Abstract
The continuous temperature rise has raised global concerns about CO2 emissions. As the country with the largest CO2 emissions, China is facing the challenge of achieving large CO2 emission reductions (or even net-zero CO2 emissions) in a short period. With the strong support and encouragement of the Chinese government, technological breakthroughs and practical applications of carbon capture, utilization, and storage (CCUS) are being aggressively pursued, and some outstanding accomplishments have been realized. Based on the numerous information from a wide variety of sources including publications and news reports only available in Chinese, this paper highlights the latest CCUS progress in China after 2019 by providing an overview of known technologies and typical projects, aiming to provide theoretical and practical guidance for achieving net-zero CO2 emissions in the future.
1. Introduction
Global climate change has become a critical challenge facing humanity on a global scale [1,2,3,4,5,6,7,8,9,10]. Growing evidence indicates that global climate change is caused by greenhouse gas emissions from human activities [6,11,12]. The main greenhouse gas in the atmosphere has been proven to be carbon dioxide (CO2), which contributes to approximately 66% of global warming [2]. The Global Carbon Project [1] has estimated that the annual anthropogenic CO2 emissions averaged around 38.9 billion tonnes worldwide between 2011 and 2020, with energy consumption and land use change contributing 89% and 11% of the total emissions [2,13], respectively. Carbon emissions from energy consumption, especially CO2 emissions from burning fossil fuels for power, heat, and transportation, are thought to be the dominant source of greenhouse gas emissions from human activities [14,15,16,17,18,19]. Global climate change driven by increased man-made emissions of heat-trapping greenhouse gas is already showing widespread effects on the environment, such as accelerated sea level rise [20,21,22], sea ice loss [23,24], melting glaciers and ice sheets [25,26], more intense heat waves [27,28], and plant and animal geographic ranges shifts [29,30,31,32,33]. The effects of human-caused global climate change are irreversible and will worsen in the decades to come.
Global greenhouse gas emissions have already reached the highest levels ever recorded in human history after the year 2010. To deal with the serious situation, at the 21st United Nations Climate Change Conference, the Paris Agreement was approved as the second binding climate agreement following the Kyoto Protocol. The Paris Agreement set a limit on global average temperature control of 2 °C below pre-industrial levels. The Sixth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC) proposed requirements for both greenhouse gas emission peaks and emission reductions. Global greenhouse gas emissions must peak before 2025 and a 43% emission reduction must occur by 2030 to limit warming to 1.5 °C, while emissions must peak before 2025 and a 25% emission must be lowered by 2030 to limit warming to 2 °C [1]. In terms of total annual CO2 emissions, China, as a major representative of the Asia-Pacific Economic Cooperation (APEC), is now ranked first, followed by the United States, the core and dominant country in North American Free Trade Area (NAFTA). To achieve its goals of carbon peak and carbon neutrality, China has implemented a variety of initiatives to generate a coordinated development plan of decarbonization and carbon reduction, emission reduction and pollution reduction, green growth and sink increase, and sustainable growth [2,34,35,36], endeavoring to gradually realize net-zero CO2 emissions [37].
At present, the world’s high energy consumption industries are shifting to developing countries [38,39,40], which results in the rising phase of CO2 emissions in developing countries. The CO2 emission reduction at the expense of reducing the amount of industrial activity harms economic development [41,42,43,44,45], generating two competing demands to keep both economic growth and substantial CO2 emission reduction for developing countries, especially for China, the major developing country in the world. China has conducted numerous studies on carbon capture, utilization, and storage (CCUS) in an effort to find a solution to the issue of balancing economic expansion and CO2 emissions. The concept of CCUS was first proposed in China [45], emphasizing the close integration of CO2 emission reduction and CO2 utilization to achieve maximum resource utilization. With the deepening understanding of CCUS as well as the continuous development of CCUS technologies, CCUS has gained international recognition recently.
The Chinese government clarified the low-carbon development goals in the “13th Five-Year Plan” and provided strong economic and policy support to China’s CCUS projects. After several years of preliminary preparation and construction, China’s CCUS projects have made significant progress after 2019, showing outstanding achievements in fundamental research [46,47], technology development [48], and engineering demonstrations [49]. Approximately 40 CCUS demonstration projects with a 3 million tonnes/year capture capacity are operating or under construction in China by 2021 [50]. Remarkable progress has been made in CCUS-enhanced oil recovery (CCUS-EOR) [51], CCUS-enhanced natural gas recovery (CCUS-EGR) [52], and CCUS-enhanced coalbed methane (CCUS-ECBM) [53]. CO2 storage in China is dominated by deep saline aquifers, with the well-developed required technology and equipment. In addition, China’s first off-shore CO2 storage demonstration project was also constructed and completed in July 2022 [54], aiming to achieve net-zero CO2 emission and green low-carbon development of off-shore oil fields.
This study provides a comprehensive review of the latest progress of CCUS development in China through the steps of information collection, classification, analysis and summary. Targeted journal publications were collected from both English and Chinese bibliographic sources, including the Web of Science (WoS) in English and the China Integrated Knowledge Resources System in Chinese. In addition, news reports on CCUS development released by Chinese media were also collected to comprehend pertinent national policies and relevant advancements that have not yet been published in paper form. The collected information was classified according to CO2 capture and transport, CO2 utilization and CO2 storage for detailed analysis and summary for each technology. Based on information from a wide variety of sources, an overview of known technologies and representative projects of CCUS in China is presented. Furthermore, the current challenges for CCUS development in China are clarified, and suggestions for future improvement are proposed.
2. Overview of CCUS in China
China’s energy system is large in scale and diverse in demand. China’s rapid economic development has resulted in an increase in CO2 emissions. According to the total annual CO2 emissions of some countries [55] from 1980 to 2020 as shown in Figure 1, China has risen to the top one in terms of annual CO2 emissions in the world. To strike a balance between the carbon neutrality goal and energy security and economic development, China has been actively constructing a modern energy system that is dominated by renewable energy and complemented by nuclear and fossil energy. However, according to China’s actual national conditions, high-carbon-based fossil fuels continue to account for up to 85% of the country’s overall energy consumption. It is predicted that China’s energy consumption will still primarily depend on fossil fuels by 2060. Therefore, CCUS is the only technology option to achieve net-zero CO2 emissions from fossil energy sources.
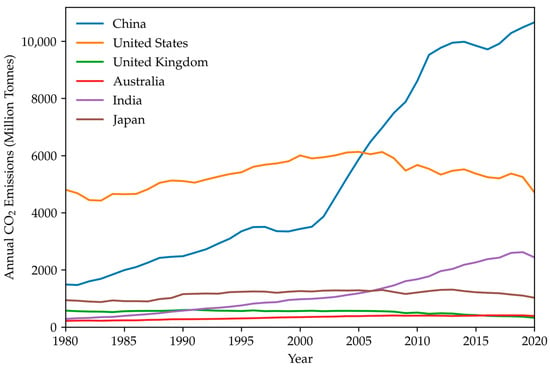
Figure 1.
Annual CO2 emissions of some countries from 1980 to 2020 [55]. Data were derived from the Global Carbon Project.
The Chinese government has consistently encouraged technical applications while prioritizing the development of CCUS technology. Research and development activities on CCUS have received substantial financial support and strong policy incentives from the government. In the mode of cooperation between industrial companies and universities or research institutes, China’s CCUS integrated technology has reached the stage of industrial demonstration. However, the demonstration scale is still limited due to the cost of the technology. With the continuous improvement and advancement of technology, China’s CCUS technology is expected to achieve low-cost large-scale demonstration applications in the near future [50].
2.1. Storage Potential in China
Despite the huge annual CO2 emissions, China also possesses a considerable potential to store CO2 compared with other countries in the world. Based on theoretical storage capacity [56] shown in Table 1, China’s CO2 storage capacity is between 1.21 and 4.13 trillion tonnes [50]. Therein, CO2-EOR and CO2-EGR, with a storage capacity of 5.1 billion tonnes and 9 billion tonnes, respectively, are dominating in terms of CO2 utilization. Deep saline aquifers can store up to 2420 billion tonnes of CO2; it is followed by depleted oil and gas reservoirs, which can store 15.3 billion tonnes of CO2 [50]. The major storage locations and their storage capacities in China are shown in Table 2.

Table 1.
CO2 emissions and CCUS theoretical storage capacity in some countries [50,55,56].

Table 2.
Dominate CO2 storage location and storage capacity in China [50].
2.2. CCUS Development Plan in China
China has approximately 40 CCUS demonstration projects in operation or under construction (Figure 2), with an annual CO2 capture capacity of 3 million tonnes [50]. Most of these projects are small-scale CO2 capture demonstrations in the electricity, coal, and petroleum industries. In China, every five years is set as one development period for CCUS, and a specific short-term development plan is designed for each development period. According to the “Overall CCUS Technology Development Roadmap in China” released in 2019, the four development periods from 2030 to 2050 are planned as follows. By 2030, the existing technologies are expected to move into the commercial stage to undergo industrialization; by 2035, certain new technologies are expected to be implemented at large scale; by 2040, breakthroughs in CCUS systemic integration and risk management technology are expected to be achieved; by 2050, the CCUS technology will be extensively deployed, and multiple industrial CCUS clusters will be completed.
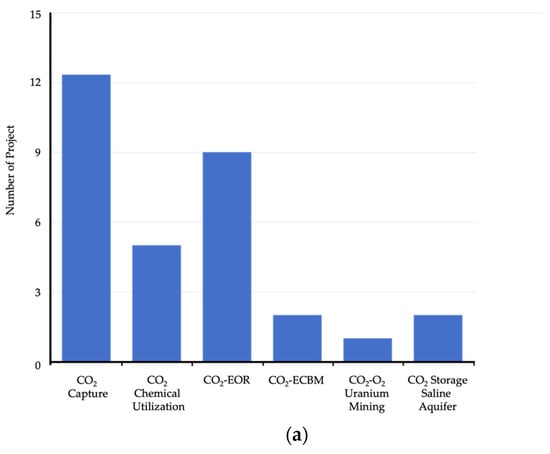
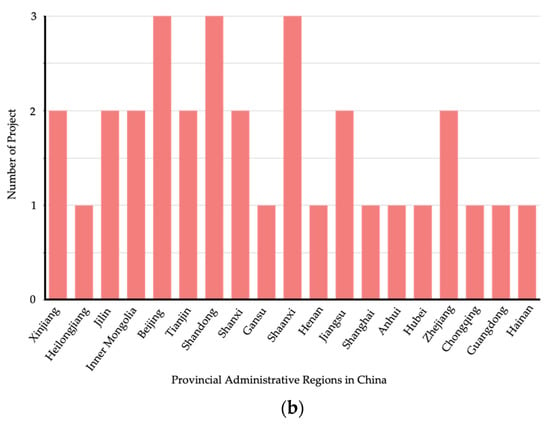
Figure 2.
Distribution of CCUS projects in China [50]. (a) CCUS project type distribution in China; (b) CCUS project location distribution in China.
3. Progress of CO2 Capture and Transport in China
3.1. Development Status of CO2 Capture in China
CO2 capture is the process of capturing and separating CO2 from the exhaust gases produced during the combustion of fossil fuels. The captured CO2 is purified to provide a stream of high-purity high-concentrated CO2 for subsequent transport, utilization and storage [57]. CO2 capture technologies are classified into three categories [58], namely post-combustion capture, pre-combustion capture, and oxy-fuel combustion capture. Schematic flow diagrams of these three capture technologies are illustrated in Figure 3. In post-combustion capture, CO2 is captured from flue gases after the combustion of the fuel [58]. In pre-combustion capture, fuel is pre-treated with steam and air (or oxygen) to generate syngas consisting mainly of CO and H2, and then the syngas undergoes the water-gas shift reaction with steam to form CO2 and more H2. After CO2 is separated, H2 can be used as a carbon-free energy carrier to generate energy. In oxy-fuel combustion capture, the fuel is burned in pure oxygen instead of air, and the generated flue gases consist of mainly CO2 and water vapor, the latter of which is easily removed through condensing, resulting in high-concentrated CO2. The applicability, advantages and disadvantages of CO2 capture technologies are summarized in Table 3.
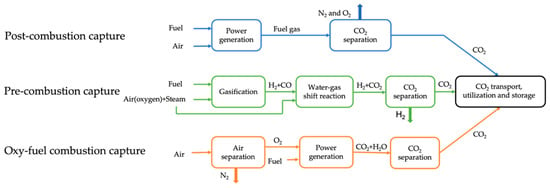
Figure 3.
Schematic flow diagrams of post-combustion capture, pre-combustion capture and oxy-fuel combustion capture.

Table 3.
Comparison of CO2 capture technologies [57].
The CO2 separation technology can be classified into two categories: physical method and chemical method [59], both of which can be further subdivided into absorption, adsorption, membrane separation, etc. Some comprehensive summaries of the principles, applicability, advantages and disadvantages of CO2 separation technologies have been provided in detail by other literature [59,60,61].
The selection of capture technology mainly depends on appliance objects, and post-combustion capture is now the most developed and common technology of CO2 capture in China. The Guohua Jinjie carbon capture demonstration project of China Energy Investment Group CO., Ltd. (CHN ENERGY), which was put into operation in June 2021, is currently the largest demonstration facility for CO2 capture employing post-combustion capture technology, with a capture capacity of 150,000 tonnes/year [61,62]. By utilizing post-combustion capture technology, the advanced low-energy carbon capture engineering project of Huaneng Longdong Base with a carbon capacity of 1.5 million tonnes/year, which is about to start construction, will become the largest coal-fired power plant carbon capture project in China once it is put into operation in 2023 [62]. Compared with post-combustion capture, pre-combustion capture has a more complicated system. Currently, IGCC-based pre-combustion CO2 capture technology is an important technology for large-scale carbon capture demonstration in the power industry [63]. The carbon capture plant of the Huaneng IGCC power plant is China’s first demonstration project with pre-combustion CO2 capture technology, and it has a capture capacity of 100,000 tonnes/year after being put into operation in July 2016 [63,64]. In addition, oxy-fuel combustion capture technology, which has the benefit of high CO2 concentration for effective capture, has made significant strides. In 2016, the first 10,000-tonne pilot system for oxy-fuel combustion capture was completed in China [59,65], and the construction of a 100,000-tonne oxy-fuel combustion industrial demonstration project has been planned [66,67]. CO2 capture projects that are currently in operation are listed in Table 4.

Table 4.
CO2 capture projects in China [50].
While existing capture technologies are gradually being put into practical large-scale application, major advancements have been achieved in the study of CO2 separation technology. In 2020, the first “phase-changing” carbon capture industrial facility with a capacity of 1000 tonnes/year, which was developed by China Huaneng Group (CHNG), successfully realized continuous and stable operation in Huaneng Changchun Thermal Power Plant. In this facility, a biphasic carbon separation technology used in the post-combustion capture process can address the issue of high energy consumption caused by the heating of the CO2 absorption liquid in the traditional method, thus reducing the cost of CO2 capture [68].
In addition, direct air capture (DAC), which can reduce the atmospheric level of CO2, has also attracted attention in China. In contrast to the above-mentioned three traditional CO2 capture technologies, which are primarily appropriate to capture CO2 emissions from industrial stationary sources, DAC is primarily designed to capture less-concentrated CO2 from distributed sources, such as small fossil combustion units and vehicles. It is regarded as a means of balancing difficult-to-avoid emissions as well as a remedy for legacy emissions [69,70]. The research focus in DAC technology is on the development and utilization of efficient and low-cost adsorption materials. Hydrogen-bonded Organic Frameworks (HOFs) [71], which are a type of solid adsorption material with an organic framework crystal structure, can achieve direct CO2 capture from the air at atmospheric pressure of 25 °C. With the help of the permanent pores, selective adsorption of CO2 can be accomplished with 1 tonne of adsorbent for 113 kg of CO2 captured [59]. Given the promising application of HOFs materials for direct air capture of CO2, Chinese research institutions and scholars have been improving the properties of HOFs materials in recent years. Hu et al. developed a type of HOFs material (HOF-TCBP) with high stability and a large specific surface area, which can be regenerated under the rotary evaporation method. The regenerated material has the same specific surface area as the original sample, so it can be repeatedly used for efficient CO2 capture [72]. Huang et al. proposed a flexible HOFs material with large-scale void-regulated permanent pores. Its maximum porosity can reach 33.2% of the total unit volume, enabling a larger volume of CO2 adsorption. These advancements in HOFs material development have laid the foundation for the future potential application of direct air capture of CO2 [73].
3.2. Development Status of CO2 Transport in China
Many of the power stations and industrial plants where CO2 capture can be implemented are not located close to potential CO2 utilization or storage sites, thus requiring significant CO2 transport as a momentous link [74,75]. At present, the main CO2 transport can be sorted into two categories. One is pipeline-based transport [76,77], which includes on-land and off-shore pipelines; the other is tank-based transport, which includes ship transport, rail train transport, and tanker truck transport. Pipeline-based transport has historically been the preferred means to transport CO2 due to its low cost and large transport capacity for low-to-medium distances [74,78]. Tank-based CO2 transport has recently garnered increased interest [79,80] due to the lower investment costs, flexibility, and shorter construction times compared to pipelines for modest amounts of long-distance transport. It is worth noting that tank-based transport only carries liquefied CO2 with strict temperature and pressure restrictions whereas pipeline-based transport can transport CO2 in various states, including gaseous, liquid, dense-phase, and supercritical states [75].
Due to the small scale of China’s current CCUS demonstration projects, tanker truck transport remains the primary mode of CO2 transport [81], while certain oil and gas fields use ship transport [82]. On-land pipeline transport is still in the pilot stage, with only limited adoptions in the Jilin oilfield and Qilu Petrochemical projects [83]. Jilin Oilfield has built a 50 km of CO2 gas-phase transport pipeline with a transport capacity of 50 × 104 tonnes/year [83]. Up to now, off-shore pipeline transport of CO2 is still in the research stage. Additionally, the supercritical CO2 long-distance pipeline construction is another challenge for CO2 transport, with no undertaken demonstration projects [81].
4. Progress of CO2 Utilization in China
CO2 can be utilized in a variety of ways, and CO2 utilization has drawn interest from all over the world due to its ability to reduce CO2 emissions and provide economic value [84,85,86,87,88]. As illustrated in Figure 4, CO2 utilization is classified into chemical [89,90], biological [91,92] and geological categories [93,94] based on the various technologies. Valuable products generated by CO2 utilization cover a wide range, including useful chemicals and materials [94], fuels [95], food, feed, and fertilizer [96].
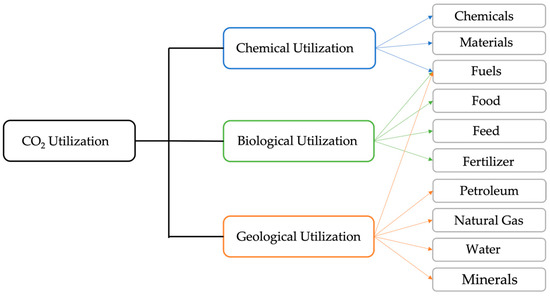
Figure 4.
CO2 utilization classification [97].
4.1. Development Status of CO2 Chemical Utilization in China
CO2 chemical utilization refers to the efficient conversion of CO2 into a variety of more valuable compounds through some complicated chemical processes such as catalyzed hydrogenation [98,99,100,101,102,103], photochemical [104,105], electrochemical [104,105,106], thermochemical [102,107,108], and hybrid methods.
China’s CO2 chemical utilization technology has advanced in recent years, yielding considerable economic benefits. Currently, approximately 10 × 104 tonnes of captured CO2 are used for synthesizing energy fuels each year, with an output value of about 100 million yuan/year; about 10 × 104 tonnes of captured CO2 are used annually in the synthesis of high-value-added chemicals, with an output value of about 400 million yuan/year; about 5 × 104 tonnes of captured CO2 are used for synthetic materials, with an output value of 200 million yuan/year [67]. It is worth noting that the methanol [59,109] and carbonate syntheses [110] using captured CO2 have realized industrialization, and the production of olefin and fuel [111,112] from CO2 has also made significant strides. Meanwhile, industrial demonstration of syngas production from captured CO2 has been accomplished [113]. CO2 chemical utilization projects in China are listed in Table 5.
Methanol synthesis from CO2. China’s first 1000-tonne liquid solar fuel synthesis demonstration project was successfully launched in January 2020 [114]. This project adopts the methanol synthesis from CO2 hydrogenation technology developed by the Dalian Institute of Physical Chemistry, Chinese Academy of Sciences (CAS) [59]. In this project, methanol can be synthesized through three fundamental components, namely, solar photovoltaic power generation, electrolysis of water to produce hydrogen, and CO2 hydrogenation. Solar energy can be converted into liquid fuel methanol with an efficiency of more than 14% [59]. The project is expected to produce 1440 tonnes of “liquid sunshine” methanol annually after being put into operation [115]. In addition, in September 2020, the world’s first 5000 tonnes/year CO2 hydrogenation to methanol industrial pilot plant, which was developed by China National Offshore Oil Corporation (CNOOC), achieved stable operation and realized industrial demonstration [116].
Carbonate synthesis from CO2. The energy required to synthesize carboxylic acids or carbonates from CO2 is comparatively low, and the corresponding energy utilization efficiency and economy are relatively high. Therefore, the synthesis of carbonates from captured CO2 is also a major chemical utilization of CO2 in China. In addition to China’s first industrial production plant for polycarbonate degradable plastic synthesis from CO2 built in 2004 [117], China’s first 100,000-tonne facility for carbonate synthesis from ionic liquid catalyzed CO2 was put into operation in March 2021 and has achieved continuous and stable operation [118]. The produced carbonate (including ethylene carbonate and DMC) products meet electronic-grade standards, and the system energy consumption has been reduced by 37%, demonstrating the remarkable carbon reduction effect [110,113].
Olefin and fuel production from CO2. The direct synthesis of low-carbon olefins, liquefied petroleum gas, aromatics, and other components from CO2 hydrogenation can be achieved by using a bifunctional catalyst system and coupling multiple reaction mechanisms [119]. Shanghai Advanced Research Institute, CAS, achieved a highly selective synthesis of gasoline fractions with high isomeric hydrocarbons (C5–C11) by direct CO2 hydrogenation in 2017 [120] and direct conversion of CO2 hydrogenation to aviation fuel under milder conditions in 2021 [121]. In May 2022, Li et al. achieved the highly selective preparation of long-chain olefins by ambient-pressure hydrogenation of CO2 [122]. In June 2022, the world’s first 10,000-tonne CO2 hydrogenation to aromatics industrial pilot project jointly constructed by Tsinghua University and Jiutai Group started construction [123], further promoting the production process of high-end chemicals from CO2.

Table 5.
CO2 chemical utilization projects in China [50,113,123].
Table 5.
CO2 chemical utilization projects in China [50,113,123].
| Project | Operation Start Year | Technology | Productivity |
|---|---|---|---|
| Lanzhou liquid solar fuel synthesis demonstration project | 2020 | Methanol synthesis from CO2 hydrogenation technology | 1440 tonnes/year |
| CNOOC methanol synthesis industrial pilot plant | 2020 | Methanol synthesis from CO2 hydrogenation technology | 5000 tonnes/year |
| Guangdong Huizhou Daya Bay carbonate synthesis project | 2021 | Carbonate synthesis from ionic liquid catalyzed CO2 | 100,000 tonnes/year |
| Erdos aromatics industrial pilot project by Tsinghua University and Jiutai Group | 2023 (expected) | Aromatics synthesis from CO2 hydrogenation | 10,000 tonnes/year |
| Shanxi Lu’an CH4-CO2 dry reforming project | 2021 | Syngas production from CH4-CO2 reforming | 10,000 m3/year |
Syngas production from CO2. As the “cornerstone” of the synthesis industry, syngas has a huge market demand [113]. The efficient production of syngas can be achieved by employing recent technological advancements such as CH4-CO2 dry reforming [124,125], CH4-CO2-H2O ternary reforming [126], and reverse water gas shift reaction (RWGS) [127]. Moreover, these technologies promote the effective utilization of natural gas while lowering CO2 emissions. In August 2021, the world’s first 10,000-cubic-meter-level CH4-CO2 dry reforming facility for syngas production [128] was completed in Changzhi, Shanxi. After achieving stable operation, it realizes the daily conversion of CO2 up to 60 tonnes [129]. In addition, this facility can realize the efficient utilization of CO2 and the flexible adjustment of H2/CO of the product syngas, and it is ready for industrial application promotion [130].
4.2. Development Status of CO2 Biological Utilization in China
CO2 biological utilization combines synthetic biology processes with CO2 to produce valuable products, including food and feed, biofertilizers, chemicals, biofuels, and gas fertilizers [131]. Products generated by CO2 biological utilization technology have high-added values and substantial economic benefits [132].
In China, the conversion of CO2 to food and feed has been commercialized on a large scale, with a CO2 utilization of 1000 tonnes/year and an output value of about 50 million yuan/year; about 50,000 tonnes of CO2 are utilized annually to create biofertilizers, with an annual production value of 500 million yuan; about 10,000 tonnes of CO2 are used annually for chemical conversion, with an annual production value of 20 million yuan; and roughly 10,000 tonnes of CO2 are used to make gas fertilizer each year, with an annual production value of about 20 million yuan [67].
In addition to the existing CO2 biological utilization technologies such as microalgae fixation [84,133] and gas fertilization [59], the research institutions and universities in China are dedicated to the development of more advantageous CO2 biological utilization technologies, and some outstanding outcomes have been obtained. Tan et al. proposed an innovative method to produce high-performance biodegradable plastics by directly employing captured CO2 in conjunction with cyanobacterial cell factories [134], which resolves the issues of plastic pollution and the replacement of non-grain raw materials in PLA production. Cai et al. realized the efficient cell-free chemoenzymatic starch synthesis from CO2, bringing the opportunity to replace the traditional starch manufacturing factory. This technical route of synthesizing starch from CO2 has achieved the first-time complete synthesis of CO2 to starch from beginning to end in the laboratory, which is a major disruptive and original breakthrough in the field of artificial starch synthesis [135]. Yu et al. constructed a biosynthetic pathway of CO2 to organic matter by using Rhodobacter sphaeroides strains [136]. Wei et al. proposed a new “genomic scalpel” that can quickly prune and knock out the genome of microalgae named “Nannochloropsis oceanica” to form an efficient cell factory, which can be combined with available CO2 and sunlight to customize the production of biomolecules such as biofuels or bioplastics [137]. The continuous development of CO2 biological utilization technology lowers the price of processing raw materials while simultaneously improving environmental friendliness, which is consistent with the concept of green ecology and accelerates the transition toward carbon neutrality.
4.3. Development Status of CO2 Geological Utilization in China
The process of injecting massive volumes of CO2 into the ground to achieve the purpose of production and enhancement of fossil fuels is known as the geological utilization of CO2. The notable advantage of this technology is its capacity to dramatically enhance energy output while fulfilling the target of reducing CO2 emissions. Therefore, it is now viewed as the most economically efficient approach for CO2 utilization.
4.3.1. CO2-Enhanced Oil Recovery (CO2-EOR) in China
CO2-EOR is injecting CO2 into the reservoir to modify the flow characteristics of the multiphase fluid in porous media, including interfacial tension reduction, oil viscosity reduction and oil permeability improvement, thereby enhancing oil recovery [113]. CO2-EOR is applicable to both conventional and unconventional reservoirs with low or ultra-low permeability.
China’s CO2-EOR technologies and apparatus are extremely advanced because CO2 flooding research and applications have been carried out in China since the 1960s [97]. Currently, China’s CO2-EOR has already progressed to the stage of industrial demonstration. The annual CO2 utilization scale for EOR is approximately 1.54 million tonnes, and the cumulative CO2 storage has exceeded more than 660 × 104 tonnes [83], still demonstrating considerable potential.
The distribution of CO2 emission sources and the geological characteristics need to be considered when selecting the location for CO2-EOR projects. China’s iron and steel enterprises are mainly distributed in the east and north, and the large amount of CO2 emissions produced during the steel production process can serve as the CO2 source for CO2-EOR projects. For the basins that are also located in the east and north of China, such as the Ordos Basin, Songliao Basin, and Bohai Bay Basin, there is a shorter CO2 transport distance between the CO2 emission sources and these basins, namely, good spatial matching. Therefore, these basins are regarded as priority areas for China’s CO2-EOR project implementation [50] due to the substantial advantage of good spatial matching. For the Tarim Basin and Junggar Basin situated in the northwest of China, although there are fewer CO2 emission sources nearby, they are still considered to be appropriate areas for CO2-EOR projects due to their favorable geological characteristics and high geological storage capacities.
Due to the close integration of CO2 with oil production in CO2-EOR, most of the CO2-EOR projects in China are undertaken by oil companies, including CNPC, Sinopec, and Shaanxi Yanchang Petroleum. By 2021, there are nine major CO2-EOR projects in operation. The CNPC Jilin oilfield CO2-EOR project is currently the largest CCUS project in Asia, and it is also the only project in China among the 21 large CCUS projects currently operating across the world. It adopts pre-combustion capture combined with associated gas separation technology and pipeline transport, with a CO2 injection capacity of 60 × 104 tonnes/year [50] and an oil production capacity of approximately 10 × 104 tonnes/year [83]. The cumulative CO2 storage has reached 2.12 million tonnes since it was put into operation in 2008 [83]. Another representative national demonstration project in China is the Daqing Oilfield CO2-EOR project. It adopts the pre-combustion method to capture CO2 generated from natural gas processing and uses a combination of tanker truck and pipeline for CO2 transport, realizing CO2 injection of 30 × 104 tonnes/year and oil production of 10 × 104 tonnes/year [83]. The current CO2-EOR projects in China are listed in Table 6. According to the results of CO2-EOR in Jilin and Daqing oilfields, oil recovery can be enhanced by 10–25% through CO2-EOR, that is, approximately 1.0 tonnes of oil increment for every 2.0–3.0 tonnes of CO2 injection, indicating obvious advantages in oil increment and CO2 storage [83].

Table 6.
CO2-EOR projects in China [50,83].
In addition, Sinopec Shengli Oilfield and Qilu Petrochemical cooperate to carry out China’s first million-tonne CO2-EOR project, which was officially completed and put into production in August 2022 [83,138]. In this project, Qilu Petrochemical is responsible for CO2 capture and transport, while Shengli Oilfield is in charge of CO2 injection into oil reservoirs for oil production. CO2 produced in the coal gasification process of the fertilizer plant is captured and transported by tanker truck; 73 injection wells and 166 production wells are deployed; and CO2 is injected into the ultra-low permeability reservoirs of Shengli Oilfield. It is expected to inject a cumulative amount of more than 10 million tonnes of CO2 over the course of 15 years [138], to achieve the purpose of increasing oil production by 3 million tonnes [83] and enhancing oil recovery by 12% [139].
4.3.2. CO2-Enhanced Natural/Shale Gas Recovery (CO2-EGR) in China
Natural gas mainly exists in a free state under natural conditions, and it is stored in underground porous formations, primarily in oil and natural gas fields. Natural gas is easy to produce in large amounts due to its free state. Although shale gas has the same composition as natural gas, it is trapped within the microcracks of shale rock. The characteristics of shale, such as the low pore pressure, porosity, and permeability, make shale gas production much more difficult. Therefore, shale gas is considered as unconventional natural gas. The utilization of CO2 has a considerable positive impact on the production of both natural gas and shale gas. The primary premise of CO2-enhanced natural gas recovery is to leverage the physical differences between the supercritical-state CO2 and the free-state natural gas to accomplish natural gas displacement by CO2 in natural gas reservoirs [140], while the CO2-enhanced shale gas recovery process mainly relies on distinctions in the adsorption characteristics of shale for CO2 and CH4. Because shale preferentially absorbs CO2 over CH4, shale gas recovery can be enhanced by replacing CH4 in the shale formation with CO2 injection [141].
The proven reserves of gas in China have rebounded since 2018 [142]. In 2019, new proven natural gas reserves of over 100 billion m3 were discovered in three gas fields, including two gas fields in the Ordos Basin and one in the Sichuan Basin [138]; the Sichuan Basin added 124.7 billion m3 of proven shale gas fields [143], and Guizhou added 642.4 billion m3 of predicted shale gas geological reserves [144]. Theoretically, China’s CO2-EGR has approximately 9 billion tonnes of CO2 storage potential, while China’s CO2-EGR technologies currently are still in the basic research stage [50].
Both natural gas and shale gas in China show great development potential [145]. The geological conditions of some gas reservoirs limit their development efficiency, especially shale gas reservoirs. China’s continental shale gas enrichment areas have typical characteristics of low porosity, low permeability, high adsorption, high clay content, and high depth. These characteristics lead to huge water consumption and clay hydration swelling by hydraulic fracturing during the shale gas production process, further causing fracture blockage and low gas recovery [146]. Given the above-mentioned characteristics of shale gas reservoirs and the difficulties in exploitation, Chinese experts and scholars have launched a series of research on CO2 utilization to enhance shale gas recovery, covering the microscopic behavior of gas molecules in shale nanopores [147] as well as the macroscopic parameter optimization and technical improvements in the production process [146,148]. Sun et al. investigated the microscopic states and behaviors of CH4 and CO2 in typical shale nanopores using molecular dynamics (MD), grand canonical Monte Carlo (GCMC) simulations, and density functional theory (DFT). The result revealed the prevalence of competitive adsorption of CO2 and CH4 in nanopores of different compositions, that is, CO2 adsorption ability is greater than CH4 while its self-diffusion is usually weaker than CH4 in the nanopores of kerogen, quartz, montmorillonite, calcite, and mixed components [147]. Shi et al. determined the appropriate CO2 injection parameters into shale oil reservoirs by employing numerical simulation, providing theoretical guidance for CO2 injection to enhance shale gas recovery [146]. Lu et al. proposed an integrated technology for the efficient development of shale gas and the geological storage of supercritical CO2 [148]. With this technology, the shale was first fractured with supercritical CO2 to promote the development of a more complex fracture grid, and then CO2 was continuously injected to replace CH4 in the reservoir to maximize shale gas recovery and geological storage of CO2. Unlike the traditional shale gas production process that requires hydraulic fracturing to increase the porosity and permeability of shales, this approach’s innovation lies in utilizing supercritical CO2 instead of water for fracturing. According to the pilot test result of this technology in Yanchang Petroleum National Continental Shale Gas Demonstration Area, a 2.5-time increase in the daily average production from a single well was achieved, demonstrating a considerable rise in shale gas production. This innovative technology offers a new alternative for massive CO2 storage while enhancing shale gas recovery and reducing water waste from the hydraulic fracturing of shales [149].
4.3.3. CO2-Enhanced Coalbed Methane Recovery (CO2-ECBM) in China
A large amount of coalbed methane in coal seams is formed and stored in the process of coalification. Unlike free-state natural gas in reservoirs, coalbed methane resides in the adsorbed state within coal seams. Different storage forms result in various production mechanisms and processes. In general, the production of coalbed methane is more complicated than that of conventional natural gas. Due to the lower permeability of coal seams, stimulation measures such as hydraulic fracturing and CO2 injection, are required during coalbed methane production [150]. The kinetic process of CO2 adsorption and displacement of CH4 is the key mechanism of CO2-ECBM. By injecting CO2 into the coal seam, the partial pressure of CH4 is reduced, which speeds up the desorption of CH4 adsorbed on the surface of coal particles. Meanwhile, when CO2 and CH4 are present in the coal seam at the same time, they compete for adsorption. Due to the higher adsorption energy of CO2 [151], the injected free-state CO2 can take over the initial adsorption position of CH4 in the coal seam so that the adsorbed-state CH4 can be converted into the free state, facilitating CH4 production and achieving coalbed methane enhancement by CO2.
China’s CO2-ECBM project has completed the pilot phase study. Although China has abundant coalbed methane resources, the development of coalbed methane is constrained by the “three lows and one high” properties of coalbed methane reservoirs, namely low pressure, low saturation, low permeability, and high adsorption [152]. Therefore, a series of studies have been conducted in China to address this situation. Fan et al. established a thermos-hydro-mechanical-chemical (THMC) coupled model to simulate the displacement of coalbed methane by CO2, providing insights into key processes controlling key factors [153]. Bai et al. analyzed the impact of CO2 injection on some key parameters in coal seams through experiments and summarized the changes in gas outflow rate, gas components, coal seam temperature, CH4 desorption rate and other displacement parameters during this process, establishing a crucial theoretical basis for the CO2 storage effect in coal seams and the enhanced development of coalbed methane [154]. Li et al. investigated the microstructure changes of coal samples after the injection of supercritical CO2 and confirmed that the injected supercritical CO2 could enlarge the pores and fractures in coal samples by dissolving the coal minerals, thus enhancing coalbed methane recovery [155]. Zhang et al. focused on the geochemical reactions that occurred after CO2 injection into coal seams and put forward the insight that anthracite may be a potential geological body for CO2 storage [156].
In addition to the above studies, there are currently two CO2-ECBM projects in operation in China, both of which are under the responsibility of China United Coalbed Methane Corp., Ltd. (CUCMC). Both the Shizhuang CO2-ECBM project and the Liulin CO2-ECBM project have a CO2 injection capacity of 1000 tonnes/year [50], as shown in Table 7.

Table 7.
CO2-ECBM projects in China [50].
4.3.4. CO2 for In-Situ Leach Uranium Mining in China
Uranium resources are not only a strategic national resource and an important energy resource, but also an important raw material for the nuclear military industry and nuclear power [157]. Sandstone-type uranium resources contribute 41.57% of all the proven uranium resources in China. More than 70% of these complex sandstone-type uranium resources have high salinity, high carbonate, low permeability and low grade, making them difficult to mine using the traditional acid or alkali in situ leach method. In response to this actual situation, the Beijing Research Institute of Chemical Engineering and Metallurgy of China National Nuclear Corporation (CNNC) has carried out a series of theoretical research and technological innovations since 2002. China has successfully mastered and industrialized the technology of CO2 and O2 in-situ leach uranium mining through three generations of improvement in mining and metallurgical technology, becoming the second nation after the United States to accomplish this advancement in the world.
In this technology, CO2 and O2 are added to the groundwater to form a leaching agent, which is then introduced into the uranium ore. In the reaction between the leaching agent and uranium ore, O2 oxidizes tetravalent uranium into hexavalent uranium, while CO2 reacts with groundwater to form bicarbonate ions. Then, bicarbonate and hexavalent uranium combine to form uranyl ions, which are dissolved in the solution and potently inhibit the precipitation of calcium ions. Finally, the uranium solution is lifted to the ground with a submersible pump for processing, and primary products of uranium can be obtained. The tail solution serves as the leaching agent for cycle use [158].
Low permeability now has become a major barrier restricting uranium mining in China. The permeability of sandstone-type uranium deposits gradually decreases with increasing mining depth. Therefore, based on the mature technology of CO2 and O2 in-situ leach uranium mining, Niu et al. proposed a method of combining CO2 blasting and CO2 in-situ leach uranium mining, where CO2 blasting can create a three-dimensional fracture grid inside the uranium deposit to improve its overall permeability, thereby improving the efficiency of subsequent CO2 in-situ leach uranium mining [159,160]. Chang et al. put forward a simulation test system for in-situ leach uranium mining in horizontal wells, where horizontal wells are used for liquid injection while vertical wells are used for liquid extraction, to investigate and enhance recovery of CO2 in-situ leach uranium mining [161].
At present, there is one CO2 and O2 in-situ leach uranium mining project in China, which is operated by CNNC. The improvement of CO2 and O2 in-situ leach uranium mining makes “dead ore” a lucrative resource that can be economically mined by resolving the technical challenges of excessive acid consumption and easy blockage in the in-situ leach uranium mining of sandstone-type ore. While addressing the issue of CO2 emissions, it greatly enhances the usage of sandstone-type uranium resources and effectively solves the problem of difficult groundwater pollution treatment [162].
5. Progress of CO2 Storage in China
CO2 storage occurs when captured CO2 is pumped into deep reservoirs employing engineering technologies to establish long-term separation of CO2 from the environment. CO2 storage is categorized into two forms based on its location: geological storage and ocean storage. Figure 5 shows CO2 storage classifications. The Summary of advantages and disadvantages of the various CO2 storage methods based on other literature [58,94,163,164,165,166,167,168,169] is listed in Table 8.
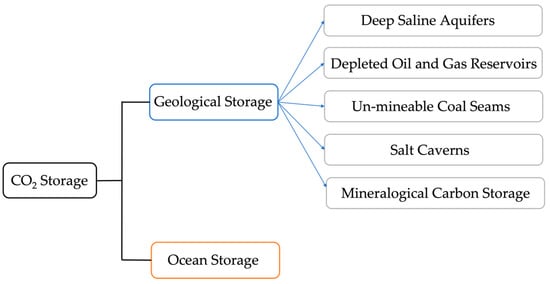
Figure 5.
CO2 storage classifications.

Table 8.
Advantages and disadvantages of CO2 storage methods [58,94,163,164,165,166,167,168,169].
5.1. Development Status of CO2 Geological Storage in China
Currently, geological storage is still the dominant method of CO2 storage [167]. The principle of CO2 geological storage is an imitation of the mechanism of fossil fuel storage in nature. The geological storage of CO2 can be achieved by transporting CO2 to an appropriate location through pipelines or vehicles and injecting it into the ground with specific geological conditions [94,170] and specific depths [170,171]. Currently, the available geological storage of CO2 includes deep saline aquifers, depleted oil and gas reservoirs, un-mineable coal seams, salt caverns, and mineralogical carbon storage.
5.1.1. CO2 Storage in Deep Saline Aquifers in China
In China, the widespread distribution of trap structures in saline aquifers matches well with high-concentration CO2 emission sources, which can reduce transport and comprehensive costs. Meanwhile, deep saline aquifers in China have a huge storage potential, with a theoretical storage capacity of up to 2417 billion tonnes [50]. Therefore, saline aquifers are currently the most widely used CO2 storage method in China. China’s current technology and equipment required for storing CO2 in saline aquifers can completely meet the demands of practical applications.
Saline aquifers located in the Ordos Basin, Bohai Bay Basin, Qaidam Basin, Tarim Basin, Junggar Basin and Songliao Basin are considered to be the most ideal areas for storing CO2 due to their excellent storage capacities and geological conditions [50]. The Ordos Basin was selected as the site for the first demonstration project of full-process CO2 capture and deep saline aquifer storage in China. The CO2 injection experiment was implemented in May 2011, and the injection was stopped in 2015. The target of 30 × 104 tonnes of CO2 injection was completed, and then the monitoring period has begun. The CO2 source of this demonstration project came from the tail gas of the conversion unit of the coal-to-hydrogen plant, and the CO2 with a purity of 88.8% was purified to over 99.99% through gas-liquid separation, oil removal, desulfurization, purification, and distillation processes. Then the CO2 was transported to the storage area by cryogenic tanker truck, firstly into the buffer tank, and later injected into the ground after pressurization and heating. Monitoring data such as buffer tanks, injection wells, monitoring wells, pressure, and temperature could be transmitted to monitoring staff in real-time [172]. Monitoring data for the past nine years show that the groundwater quality, pressure, temperature, land subsidence, surface CO2 concentration, and other indicators in the storage area have not changed significantly, and no CO2 leakage has been monitored using tracer technology [173]. Another demonstration project of full-process CO2 capture and deep saline aquifer storage, operated by CHN ENERGY, began construction in 2019 and is currently in progress. It will be utilized for CO2 storage in saline aquifers after completion with an estimated annual CO2 storage capacity of 15 × 104 tonnes [174]. China’s CO2 storage projects in deep saline aquifers are listed in Table 9.

Table 9.
CO2 storage projects in deep saline aquifers in China [50].
5.1.2. CO2 Storage in Depleted Oil and Gas Fields in China
Depleted oil and gas reservoirs have ideal geological characteristics to retain hydrocarbons and prevent their leakage for millions of years, so they are regarded as another promising site for CO2 storage [175]. Additionally, since these reservoirs have gone through the production stage, a variety of historical data such as logging data, drilling and completion data, formation parameters, and production history, is sufficient to provide a detailed basis for assessing the suitability of CO2 storage [175].
Oil and gas fields in the eastern and northern areas of China that have reached late production phases and are no longer economically feasible for production are expected to be excellent depleted oil and gas field sites for CO2 storage. It is anticipated that the overall CO2 storage capacity of these areas can reach 30% of the total demand for CCUS. The obvious benefits of CO2 storage in depleted oil and gas fields include less additional costs to implement, mature technical processes and ease of management. However, it is worth noting that the leakage of stored CO2 may occur due to fractures created by the design and production of oil and gas fields [176]. Up to now, there are still only a few large-scale projects worldwide using depleted oil and gas fields to store CO2 [177]. Although China possesses the required advanced technology, there is currently no demonstration project for storing CO2 in depleted oil and gas fields since such projects are often launched after the completion of CO2-EOR and CO2-EGR projects.
5.1.3. CO2 Storage in Um-Mineable Coal Seams in China
When a coal mine reaches the late stage of mining and the coalbed methane content is too low to be easily produced, the mine may be considered un-mineable. Although such coal mines are no longer suitable for coalbed methane mining for economic reasons, the capability of coal seams to absorb CO2 enables this type of un-mineable coal seams to function as appropriate sites for CO2 storage.
China is relatively rich in coal resources. Currently, there are few un-mineable coal mines, so China has not yet carried out any CO2 storage projects in un-mineable coal seams. However, some studies on technologies and methods of CO2 storage in um-mineable coal seams and coal mine goafs have been conducted, and the research patent has been released [178], which introduces the key technologies and precautions of the implementation process in detail, laying the foundation for future CO2 storage in un-mineable coal seams in China.
5.1.4. CO2 Storage in Salt Caverns in China
Salt caverns are artificial cavities constructed by dissolving salt deposits using water in deep underground salt formations [168]. The low permeability and visco-plastic properties of rock salt [179] allow salt caverns for the safe containment and storage of large volumes of liquids and gases under high pressure. Therefore, salt caverns have been widely used to store natural gas [180] and hydrogen [181]. The extensive successful track records of salt caverns for natural gas storage suggest that they may serve as a potential option for CO2 geological storage [182].
China’s rock salt has a bedded structure with thin salt layers and interlayers [180], and this brings great challenges for salt cavern construction. Currently, CO2 storage in salt caverns in China is still in the research stage. Zhang et al. carried out the safety and applicability evaluation of CO2 storage in typical bedded rock salt caverns of China [179] by using numerical simulation to preliminary determine the appropriate cavern pressure, volume variation, displacement and other parameters, so as to provide theoretical support for subsequent practical applications.
5.1.5. Mineralogical Carbon Storage in China
Mineralogical carbon storage, also known as carbon mineralization or mineral carbonation, is a process, in which injected CO2 reacts with geological rocks or soil containing high concentrations of divalent cations to form stable carbonate minerals [169,183]. It results in carbon permanently stored with a negligible risk of returning to the atmosphere while generating carbonate products [184,185] that can be beneficially utilized. Therefore, it is considered to be a reliable technology with excellent potential for CO2 storage [185].
Currently, mineralogical carbon storage is still in the stage of laboratory-based and field-based experiments and has not yet been implemented on a large scale throughout the world [169]. The requirement for a significant quantity of fresh water for CO2 dissolution to accelerate the carbonation reaction is one of the primary reasons preventing the large-scale implementation of this technology. Because of this, the research focus of this technology in China is mainly on increasing carbonation reaction speed. The results show that carbonation reactions can be accelerated by optimizing reaction conditions [186,187,188], such as pressure, temperature and solid–liquid ratio, and employing pretreatments [189,190], such as heat treatment and mechanical activation. China has abundant mineral resources of wollastonite [183] and serpentine [191], which are favorable raw materials for carbonation reactions. Therefore, mineralogical carbon storage theoretically has a huge potential for CO2 storage in China.
5.2. Development Status of CO2 Ocean Storage in China
The ocean, which makes up over 71% of the planet’s surface, has the largest potential capacity to store CO2. However, CO2 ocean storage is still subject to some uncertainties, such as the isolation prediction of CO2 emitted to the seafloor, the risk of CO2 leakage during storage in marine sedimentary strata, and the influence of CO2 on pH value change of the seawater, which have limited the deployment of CO2 ocean storage [94,163]. Therefore, no CO2 ocean storage project has been implemented in China before 2021.
In recent years, China has strengthened the progressive exploration of marine geological conditions and continuously improved the CO2 ocean storage technology. China’s first off-shore large-scale CO2 storage demonstration project has all manufacturing and installation completed in June 2022 [54] and began to officially launch [192]. Since 2021, CNOOC has been conducting research on critical technologies for the integration of geological reservoirs, drilling and completion, and engineering appropriate for CO2 ocean storage, and has successfully developed a complete set of technology and equipment systems for CO2 capture, treatment, injection, storage, and monitoring on off-shore platforms, filling the gap in China’s CO2 ocean storage technology [193]. The project is situated in the Enping 15-1 oilfield group in the Pearl River Estuary Basin. It is planned to permanently store 1.46 million tonnes of CO2 in total at an injection rate of about 30 × 104 tonnes/year [194]. In the project, the CO2 associated with the oil field development is injected into the saline aquifer at a depth of 800 m on the seabed. CO2 can be retained effectively and gas leakage can be prevented by making use of the saline aquifer’s dome structure in conjunction with the muddy protective layer of sufficient thickness [194]. The successful implementation of this project will create new industries and new formats of CO2 storage in China, showing important demonstration significance for the environmentally friendly development of offshore oil and gas resources [194].
6. Challenges and Suggestions for Future Development of CCUS in China
Although significant progress has been made in China’s CCUS projects, there are still issues and challenges that limit the large-scale implementation of China’s CCUS projects.
Currently, post-combustion capture, pre-combustion capture and oxy-fuel combustion as traditional CO2 capture technologies have achieved mature development. However, their large-scale demonstration applications have been constrained due to the enormous energy consumption caused by CO2 capture devices [57,58] as well as the high economic cost of CO2 compression [57,58] during the capture process. Therefore, the reduction of energy consumption and cost is the primary research focus of traditional CO2 capture technologies in the future.
In addition, future research on capture technology should concentrate on negative emission technologies, such as direct air carbon capture and storage (DACCS) and bioenergy with carbon capture and storage (BECCS). The development of adsorption materials appropriate for DAC technology has advanced significantly in recent years, and the constant enhancement of the properties of adsorption materials has improved the adsorption efficiency of DAC [71,72,73]. However, the high price of existing adsorption materials and the low concentration of CO2 in the air lead to the high application cost of DAC, making its implementation difficult despite its potential for negative emission [195]. Therefore, accelerating the development of cost-effective adsorption materials should be the research focus of DAC.
BECCS is a greenhouse gas reduction technology that combines carbon capture and storage with the use of biomass to create negative carbon emissions. The basic principle of BECCS is using biomass (CO2-absorbing plants) to generate energy while capturing and storing the resulting CO2 during biomass combustion [196,197]. The most obvious advantage of BECCS is that biomass serves as a renewable energy source to generate bioenergy, and it also acts as a carbon sink by absorbing CO2 during the growth process through photosynthesis [195,198,199]. However, there are several challenges to implementing BECCS in China. One major challenge is the high cost [197,198,199,200,201,202,203] of technology development and implementation. Additionally, there are some environmental challenges [203], such as the waste of land caused by growing biomass plants instead of food, and the drinking water contamination caused by the large-scale growth of biomass, especially algae. Despite these challenges, BECCS has the potential to play a significant role in CO2 reduction in China. To fully realize this potential, it is important to continue investing in research and development, as well as working to address the challenges associated with the technology.
In terms of CO2 transport technology, tank-based transport is the primary method of CO2 transport in China. Although pipeline-based transport is still in the research and pilot stage, it will be the most promising and economical technology for CO2 transport in future large-scale CCUS demonstration projects due to its outstanding advantages and the gradual expansion of CCUS projects. Currently, the implementation of CO2 pipeline transport is constrained by many challenges. One major challenge is that pipeline facilities are usually large and influenced by geographical conditions, which leads to long construction times and high investment costs for pipelines. In addition, pipeline design is affected by the transport states of CO2. There are different requirements [75] for CO2 transport in different states. For example, less than critical pressure is required for gaseous CO2 transport; temperature control is required for liquid CO2 transport; both temperature control and fixed pressure range are required for dense-phase CO2 transport; and above critical temperature and pressure are required for supercritical state CO2 transport. Therefore, it is necessary to comprehensively consider these influencing factors in the pipeline design. The potential improvements in future research should focus on optimizing the pipeline design, developing precise design specifications, and using suitable construction materials for pipelines, so as to achieve the cost-effective and safe transport of CO2 in the pipeline.
The potential for CO2 leakages is another challenge for CO2 transport and subsequent utilization and storage in China. Generally, CO2 leakages are less likely to occur in tank-based transport, because tanker trucks are equipped with reliable safety features to minimize the risk of spills, but CO2 leakages may still occur due to the accident of the truck or the failure of the equipment. CO2 leakages during transport by pipeline are usually caused by CO2 corrosion [204,205] or mechanical damage to pipelines [206,207]. Therefore, it is essential to employ high-quality equipment and advanced materials [208,209] for the transport system to avoid or minimize the potential risk of CO2 leakages during transport. Meanwhile, it is necessary to implement monitoring and leakage detection systems [210,211] to rapidly identify and address any leakages.
The potential reasons for leakages in CO2 utilization and storage may include unstable underground reservoir conditions and inappropriate injection equipment. Therefore, a comprehensive evaluation of geological conditions is required in the site selection for CO2 utilization and storage, and high-quality corrosion-resistant injection equipment is necessary. In addition, the implementation of reliable monitoring and leakage detection systems is also crucial. However, immature technologies of monitoring, leakage prevention and control, and corrosion prevention for post-CO2 injection are the main problems [50] faced by existing CO2-EOR projects in China. Furthermore, the challenge of accurate monitoring is even more prominent in China’s CO2-ECBM projects since accurate monitoring of the movement of one gas is more difficult in a gas-gas system than in a gas-liquid system. Therefore, future research on CO2 utilization and storage in China should focus on improving accurate monitoring and leakage detection technologies, leakage prevention and control technology, and anti-corrosion technology, to achieve effective utilization and safe storage of CO2.
7. Conclusions
Based on a large number of literature, this study demonstrates the latest progress of CCUS development in China after 2019. As a major member of developing countries, China has placed a high priority on the issue of CO2 emissions. With the great support of the Chinese government, a series of scientific research and demonstration projects have been conducted and some remarkable results have been obtained.
Currently, post-combustion capture is the dominant technology for CO2 capture in China, while pre-combustion capture and oxy-fuel combustion are being improved and gradually promoted. Additionally, direct air capture, as a negative carbon emission technology, has attracted more attention. The development of cost-effective adsorption materials used in this technology is becoming a new research focus of capture technology in China.
Tanker truck transport remains the primary mode of CO2 transport due to the limited scale of China’s current CCUS projects. Long-distance pipeline construction is a challenge for CO2 transport, which requires more technical improvement.
China’s CO2 utilization technology has advanced significantly in recent years, yielding considerable benefits. CO2 chemical utilization has realized industrial demonstration, while CO2 biological utilization has achieved significant technological innovations. China’s CO2-EOR has reached the commercial application stage, realizing the obvious enhancement in oil recovery with remarkable economic benefits. CO2 in-situ leach uranium mining is another CO2 utilization technology that is ready for commercial application in China, and it is going to serve as the cornerstone for China’s military development. Deep saline aquifers are the dominant site for CO2 storage, while China’s first off-shore large-scale CO2 storage demonstration project in the Enping oilfield also demonstrates promising prospects of CO2 ocean storage in China.
Author Contributions
All the authors (J.Y., H.H., Y.Y., Y.S. and G.L.) have made substantial contributions to this work; J.Y.: conceptualization and original draft writing; H.H.: original draft writing; Y.Y. and Y.S.: resources and visualization; G.L.: review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Yueliang Liu from the College of Petroleum Engineering, China University of Petroleum (Beijing), for his valuable suggestions and guidance. The authors are also grateful to the Research Institute of Exploration and Development, Liaohe Oilfield Company, CNPC, for the support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Climate Change 2021: The Physical Science Basis; Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar]
- Yu, G.R.; Hao, T.X.; Zhu, J.X. Discussion on action strategies of China’s carbon peak and carbon neutrality. Bull. Chin. Acad. Sci. 2022, 37, 423–434. [Google Scholar]
- Karl, T.R.; Kevin, E.T. Modern global climate change. Science 2003, 302, 1719–1723. [Google Scholar] [CrossRef]
- Clayton, S.; Devine-Wright, P.; Stern, P.C.; Whitmarsh, L.; Carrico, A.; Steg, L.; Swim, J.; Bonnes, M. Psychological research and global climate change. Nat. Clim. Change 2015, 5, 640–646. [Google Scholar] [CrossRef]
- Bodansky, D. The history of the global climate change regime. Int. Relat. Glob. Clim. Change 2001, 23, 505. [Google Scholar]
- McMichael, A.J.; Haines, A. Global climate change: The potential effects on health. Bmj 1997, 315, 805–809. [Google Scholar] [CrossRef]
- Dessler, A.E.; Edward, A.P. The Science and Politics of Global Climate Change: A Guide to the Debate; Cambridge University Press: Cambridge, UK, 2019. [Google Scholar]
- Bord, R.J.; Robert, E.O.; Fisher, A. In what sense does the public need to understand global climate change? Public Underst. Sci. 2000, 9, 205. [Google Scholar] [CrossRef]
- Janssens-Maenhout, G.; Crippa, M.; Guizzardi, D.; Muntean, M.; Schaaf, E.; Dentener, F.; Bergamaschi, P.; Pagliari, V.; Olivier, J.G.; Peters, J.A.; et al. EDGAR v4.3.2 Global Atlas of the three major greenhouse gas emissions for the period 1970–2012. Earth Syst. Sci. Data 2019, 11, 959–1002. [Google Scholar] [CrossRef]
- Mikhaylov, A.; Moiseev, N.; Aleshin, K.; Burkhardt, T. Global climate change and greenhouse effect. Entrep. Sustain. Issues 2020, 7, 2897. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.; Qin, D.; Manning, M.; Averyt, K.; Marquis, M. (Eds.) Climate Change 2007—The Physical Science Basis: Working Group I Contribution to the Fourth Assessment Report of the IPCC; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Hegerl, G.C.; Ulrich, C. Greenhouse gas induced climate change. Environ. Sci. Pollut. Res. 1996, 3, 99–102. [Google Scholar] [CrossRef]
- Lu, Y.; Cui, P.; Li, D. Carbon emissions and policies in China’s building and construction industry: Evidence from 1994 to 2012. Build. Environ. 2016, 95, 94–103. [Google Scholar] [CrossRef]
- Karnauskas, K.B.; Miller, S.L.; Schapiro, A.C. Fossil fuel combustion is driving indoor CO2 toward levels harmful to human cognition. GeoHealth 2020, 4, e2019GH000237. [Google Scholar] [CrossRef] [PubMed]
- Perera, F. Pollution from fossil-fuel combustion is the leading environmental threat to global pediatric health and equity: Solutions exist. Int. J. Environ. Res. Public Health 2018, 15, 16. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Li, G.; Wu, J. Application of In-situ combustion for heavy oil production in China: A Review. J. Oil Gas Petrochem Sci. 2018, 1, 69–72. [Google Scholar] [CrossRef]
- Shan, Y.; Huang, Q.; Guan, D.; Hubacek, K. China CO2 emission accounts 2016–2017. Sci. Data 2020, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Song, Y. Dynamic analysis approach to evaluate in-situ combustion performance for heavy oil production. J. Oil Gas Petrochem Sci. 2019, 2, 42–47. [Google Scholar] [CrossRef]
- Shan, Y.; Guan, D.; Zheng, H.; Ou, J.; Li, Y.; Meng, J.; Mi, Z.; Liu, Z.; Zhang, Q. China CO2 emission accounts 1997–2015. Sci. Data 2018, 5, 1–14. [Google Scholar] [CrossRef]
- Nerem, R.S.; Beckley, B.D.; Fasullo, J.T.; Hamlington, B.D.; Masters, D.; Mitchum, G.T. Climate-change–driven accelerated sea-level rise detected in the altimeter era. Proc. Natl. Acad. Sci. USA 2018, 115, 2022–2025. [Google Scholar] [CrossRef]
- Lindsey, R. Climate Change: Global Sea Level. Available online: http://arizonaenergy.org/News_17/News_Sep17/ClimateChangeGlobalSeaLevel.html (accessed on 11 September 2017).
- Kulp, S.A.; Strauss, B.H. New elevation data triple estimates of global vulnerability to sea-level rise and coastal flooding. Nat. Commun. 2019, 10, 1–12. [Google Scholar]
- Ogawa, F.; Keenlyside, N.; Gao, Y.; Koenigk, T.; Yang, S.; Suo, L.; Wang, T.; Gastineau, G.; Nakamura, T.; Cheung, H.N.; et al. Evaluating impacts of recent Arctic sea ice loss on the northern hemisphere winter climate change. Geophys. Res. Lett. 2018, 45, 3255–3263. [Google Scholar] [CrossRef]
- Ouyang, Z.; Qi, D.; Chen, L.; Takahashi, T.; Zhong, W.; DeGrandpre, M.D.; Chen, B.; Gao, Z.; Nishino, S.; Murata, A.; et al. Sea-ice loss amplifies summertime decadal CO2 increase in the western Arctic Ocean. Nat. Clim. Change 2020, 10, 678–684. [Google Scholar] [CrossRef]
- Rignot, E. Sea level rise from melting glaciers and ice sheets caused by climate warming above pre-industrial levels. Phys. –Uspekhi 2022, 65, 1. [Google Scholar]
- Hock, R.; Huss, M. Glaciers and climate change. Climate Change, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 157–176. [Google Scholar]
- Stillman, J.H. Heat waves, the new normal: Summertime temperature extremes will impact animals, ecosystems, and human communities. Physiology 2019, 34, 86–100. [Google Scholar] [CrossRef]
- Dosio, A.; Mentaschi, L.; Fischer, E.M.; Wyser, K. Extreme heat waves under 1.5 C and 2 C global warming. Environ. Res. Lett. 2018, 13, 054006. [Google Scholar] [CrossRef]
- Lawler, J.J.; White, D.; Neilson, R.P.; Blaustein, A.R. Predicting climate-induced range shifts: Model differences and model reliability. Glob. Change Biol. 2006, 12, 1568–1584. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts–a global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Doak, D.F.; Morris, W.F. Demographic compensation and tipping points in climate-induced range shifts. Nature 2010, 467, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Spence, A.R.; Tingley, M.W. The challenge of novel abiotic conditions for species undergoing climate-induced range shifts. Ecography 2020, 43, 1571–1590. [Google Scholar] [CrossRef]
- Seersholm, F.V.; Werndly, D.J.; Grealy, A.; Johnson, T.; Keenan Early, E.M.; Lundelius, E.L.; Winsborough, B.; Farr, G.E.; Toomey, R.; Hansen, A.J.; et al. Rapid range shifts and megafaunal extinctions associated with late Pleistocene climate change. Nat. Commun. 2020, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Deng, Z.; He, G.; Wang, H.; Zhang, X.; Lin, J.; Qi, Y.; Liang, X. Challenges and opportunities for carbon neutrality in China. Nat. Rev. Earth Environ. 2022, 3, 141–155. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, H.; Ahmad, M.; Xue, C. Analysis of influencing factors of carbon emissions in resource-based cities in the Yellow River basin under carbon neutrality target. Environ. Sci. Pollut. Res. 2022, 29, 23847–23860. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Wei, J.; Wang, R.; Chen, L.; Zhang, Y.; Yang, Z. Exploring Potential Ways to Reduce the Carbon Emission Gap in an Urban Metabolic System: A Network Perspective. Int. J. Environ. Res. Public Health 2022, 19, 5793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ma, X.; Chen, B.; Shang, Y.; Song, M. Challenges toward carbon neutrality in China: Strategies and countermeasures. Resour. Conserv. Recycl. 2022, 176, 105959. [Google Scholar] [CrossRef]
- Sarkodie, S.A.; Strezov, V. Effect of foreign direct investments, economic development and energy consumption on greenhouse gas emissions in developing countries. Sci. Total Environ. 2019, 646, 862–871. [Google Scholar] [CrossRef]
- Muhumuza, R.; Zacharopoulos, A.; Mondol, J.D.; Smyth, M.; Pugsley, A. Energy consumption levels and technical approaches for supporting development of alternative energy technologies for rural sectors of developing countries. Renew. Sustain. Energy Rev. 2018, 97, 90–102. [Google Scholar] [CrossRef]
- Khan, M.K.; Teng, J.Z.; Khan, M.I.; Khan, M.O. Impact of globalization, economic factors and energy consumption on CO2 emissions in Pakistan. Sci. Total Environ. 2019, 688, 424–436. [Google Scholar] [CrossRef]
- Adedoyin, F.F.; Gumede, M.I.; Bekun, F.V.; Etokakpan, M.U.; Balsalobre-Lorente, D. Modelling coal rent, economic growth and CO2 emissions: Does regulatory quality matter in BRICS economies? Sci. Total Environ. 2020, 710, 136284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, Y. Tourism, economic growth, energy consumption, and CO2 emissions in China. Tour. Econ. 2021, 27, 1060–1080. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Kirikkaleli, D.; Adebayo, T.S.; Adeshola, I.; Akinsola, G.D. Modeling CO2 emissions in Malaysia: An application of Maki cointegration and wavelet coherence tests. Environ. Sci. Pollut. Res. 2021, 28, 26030–26044. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Dong, S.; Li, Z.; Li, Y.; Li, S.; Wan, Y. The improvement of CO2 emission reduction policies based on system dynamics method in traditional industrial region with large CO2 emission. Energy Policy 2012, 51, 683–695. [Google Scholar] [CrossRef]
- Qin, J.; Han, H.; Liu, X. Application and enlightenment of CO2 flooding technology in the United States. Pet. Explor. Dev. 2015, 42, 209–216. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, J. Selective adsorption of CO2/CH4 mixture on clay-rich shale using molecular simulations. J. CO2 Util. 2020, 39, 101143. [Google Scholar] [CrossRef]
- Liu, Y.; Rui, Z.; Yang, T.; Dindoruk, B. Using propanol as an additive to CO2 for improving CO2 utilization and storage in oil reservoirs. Appl. Energy 2022, 311, 118640. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, R.; Pan, Y.; Sun, L.; Tang, Y. Experimental Analysis and Numerical Simulation of the Stability of Geological Storage of CO2: A Case Study of Transforming a Depleted Gas Reservoir into a Carbon Sink Carrier. ACS Omega 2021, 6, 34832–34841. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, R.; Bian, X. A review of sequestration projects and application in China. Sci. World J. 2014, 2014, 381854. [Google Scholar] [CrossRef]
- Cai, B.; Li, Q.; Zhang, X. 2021 Annual Report on CO2 Capture Utilization and Storage in China: China’s CCUS Pathways; Ministry of Ecology and Environment of China: Beijing, China, 2021.
- Liu, Y.; Rui, Z. A storage-driven CO2 EOR for a net-zero emission target. Engineering, 2022; in press. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Q.; Li, J.; Han, H. Technology progress and prospects of enhanced oil recovery by gas injection. Acta Pet. Sin. 2020, 41, 1623. [Google Scholar]
- Zhang, T.; Zhang, W.; Yang, R.; Cao, D.; Chen, L.; Li, D.; Meng, L. CO2 injection deformation monitoring based on UAV and InSAR technology: A case study of Shizhuang Town, Shanxi Province, China. Remote Sens. 2022, 14, 237. [Google Scholar] [CrossRef]
- Xinhua Silk Road. Available online: http://stic.sz.gov.cn/gzcy/msss/ztzlstyhj/content/post_9954290.html (accessed on 18 June 2022).
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.; Hauck, J.; Zeng, J. Global carbon budget 2021. Earth Syst. Sci. Data 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- Bachu, S.; Bonijoly, D.; Bradshaw, J.; Burruss, R.; Holloway, S.; Christensen, N.P.; Mathiassen, O.M. CO2 storage capacity estimation: Methodology and gaps. Int. J. Greenh. Gas Control 2007, 1, 430–443. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Zeng, M.; Ouyang, S.; Zhang, Y.; Shi, H. CCS technology development in China: Status, problems and countermeasures—Based on SWOT analysis. Renew. Sustain. Energy Rev. 2014, 39, 604–616. [Google Scholar]
- SISD. Available online: https://sisd.org.cn/express/express598.html (accessed on 24 November 2021).
- Figueroa, J.D.; Fout, T.; Plasynski, S.; McIlvried, H.; Srivastava, R.D. Advances in CO2 capture technology—The US Department of Energy’s Carbon Sequestration Program. Int. J. Greenh. Gas Control 2008, 2, 9–20. [Google Scholar] [CrossRef]
- Liu, Z. Research and Process Optimization Operation Simulation of Carbon Dioxide Capture Absorbent for Coal-Fired Flue Gas. Master’s Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar]
- Wang, L.; Jing, R.; Wang, R.; Li, Q. Research on the CO2 emission reduction technology path of coal-fired flue gas in China’s thermal power industry. J. Beijing Inst. Technol. (Soc. Sci. Ed.) 2022, 24, 4. [Google Scholar]
- Liu, K.; Xu, S.; Li, G.; Ren, Y. Technological process and system analysis of pre-combustion CO2 capture based on IGCC. Chem. Ind. Eng. Prog. 2018, l37, 4897–4907. [Google Scholar]
- Ma, S.; Fan, S.; Wu, K.; Yang, P.; Chen, L. CCUS technology development in coal-fired power plants under the background of dual-carbon strategy: Challenges and responses. Clean Coal Technol. 2022, 28, 1–13. [Google Scholar]
- Li, H.; Dang, C.; Yang, G.; Cao, Y.; Wang, H.; Peng, F.; Yu, H. Process intensification techniques towards carbon dioxide capture: A review. Chem. Ind. Eng. Prog. 2020, 39, 4919–4939. [Google Scholar]
- Guo, J.; Hu, F.; Jiang, X.; Huang, X.; Li, P.; Liu, Z.; Zheng, C. Experimental and numerical investigations on heat transfer characteristics of a 35MW oxy-fuel combustion boiler. Energy Procedia 2017, 114, 481–489. [Google Scholar] [CrossRef]
- China Atmosphere Network. Available online: https://www.chndaqi.com/news/327245.html (accessed on 30 August 2021).
- Liu, L.; Fang, M.; Xu, S.; Wang, J.; Guo, D. Development and testing of a new post-combustion CO2 capture solvent in pilot and demonstration plant. Int. J. Greenh. Gas Control 2022, 113, 103513. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Direct Air Capture: A Key Technology for Net Zero, IEA, Paris. 2022. Available online: https://www.iea.org/reports/direct-air-capture-2022 (accessed on 20 April 2022).
- Ccidmedia. Available online: https://www.ahszyd.com/home/periodical/articleDetail?articleId=3676180 (accessed on 24 March 2022).
- Lin, Z.; Cao, R. Porous Hydrogen-bonded Organic Frameworks (HOFs): Status and Challenges. Acta Chim. Sin. 2020, 78, 1309–1335. [Google Scholar] [CrossRef]
- Hu, F.; Liu, C.; Wu, M.; Pang, J.; Jiang, F.; Yuan, D.; Hong, M. An ultrastable and easily regenerated hydrogen-bonded organic molecular framework with permanent porosity. Angew. Chem. Int. Ed. 2017, 56, 2101–2104. [Google Scholar] [CrossRef]
- Huang, Q.; Li, W.; Mao, Z.; Qu, L.; Li, Y.; Zhang, H.; Yu, T.; Yang, Z.; Zhao, J.; Zhang, Y.; et al. An exceptionally flexible hydrogen-bonded organic framework with large-scale void regulation and adaptive guest accommodation abilities. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Morbee, J.; Serpa, J.; Tzimas, E. Optimised deployment of a European CO2 transport network. Int. J. Greenh. Gas Control 2012, 7, 48–61. [Google Scholar] [CrossRef]
- Lu, H.; Ma, X.; Huang, K.; Fu, L.; Azimi, M. Carbon dioxide transport via pipelines: A systematic review. J. Clean. Prod. 2020, 266, 121994. [Google Scholar] [CrossRef]
- Wilkes, M.D.; Mukherjee, S.; Brown, S. Linking CO2 capture and pipeline transportation: Sensitivity analysis and dynamic study of the compression train. Int. J. Greenh. Gas Control 2021, 111, 103449. [Google Scholar] [CrossRef]
- Jatmoko, F.A.; Kusrini, E. Analysis of CO2 transmission pipelines for CO2 enhanced oil recovery networks: Gas field X to oil field Y. InE3S Web Conf. 2018, 67, 04009. [Google Scholar] [CrossRef]
- Roussanaly, S.; Deng, H.; Skaugen, G.; Gundersen, T. At what pressure shall CO2 be transported by ship? an in-depth cost comparison of 7 and 15 barg shipping. Energies 2021, 14, 5635. [Google Scholar] [CrossRef]
- Roussanaly, S.; Berghout, N.; Fout, T.; Garcia, M.; Gardarsdottir, S.; Nazir, S.M.; Ramirez, A.; Rubin, E.S. Towards improved cost evaluation of carbon capture and storage from industry. Int. J. Greenh. Gas Control 2021, 106, 103263. [Google Scholar] [CrossRef]
- Deng, H.; Roussanaly, S.; Skaugen, G. Techno-economic analyses of CO2 liquefaction: Impact of product pressure and impurities. Int. J. Refrig. 2019, 103, 301–315. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Zhang, J.; Cao, D. Toward a framework of environmental risk management for CO2 geological storage in China: Gaps and suggestions for future regulations. Mitig. Adapt. Strateg. Glob. Change 2016, 21, 191–207. [Google Scholar] [CrossRef]
- Gao, L.; Fang, M.; Li, H.; Hetland, J. Cost analysis of CO2 transportation: Case study in China. Energy Procedia 2011, 4, 5974–5981. [Google Scholar] [CrossRef]
- Yuan, S.; Ma, D.; Li, J.; Zhou, T.; Ji, Z.; Qi, X.; Han, H. Progress and prospects of carbon dioxide capture, EOR-utilization and storage industrialization. Pet. Explor. Dev. 2022, 49, 1–7. [Google Scholar] [CrossRef]
- Huang, C.H.; Tan, C.S. A review: CO2 utilization. Aerosol Air Qual. Res. 2014, 14, 480–499. [Google Scholar] [CrossRef]
- Zhu, Q. Developments on CO2-utilization technologies. Clean Energy 2019, 3, 85–100. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Meylan, F.D.; Moreau, V.; Erkman, S. CO2 utilization in the perspective of industrial ecology, an overview. J. CO2 Util 2015, 12, 101–108. [Google Scholar] [CrossRef]
- Song, C. Global challenges and strategies for control, conversion and utilization of CO2 for sustainable development involving energy, catalysis, adsorption and chemical processing. Catal. Today 2006, 115, 2–32. [Google Scholar] [CrossRef]
- Leitner, W.; Franciò, G.; Scott, M.; Westhues, C.; Langanke, J.; Lansing, M.; Hussong, C.; Erdkamp, E. Carbon2Polymer–Chemical Utilization of CO2 in the Production of Isocyanates. Chem. Ing. Tech. 2018, 90, 1504–1512. [Google Scholar] [CrossRef]
- Ampelli, C.; Perathoner, S.; Centi, G. CO2 utilization: An enabling element to move to a resource-and energy-efficient chemical and fuel production. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2015, 373, 20140177. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. The contribution of the utilization option to reducing the CO2 atmospheric loading: Research needed to overcome existing barriers for a full exploitation of the potential of the CO2 use. Catal. Today 2004, 98, 455–462. [Google Scholar] [CrossRef]
- Otsuki, T. A study for the biological CO2 fixation and utilization system. Sci. Total Environ. 2001, 277, 21–25. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Miao, X.; Gan, M.; Li, X. Geochemistry in geologic CO2 utilization and storage: A brief review. Adv. Geo-Energy Res. 2019, 3, 304–313. [Google Scholar] [CrossRef]
- Li, L.; Zhao, N.; Wei, W.; Sun, Y. A review of research progress on CO2 capture, storage, and utilization in Chinese Academy of Sciences. Fuel 2013, 108, 112–130. [Google Scholar] [CrossRef]
- Daneshvar, E.; Wicker, R.J.; Show, P.L.; Bhatnagar, A. Biologically-mediated carbon capture and utilization by microalgae towards sustainable CO2 biofixation and biomass valorization–a review. Chem. Eng. J. 2022, 427, 130884. [Google Scholar] [CrossRef]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The use of microalgae for coupling wastewater treatment with CO2 biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Wu, D.; Weng, H.; Wang, G. CCUS global progress and China’s policy suggestions. Editor. Dep. Pet. Geol. Recovery Effic. 2020, 27, 20–28. [Google Scholar]
- Alper, E.; Orhan, O.Y. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Meiri, N.; Radus, R.; Herskowitz, M. Simulation of novel process of CO2 conversion to liquid fuels. J. CO2 Util. 2017, 17, 284–289. [Google Scholar] [CrossRef]
- Landau, M.V.; Vidruk, R.; Herskowitz, M. Sustainable production of green feed from carbon dioxide and hydrogen. ChemSusChem 2014, 7, 785–794. [Google Scholar] [CrossRef]
- Riedel, T.; Schaub, G.; Jun, K.W.; Lee, K.W. Kinetics of CO2 hydrogenation on a K-Promoted Fe catalyst. Ind. Eng. Chem. Res 2001, 40, 1355–1363. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, S.; Lee, S.B.; Choi, M.J.; Lee, K.W. Performance of catalytic reactors for the hydrogenation of CO2 to hydrocarbons. Catal. Today 2006, 115, 228–234. [Google Scholar] [CrossRef]
- Sun, K.; Fan, Z.; Ye, J.; Yan, J.; Ge, Q.; Li, Y.; He, W.; Yang, W.; Liu, C.J. Hydrogenation of CO2 to methanol over In2O3 catalyst. J. CO2 Util. 2015, 12, 1–6. [Google Scholar] [CrossRef]
- Cao, Y.; He, X.; Wang, N.; Li, H.R.; He, L.N. Photochemical and electrochemical carbon dioxide utilization with organic compounds. Chin. J. Chem. 2018, 36, 644–659. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.J.; Saravanan, A. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J. CO2 Util. 2019, 33, 131–147. [Google Scholar] [CrossRef]
- Whipple, D.T.; Kenis, P.J. Prospects of CO2 utilization via direct heterogeneous electrochemical reduction. J. Phys. Chem. Lett. 2010, 1, 3451–3458. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bhoi, P.R. An overview of non-biodegradable bioplastics. J. Clean. Prod. 2021, 294, 126218. [Google Scholar] [CrossRef]
- Hu, B.; Guild, C.; Suib, S.L. Thermal, electrochemical, and photochemical conversion of CO2 to fuels and value-added products. J. CO2 Util. 2013, 1, 18–27. [Google Scholar] [CrossRef]
- Dang, S.; Qin, B.; Yang, Y.; Wang, H.; Cai, J.; Han, Y.; Li, S.; Gao, P.; Sun, Y. Rationally designed indium oxide catalysts for CO2 hydrogenation to methanol with high activity and selectivity. Sci. Adv. 2020, 6, eaaz2060. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, M.; Sun, J.; Fang, C. Discussion on the utilization of carbon dioxide carboxylation. Prog. Chem. Ind. 2019, 38, 229–243. [Google Scholar]
- Chinese Science News. Available online: https://www.cas.cn/zt/kjzt/sswcj/mt/202112/t20211217_4818570.shtml (accessed on 15 December 2021).
- Science Net. Available online: https://news.sciencenet.cn/htmlnews/2017/6/379127.shtm (accessed on 13 June 2017).
- China Petroleum News Center. Available online: http://news.cnpc.com.cn/system/2022/06/28/030072124.shtml (accessed on 28 June 2022).
- Dalian Tianjian.com. Available online: https://dalian.runsky.com/2020-01/17/content_6001709.html (accessed on 17 January 2020).
- Sohu. Available online: https://www.sohu.com/a/426928600_747560 (accessed on 24 October 2020).
- Sohu. Available online: https://www.sohu.com/a/421816142_100273878 (accessed on 30 September 2020).
- China Polymer Network. Available online: http://www.polymer.cn/polymernews/2010-6-29/_2010629103605335_2.htm (accessed on 29 June 2010).
- Sina Finance. Available online: http://www.ccin.com.cn/detail/e4e9bb5ca82156bf5956ff76d873c1d7 (accessed on 24 May 2022).
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO 2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef]
- Gao, P.; Cui, X.; Zhong, L. CO/CO2 hydrogenation to chemicals and liquid fuels with high selectivity. Chem. Ind. Eng. Prog. 2019, 38, 183–195. [Google Scholar]
- Wang, T.; Yang, C.; Gao, P.; Zhou, S.; Li, S.; Wang, H.; Sun, Y. ZnZrOx integrated with chain-like nanocrystal HZSM-5 as efficient catalysts for aromatics synthesis from CO2 hydrogenation. Appl. Catal. B Environ. 2021, 286, 119929. [Google Scholar] [CrossRef]
- Li, Z.; Wu, W.; Wang, M.; Wang, Y.; Ma, X.; Luo, L.; Chen, Y.; Fan, K.; Pan, Y.; Li, H.; et al. Ambient-pressure hydrogenation of CO2 into long-chain olefins. Nat. Commun. 2022, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- People.cn. Available online: http://nm.people.com.cn/n2/2022/0605/c347194-35301488.html (accessed on 5 June 2022).
- Mi, J.; Liu, D.; Zhang, X.; He, W. Research progress on catalysts for methane and carbon dioxide dry gas reforming. Coal Chem. Ind. 2019, 47, 6–9. [Google Scholar]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 69, 199–206. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, K.; Pan, M. Research progress of ternary thermodynamics of CHO in the study of carbon deposition in methane reforming. Prog. Chem. Ind. 2015, 34, 60–65. [Google Scholar]
- Sun, F.M.; Yan, C.F.; Guo, C.Q.; Huang, S.L. Ni/Ce–Zr–O catalyst for high CO2 conversion during reverse water gas shift reaction (RWGS). Int. J. Hydrog. Energy 2015, 40, 15985–15993. [Google Scholar] [CrossRef]
- Shanghai Advanced Research Institute. Available online: http://www.shb.cas.cn/kjjz2016/201708/t20170814_4844263.html (accessed on 14 August 2017).
- Chen, Q.; Gu, Y.; Tang, Z.; Sun, Y. A carbon emission reduction scheme centered on the large-scale utilization of carbon dioxide. Proc. Chin. Acad. Sci. 2019, 34, 478–487. [Google Scholar]
- Shao, B.; Sun, Z.; Zhang, Y. Research progress on the conversion of carbon dioxide into syngas and high value-added products. Prog. Chem. Ind. 2022, 41, 1136–1151. [Google Scholar]
- NetEase. Available online: https://www.163.com/dy/article/GOT6MLKQ0532PL1J.html (accessed on 16 November 2021).
- Jiang, J. Research Status and Development Prospects of Biomass Energy Application. For. Prod. Chem. Ind. 2002, 22, 75–80. [Google Scholar]
- Chauvy, R.; De Weireld, G. CO2 utilization technologies in Europe: A short review. Energy Technol. 2020, 8, 2000627. [Google Scholar] [CrossRef]
- Tan, C.; Tao, F.; Xu, P. Direct carbon capture for the production of high-performance biodegradable plastics by cyanobacterial cell factories. Green Chem. 2022, 24, 4470–4483. [Google Scholar] [CrossRef]
- Cai, T.; Sun, H.; Qiao, J.; Zhu, L.; Zhang, F.; Zhang, J.; Tang, Z.; Wei, X.; Yang, J.; Yuan, Q.; et al. Cell-free chemoenzymatic starch synthesis from carbon dioxide. Science 2021, 373, 1523–1527. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Shao, M.; Li, D.; Fan, F.; Xu, H.; Lu, F.; Bi, C.; Zhu, X.; Zhang, X. Construction of a carbon-conserving pathway for glycolate production by synergetic utilization of acetate and glucose in Escherichia coli. Metab. Eng. 2020, 61, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; El, H.M.; Shen, C.; You, W.; Lu, Y.; Li, J.; Jing, X.; Hu, Q.; Zhou, W.; Poetsch, A.; et al. Transcriptomic and proteomic responses to very low CO2 suggest multiple carbon concentrating mechanisms in Nannochloropsis oceanica. Biotechnol. Biofuels 2019, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Chen, W. Building a carbon cycle model and improving the carbon emission reduction capacity-Sinopec started building China’s first million-ton CCUS project. Environ. Econ. 2021, 13, 58–59. [Google Scholar]
- World Wide Web. Available online: https://china.huanqiu.com/article/49RFnbUCpmi (accessed on 29 August 2022).
- Shi, Y.; Jia, Y.; Pan, W.; Yan, J.; Huang, L. Mechanism of supercritical CO2 flooding in low-permeability tight gas reservoirs. Oil Gas Geol. 2017, 38, 7. [Google Scholar]
- Zhou, J. Feasibility analysis of CO2-enhanced shale gas exploitation and geological storage. In Proceedings of the National Symposium on Special Gas Reservoir Development Technology, Beijing, China, 26–28 March 2013. [Google Scholar]
- State-Owned Assets Supervision and Administration Commission of the State Council Website. Available online: https://news.sina.com.cn/c/2020-08-03/doc-iivhuipn6486866.shtml (accessed on 3 August 2020).
- Chinanews. Available online: https://www.chinanews.com.cn/sh/2019/03-26/8790938.shtml (accessed on 26 March 2019).
- Petroleum Link. Available online: https://www.jiemian.com/article/5411851.html (accessed on 16 December 2020).
- Chen, S.; Zhu, Y.; Wang, H.; Liu, H.; Wei, W.; Luo, Y.; Li, W.; Fang, J. Research status and development trend of shale gas in China. Chin. J. Pet. 2010, 31, 689–694. [Google Scholar]
- Shi, X.; Zhu, D.; Yang, L.; Li, D. Numerical simulation of CO2 injection for enhanced shale gas recovery. In Proceedings of the China Mechanics Conference 2017, Beijing, China, 13 August 2017. [Google Scholar]
- Sun, H.; Zhao, H.; Qi, N.; Zhang, X.; Li, Y. Exploration of capturing CO2 from flue gas by calcite slit-nanopores: A computational investigation. Energy Technol. 2018, 9, 1732–1738. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, J.; Xian, X.; Tang, J.; Zhou, L.; Jiang, Y.; Xia, B.; Wang, X.; Kang, Y. Research progress and prospect of integration of supercritical CO2 enhanced shale gas exploitation and geological storage. Nat. Gas Ind. 2021, 41, 60–73. [Google Scholar]
- Xinhuanet. Available online: http://www.xinhuanet.com/science/2021-04/15/c_139881335.htm (accessed on 15 April 2021).
- Wang, J.; Wang, H.; Yu, H. Feasibility analysis of enhanced coalbed methane recovery (CO2-ECBM) by flue gas injection. J. Anhui Norm. Univ. Nat. Sci. Ed. 2005, 28, 344–347. [Google Scholar]
- Gan, M.; Zhang, L.; Miao, X.; Oladyshkin, S.; Cheng, X.; Wang, Y.; Shu, Y.; Su, X.; Li, X. Application of computed tomography (CT) in geologic CO2 utilization and storage research: A critical review. J. Nat. Gas Sci. Eng. 2020, 83, 103591. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Guo, H. The application and development prospect of supercritical CO2 fracturing in coal-rock reservoir to stimulate coalbed methane. China Min. 2021, 30, 160–167. [Google Scholar]
- Fan, C.; Elsworth, D.; Li, S.; Zhou, L.; Yang, Z.; Song, Y. Thermo-hydro-mechanical-chemical couplings controlling CH4 production and CO2 sequestration in enhanced coalbed methane recovery. Energy 2019, 173, 1054–1077. [Google Scholar] [CrossRef]
- Bai, G.; Su, J.; Zhang, Z.; Lan, A.; Zhou, X.; Gao, F.; Zhou, J. Effect of CO2 injection on CH4 desorption rate in poor permeability coal seams: An experimental study. Energy 2022, 238, 121674. [Google Scholar] [CrossRef]
- Li, R.; Ge, Z.; Wang, Z.; Zhou, Z.; Zhou, J.; Li, C. Effect of Supercritical Carbon Dioxide (ScCO2) on the Microstructure of Bituminous Coal with Different Moisture Contents in the Process of ScCO2 Enhanced Coalbed Methane and CO2 Geological Sequestration. Energy Fuels 2022, 36, 3680–3694. [Google Scholar] [CrossRef]
- Zhang, K.; Sang, S.; Liu, C.; Ma, M.; Zhou, X. Experimental study the influences of geochemical reaction on coal structure during the CO2 geological storage in the deep coal seam. J. Pet. Sci. Eng. 2019, 178, 1006–1017. [Google Scholar] [CrossRef]
- Observer Network. Available online: http://inews.ifeng.com/yidian/42939113/news.shtml?ch=ref_zbs_ydzx_news (accessed on 15 January 2015).
- Shen, N.; Li, J.; Guo, Y.; Li, X. Thermodynamic modeling of in situ leaching of sandstone-type uranium minerals. J. Chem. Eng. Data 2020, 65, 2017–2031. [Google Scholar] [CrossRef]
- Niu, Q.; Wang, W.; Zheng, Y.; Su, X.; Zhou, G.; Zhao, L.; Li, S.; Zhou, X.; Yuan, W.; Wen, L.; et al. A Low Permeability Sandstone Uranium Ore In-Situ Leaching Mining Method, Device, and Terminal Equipment. CN114183118A, 15 March 2022. [Google Scholar]
- Niu, Q.; Wang, W.; Su, X.; Zhou, G.; Zhao, L.; Li, S.; Yuan, W.; Wen, L.; Chang, J.; Zheng, Y.; et al. A Test Device for Carbon Dioxide Blasting and In-Situ Leaching of Carbon Dioxide and Oxygen in Uranium Mines. CN114152731 A, 8 March 2022. [Google Scholar]
- Chang, J.; Wang, W.; Su, X.; Zhou, M.; Niu, Q.; Li, S.; Liu, J.; Yuan, W.; Wen, L. Sandstone Uranium Mine Horizontal Well In-Situ Leaching Mining Simulation Test System. CN216665589U, 3 June 2022. [Google Scholar]
- Su, X.; Du, Z. Development status and prospect of in-situ leaching uranium mining technology in China. China Min. Ind. 2012, 21, 79–83. [Google Scholar]
- Metz, B.; Davidson, O.; De Coninck, H.C.; Loos, M.; Meyer, L. IPCC Special Report on Carbon Dioxide Capture and Storage; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Heddle, G.; Herzog, H.; Klett, M. The Economics of CO2 Storage. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, August 2003. [Google Scholar]
- Li, X.; Fang, Z.; Wei, N.; Bai, B. Discussion on technical roadmap of CO2 capture and storage in China. Rock Soil Mech 2009, 30, 2674–2678. [Google Scholar]
- Shi, J.Q.; Durucan, S. CO2 storage in deep unminable coal seams. Oil Gas Sci. Technol. 2005, 60, 547–558. [Google Scholar] [CrossRef]
- Baines, S.J.; Worden, R.H. Geological storage of carbon dioxide. Geol. Soc. Lond. Spec. Publ. 2004, 233, 1–6. [Google Scholar] [CrossRef]
- Małachowska, A.; Łukasik, N.; Mioduska, J.; Gębicki, J. Hydrogen storage in geological formations—The potential of salt caverns. Energies 2022, 15, 5038. [Google Scholar] [CrossRef]
- Snæbjörnsdóttir, S.Ó.; Sigfússon, B.; Marieni, C.; Goldberg, D.; Gislason, S.R.; Oelkers, E.H. Carbon dioxide storage through mineral carbonation. Nat. Rev. Earth Environ. 2020, 1, 90–102. [Google Scholar] [CrossRef]
- Ha-Duong, M.; Keith, D.W. Carbon storage: The economic efficiency of storing CO2 in leaky reservoirs. In Technological Choices for Sustainability; Springer: Berlin/Heidelberg, Germany, 2004; pp. 165–182. [Google Scholar]
- Zhou, D.; Zhao, Z.; Liao, J.; Sun, Z. A preliminary assessment on CO2 storage capacity in the Pearl River Mouth Basin offshore Guangdong, China. Int. J. Greenh. Gas Control 2011, 5, 308–317. [Google Scholar] [CrossRef]
- Carbon Trading Network. Available online: http://www.tanpaifang.com/CCUS/202004/0269674.html (accessed on 2 April 2020).
- Xinhuanet. Available online: http://www.xinhuanet.com/politics/2019-04/30/c_1124439694.htm (accessed on 30 April 2019).
- Polaris Carbon Stewardship Network. Available online: https://news.bjx.com.cn/html/20220906/1253394.shtml (accessed on 6 September 2022).
- Callas, C.; Saltzer, S.D.; Davis, J.S.; Hashemi, S.S.; Kovscek, A.R.; Okoroafor, E.R.; Wen, G.; Zoback, M.D.; Benson, S.M. Criteria and workflow for selecting depleted hydrocarbon reservoirs for carbon storage. Appl. Energy 2022, 324, 119668. [Google Scholar] [CrossRef]
- Hannis, S.; Lu, J.; Chadwick, A.; Hovorka, S.; Kirk, K.; Romanak, K.; Pearce, J. CO2 storage in depleted or depleting oil and gas fields: What can we learn from existing projects? Energy Procedia 2017, 114, 5680–5690. [Google Scholar] [CrossRef]
- China Energy Network. Available online: https://www.hxny.com/nd-69390-0-17.html (accessed on 26 April 2022).
- Chen, W.; Ni, X.; Liu, M.; Wang, Q.; Li, Q. Method for Sequestering Carbon Dioxide. CN102942006a, 27 February 2013. [Google Scholar]
- Zhang, X.; Liu, W.; Chen, J.; Jiang, D.; Fan, J.; Daemen, J.J.K.; Qiao, W. Large-scale CO2 disposal/storage in bedded rock salt caverns of China: An evaluation of safety and suitability. Energy 2022, 249, 123727. [Google Scholar] [CrossRef]
- Yang, C.; Wang, T.; Li, Y.; Yang, H.; Li, J.; Qu, D.; Xu, B.; Yang, Y.; Daemen, J.J.K. Feasibility analysis of using abandoned salt caverns for large-scale underground energy storage in China. Appl. Energy 2015, 137, 467–481. [Google Scholar] [CrossRef]
- Bai, M.; Song, K.; Sun, Y.; He, M.; Li, Y.; Sun, J. An overview of hydrogen underground storage technology and prospects in China. J. Pet. Sci. Eng. 2014, 124, 132–136. [Google Scholar] [CrossRef]
- Shi, J.Q.; Durucan, S. CO2 storage in caverns and mines. Oil Gas Sci. Technol. 2005, 60, 569–571. [Google Scholar] [CrossRef]
- Liu, W.; Teng, L.; Rohani, S.; Qin, Z.; Zhao, B.; Xu, C.C.; Ren, S.; Liu, Q.; Liang, B. CO2 mineral carbonation using industrial solid wastes: A review of recent developments. Chem. Eng. J. 2021, 416, 129093. [Google Scholar] [CrossRef]
- Yadav, S. Carbon storage by mineral carbonation and industrial applications of CO2. Mater. Sci. Energy Technol. 2020, 3, 494–500. [Google Scholar]
- Hills, C.D.; Tripathi, N.; Carey, P.J. Mineralization technology for carbon capture, utilization, and storage. Front. Energy Res. 2020, 8, 142. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Bai, X.; Luo, W.; Tang, H.; Cao, Y.; Wu, L.; Chen, F.; Li, Q.; Zeng, C.; et al. Spatiotemporal distribution and national measurement of the global carbonate carbon sink. Sci. Total Environ. 2018, 643, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, S.; Bai, X.; Cao, Y.; Wu, L. Spatiotemporal evolution of carbon sequestration of limestone weathering in China. Sci. China Earth Sci. 2019, 62, 974–991. [Google Scholar] [CrossRef]
- Li, H.W.; Wang, S.J.; Bai, Y.X.; Cao, Y.; Tian, Y.C.; Luo, G.J.; Chen, F.; Li, Q.; Wu, L.H.; Wang, J.F. Effects of climate change and ecological restoration on carbonate rock weathering carbon sequestration in the karst valley of Southwest China. Acta Ecol. Sin. 2019, 39, 6158–6172. [Google Scholar]
- Li, Z.W.; Li, W.; Bai, Z.Q.; Li, B.Q. Sequest ration of carbon dioxide with olivine promoted by an electrochemical method. J.-China Univ. Min. Technol. 2010, 39, 38–42. [Google Scholar]
- Li, W.; Li, W.; Li, B. Using Electrolytic Method to Promote CO2 Sequestration in Serpentine by Mineral Carbonation. J.-China Univ. Min. Technol. 2007, 36, 817. [Google Scholar]
- Liu, Z.; Wu, Y. Geological characteristics and development and utilization status of serpentinite deposits in China. Chem. Miner. Geol. 2015, 37, 171–179. [Google Scholar]
- Xinhua Finance. Available online: https://www.cnfin.com/qy-lb/detail/20220628/3649217_1.html (accessed on 28 June 2022).
- Polaris Carbon Butler Network. Available online: https://news.bjx.com.cn/html/20210830/1173445.shtml (accessed on 30 August 2021).
- Xinhua Silk Road. Available online: https://www.imsilkroad.com/news/p/462448.html (accessed on 1 September 2021).
- Babin, A.; Vaneeckhaute, C.; Iliuta, M.C. Potential and challenges of bioenergy with carbon capture and storage as a carbon-negative energy source: A review. Biomass Bioenergy 2021, 146, 105968. [Google Scholar] [CrossRef]
- Bui, M.; Fajardy, M.; Mac Dowell, N. Bio-Energy with CCS (BECCS) performance evaluation: Efficiency enhancement and emissions reduction. Appl. Energy 2017, 195, 289–302. [Google Scholar] [CrossRef]
- Favero, A.; Mendelsohn, R. Using markets for woody biomass energy to sequester carbon in forests. J. Assoc. Environ. Resour. Econ. 2014, 1, 75–95. [Google Scholar] [CrossRef]
- Moreira, J.R.; Romeiro, V.; Fuss, S.; Kraxner, F.; Pacca, S.A. BECCS potential in Brazil: Achieving negative emissions in ethanol and electricity production based on sugar cane bagasse and other residues. Appl. Energy 2016, 179, 55–63. [Google Scholar] [CrossRef]
- Kirubakaran, V.; Sivaramakrishnan, V.; Nalini, R.; Sekar, T.; Premalatha, M.; Subramanian, P. A review on gasification of biomass. Renew. Sustain. Energy Rev. 2009, 13, 179–186. [Google Scholar] [CrossRef]
- Al-Qayim, K.; Nimmo, W.; Hughes, K.; Pourkashanian, M. Kinetic parameters of the intrinsic reactivity of woody biomass and coal chars via thermogravimetric analysis. Fuel 2017, 210, 811–825. [Google Scholar] [CrossRef]
- Keller, M.; Kaibe, K.; Hatano, H.; Otomo, J. Techno-economic evaluation of BECCS via chemical looping combustion of Japanese woody biomass. Int. J. Greenh. Gas Control 2019, 83, 69–82. [Google Scholar] [CrossRef]
- Buss, W.; Jansson, S.; Wurzer, C.; Mašek, O. Synergies between BECCS and biochar—Maximizing carbon sequestration potential by recycling wood ash. ACS Sustain. Chem. Eng. 2019, 7, 4204–4209. [Google Scholar] [CrossRef]
- Johnson, N.; Parker, N.; Ogden, J. How negative can biofuels with CCS take us and at what cost? Refining the economic potential of biofuel production with CCS using spatially-explicit modeling. Energy Procedia 2014, 63, 6770–6791. [Google Scholar] [CrossRef]
- Cui, G.; Yang, Z.; Liu, J.; Li, Z. A comprehensive review of metal corrosion in a supercritical CO2 environment. Int. J. Greenh. Gas Control 2019, 90, 102814. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, K. Influence of SO2 on the corrosion and stress corrosion cracking susceptibility of supercritical CO2 transportation pipelines. Corros. Sci. 2020, 165, 108404. [Google Scholar] [CrossRef]
- Gu, S.; Li, Y.; Teng, L.; Wang, C.; Hu, Q.; Zhang, D.; Ye, X.; Wang, J.; Iglauer, S. An experimental study on the flow characteristics during the leakage of high pressure CO2 pipelines. Process Saf. Environ. Prot. 2019, 125, 92–101. [Google Scholar] [CrossRef]
- Li, Y.; Gu, S.; Zhang, D.; Hu, Q.; Teng, L.; Wang, C. An experimental study on the choked flow characteristics of CO2 pipelines in various phases. Chin. J. Chem. Eng. 2021, 32, 17–26. [Google Scholar] [CrossRef]
- Sun, C.; Liu, S.; Li, J.; Zeng, H.; Luo, J.L. Insights into the interfacial process in electroless Ni–P coating on supercritical CO2 transport pipeline as relevant to carbon capture and storage. ACS Appl. Mater. Interfaces 2019, 11, 16243–16251. [Google Scholar] [CrossRef] [PubMed]
- Zargarnezhad, H.; Asselin, E.; Wong, D.; Lam, C.C. A critical review of the time-dependent performance of polymeric pipeline coatings: Focus on hydration of epoxy-based coatings. Polymers 2021, 13, 1517. [Google Scholar] [CrossRef]
- Sleiti, A.K.; Al-Ammari, W.A.; Vesely, L.; Kapat, J.S. Carbon dioxide transport pipeline systems: Overview of technical characteristics, safety, integrity and cost, and potential application of digital twin. J. Energy Resour. Technol. 2022, 144, 092106. [Google Scholar] [CrossRef]
- Kim, Y.J.; He, W.; Yoo, G. Suggestions for plant parameters to monitor potential CO2 leakage from carbon capture and storage (CCS) sites. Greenh. Gases Sci. Technol. 2019, 9, 387–396. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).