The Influencing Factors of CO2 Utilization and Storage Efficiency in Gas Reservoir
Abstract
1. Introduction
2. Experiments
2.1. Materials
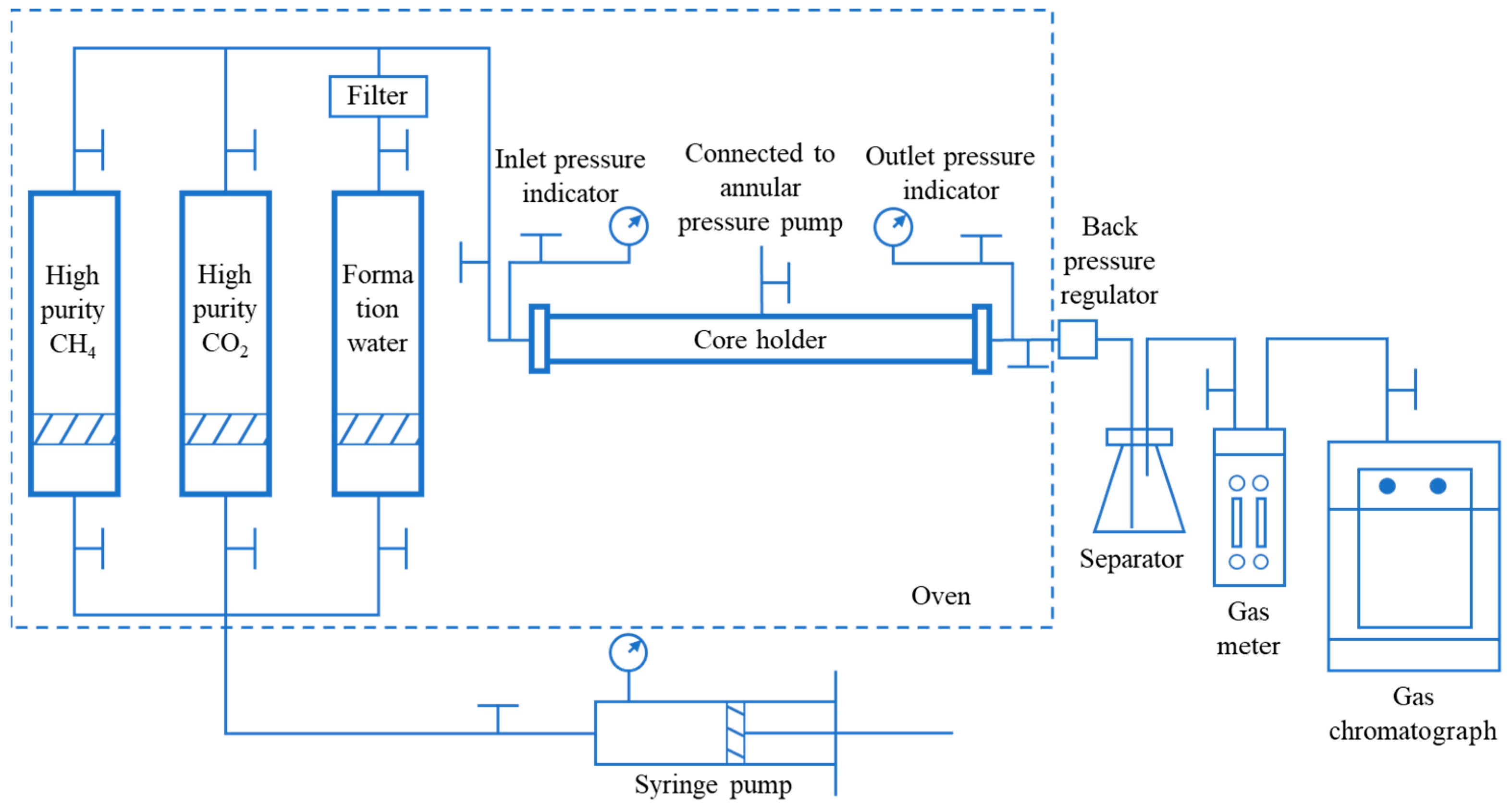
2.2. Experimental Set-Up
2.3. Procedure
- The short cores were loaded into the long core holder in sequence and put into the convection oven with a certain dip angle according to the experimental design. Then, the hold system was connected to the injection and production systems;
- The long cores were displaced with the mixture of petroleum ether and absolute ethanol for cleaning until the outlet fluid has no discolorations and impurities when the confining pressure was improved to 10 MPa, and the speed of displacement pump was set to 5 mL/min with a max pressure of 5 MPa;
- The long cores were displaced with nitrogen at a constant pressure of 2 MPa for 12 h and vacuumized for 5 h after drying;
- The long cores were saturated by high purity CH4 with 8 MPa displacing pressure and 12 MPa confining pressure till the inlet pressure stabilized at 8 MPa. The outlet valve was closed during the saturating process;
- The CH4 contained in the cores were displaced by connecting the CO2 container until the CO2 content of outlet reached more than 98% or until the recovery rate of CH4 did not increase. The displacement speed was set as experimental design; the back pressure was set to 8 MPa; and the composition of produced gas was recorded every 0.1 PV. Then, the CH4 recovery was calculated by analyzing the contents of CH4 and CO2 measured through chromatograph;
- The cores were vacuumized and saturated with CH4 again to conduct next displacement experiment as the experimental design after this experiment was conducted;
- Different dip angles represent for “high injection and low production” and “low injection and high production” could be achieved by changing the angle of the long core holder;
- After the cores were saturated with formation water, the original irreducible water saturation could be established by displacing formation water with CH4 under the experimental conditions until no water produced.
2.4. Measure Tools and Error Estimation
3. Numerical Simulations
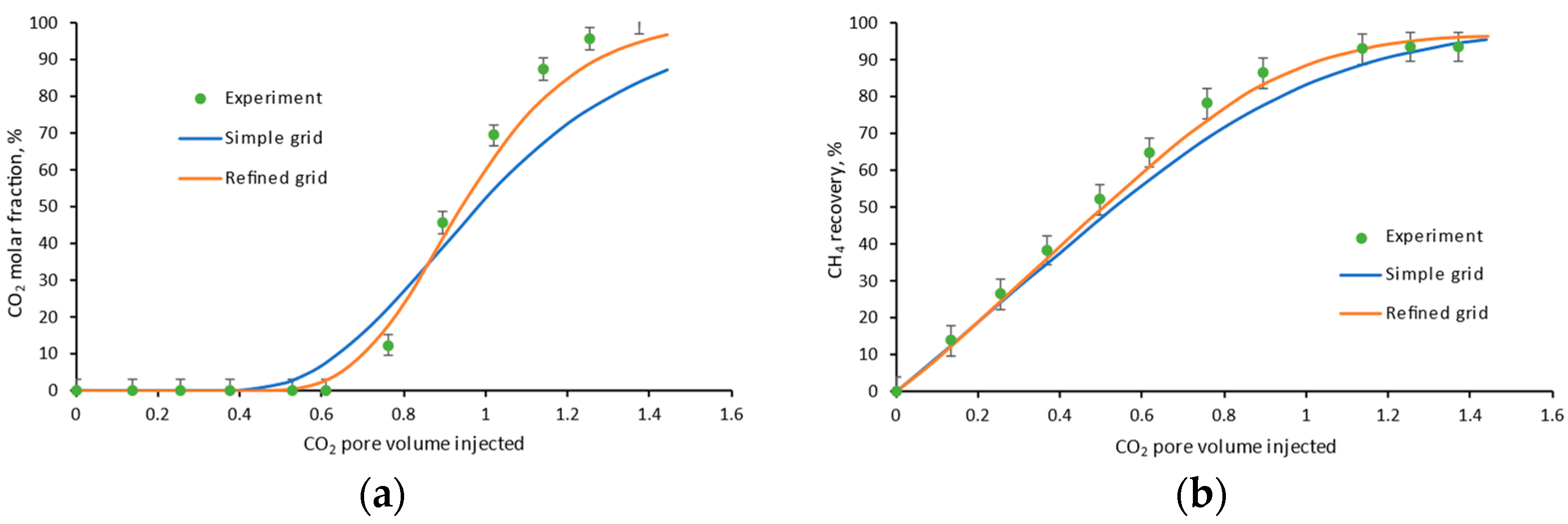
3.1. One-Dimensional Simulations for Long Core Displacement
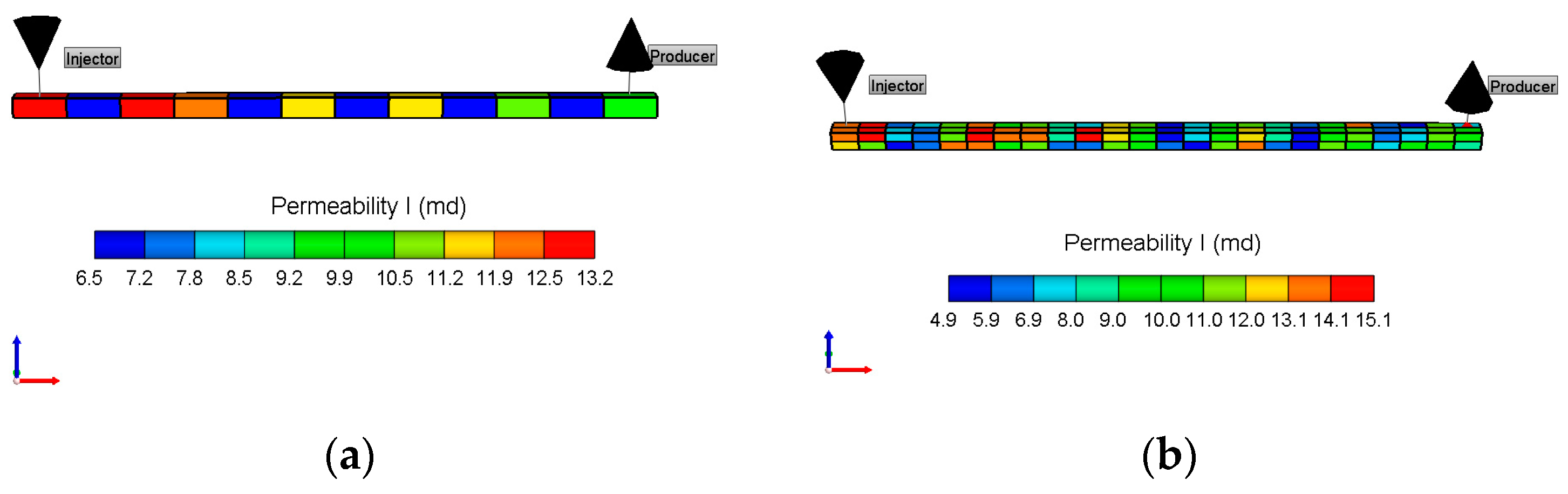
3.2. Two-Dimensional Simulations for Heterogeneous Analyses
3.3. Three-Dimensional Simulations for Storage Mechanism Analyses
4. Results
4.1. Fluid Property
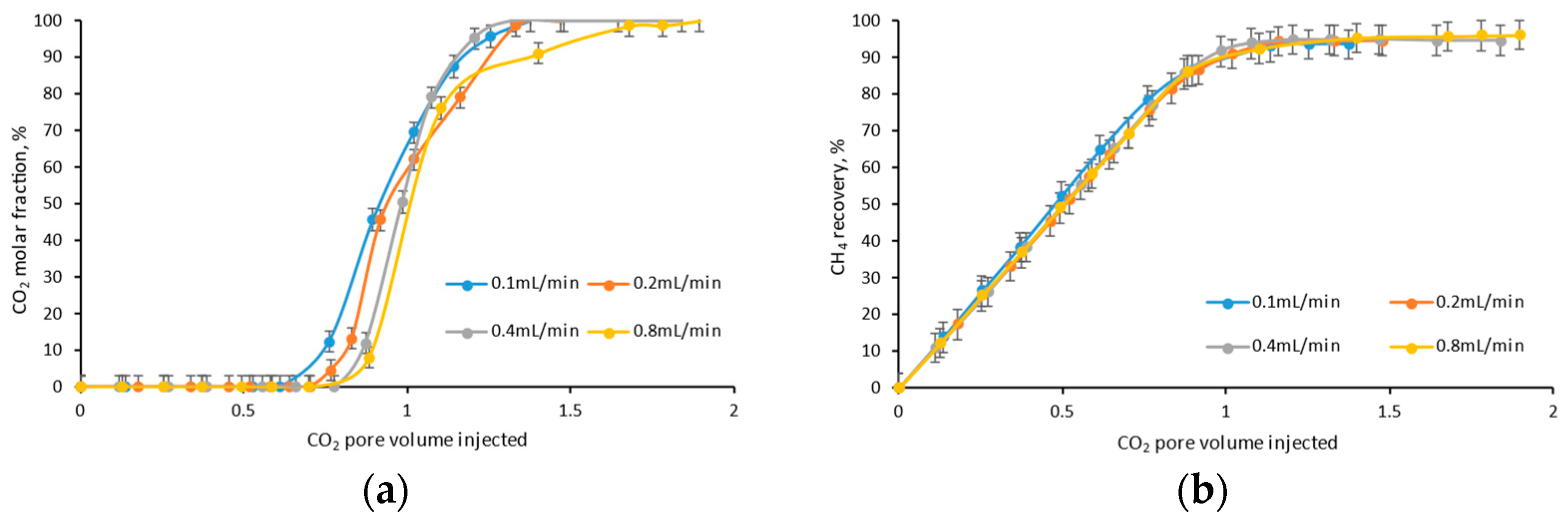
4.2. Injection Rate
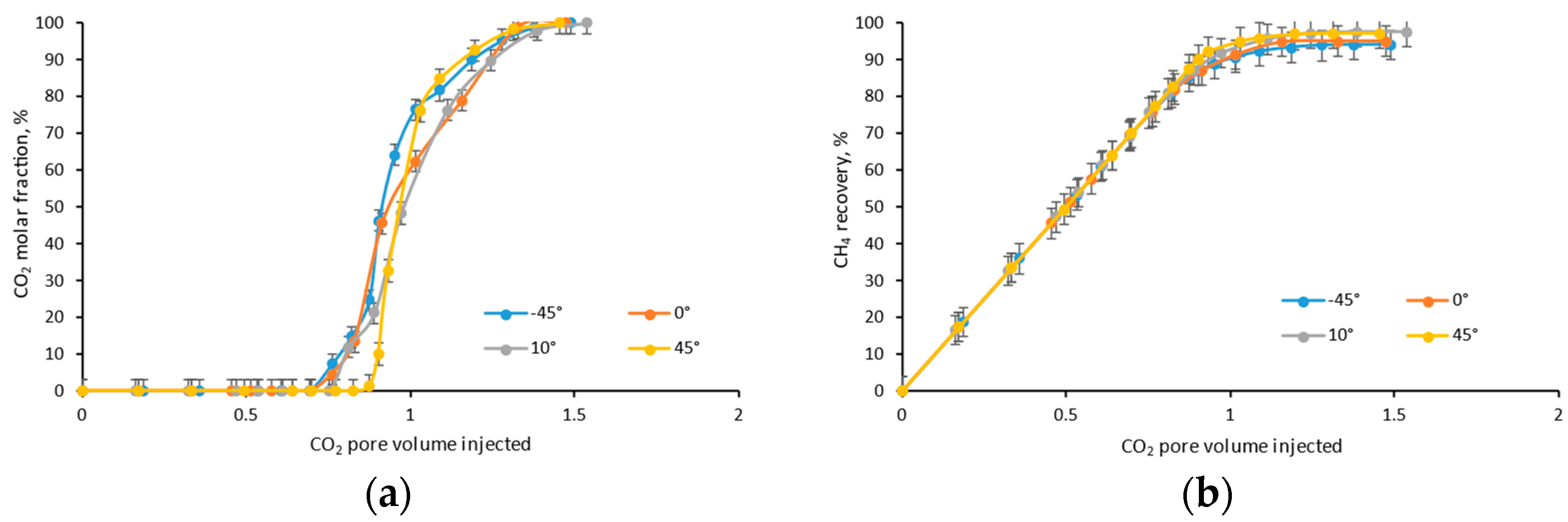
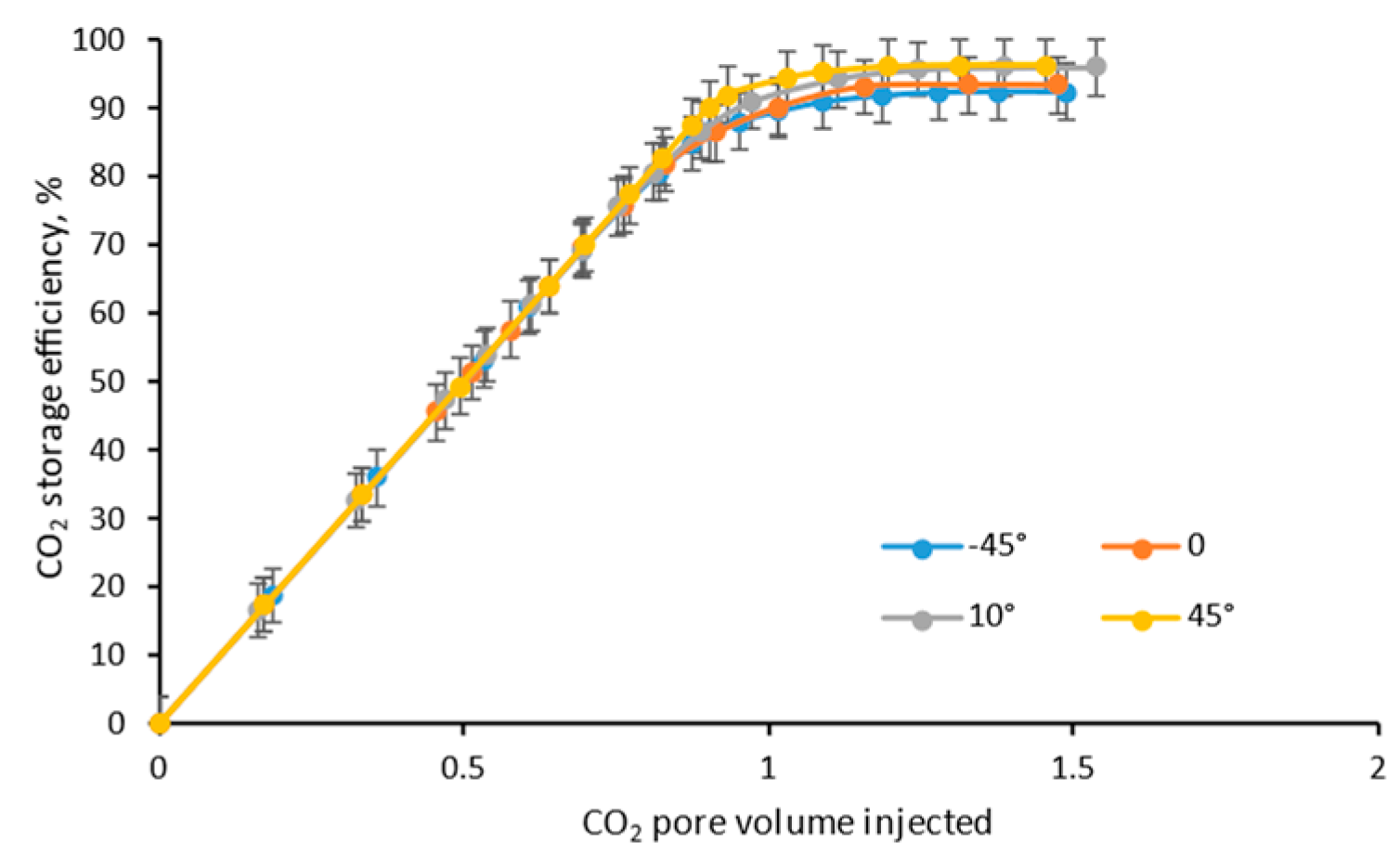
4.3. Dip Angle
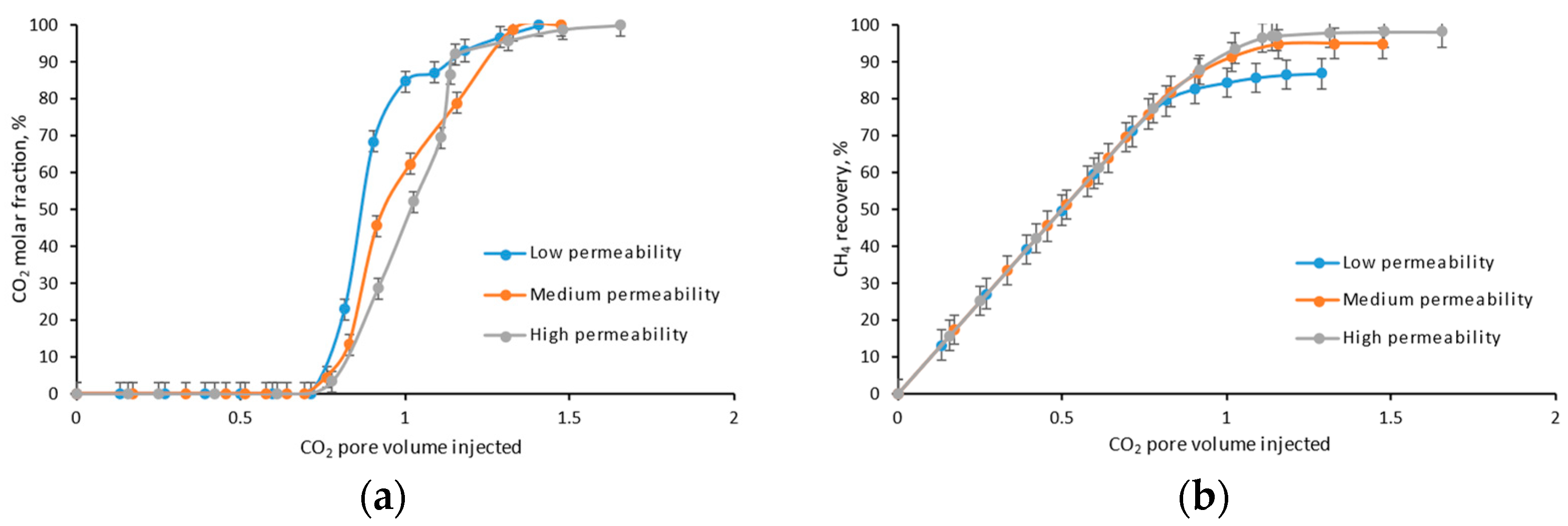
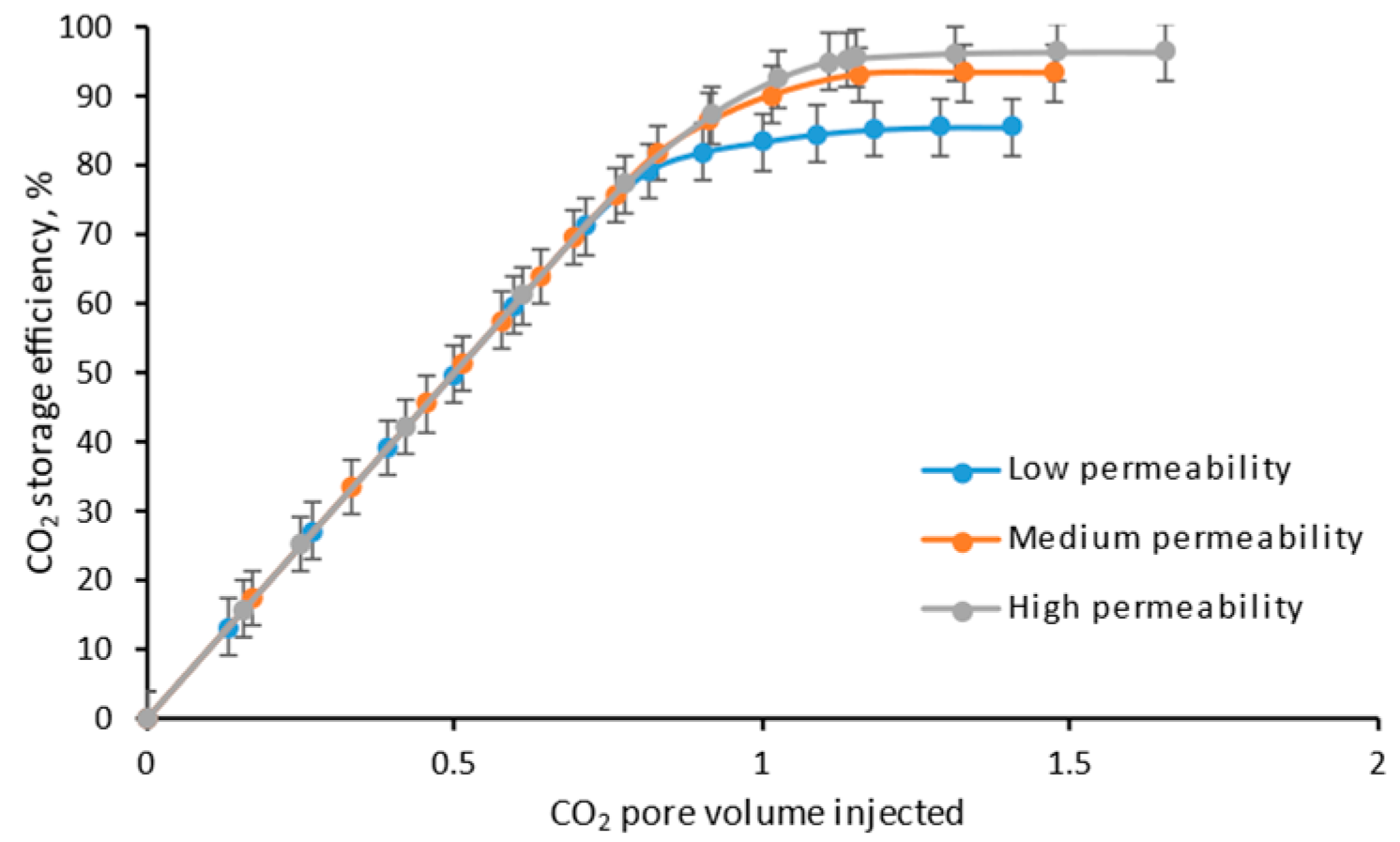
4.4. Permeability
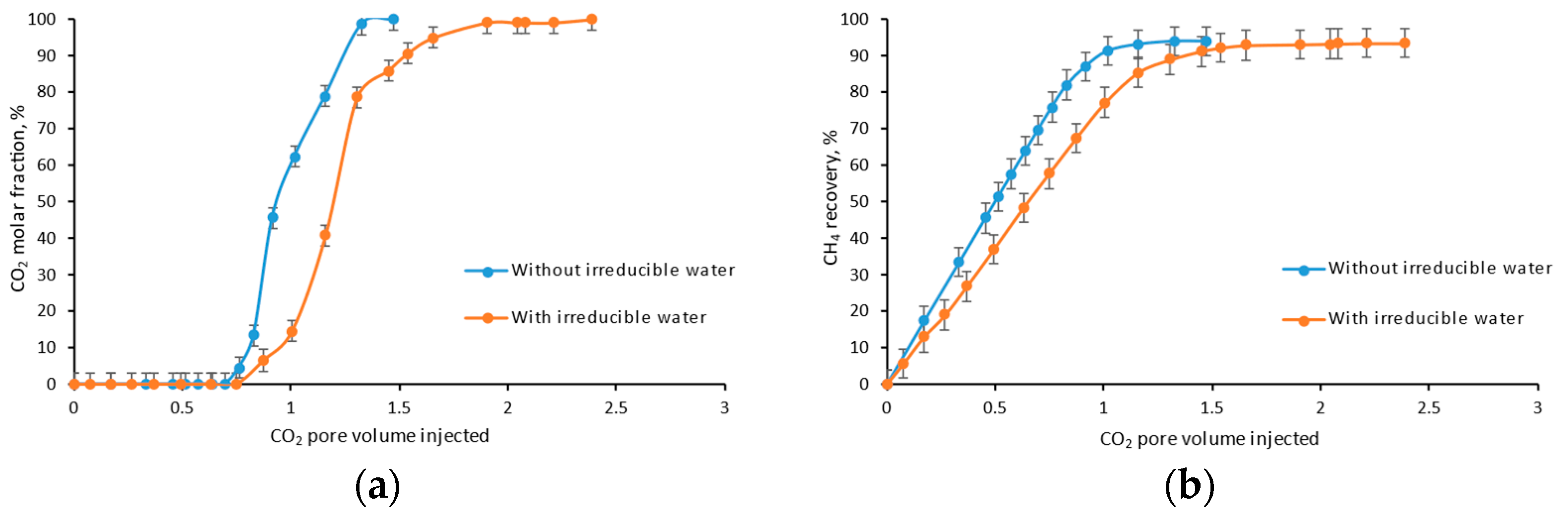
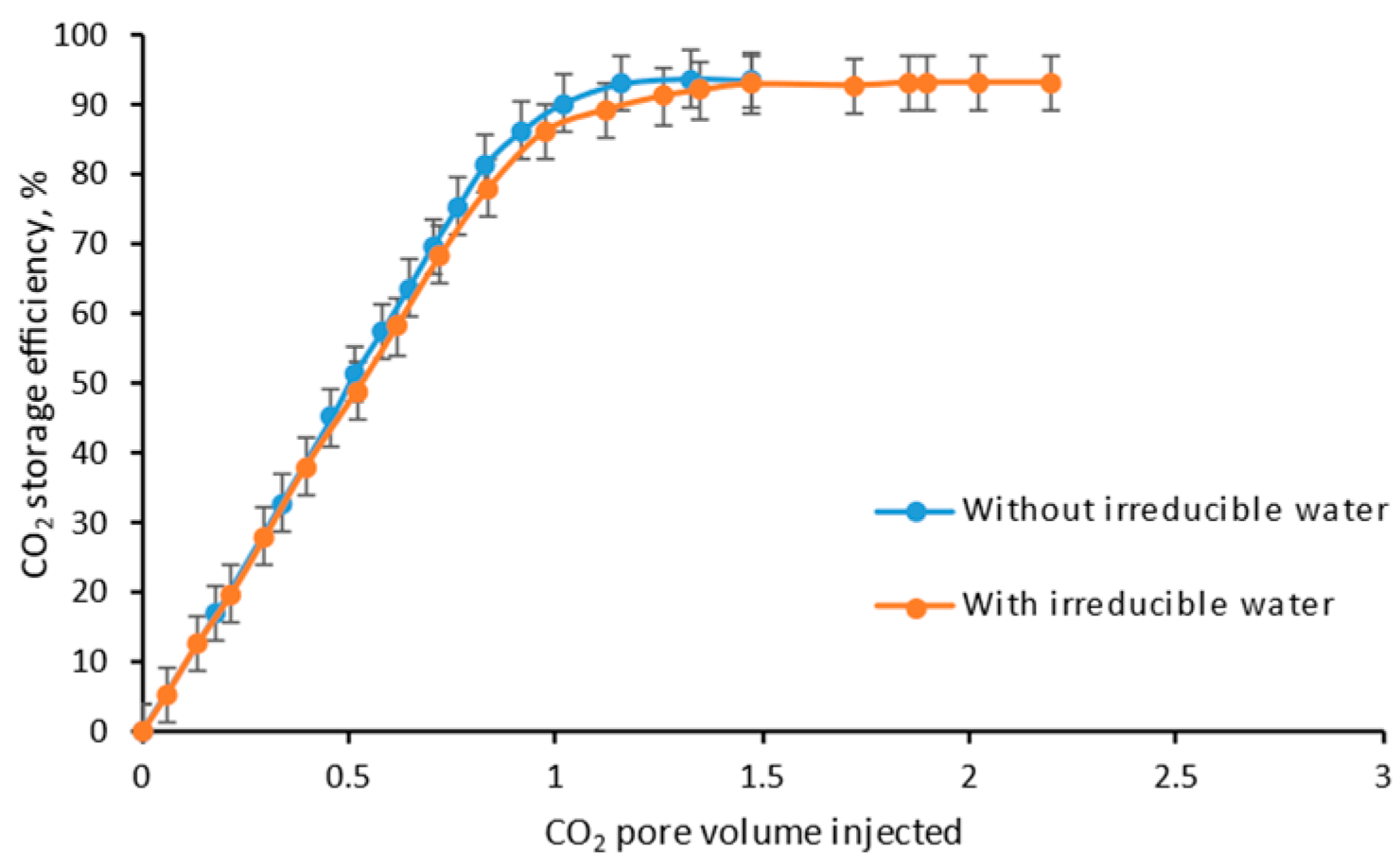
4.5. Irreducible Water
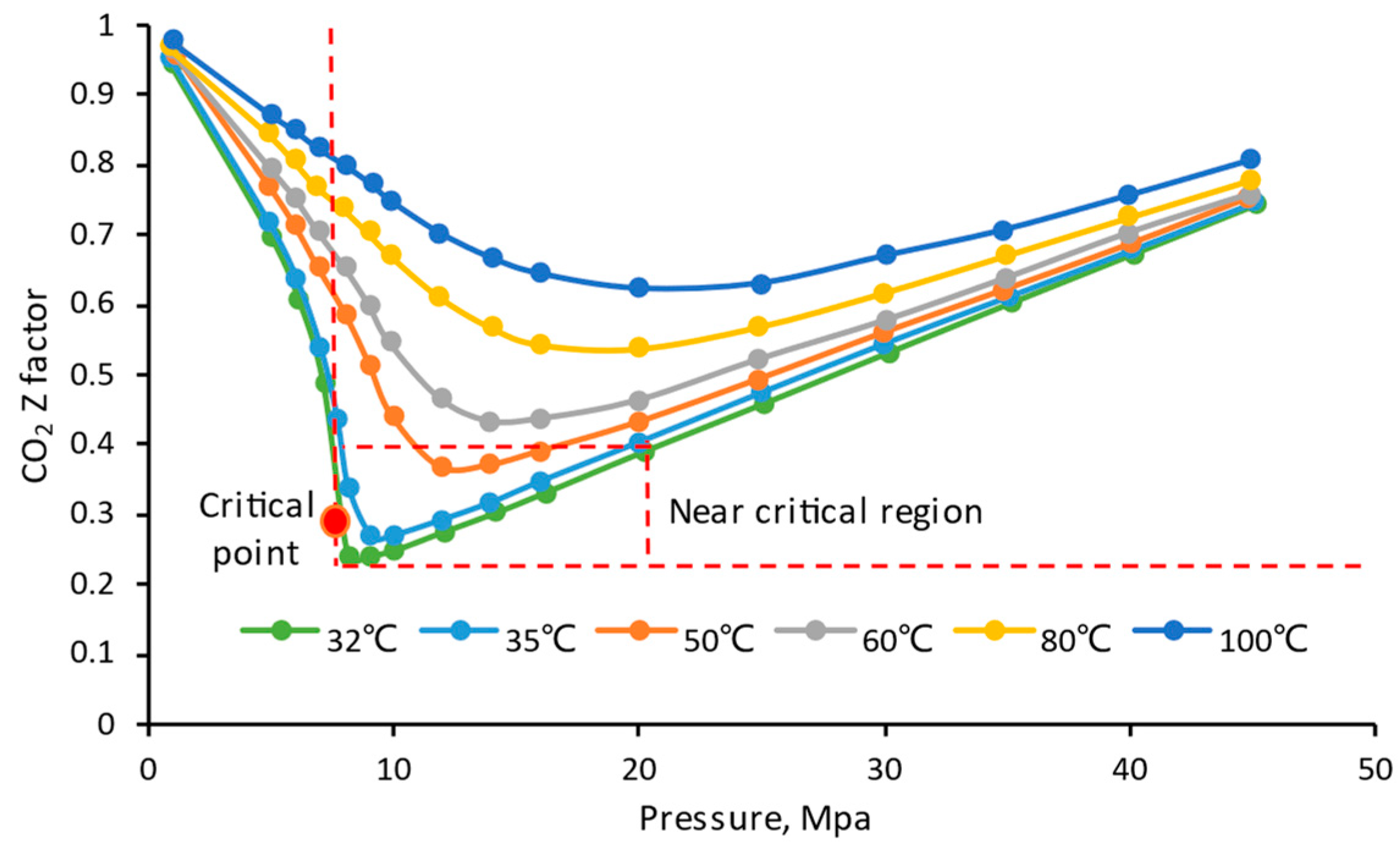
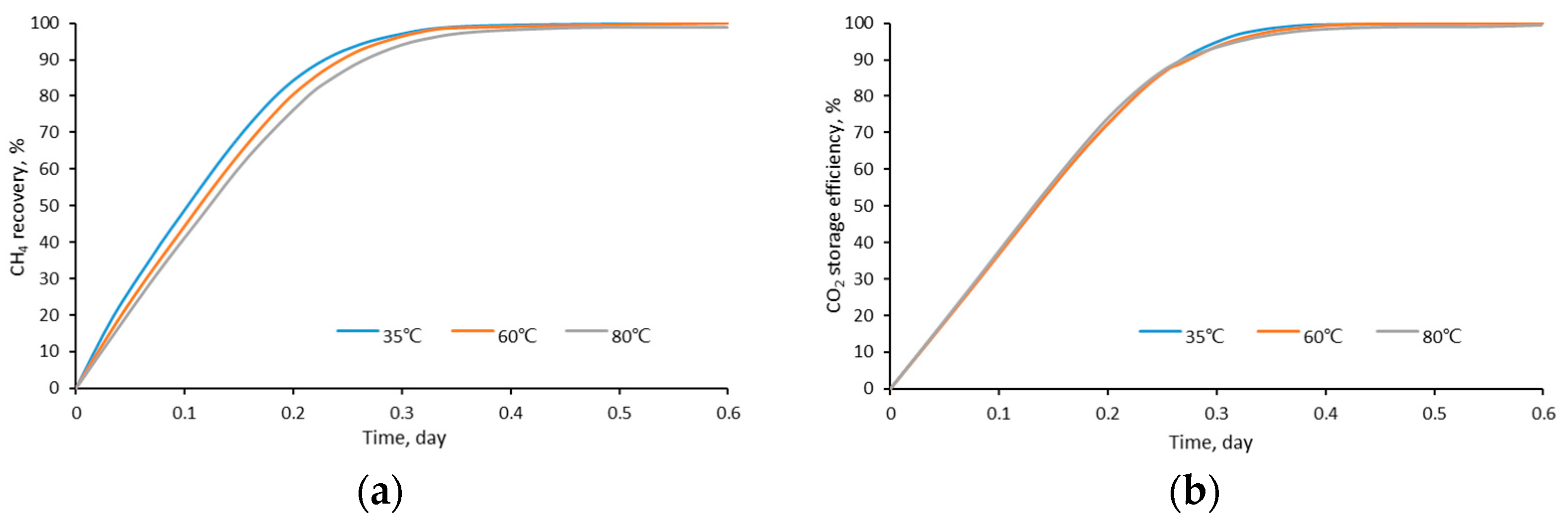
4.6. Temperature
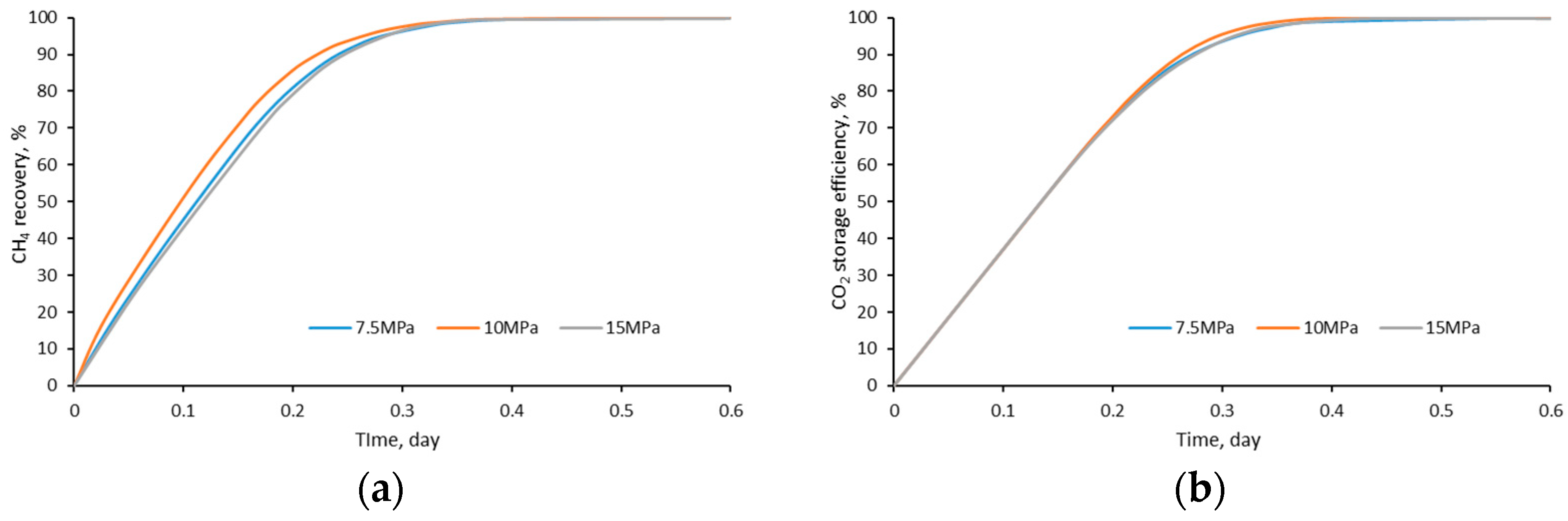
4.7. Pressure
4.8. CO2 Purity
4.9. Heterogeneity
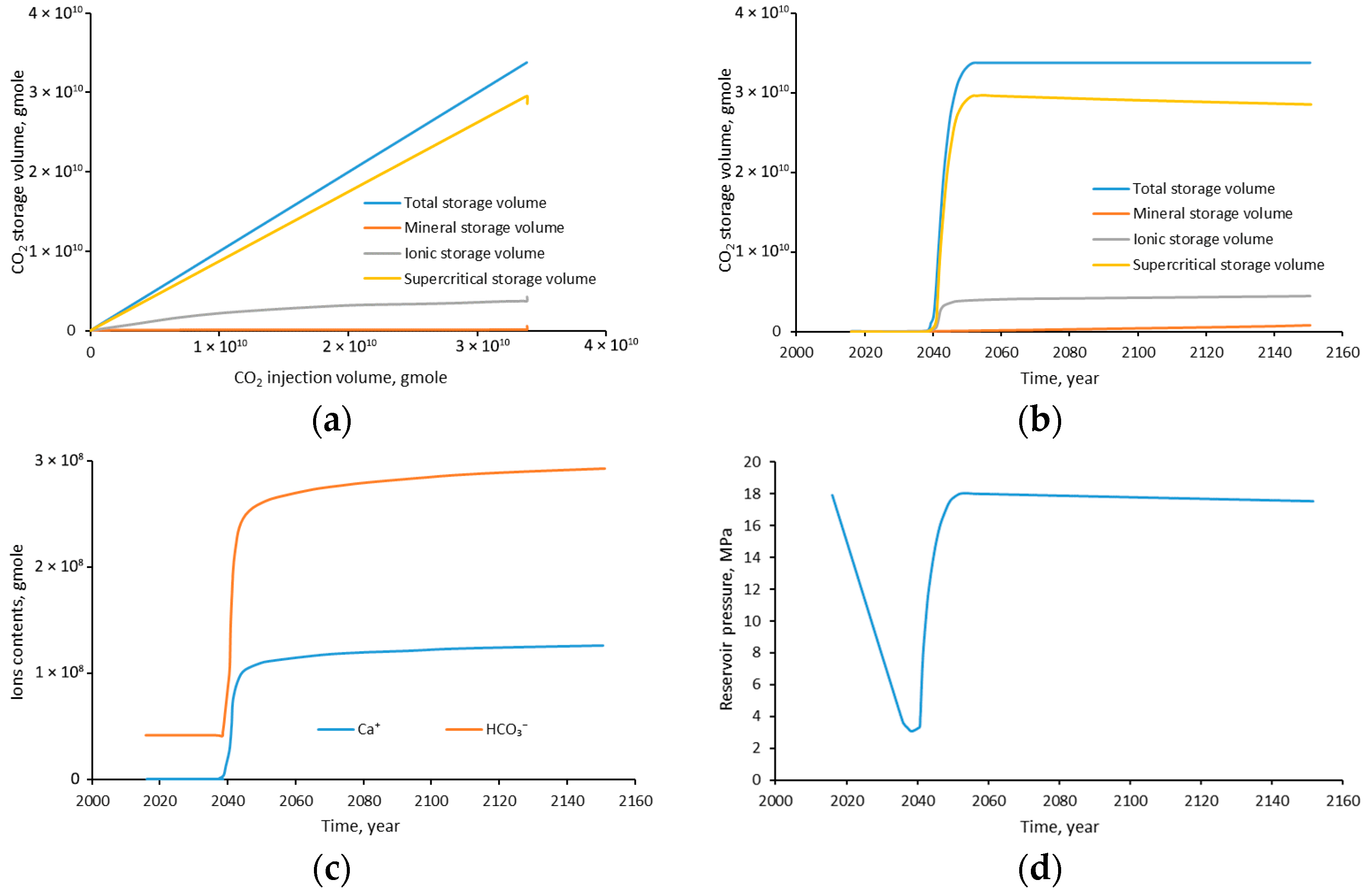
4.10. Storage Mechanisms
5. Discussions
6. Conclusions
- In the same injection pore volume, the lower the injection rate, the longer the injection and diffusion duration, the earlier the CO2 breakthrough, and the lower the ultimate CH4 recovery efficiency;
- The lower the core permeability, the earlier the CO2 breakthrough, and the lower the ultimate CH4 recovery efficiency, due to the stronger fingering between CO2 and CH4;
- The higher the injection spot (according to the dip angle), the earlier the CO2 breakthrough, and the lower the ultimate CH4 recovery efficiency, due to the stronger gravity segregation;
- As the existence of irreducible water, the ultimate recovery and storage efficiency become higher due to the delay of CO2 breakthrough caused by dissolution.
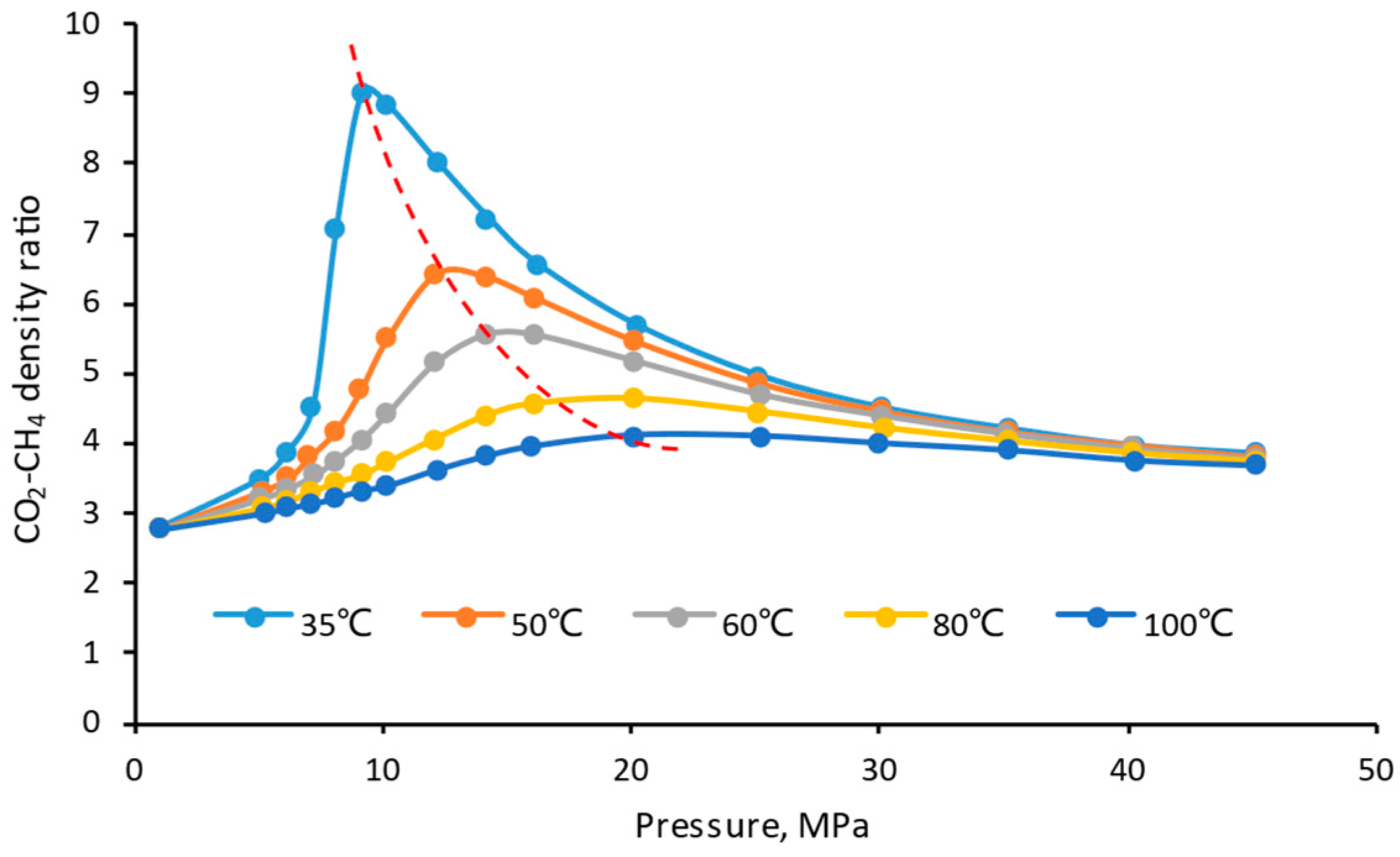
- The density and viscosity ratio of CO2/CH4 reach the maximum when the temperature is close to the critical CO2 temperature (31 °C) and when the pressure is about 11 MPa. Therefore, the closer the reservoir condition to this temperature and pressure, the higher the CO2 displacement and storage efficiency;
- The higher purity CO2 is injected, the more CH4 is displaced and produced, so there is more space for CO2 in the reservoir, and higher CO2 storage efficiency will be achieved. When lower purity CO2 is injected, the gas diffusion and fingering become stronger due to the lower compressibility, viscosity and dissolution of the impurities;
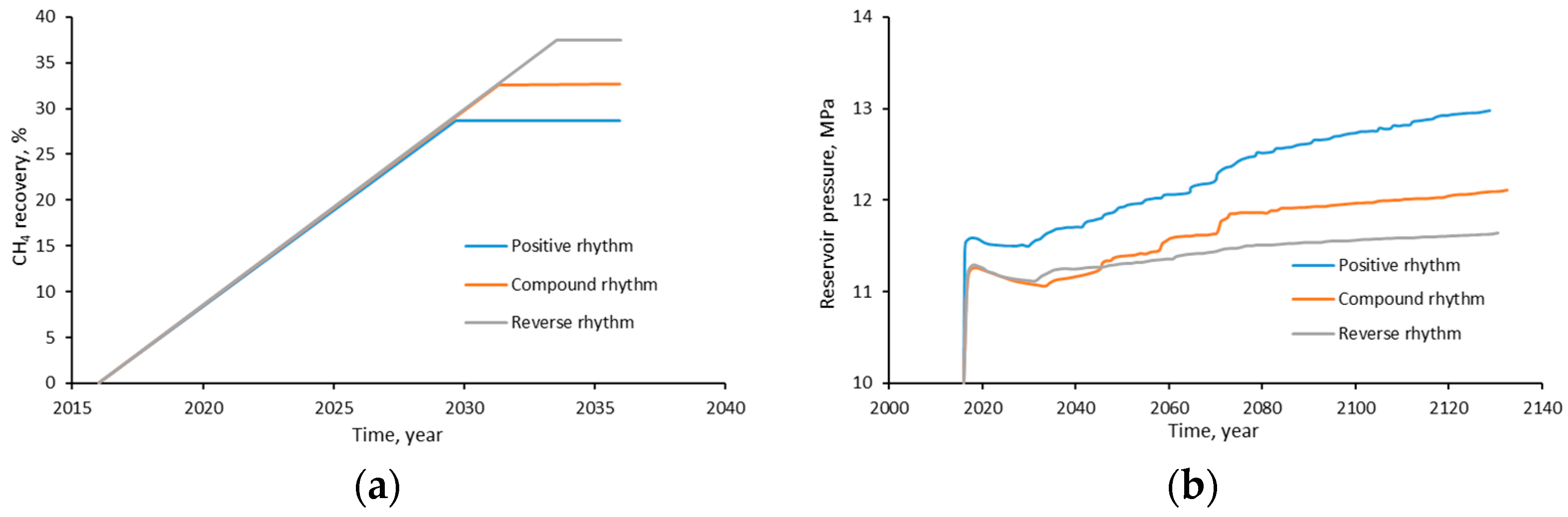
- In comparison to the positive and compound rhythm reservoirs, the reverse rhythm reservoir has the greatest storage potential since it has the most recent CO2 breakthrough, the highest CH4 recovery efficiency, and the lowest storage stage pressure increase;
- The CO2 injection enhances the CH4 recovery efficiency by 5.8%. Among different storage mechanisms, the CO2 supercritical storage accounts for 83.78%; the CO2 ionic storage accounts for 12.67%; and the CO2 mineral storage accounts for 3.85%. Furthermore, part of supercritical storage will transform to mineral storage over time.
- The urgent need for CO2 emission reduction was revealed after COP27. With the continuous advancement of subsidy policies and CO2 commercialization, CCUS should not be limited to oil reservoirs. Through the above studies, CCUS can also achieve fine recovery and storage effects in tilted gas reservoirs with good geological properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrier, J.R.; Pultar, A.; Maroto-Valer, M. CCUS: Paving the way from COP26 to net zero. Greenh. Gas Sci. Technol. 2022, 12, 335–338. [Google Scholar] [CrossRef]
- Victor, N.; Nichols, C. CCUS deployment under the U.S. 45Q tax credit and adaptation by other North American Governments: MARKAL modeling results. Comput. Ind. Eng. 2022, 169, 108269. [Google Scholar] [CrossRef]
- Bazhenov, S.; Chuboksarov, V.; Maximov, A.; Zhdaneev, O. Technical and economic prospects of CCUS projects in Russia. Sustain. Mater. Technol. 2022, 33, e00452. [Google Scholar] [CrossRef]
- Song, X.; Ge, Z.; Zhang, W.; Wang, Z.; Huang, Y.; Liu, H. Study on multi-subject behavior game of CCUS cooperative alliance. Energy 2023, 262, 125229. [Google Scholar] [CrossRef]
- Lukoye, A.; Gregory, E.E.; Aiah, A.G. COP27 Climate Change Conference: Urgent action needed for Africa and the world. Pak. J. Med. Sci. 2022, 38, 2058–2060. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, L.; Chang, D.; Tsimplis, M.; Greig, C.; Wright, S. International chains of CO2 capture, utilization and storage (CCUS) in a carbon-neutral world. Resour. Conserv. Recycl. 2021, 167, 105433. [Google Scholar] [CrossRef]
- Rakhiemah, A.N.; Xu, Y. Economic viability of full-chain CCUS-EOR in Indonesia. Resour. Conserv. Recycl. 2022, 179, 106069. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ma, Q.; Liu, L. Development of Carbon Capture, Utilization and Storage Technology in China. Strateg. Study CAE 2021, 33, 70–80. [Google Scholar] [CrossRef]
- Terdal, R.M.; Steeghs, N.; Craig, W. Carbon, Capture, Utilization and Storage CCUS: How to Commercialize a Business with No Revenue. In Proceedings of the Canadian Energy Technology Conference, Calgary, AB, Canada, 16–17 March 2022. [Google Scholar] [CrossRef]
- Betiku, A.; Bassey, O.B. Exploring the Barriers to Implementation of Carbon Capture, Utilisation and Storage in Nigeria. In Proceedings of the International Petroleum Technology Conference, Riyadh, Saudi Arabia, 21–23 February 2022. [Google Scholar] [CrossRef]
- Derse, O. CO2 capture, utilization, and storage (CCUS) storage site selection using DEMATEL-based Grey Relational Analysis and evaluation of carbon emissions with the ARIMA method. Environ. Sci. Pollut. Res. Int. 2022, 30, 14353–14364. [Google Scholar] [CrossRef]
- Magzymov, D.; Dindoruk, B.; Johns, R.T. Carbon Capture, Utilization, and Storage in the Context of Petroleum Industry: A State-of-the-art Review. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 25–29 April 2022. [Google Scholar]
- Wendt, A.; Sheriff, A.; Shih, C.Y.; Vikara, D.; Grant, T. A multi-criteria CCUS screening evaluation of the Gulf of Mexico, USA. Int. J. Greenh. Gas Control 2022, 118, 103688. [Google Scholar] [CrossRef]
- Guo, H.; Liu, X.; Meng, E.; Xu, Y.; Zhang, M.; Fu, H.; Zhang, Y.; Song, K. CCUS in China: Challenges and Opportunities. In Proceedings of the SPE Improved Oil Recovery Conference, Virtual, 25–29 April 2022. [Google Scholar] [CrossRef]
- Oldenburg, C.M.; Stevens, S.H.; Benson, S.M. Economic feasibility of carbon sequestration with enhanced gas recovery (CSEGR). Energy 2004, 29, 1413–1422. [Google Scholar] [CrossRef]
- Guo, P.; Xu, Q.; Sun, Z.; Du, J.; Wang, Z. Research progress of CO2 flooding and geological storage in gas reservoirs. Lithol. Reserv. 2016, 28, 6–11. [Google Scholar]
- Zangeneh, H.; Jamshidi, S.; Soltanieh, M. Coupled optimization of enhanced gas recovery and carbon dioxide sequestration in natural gas reservoirs: Case study in a real gas field in the south of Iran. Int. J. Greenh. Gas Control 2013, 17, 515–522. [Google Scholar] [CrossRef]
- Marbun, B.T.H.; Zulkhifly, S.; Lie, H.S.; Promediaz, A. Northwest Java and East Natuna Field: Perspective to Apply Carbon Capture And Storage (CCS) In Indonesia. In Proceedings of the Carbon Management Technology Conference, Orlando, FL, USA, 7–9 February 2012. [Google Scholar] [CrossRef]
- Andersen, P.Ø.; Brattekås, B.; Zhou, Y.; Nadeau, P.; Nermoen, A.; Yu, Z.; Fjelde, I.; Oelkers, E.H. Carbon capture utilization and storage (CCUS) in tight gas and oil reservoirs. J. Nat. Gas Sci. Eng. 2020, 81, 103458. [Google Scholar] [CrossRef]
- Sun, Y.; Cui, F.; Sun, L.; Du, Z.; Tang, Y. Influence of gravity differentiation and heterogeneity on CO2 sequestration in gas reservoirs: A case of the XC Gas Reservoir in South China. Nat. Gas Ind. 2014, 34, 82–86. [Google Scholar]
- Liu, J.; Xie, L.; He, B.; Gan, Q.; Zhao, P. Influence of anisotropic and heterogeneous permeability coupled with in-situ stress on CO2 sequestration with simultaneous enhanced gas recovery in shale: Quantitative modeling and case study. Int. J. Greenh. Gas Control 2021, 104, 103208. [Google Scholar] [CrossRef]
- Liu, S.; Lv, J.; Li, G.; Song, Y.; Zhang, Y. Visulized and Quantitative Investigation of CO2-CH4 Diffusion Using X-ray Computed Tomography. In Proceedings of the 14th Greenhouse Gas Control Technologies Conference Melbourne, Cheltenham, UK, 21–26 October 2018. [Google Scholar]
- Sun, H.; Yao, J.; Gao, S.; Fan, D.; Wang, C.; Sun, Z. Numerical study of CO2 enhanced natural gas recovery and sequestration in shale gas reservoirs. Int. J. Greenh. Gas Control 2013, 19, 406–419. [Google Scholar] [CrossRef]
- Jun, Y.; Yeon, J.; Tae, S. Effects of Anisotropy and CO2 Wettability on CO2 Storage Capacity in Sandstone. Geofluids 2022, 2022, 5900255. [Google Scholar] [CrossRef]
- Amin, S.M.; Tiwari, R.D.; Widyanita, A.; Chidambaram, P.; Ali, S.S.M.; Mazeli, A.H.; Tan, C.P.; Hamid, M.K.A. Impact of karstification in trapping mechanisms of CO2 storage. J. Pet. Explor Prod. Technol. 2022, 12, 2817–2831. [Google Scholar] [CrossRef]
- Li, D.; Zhang, H.; Li, Y.; Xu, W.; Jiang, X. Effects of N2 and H2S binary impurities on CO2 geological storage in stratified formation—A sensitivity study. Appl. Energy 2018, 229, 482–492. [Google Scholar] [CrossRef]
- Al-Abri, A.S.; Sidiq, H.; Amin, R. Mobility ratio, relative permeability and sweep efficiency of supercritical CO2 and methane injection to enhance natural gas and condensate recovery: Coreflooding experimentation. J. Nat. Gas Sci. Eng. 2012, 9, 166–171. [Google Scholar] [CrossRef]
- Liu, S.; Ren, B.; Li, H.; Yang, Y.; Wang, Z.; Wang, B.; Xu, J.; Agarwal, R. CO2 storage with enhanced gas recovery (CSEGR): A review of experimental and numerical studies. Pet. Sci. 2021, 19, 594–607. [Google Scholar] [CrossRef]
- Cao, C.; Hou, Z.; Li, Z.; Pu, X.; Liao, J.; Wang, G. Numerical modeling for CO2 storage with impurities associated with enhanced gas recovery in depleted gas reservoirs. J. Nat. Gas Sci. Eng. 2022, 102, 104554. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, L.; Liu, W.; Zhao, C.; Zhang, Y.; Song, Y. Study on the influence of various factors on dispersion during enhance natural gas recovery with CO2 sequestration in depleted gas reservoir. J. Nat. Gas Sci. Eng. 2022, 103, 104644. [Google Scholar] [CrossRef]
- Ding, J.; Yan, C.; He, Y.; Wang, C. Supercritical CO2 sequestration and enhanced gas recovery in tight gas reservoirs: Feasibility and factors that influence efficiency. Int. J. Greenh. Gas Control 2021, 105, 103234. [Google Scholar] [CrossRef]
- Honari, A.; Bijeljic, B.; Johns, M.L.; May, E.F. Enhanced gas recovery with CO2 sequestration: The effect of medium heterogeneity on the dispersion of supercritical CO2–CH4. Int. J. Greenh. Gas Control 2015, 39, 39–50. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, Y.; Yan, J. The Feasibility Appraisal for CO2 Enhanced Gas Recovery of Tight Gas Reservoir: Case Analysis and Economic Evaluation. In Proceedings of the International Petroleum Technology Conference, Virtual, 23 March–1 April 2021. [Google Scholar] [CrossRef]
- Guo, P.; Li, X.; Sun, Z.; Wang, S.; Du, J. The Numerical Simulation of CO2 Displacement and Storage in Low-permeability Gas Reservoir. Sci. Technol. Eng. 2019, 19, 68–76. [Google Scholar]
- Gosset, W.S. The probable error of a mean. Biometrika 1908, 6, 1–25. [Google Scholar]







| K+ + Na+ | Mg2+ | Ca2+ | Cl− | SO32− | HCO3− | CO32− | Salinity (mg/L) |
|---|---|---|---|---|---|---|---|
| 12,160 | 27 | 32 | 17,305 | 263 | 2374 | 0 | 32,161 |
| Core Number | Core Length (cm) | Core Diameter (cm) | Pore Volume (cm3) | Porosity (%) | Permeability (10−3 μm2) |
|---|---|---|---|---|---|
| 20 | 4.279 | 2.502 | 2.248 | 10.71 | 1.80 |
| 14 | 4.995 | 2.502 | 2.745 | 11.20 | 1.80 |
| 5 | 5.128 | 2.498 | 2.929 | 11.64 | 2.27 |
| 27 | 5.500 | 2.498 | 3.138 | 11.63 | 2.42 |
| 31 | 5.934 | 2.512 | 3.668 | 12.60 | 2.49 |
| 15 | 4.400 | 2.498 | 2.461 | 11.40 | 2.50 |
| 78 | 5.400 | 2.502 | 2.567 | 9.69 | 1.17 |
| 25 | 5.750 | 2.502 | 3.320 | 11.77 | 2.53 |
| 18 | 5.051 | 2.504 | 3.214 | 12.97 | 2.58 |
| 77 | 4.534 | 2.511 | 2.209 | 9.93 | 0.99 |
| 9 | 5.257 | 2.502 | 3.149 | 12.21 | 2.60 |
| 17 | 4.971 | 2.500 | 3.012 | 12.35 | 2.67 |
| 22 | 4.763 | 2.498 | 2.979 | 12.75 | 2.68 |
| 16 | 5.675 | 2.512 | 2.940 | 10.56 | 0.93 |
| 10 | 4.846 | 2.500 | 2.943 | 12.38 | 2.70 |
| 8 | 5.095 | 2.498 | 2.910 | 11.64 | 2.74 |
| Σ | 81.875 | 2.502 | 46.432 | 11.58 | 2.18 |
| Core Number | Core Length (cm) | Core Diameter (cm) | Pore Volume (cm3) | Porosity (%) | Permeability (10−3 μm2) |
|---|---|---|---|---|---|
| 2–12 | 6.903 | 2.496 | 8.305 | 24.60 | 9.56 |
| 5–6 | 6.962 | 2.496 | 4.266 | 12.53 | 7.13 |
| 2–13 | 7.120 | 2.496 | 8.740 | 25.10 | 11.10 |
| 2–9 | 7.056 | 2.498 | 2.689 | 7.78 | 6.91 |
| 4–4 | 7.282 | 2.512 | 8.729 | 24.20 | 11.40 |
| 1–15 | 6.892 | 2.496 | 6.842 | 20.30 | 6.87 |
| 4–3 | 7.210 | 2.498 | 3.055 | 8.65 | 11.49 |
| 2–2 | 6.876 | 2.497 | 6.866 | 20.40 | 6.78 |
| 1–1 | 6.964 | 2.513 | 8.562 | 24.80 | 11.90 |
| 2–8 | 6.992 | 2.520 | 8.505 | 24.40 | 13.10 |
| 1–10 | 7.156 | 2.496 | 6.684 | 19.10 | 6.51 |
| 2–3 | 7.114 | 2.496 | 8.872 | 25.50 | 13.20 |
| Σ | 84.527 | 2.501 | 82.115 | 19.78 | 9.66 |
| Core Number | Core Length (cm) | Core Diameter (cm) | Pore Volume (cm3) | Porosity (%) | Permeability (10−3 μm2) |
|---|---|---|---|---|---|
| 20 | 6.400 | 2.5 | 4.245 | 13.52 | 99.32 |
| 14 | 6.647 | 2.5 | 4.471 | 13.71 | 96.55 |
| 5 | 6.287 | 2.5 | 4.075 | 13.21 | 96.49 |
| 27 | 5.739 | 2.5 | 3.810 | 13.53 | 95.19 |
| 31 | 6.513 | 2.5 | 4.461 | 13.96 | 103.26 |
| 15 | 4.461 | 2.5 | 4.629 | 21.15 | 93.14 |
| 78 | 5.845 | 2.5 | 4.124 | 14.38 | 106.23 |
| 25 | 6.198 | 2.5 | 4.437 | 14.59 | 110.44 |
| 18 | 6.475 | 2.5 | 4.238 | 12.35 | 118.75 |
| 77 | 6.854 | 2.5 | 4.153 | 13.54 | 72.49 |
| 9 | 6.567 | 2.5 | 4.362 | 23.11 | 129.57 |
| 17 | 4.700 | 2.5 | 5.329 | 12.76 | 68.44 |
| 22 | 5.981 | 2.5 | 3.744 | 24.99 | 176.67 |
| Σ | 83.442 | 2.5 | 61.932 | 15.581 | 103.63 |
| Experiment Number | Sensitive Parameter | Parameter Value |
|---|---|---|
| 1 | Displacement velocity | 0.1 mL/min velocity, 0° dip angle, medium permeability |
| 2 | 0.2 mL/min velocity, 0° dip angle, medium permeability | |
| 3 | 0.4 mL/min velocity, 0° dip angle, medium permeability | |
| 4 | 0.8 mL/min velocity, 0° dip angle, medium permeability | |
| 5 | Dip angle | 0.2 mL/min velocity, +45° dip angle, medium permeability |
| 6 | 0.2 mL/min velocity, −10° dip angle, medium permeability | |
| 7 | 0.2 mL/min velocity, −45° dip angle, medium permeability | |
| 8 | Permeability | 0.2 mL/min velocity, 0° dip angle, low permeability |
| 9 | 0.2 mL/min velocity, 0° dip angle, high permeability | |
| 10 | Irreducible water | 0.2 mL/min velocity, 0° dip angle, medium permeability, with irreducible water |
| Measure Tools and Error Events | Error Value |
|---|---|
| Pore permeability instrument | Systematic error: ±1% |
| Flowmeter of syringe pump | Systematic error: ±1% |
| Gas flowmeter at outlet | Systematic error: ±1% |
| Gas chromatograph | Systematic error: ±0.5% |
| Annular pressure indicator | Systematic error: ±0.08% |
| Inlet pressure indicator | Systematic error: ±0.08% |
| Outlet pressure indicator | Systematic error: ±0.08% |
| Fluid retention in pipeline | Systematic error: −1.5% max recovery |
| Air doping in gas chromatograph | Systematic error: ±1% |
| Timing of stopping injection | Systematic error: ±1% for last point |
| Simulation Number | Sensitive Parameter | Simulation Design |
|---|---|---|
| 1 | Temperature | 8 MPa, 35 °C |
| 2 | 8 MPa, 60 °C | |
| 3 | 8 MPa, 80 °C | |
| 4 | Pressure | 7.5 MPa, 80 °C |
| 5 | 10 MPa, 80 °C | |
| 6 | 15 MPa, 80 °C | |
| 7 | CO2 purity | 100%CO2 |
| 8 | 90%CO2 + 10%N2 | |
| 9 | 80%CO2 + 20%N2 | |
| 10 | 70%CO2 + 30%N2 |
| Layer Number | Thickness (m) | Porosity | Permeability (10−3 μm2) |
|---|---|---|---|
| 1 | 15 | 0.1 | 20 |
| 2 | 15 | 0.1 | 20 |
| 3 | 15 | 0.15 | 40 |
| 4 | 15 | 0.15 | 40 |
| 5 | 15 | 0.2 | 60 |
| 6 | 15 | 0.2 | 60 |
| 7 | 15 | 0.25 | 80 |
| 8 | 15 | 0.25 | 80 |
| 9 | 15 | 0.3 | 100 |
| 10 | 15 | 0.3 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Qin, J.; Cai, J.; Tang, Y. The Influencing Factors of CO2 Utilization and Storage Efficiency in Gas Reservoir. Appl. Sci. 2023, 13, 3419. https://doi.org/10.3390/app13063419
Luo Y, Qin J, Cai J, Tang Y. The Influencing Factors of CO2 Utilization and Storage Efficiency in Gas Reservoir. Applied Sciences. 2023; 13(6):3419. https://doi.org/10.3390/app13063419
Chicago/Turabian StyleLuo, Yulong, Jiazheng Qin, Jianqin Cai, and Yong Tang. 2023. "The Influencing Factors of CO2 Utilization and Storage Efficiency in Gas Reservoir" Applied Sciences 13, no. 6: 3419. https://doi.org/10.3390/app13063419
APA StyleLuo, Y., Qin, J., Cai, J., & Tang, Y. (2023). The Influencing Factors of CO2 Utilization and Storage Efficiency in Gas Reservoir. Applied Sciences, 13(6), 3419. https://doi.org/10.3390/app13063419








