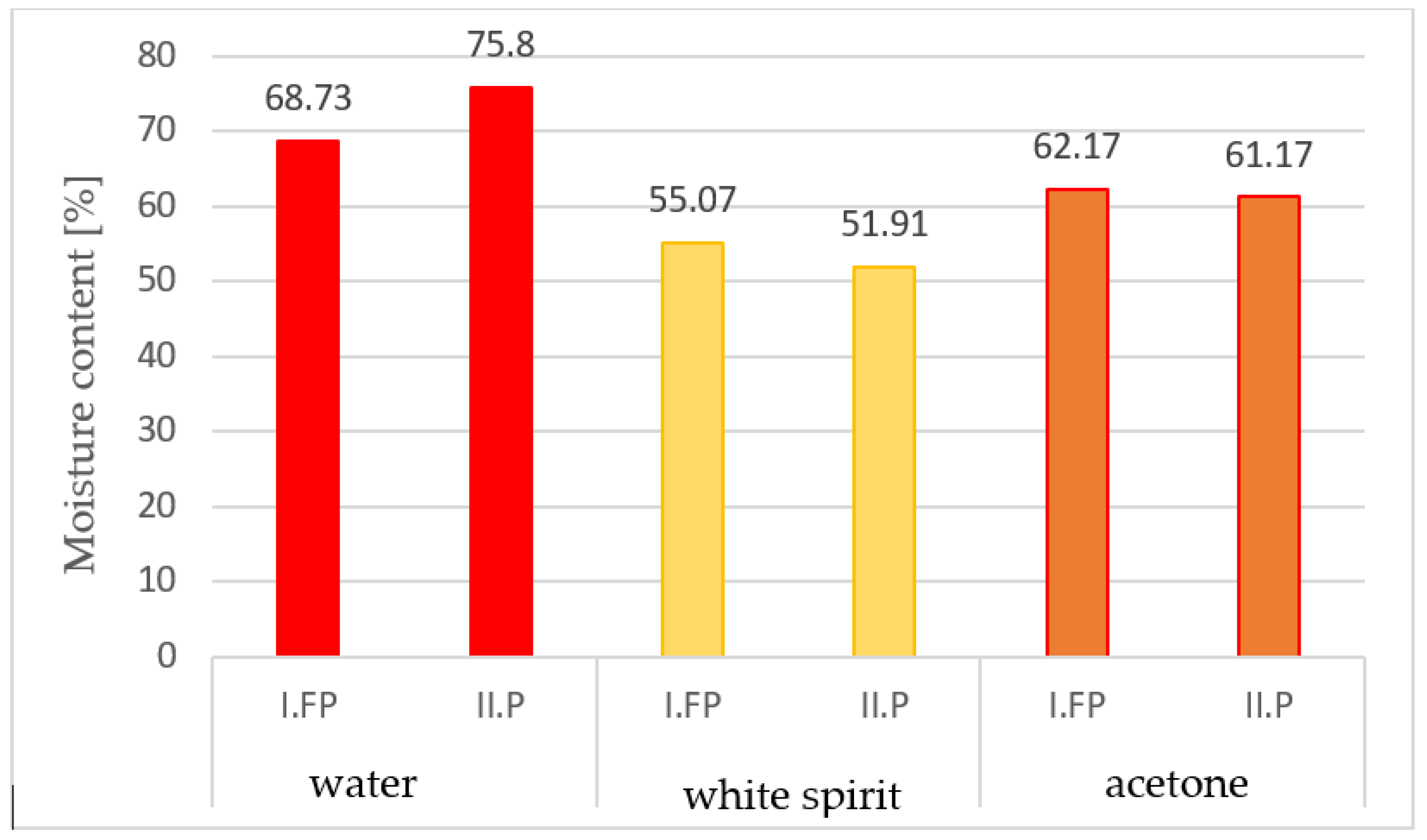
The absorption of the amount of solvent was determined, both in grams and in cm3, and it was found that the highest absorbed volume was of acetone, followed by water, and then by white spirit (according to Table 2). It is found that the absorption in grams is higher in the case of water, compared to acetone and white spirit, and the humidity of the samples extracted from water had the highest values (Figure 6).

Table 2.
Means obtained from the determinations for the 12-millimeter-thick sectioned samples.
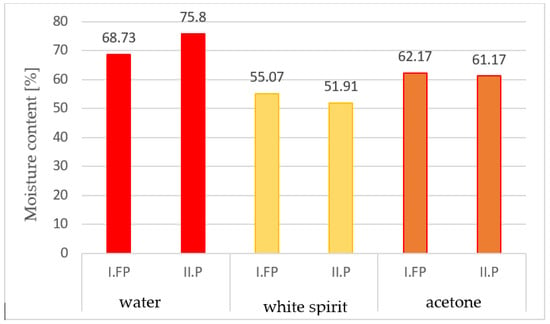
Figure 6.
The moisture contents of the samples as a percentage, after immersion in solvents for 15 min.
The moisture content, obtained mathematically, can be attributed to the fact that the samples experienced an intense xylophagous attack that caused galleries in the wood. The wooden substance was lost and was replaced by the liquid absorbed by the wood.
The results regarding the determinations of mass, volume, solvent absorption, and density, both before and after immersion of the thin specimens, can be seen in Table 2.
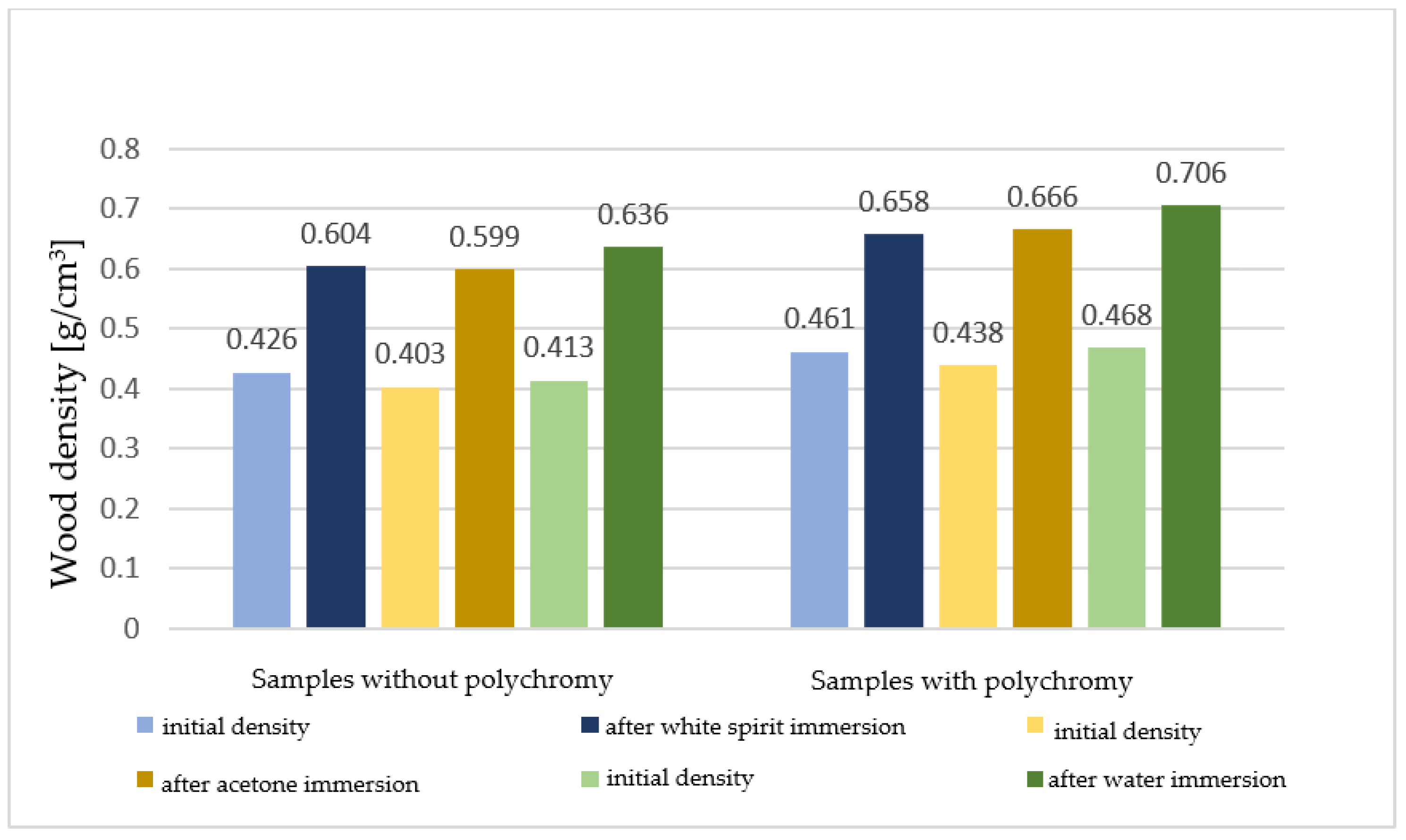
The densities reached by the samples after immersion can be seen in Figure 7.
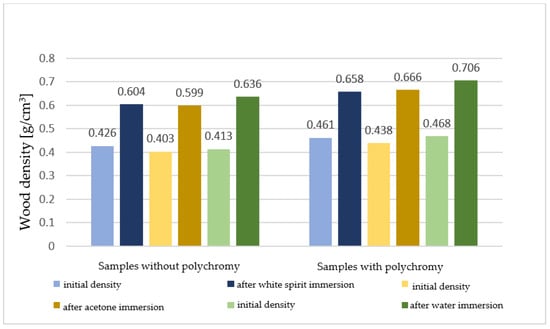
Figure 7.
Densities (g/cm3) of the sectioned samples before treatment and after extraction from the solvents.
The same methodology was applied to the samples with a thickness of 27 mm, and the results thus obtained can be seen in Table 3. Observing the results obtained in Table 3 and comparing them with those in Table 2, it can be seen that the behavior of the wood is different depending on its thickness, the type of liquid, and the length of time during which the liquid acts on the wood.

Table 3.
Means obtained from the determinations for the samples with a thickness of 27 mm.
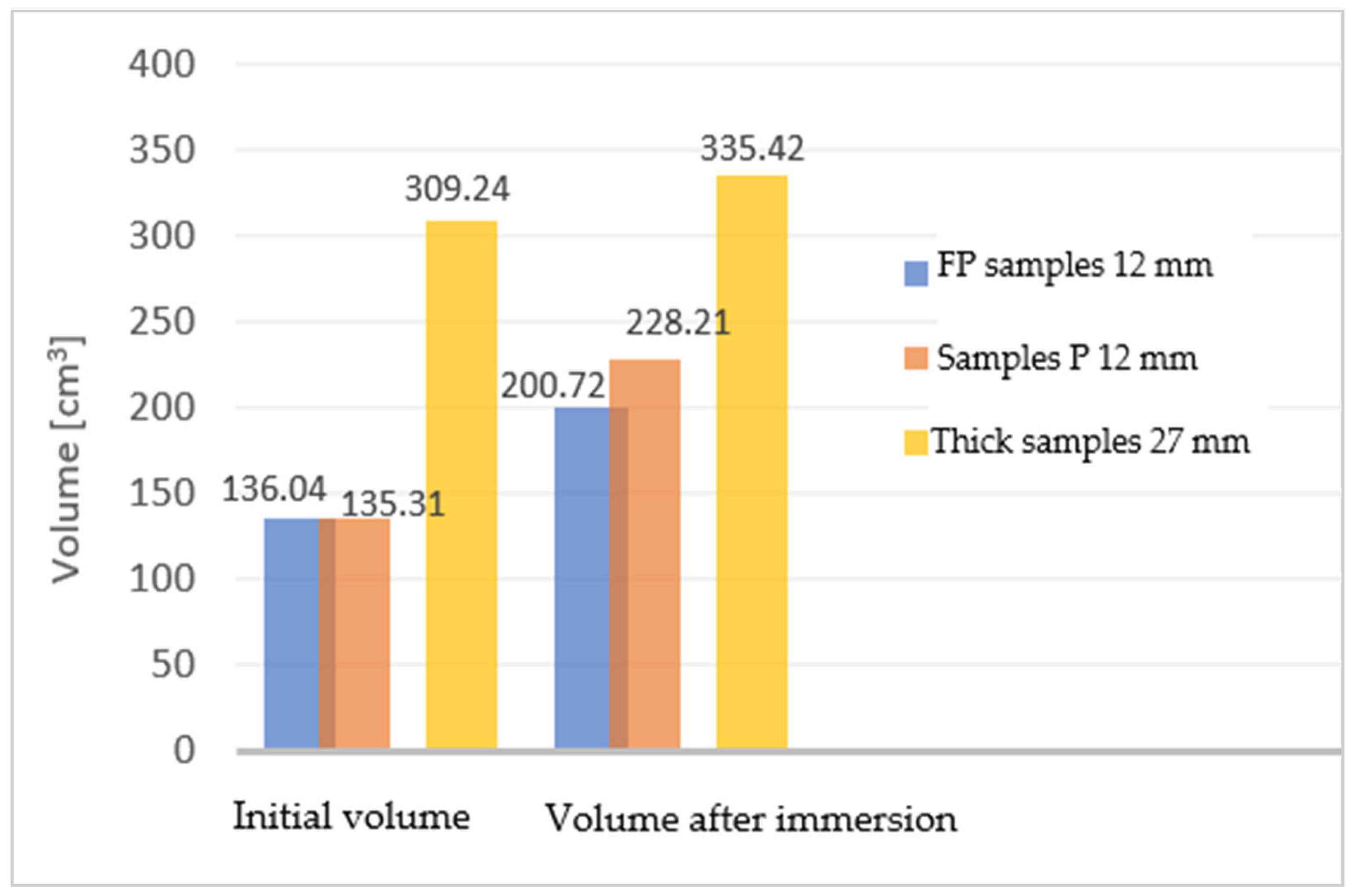
Comparing the two types of samples with different thicknesses (27 mm and 12 mm), the difference in volume growth can be seen in Figure 8.
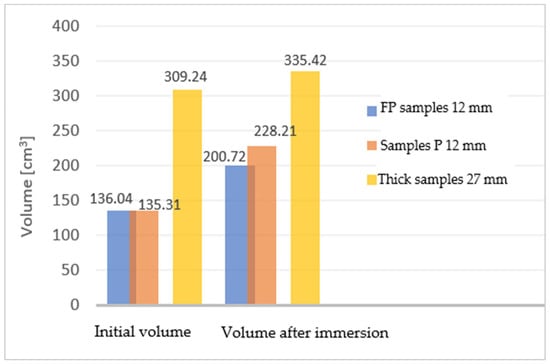
Figure 8.
The difference in volume growth for the two types of samples.
For this experiment, we wanted to report the changes regarding swelling in the tangential direction because this section produces the greatest change, according to the specialized literature [12,13]. These changes are noted in Table 4 and Table 5. Moreover, the research refers to and applies to the specific case of painted panels, representing art objects that require impregnation treatments, and these panels were usually developed on the tangential section.

Table 4.
Dimensional changes in tangential average values with swelling.

Table 5.
Tangential swellings in the case of specimens with a thickness of 27 mm.
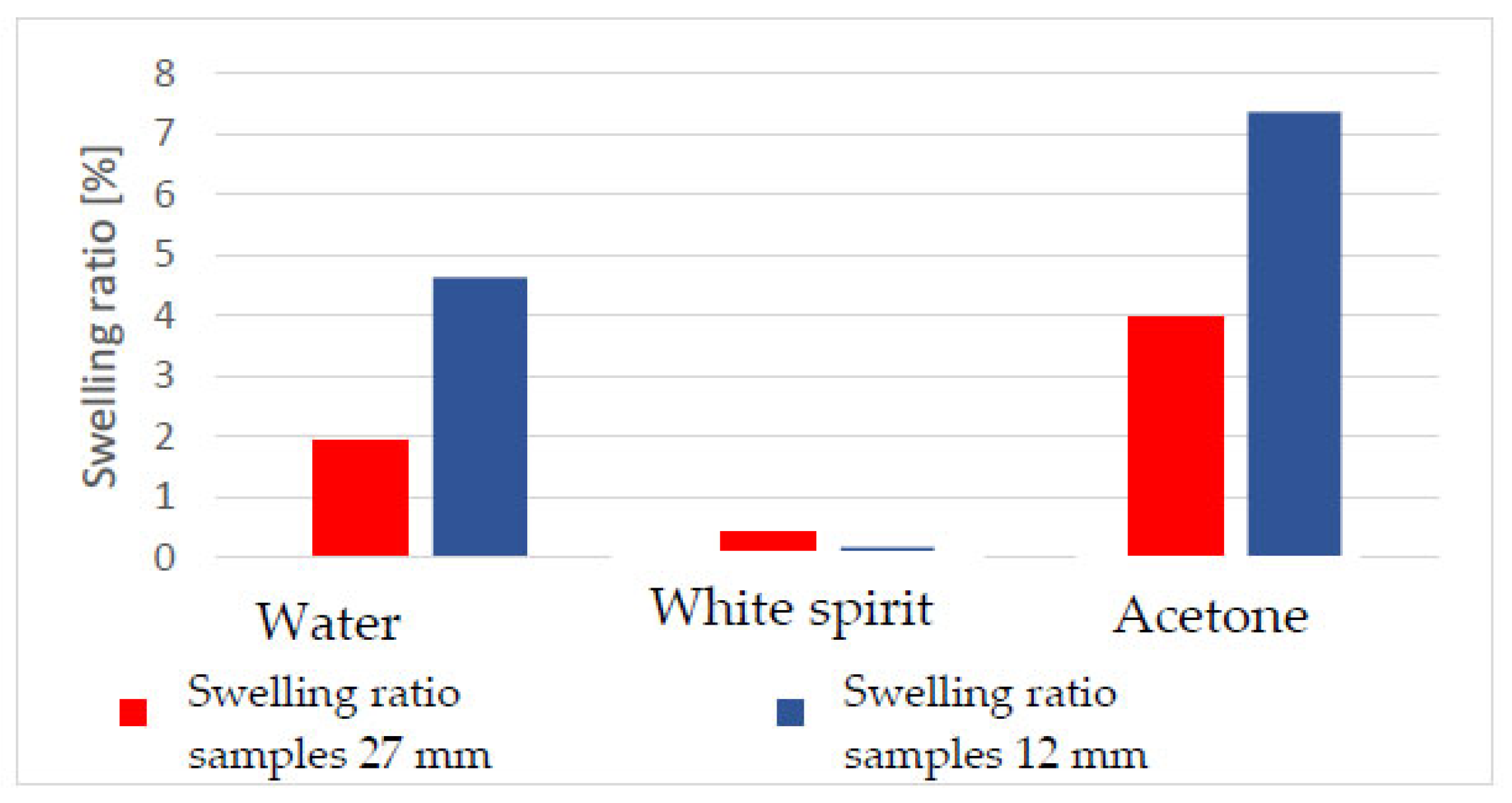
Acetone was observed to cause the most significant swelling of damaged wood.
For example, in the case of samples without polychromy, the swelling was 65.61% greater in the case of immersion in acetone than in water.
Regarding the polychromy samples, the swelling variation was 52.31% greater in the case of immersion in acetone compared to water.
Comparing the white spirit solvent with acetone, it was observed that the swelling in acetone was 2127% higher for the samples without polychromy and 20,340% higher for the polychromy samples.
The swelling ratio in the tangential direction was also determined for the thicker specimens. It was found that in these cases, acetone also produced the greatest swelling, but this was smaller compared to the sectioned pieces (12 mm thick).
It can be concluded that, depending on the thickness of the wood and the time it remains under the action of the solvent, the swelling ratio is higher or lower.
3.2. Dimensional Changes—Wood Deformation Produced by Solvents
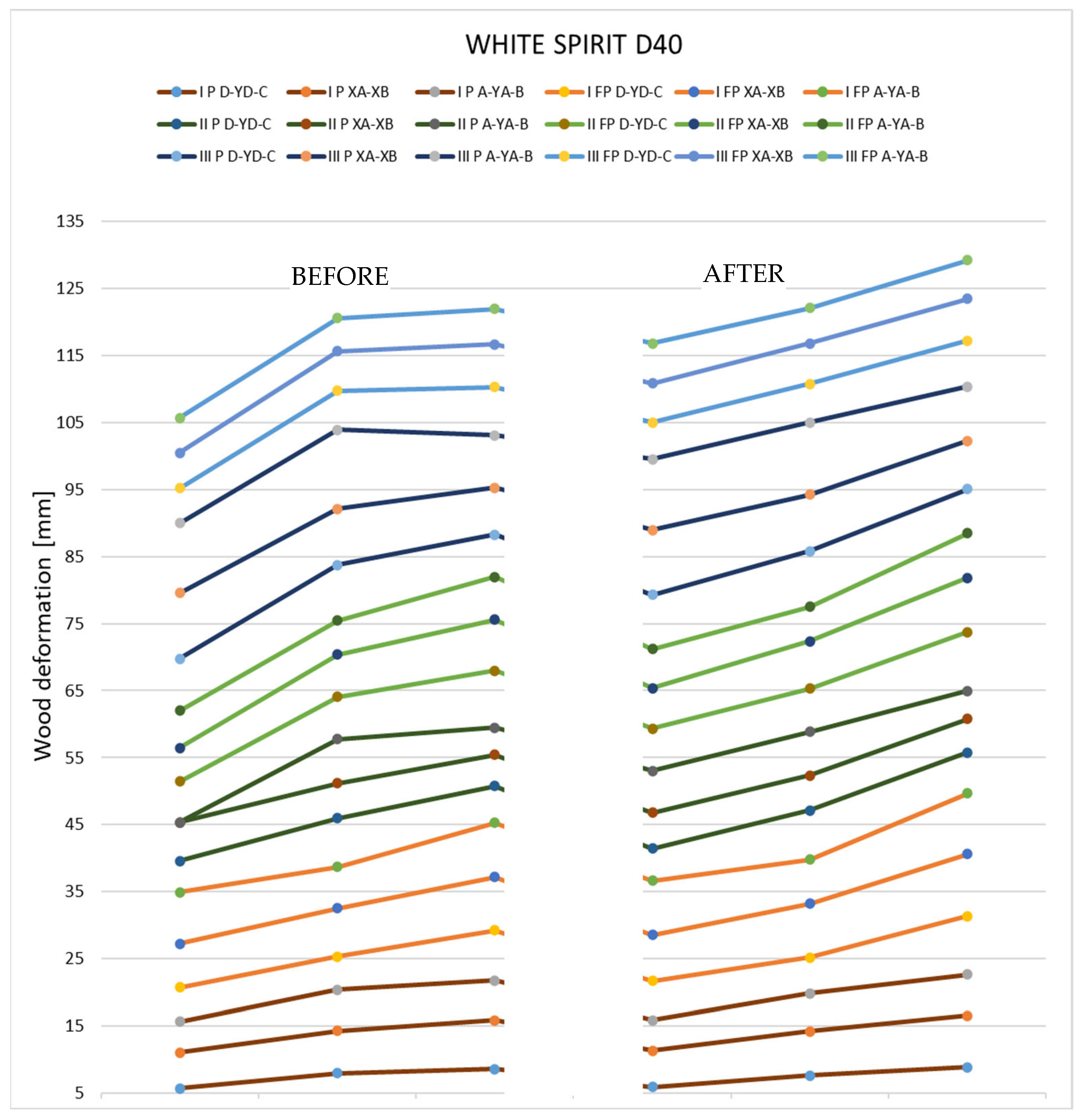
As a result of the experiment, it is evident that there are changes in the flatness of the samples. The deformation could be observed visually and was also determined using a dial gauge and digital depth gauge measurements. The values obtained for the three solvents used in the experiment, for both thin and thick samples, can be seen in Table 6, Table 7 and Table 8.

Table 6.
Deformations produced in the samples that were partially immersed in water (in mm).

Table 7.
Deformations produced in the samples that were partially immersed in white spirit.

Table 8.
Deformations produced in the samples that were partially immersed in acetone.
3.2.1. Results for Water Samples
The water-immersed specimens, both the thin (12 mm) and the thick (27 mm) samples, curved either concavely or convexly. Where the values are negative as a result of the immersion, a change is produced that is in a direction lower than the initial value, resulting in deformation in the form of either bends or twists.
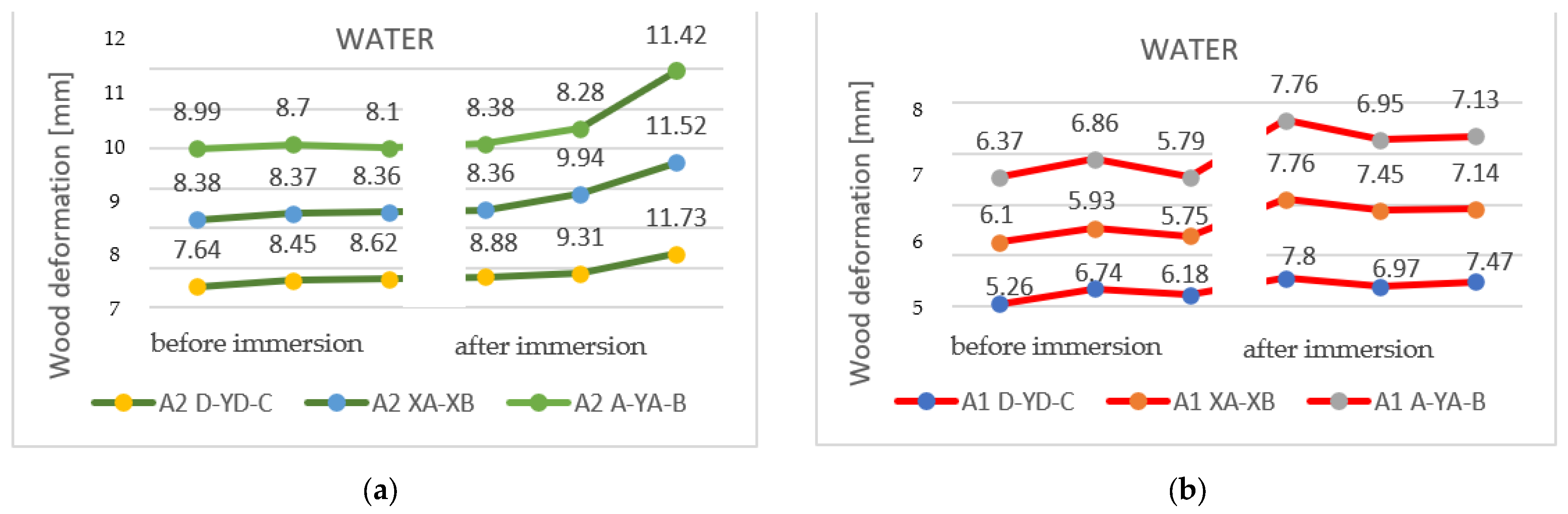
Figure 9 identifies the changes that occurred after immersion in water in the samples with a section of 27 mm, as shown on the three horizontal axes. Pronounced deformation can be observed, compared to the original shape of the specimen.
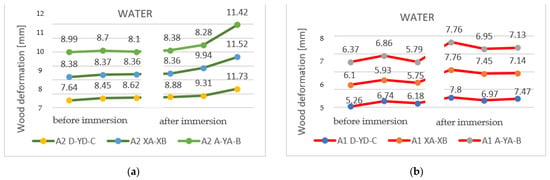
Figure 9.
The deformations on the horizontal axis, shown on the three measuring lines, for the specimens with a thickness of 27 mm: (a) sample A2 (2P); (b) sample A1 (1P).
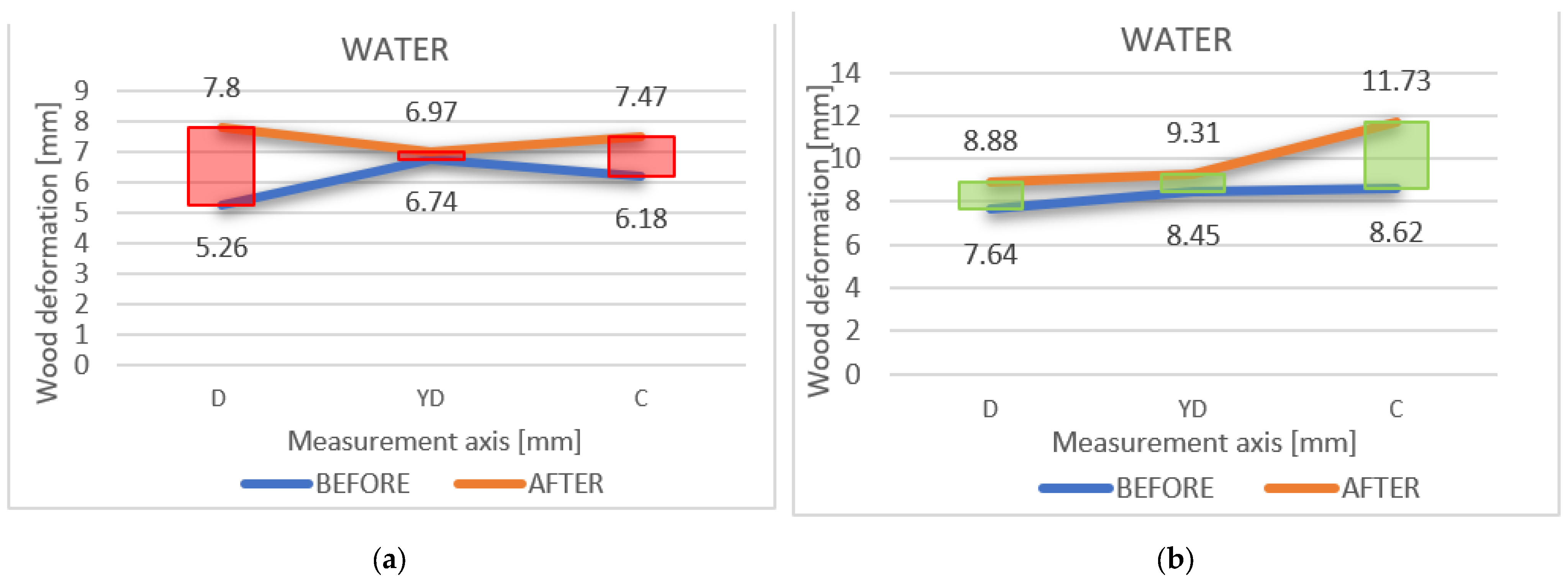
The different deformation variations between the points were measured initially and after, as shown on the axis D-DY-C; D1-YD1-C1 is also highlighted in Figure 10a,b.
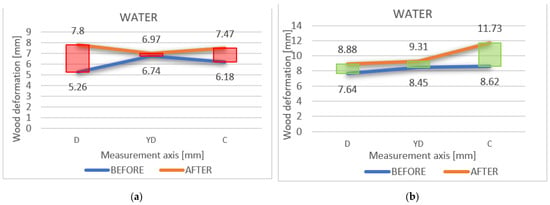
Figure 10.
(a,b) The different variations in deformation between the points, measured initially and after immersion, on the axis D-DY-C and D1-YD1-C1, respectively.
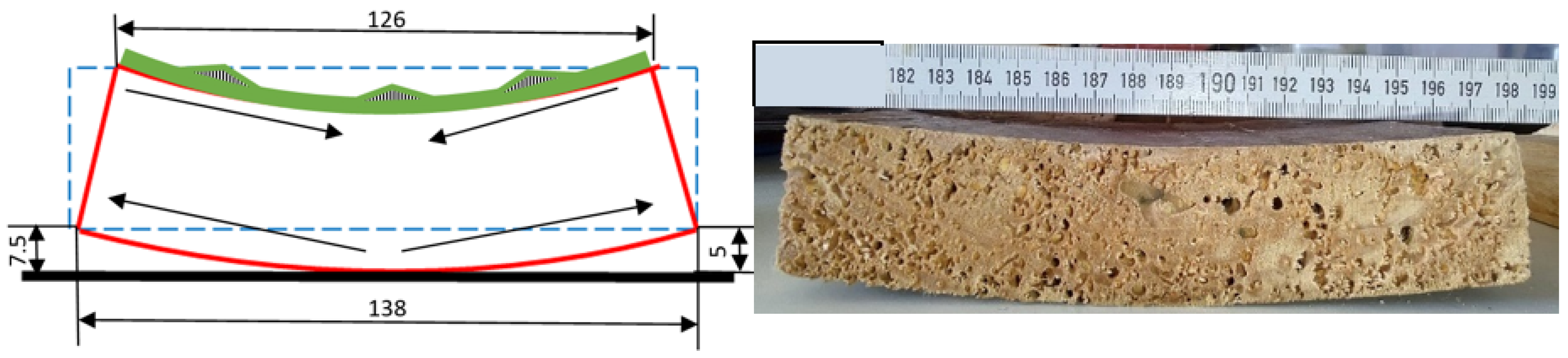
The thin samples changed after being extracted from the water and strong curvatures were found, with a curvature radius of between 4 and 10 mm (Figure 11 and Figure 12a) In their case, unlike with the thick samples, torsions (Figure 12b) were present with values of between 4 and 7 mm.

Figure 11.
Strong curvatures are visible after extracting the sectioned samples from the water.

Figure 12.
(a) Curvature of 10 mm after extraction from water; (b) torsion after extraction from water.
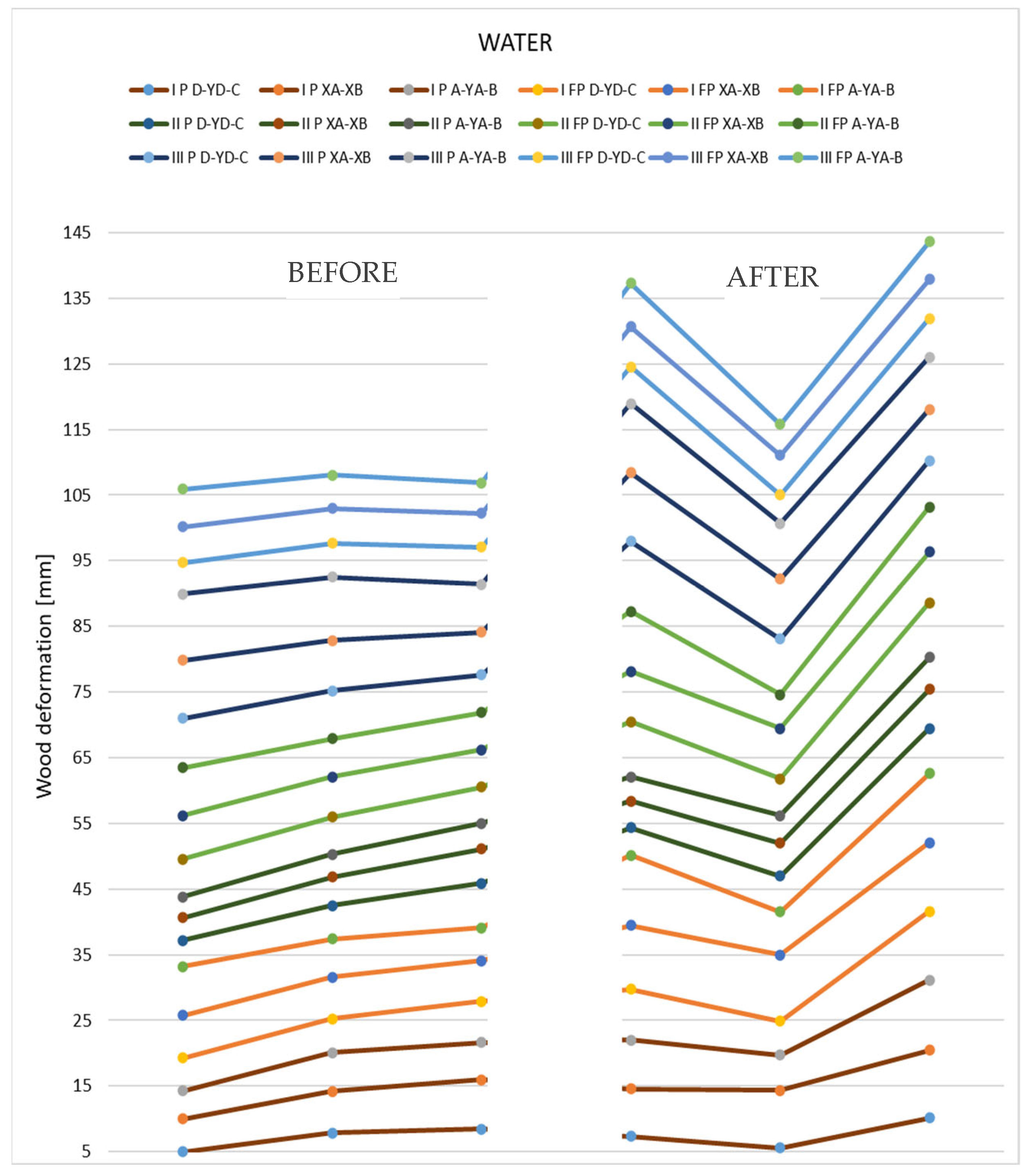
Figure 13 shows the changes that occurred after immersion in water for the tested thin samples.
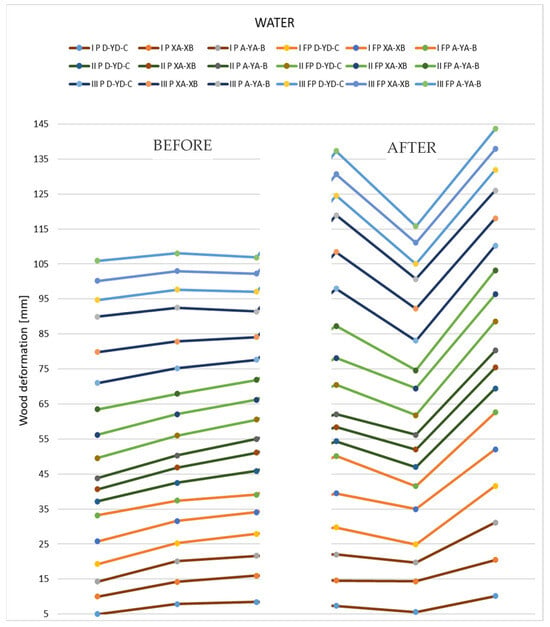
Figure 13.
Representation of the deformation values obtained from measurements of thin 12 mm specimens.
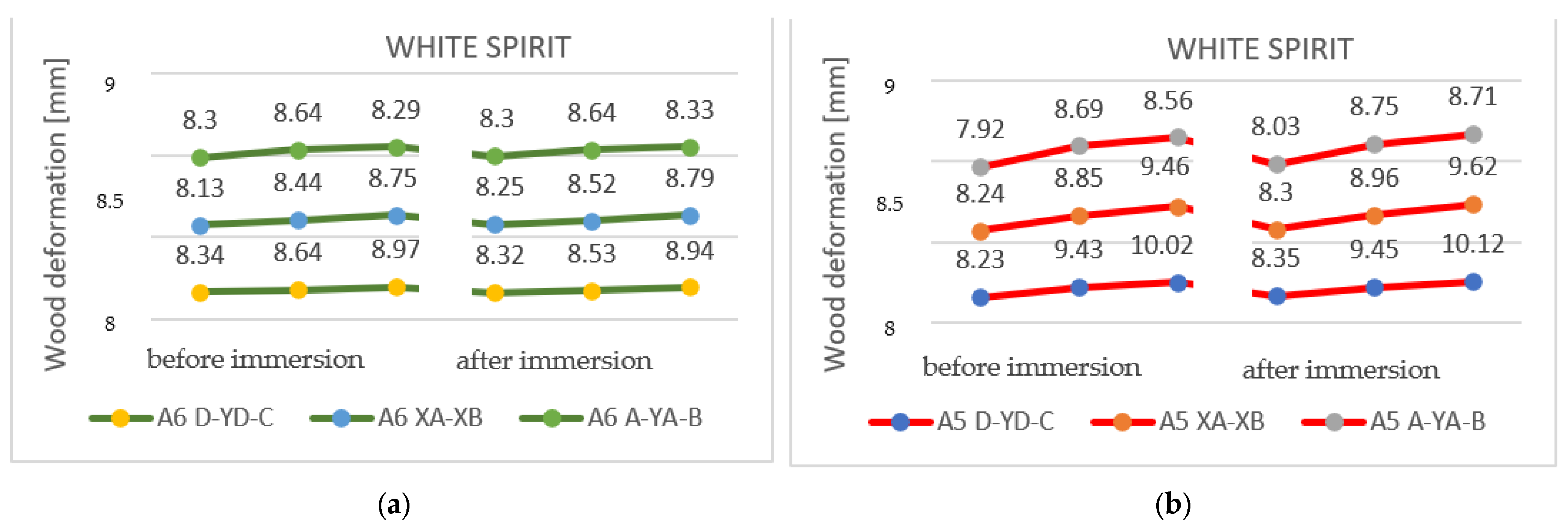
3.2.2. Results Obtained with White Spirit
In the case of samples immersed in white spirit, it is evident that the wood absorbed a significant amount of solvent (31.89 g and 40.88 cm3). However, it is clear that the tested samples showed fewer twists and bends compared to the other two solvents. The results obtained from the experiment can be seen in Table 7.
In the case of specimens with a thickness of 27 mm, it can be observed that the deformations identified in Figure 14 were uniform and were greatly reduced throughout the samples.
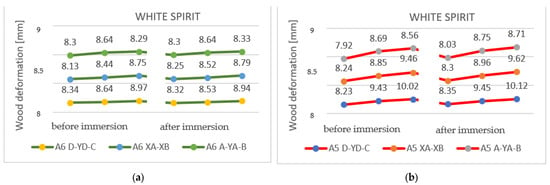
Figure 14.
Dimensional variations after immersion in white spirit: (a) sample A4 (4P); (b) sample A3 (3P).
The resulting deformations in the thin samples (12 mm) were more obvious, and, to exemplify them on the surface, they were marked on the measuring axes in Figure 15.
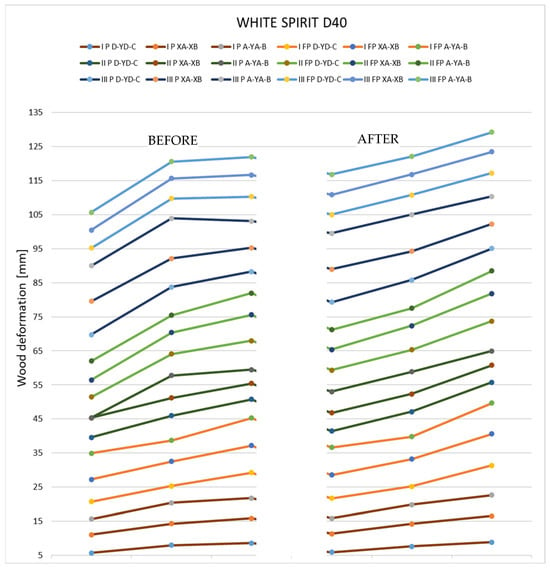
Figure 15.
Deformation after immersion in white spirit.
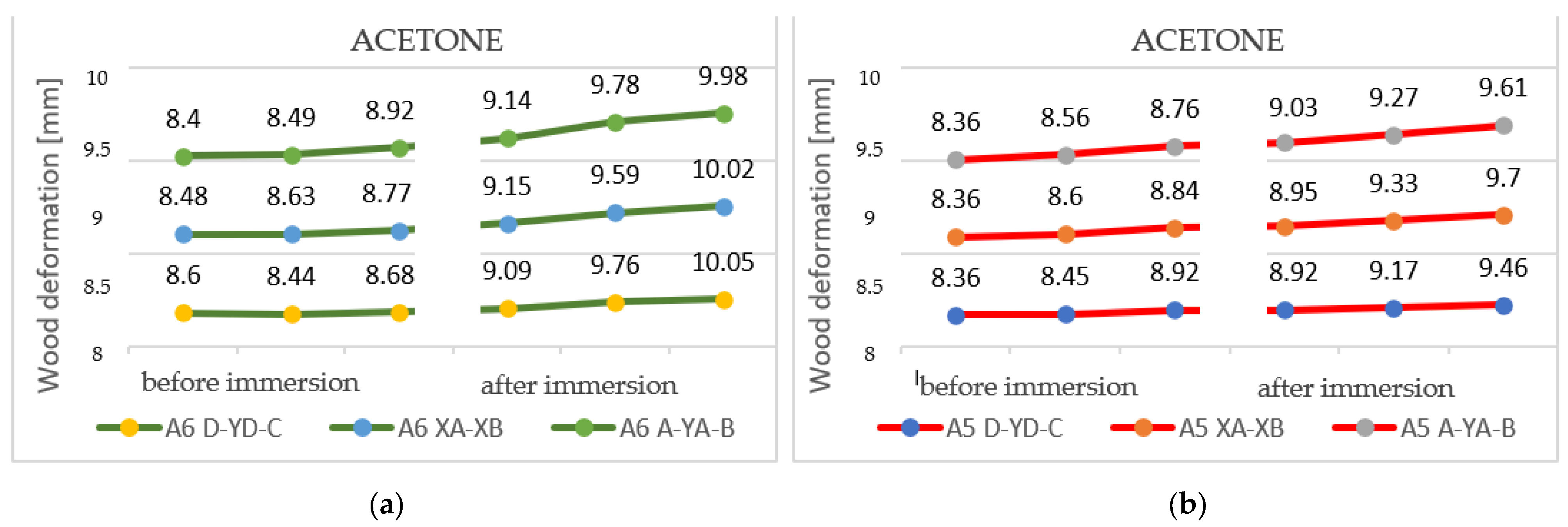
3.2.3. Results Obtained for Acetone
After immersing the 27-millimeter samples in acetone, it was evident that two types of deformations occurred: pronounced curvatures and torsions with reduced values. These can be seen in Figure 16a,b.
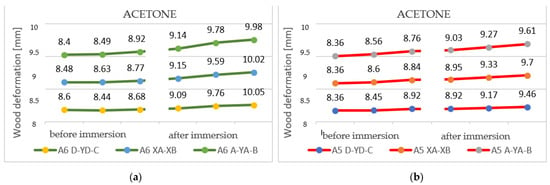
Figure 16.
Changes following the immersion in acetone of thick samples: (a) sample A6 (6P); (b) sample A5 (5P).
The 12-millimeter sectioned samples show different and non-uniform curvatures and torsions; these deformations are noticeable for both types of samples (with polychromy and without polychromy).
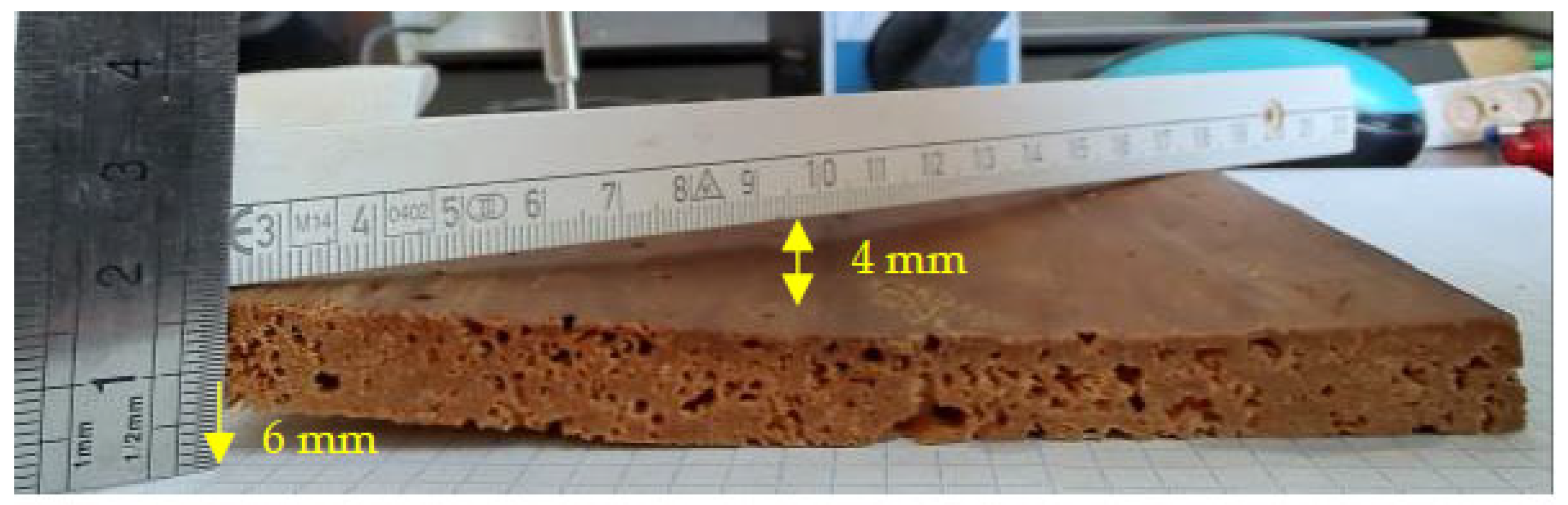
For example, for the thin sample without painting (1FP), deformations of the convex curvature type, with approximately 3–4 mm of curvature (arrow) and torsion of 6 mm are evident (Figure 17).
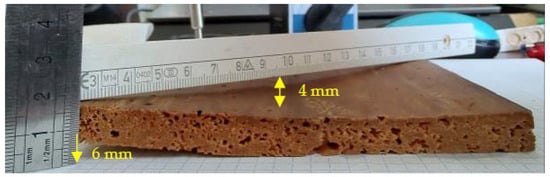
Figure 17.
Thin specimen without painting, with strong twisting.
In the case of the polychromy specimen (3P), there are torsional deformations of approximately 8–12 mm, and a curvature of 4 mm (Figure 18), while the specimen without painting, 1FP, does not show significant deformations, with only a slight torsion of about 1–1.5 mm (Figure 19).

Figure 18.
Sample 3P shows significant twists and bends after extraction from the solvent.

Figure 19.
Sample 1FP: after extraction from the acetone, a slight torsion is observed, compared to the other samples extracted from the same solvent.
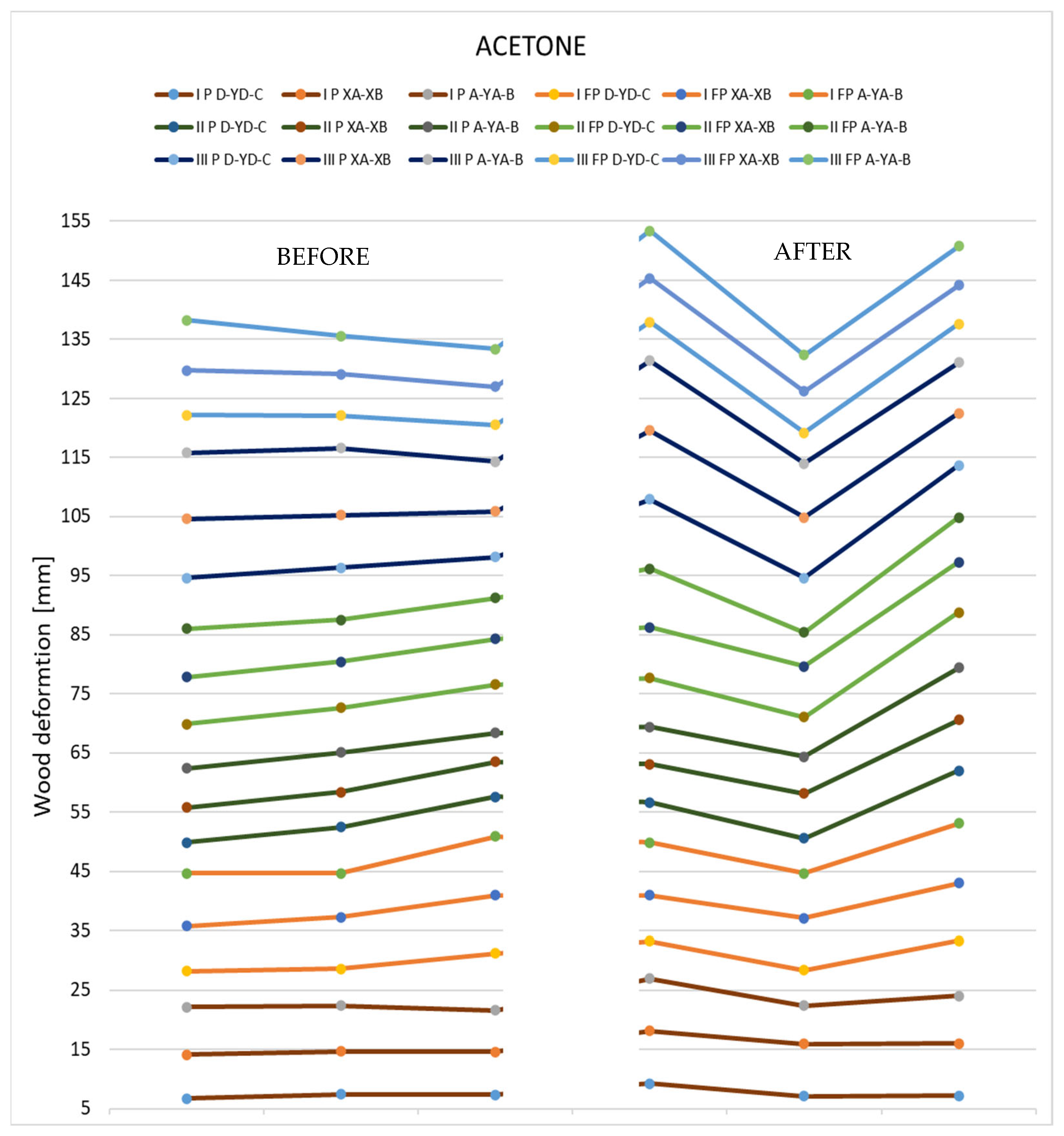
In the following graph (Figure 20), it is possible to see the deformations on the three measuring axes for the sectioned samples (three with polychromy and three without polychromy).
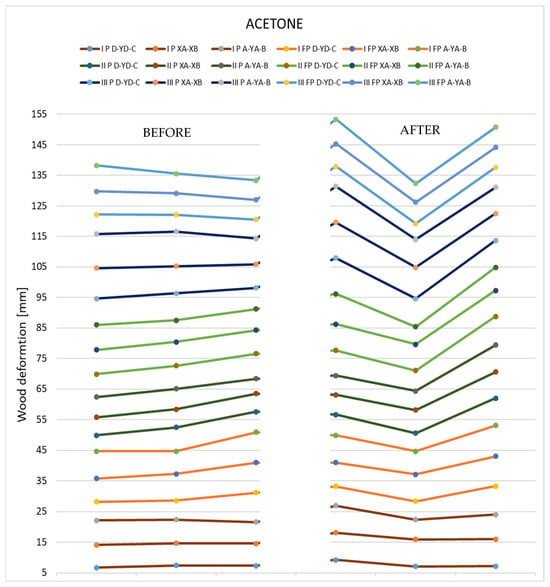
Figure 20.
Deformations produced by acetone in the thin specimens.
4. Discussion
Before applying a solvent treatment to a damaged wood object, it is necessary and important to first determine its volume, in order to be able to determine the amount of solvent to be introduced. In the case of art objects that have been severely damaged by xylophagous insects, where the damage is not uniform, the use of an excessive amount of solvent for a treatment application is difficult to control, and consequences may occur for the support but especially for the polychromy layer. The migration of the solvent to the surface with the painting, in relation to the heavily damaged wood, can happen in a very short time, thereby destabilizing it. The choice of a fast-evaporating solvent will also cause dimensional changes to the wooden support, as well as cracks and fractures. In the case of solvents with a slow evaporation rate, there is both the advantage of a deep treatment and the disadvantage of increasing the carrier mass, with the evaporation time being long.
For this experiment, the amount of solvent was limited because total immersion of the samples was not desired since polychromic art objects cannot be fully immersed. Restorative treatments, whether preventive or curative, that are applied to prevent or stop bio-pests, or for the purposes of consolidation, will generally require multiple, repeated sessions. In this context, wood undergoes dimensional changes and deformations through repeated swelling and contraction. This stress will inevitably transfer to the layers of polychromy.
In restoration laboratory practice, during strengthening treatments with synthetic resins, deformations of geometry and dimensions appeared on the panels showing severe damage caused by wood-eating insects. The treatment was performed with Paraloid B72, at 10%, in acetone. The changes observed were mainly in the geometry of the panel, when, after introducing the consolidant, the panel curves according to its constraints and the positioning of the work surface (Figure 21). The curvature radius was 7.93 mm for a width of 630 mm [28].

Figure 21.
(a) Panel flatness before treatment; (b) changes produced during treatment.
From this observation, it is clear that the panel changed not only its geometry but also its dimensions during the treatment. The present study was developed to ascertain the causes of these deformations, with a return to the original shape after the completion of the treatment and the evaporation of the solvent that solubilized the consolidant. This warping and the changes in geometry and dimension effects, although only 1% to 3%, actually cause stress to the transfer media on the paint layer, resulting in cracks, delamination, and gaps.
4.1. Fluid Absorption and Swelling Dimensional Changes
Regarding the swelling of historical wood damaged by xylophagous insects, it can be seen that depending on the level of damage [10,28], the thickness of the wood, and the time in which it is in contact with a solvent, it will dimensionally change differently (Figure 22).
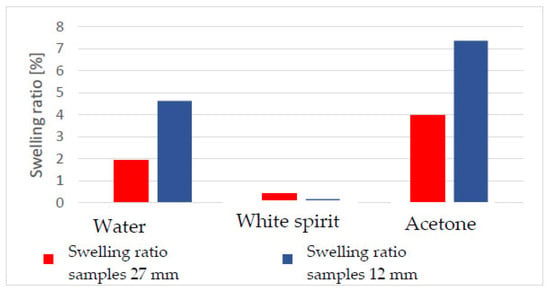
Figure 22.
The swelling ratio according to the thickness of the specimens and the immersion time (15 min for both types).
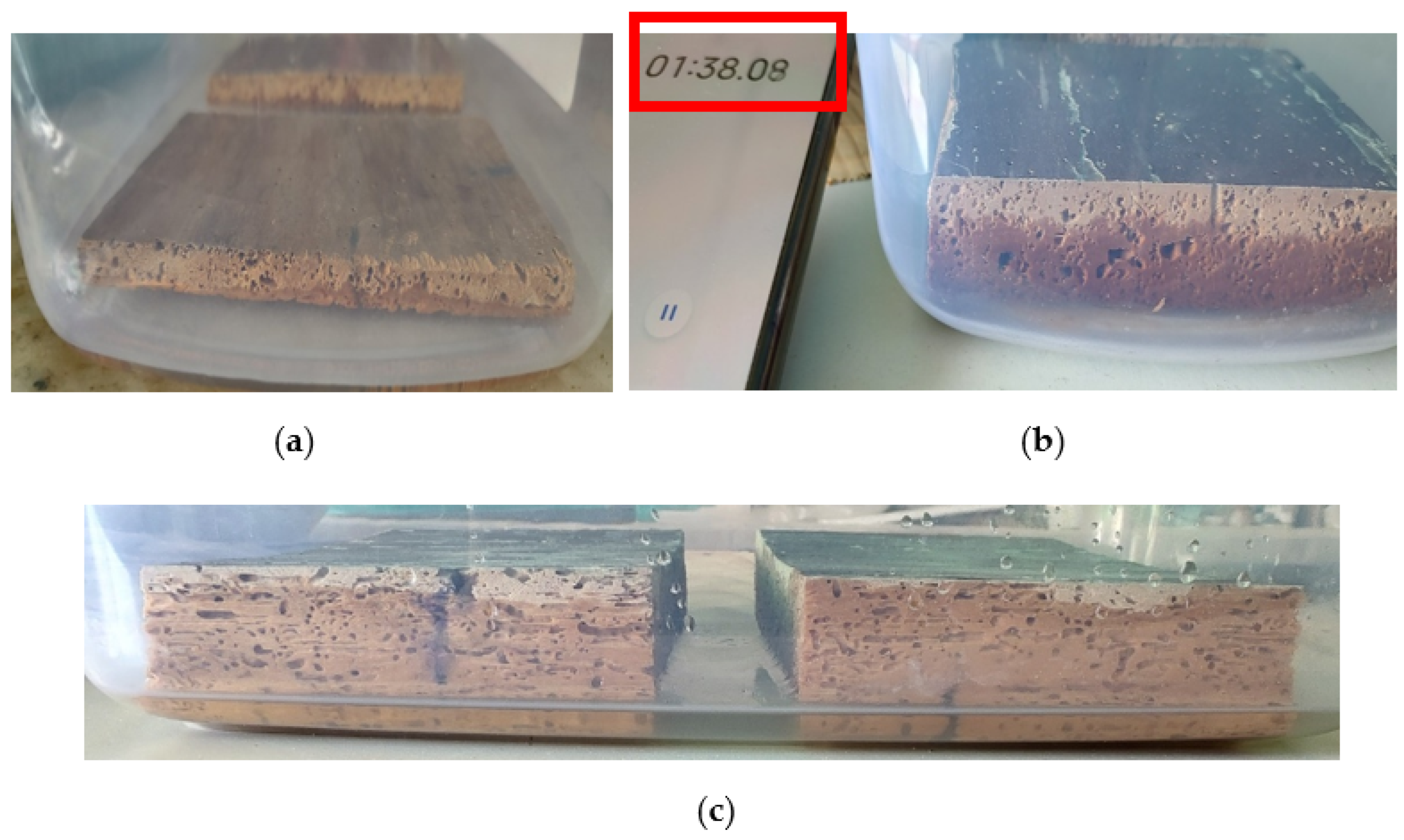
In the case of less polar solvents, the migration inside the samples was very fast, at less than 2 min for acetone and up to 5 min for white spirit, compared to water, where, due to the increased polarity, the absorption occurred more slowly; the absorption/migration time increased to 10 min (Figure 23).
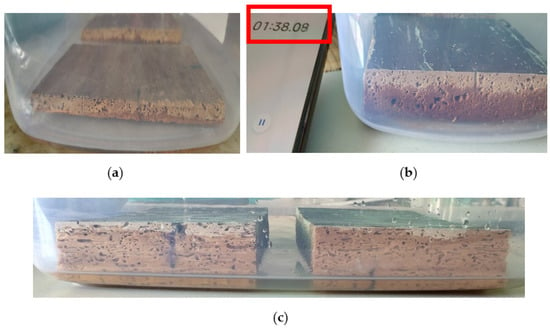
Figure 23.
Migration of the solvent into the wood after the first 2 min: (a) water; (b) white spirit; (c) acetone.
The solvents were absorbed in different amounts and volumes (g and cm3). Along with this absorption, different degrees of swelling occurred, as exemplified in Table 5 and Table 6, which show that the largest volume is represented by the swelling in acetone, at approximately 68.65%. If we refer to the initial mass of the samples and the maximum volume obtained after extraction, it can be concluded that the mass and volume after immersion do not increase equally or in the same proportions with any of the solvents used.
During the experiments observing the changes in the wood that were produced by the solvents, it was found that the greatest absorption occurred in the samples that were partially immersed in water; however, the greatest level of swelling occurred with the acetone solvent.
There are different mass increases in all three solvents. In the case of water and white spirit, evaporation is slower and remains longer in the volume of wood, while acetone, being very volatile, evaporates much faster.
During the research, it was found that in the case of acetone, once it evaporated from the wood, the initial mass of the sample decreased from 63 g to 58 g. This phenomenon can be explained by the fact that the rapid evaporation of acetone also involves water from the initial moisture content of the wood in the evaporation process, thereby reducing the density of the wood.
In the case of the white spirit solvent, after immersion, the mass of the sample increased to 212 g, resulting in a quantity of 77 g of remaining solvent in the wood. After 24 h of slow evaporation, the mass reached 182 g, including 47 g of solvent, an additional 30 g having evaporated. It was found that after 24 h, approx. 38.96% of the absorbed amount had evaporated.
Analyzing the samples with polychromy, it was found that white spirit, even if it migrates to the painted surface, does not then destabilize the polychrome layer, does not wet it, and does not produce additional cracking, as seen in the case of acetone or water.
Regarding the samples immersed in acetone, it was observed that micro-cracks appeared on the wooden surface and on the polychrome layer, where the already existing micro-cracks became more pronounced, making the layer unstable and brittle (Figure 24 and Figure 25).

Figure 24.
Cracks produced in the wood under the polychrome layer, with accentuation of the originally existing cracks.

Figure 25.
Magnified view of the cracks and swelling after immersion in acetone.
On the polychrome samples that were in contact with water, it was found that after migrating to the polychrome surface, the water caused wetting and destabilized the layer. On the reverse side, the initial deep cracks stood out, an accentuated cracking of approximately 0.8 to 1 mm occurred, and this continued throughout the thickness of the specimen (Figure 26).

Figure 26.
Cracking of wood in water: (a) wood crack before immersion; (b) crack spacing after immersion; (c) the freshly detached woody fraction can be seen.
4.2. Dimensional Changes—Wood Deformations Produced by Solvents
Deformations following immersion in water. Once the liquid is absorbed, the wood increases in volume and deforms differently, depending on the solvent.
Concave or convex curvatures, as well as twists, can be seen to occur. It is clear that the deformations are not always influenced by the annual rings, and the curvature does not occur only in the opposite direction to the annual rings.
In Figure 27, we can see an example of a situation with concave curvature toward the face with polychromy, producing an agglomeration of the painted surface, detachments in the “roof” (exfoliated painted layer), and, eventually, gaps in the surface. When the curvature is convex to the polychromy layer, it will stretch with the curvature of the wood, but since it does not have the same elasticity, cracks and gaps will occur.
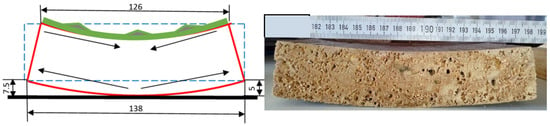
Figure 27.
Graphic representation of concave deformation relative to the surface with polychromy, producing detachments in the roof.
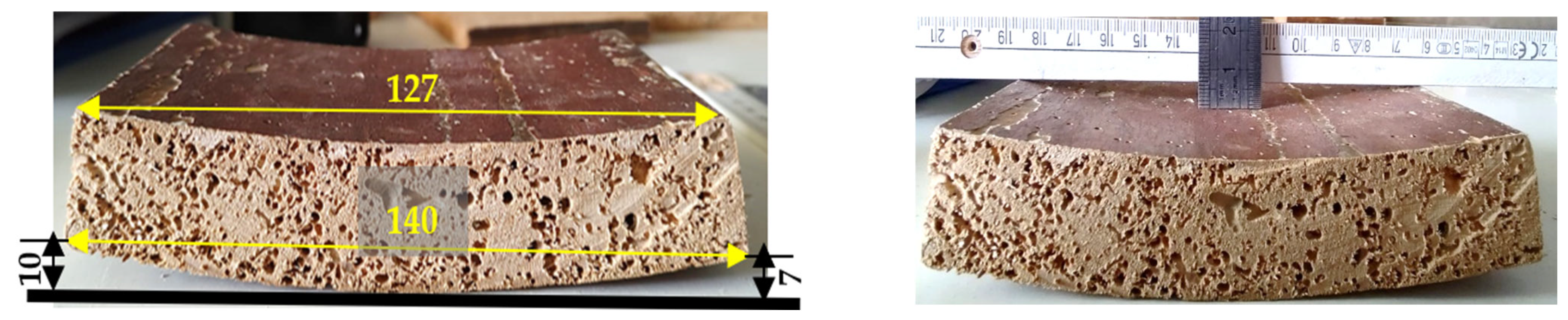
In the case of samples with a thickness of 27 mm, extracted from the water and left to dry in the open air for 24 h, on the polychromy face, deformations of the concave curvature type are present, sample 1P having a curvature radius of approximately 6–7 mm (Figure 27). It can be seen that the deformation continues; the curvature on the convex side increases to 140 mm, and the bend increases from 7.5 mm (Figure 27 and Figure 28) to 10 mm.

Figure 28.
The test piece after 24 h of immersion in water.
Deformations following immersion in white spirit. Analyzing the results that were obtained after immersing the samples in white spirit, it can be seen that in the case of pieces with a thickness of 27 mm, the deformations are very reduced, almost insignificant. In the case of thin specimens, they show a tendency to deform in the direction opposite to the initial shape.
Deformations following immersion in acetone. Analyzing all the samples immersed in acetone, it is evident that the deformations produced by the solvent are non-unitary. It can be seen that in the case of thick specimens, more pronounced bends and fewer torsions occur, while, in the case of thin specimens, the bends are slightly smaller, but the torsions are more pronounced.
The curvatures are, on average, between 1.5 and 5 mm, while the twists have values of between 4 and 12 mm.
In sample 1 FP and sample 1P, a strong deformation appeared, which changed the sample from the initial state, with a convex curvature, to a reverse deformation, with a curvature in the concave plane. Point D was initially 6.75 mm; after immersion, it reached 9.23 mm. All this must also be correlated with the swelling ratio, as well as the effects on the polychromy, highlighted in Section 4.1, regarding absorption and swelling in acetone, as well as is shown in Figure 25 and Figure 26, where the cracks produced in the polychromy layer are revealed.
5. Future Research Directions
The current research may extend to other solvents used in the restoration of wooden art objects. Dimensional changes and deformations produced during consolidation treatments with Paraloid B72 can also be studied comparatively.
6. Conclusions
This study focused on understanding the changes and deformations occurring in polychrome panels from heritage assets during restoration treatments.
The data obtained give us a better understanding of the mechanisms behind these changes. This information is essential for developing an intervention process that reduces or limits impairments without exacerbating them through the treatment response.
We wished to observe the changes produced by solvents on the wood, having a limited time and a reduced amount of solvent, respectively, at 10% of the volume of the tested pieces.
Wet treatments were applied to polychrome panels that show significant degradation caused by the attack of xylophagous insects; the treatments must be prepared and applied rigorously.
Liquid solutions used to perform such treatments can produce:
- Dimensional changes—swelling during treatment and contraction after the evaporation and drying of the wood.
- Deformations—concave/convex curvature and twists.
Changes and deformations occurred simultaneously throughout the composite of the work of art.
The elasticity of the components of the matrix of such cultural goods is not uniform and similar and, as such, behaves differently from the stresses and forces to which the goods are subjected during their treatments. Not knowing the limits and, especially, the impossibility of controlling the stresses, causes fissures, gaps, fractures, and damage to the art object.
Author Contributions
Conceptualization, A.M.-A. and C.Ș.I.; methodology, C.Ș.I.; software, A.M.-A. and C.Ș.I.; validation, A.L. and C.Ș.I.; formal analysis, C.Ș.I.; investigation, A.M.-A.; resources, A.M.-A.; data curation, A.L.; writing—original draft preparation, A.M.-A.; writing—review and editing, A.L. and C.Ș.I.; visualization, C.Ș.I.; supervision, C.Ș.I.; project administration, C.Ș.I.; funding acquisition, C.Ș.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Transylvania University of Brasov for the technical and logistical support provided; the authors also thank the Research and Conservation Laboratory for Cultural Heritage RESTAURARE IONESCU CONSTANTIN, Sibiu, Romania.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parliament of Romania—Law No. 182 of 25 October 2000 Regarding the Protection of the National Cultural Heritage Object. Published in the Official Gazette Number 10 of 8 January 2007. Available online: https://en.unesco.org/sites/default/files/rom_lege_182_romorof.pdf (accessed on 29 September 2023).
- Code of Ethics Document Promoted by the European Confederation of Conservator-Restoration Organizations and Adopted by the General Assembly, Brussels, 03/01/2002 Professional Guide (II) Promoted by the European Confederation of Conservator-Restorer Organizations and Approved in Its General Assembly of 7 March 2003. Available online: https://www.academia.edu/2905696/Cod_deontologic_E_C_C_O_E_C_C_O_PROFESSIONAL_GUIDELINES (accessed on 20 April 2022).
- Castelli, C. The Restoration of Panel Painting Supports Some Case Histories în Current Approaches to the Structural Conservation of Panel Paintings. In The Structural Conservation of Panel Paintings: Proceedings of a Symposium at the J. Paul Getty Museum, 24–28 April 1995; Getty Publications: Los Angeles, CA, USA, 1998; pp. 316–340. [Google Scholar]
- Caggiani, M.C.; Cosentino, A.; Mangone, A. Pigments Checker version 3.0, a handy set for conservation scientists: A free online Raman spectra database. Microchem. J. 2016, 129, 123–132. [Google Scholar] [CrossRef]
- Janas, A.; Fuster-López, L.; Andersen, C.K. Mechanical properties and moisture-related dimensional change of canvas paintings–canvas and glue sizing. Herit. Sci. 2022, 10, 160. [Google Scholar] [CrossRef]
- Sargent, R. Evaluating dimensional stability in solid wood: A review of current practice. J. Wood Sci. 2019, 65, 36. [Google Scholar] [CrossRef]
- Filipovici, J. Studiul Lemnului, 1st ed.; Didactică și Pedagogică: București, Romania, 1964; Volume 1, pp. 68–96. [Google Scholar]
- Pescăruș, P. Proprietățile Fizice ale Lemnului. In Tehnologia Prelucrării Lemnului; Râmbu, I., Ed.; Tehnică: București, Romania, 1978; pp. 64–89. [Google Scholar]
- Wadum, J. Microclimate Boxes for Panel Paintings in Approaches to the Structural Conservation of Panel Paintings. In The Structural Conservation of Panel Paintings: Proceedings of a Symposium at the J. Paul Getty Museum, 24–28 April 1995; Getty Publications: Los Angeles, CA, USA, 1998; pp. 497–524. [Google Scholar]
- Ionescu, C.Ș. Stabilizarea și Consolidarea Suportului Lemnos Pentru Obiectele de Patrimoniu, Utilizând Materiale Clasice și Modern/Stabilization and Consolidation of Wooden Support for Heritage Objects, Using Classic and Modern Materials. Ph.D. Thesis, Transilvania University of Brașov, Brașov, România, 2020. [Google Scholar]
- Avram, A.; Ionescu, C.S.; Lunguleasa, A. A consolidation of degraded lime wooden support from heritage objects using two types of consolidant. BioResources 2023, 18, 4580–4597. [Google Scholar] [CrossRef]
- Arzola-Villegas, X.; Lakes, R.; Plaza, N.Z.; Jakes, J.E. Wood Moisture-Induced Swelling at the Cellular Scale—Ab Intra. Forests 2019, 10, 996. [Google Scholar] [CrossRef]
- Zhan, T.; Lyu, J.; Eder, M. In situ observation of shrinking and swelling of normal and compression Chinese fir wood at the tissue, cell and cell wall level. Wood Sci. Technol. 2021, 55, 1359–1377. [Google Scholar] [CrossRef]
- Callum, A.S. Hill Wood Modification: Chemical, Thermal and Other Processes; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 19–44. [Google Scholar]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites; CRC Press: Boca Raton, FL, USA, 2005; Chapter 4; pp. 77–97. [Google Scholar]
- Lunguleasa, A. Wood Physics and Mechanics; Editura Universiatii Transilvania: Brasov, Romania, 2007; pp. 18–43. [Google Scholar]
- Tiryaki, S.; Bardak, S.; Aydin, A.; Nemli, G. Analysis of volumetric swelling and shrinkage of heattreated woods: Experimental and artificial neural network modeling approach. Maderas Cienc. Technol. 2016, 18, 477–492. [Google Scholar] [CrossRef]
- Filipovici, J. Studiul Lemnului, 2nd ed.; Didactică și Pedagogică: București, Romania, 1965; Volume 2, p. 77. [Google Scholar]
- Ispas, M. Experimental investigations on swelling pressure of natural and heat-treated ash wood. Bull. Transilv. Univ. Brasov. Ser. II For. Wood Ind. Agric. Food Eng. 2013, 6, 55. [Google Scholar]
- Krauss, A. Swelling Pressure of Wood Determined from Hygro-Mechanical Creep Measurements. 2004. Available online: http://www.ejpau.media.pl/volume7/issue2/wood/art-01.html (accessed on 21 February 2023).
- Zelinka, S.L.; Altgen, M.; Emmerich, L.; Guigo, N.; Keplinger, T.; Kymäläinen, M.; Thybring, E.E.; Thygesen, L.G. Review of Wood Modification and Wood Functionalization Technologies. Forests 2022, 13, 1004. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Glass, S.V.; Jakes, J.E. A solution thermodynamics definition of the fiber saturation point and the derivation of a wood–water phase (state) diagram. Wood Sci. Technol. 2016, 50, 443–462. [Google Scholar] [CrossRef]
- Thybring, E.E.; Fredriksson, M. Wood Modification as a Tool to Understand Moisture in Wood. Forests 2021, 12, 372. [Google Scholar] [CrossRef]
- Maher, Z.A. Evaluation of Moisture Content in Wood Fiber and Recommendation of the Best Method for Its Determination, Submitted in Partial Fulfillment of the Requirements for the Degree of Master of Science at Helwan University Ain Helwan, Cairo. 2006. Available online: https://maher2100.tripod.com/msc1.pdf (accessed on 21 February 2023).
- Lahtela, V.; Kärki, T. Effects of impregnation and heat treatment on the physical and mechanical properties of Scots pine (Pinus sylvestris) wood. Wood Mater. Sci. Eng. 2016, 11, 217–227. [Google Scholar] [CrossRef]
- Wu, G.; Shah, U.D.; Janeček, E.-R.; Burridge, H.C.; Reynolds, T.P.S.; Fleming, P.H.; Linde, P.F.; Ramage, M.H.; Scherman, O.A. Predicting the pore-filling ratio in lumen-impregnated wood. Wood Sci. Technol. 2017, 51, 1277–1290. [Google Scholar] [CrossRef]
- Ionescu, C.Ş.; Lunguleasa, A.; Avram, A.; Spîrchez, C. Evaluation of the efficiency of the consolidation treatment with Paraloid B72, performed on artworks with degraded wood support. MATEC Web Conf. 2021, 343, 1–10. [Google Scholar] [CrossRef]
- Avram, A.; Ionescu, C.Ș.; Lunguleasa, A. Some Methods for the Degradation-Fragility Degree Determination and for the Consolidation of Treatments with Paraloid B72 of Wood Panels from Icon-Type Heritage Objects. Forests 2022, 13, 801. [Google Scholar] [CrossRef]
- Darwish, S.S.; El-Hadidi, N.M.N. The effect of solvents on the chemical composition of archaeological wood. In Proceedings of the International Conference on Giza through the Ages, Giza, Egypt, 4–6 March 2008. [Google Scholar]
- Bossu, J.; Le Moigne, N.; Corn, S.; Trens, P.; Di Renzo, F. Sorption of water–ethanol mixtures by poplar wood: Swelling and viscoelastic behaviour. Wood Sci. Technol. 2018, 52, 987–1008. [Google Scholar] [CrossRef]
- Chang, S.S.; Quignard, F.; Di Renzo, F.; Clair, B. Solvent polarity and internal stresses control the swelling behavior of green wood during dehydration in organic solution. BioResources 2012, 7, 2418–2430. [Google Scholar] [CrossRef]
- Mantanis, G.I.; Young, R.A.; Rowell, R.M. Swelling of wood, Part 1. Swelling in water. Wood Sci. Technol. 1994, 28, 119–134. [Google Scholar]
- Mantanis, G.I.; Raymond, A.Y.; Rowell, R.M. Swelling of compressed cellulose fiber webs in organic liquids. Cellulose 1995, 2, 1–22. [Google Scholar] [CrossRef]
- Meier, P.; Kaps, T.; Kallavus, U. Swelling of pinewood (Pinus sylvestris) in binary aqueous solutions of organic substances. Mater. Sci.-Medzg. 2005, 11, 140–145. [Google Scholar]
- Rowell, R.M.; Dale, E.W. Determination of dimensional stabilization of wood using the water soak method. Wood Fiber Sci. 1978, 2, 104–111. Available online: https://wfs.swst.org/index.php/wfs/article/view/1004/1004 (accessed on 24 September 2023).
- Rafsanjani, A.; Stiefel, M.; Jefimovs, K.; Mokso, R.; Derome, D.; Carmeliet, J. Hygroscopic swelling and shrinkage of latewood cell wall micropillars reveal ultrastructural anisotropy. J. R. Soc. Interface 2014, 11. [Google Scholar] [CrossRef]
- International Standard ISO 3131-1975; Wood Determination of Density for Physical and Mechanical Tests. International Organization for Standardization: Geneva, Switzerland, 1975.
- European Standard EN 13183-1/2002; Moisture Content of a Piece of Sawn Timber. European Committee for Standardization: Brussels, Belgium, 2002.
- International Standard ISO 4859-1982; Wood Determination of Radial and Tangential Swelling. International Organization for Standardization: Geneva, Switzerland, 1982.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).