Featured Application
The production of lipids by microorganisms is always associated with post-extraction residues, a large part of which are proteins. The thermal properties of oleaginous yeast biomass indicate its stability over a wide range of temperatures, which may facilitate the design of technology and versatile use of its components, especially since its good amino acid composition indicates the nutritional potential of the protein. These could have applications in feed and food products.
Abstract
The biotechnological processing of oleaginous yeast biomass should be comprehensively managed using the zero-waste policy. This study focused on the biomass of the red yeast Rhodotorula obtained from a medium containing waste nitrogen. The cells accumulate lipids in intracellular lipid droplets; however, they are also rich in protein. Therefore, the nutritional value of lipid and protein, according to their fatty acid and amino acid composition, is a necessary step for practical application. For the very first time, this study focused on understanding the influence of temperature on powdered red yeast biomass to study components phase transition or chemical reactions by using DSC. Rhodotorula glutinis var. rubescens was cultivated in a glucose fed-batch in a potato wastewater medium, where the biomass yield was powdered, and lipid and fatty acid, protein, and amino acid compositions were determined. The DSC diagrams of red yeast biomass were characterized by two small and mild endothermic peaks, indicating the presence of fat and the presence of low molecular weight carbohydrates and a distinct peak associated with the presence of crystalline sugars. The nutritional quality of the lipid fraction as atherogenicity (0.223), thrombogenicity index (0.438), PUFA/SFA (0.24), and the n-6/n-3 ratio (3.275) was adequate for the recommendation and resulted from the fatty acid composition. The yeast protein was characterized by a high content of glutamic acid (99 mg/1 g of protein), and a value of essential amino acid index of protein suggested a superior amino acid composition compared to the FAO/WHO standard. Despite a high essential amino acid index (>120), yeast protein was characterized by a low content of Lys or Met.
Keywords:
yeast biomass; DSC; lipid; fatty acids; protein; amino acids composition; nutritional characteristic; SCO; SCP; fed-batch; wastewater 1. Introduction
The growing number of people in the world, climate change caused by environmental pollution, and the dynamically changing geopolitical situation influencing the food supply chain require the search for environmentally and economically friendly food production technologies. This applies to both plant products given soil eutrophication, droughts, or floods ruining crops, as well as animal products (competition in the use of fields for the cultivation of fodder and food plants, emission of greenhouse gases, unethical). Microbial fat and protein can be valuable products for the food industry. The idea of using microorganism biomass as a source of protein (SCP) [1,2] or fat (SCO) [3] is not new, but it has recently become more realistic, especially when it meets the requirements of modern technologies. Biotechnological processing is certainly faster, and more environment-friendly compared to standard oilseeds, high-protein crops, or animal breeding. Components of the biomass of many species of fungi and unicellular algae are already used in practice as a source of protein, including spirulina and other bioactive compounds. And their fat, rich in polyunsaturated fatty acid, is used to supplement milk replacers or feed [4,5,6].
Rhodotorula spp. is red oleaginous yeast, the cells of which are able to accumulate lipids (>20% d.w.) [7]. The composition of fatty acids influences the intended use, so if the goal is to produce biodiesel, there should be a high proportion of SFAs. Then the final product has appropriate properties, such as cetane number (CN), flash point (FP), and viscosity (μ) [8]. If SCO has a nutritionally proper fatty acid content, it can be used for food production [9]. The similarity of the fatty acid composition of red yeast lipids to valuable vegetable or fish oils predisposes them to be profitable as a food ingredient. Previous research showed that strain Rhodotorula glutinis var. rubescens can grow efficiently (biomass yield 39.52 g L−1) in deproteinated potato wastewater and glucose medium [8], and after fed-batch cultivation, the content of lipids in biomass exceed >34% d.w. [10]. Except for accumulated fat in intracellular lipid droplets, the cells are also rich in proteins, carbohydrates, and carotenoids [11]. These valuable substances are post-extraction residues and should be utilized following the zero-waste policy. The comprehensive management of biomass, without the need to extract or separate individual substances, as is the case with fodder yeast or dried or flaked baker’s yeast, can be another concept. Because protein constitutes a large share of oleaginous biomass, the analysis of its composition should allow us to determine its nutritional suitability. After the cultivation process, the wet biomass is separated from the medium and then dried or lyophilized for storage or lipid extraction. The temperature influences the phase transitions and chemical reactions, so the thermal properties of biomass are valuable for processing. Differential scanning calorimetry (DSC) can be used to determine qualitative parameters, as well as the parameters of certain types of phase transitions (e.g., melting and crystallization) and oxidative stability of lipid-rich samples [10,12]. Regardless of the next technological steps after the cultivation of red yeast, the quantitative and qualitative characteristics of biomass components are necessary for the prediction of the nutritional properties.
This study focused on the thermal characterization of dried oleaginous yeast biomass in terms of lipids and proteins and their characteristics, including the nutritional characteristics. Such research has not been conducted so far.
2. Materials and Methods
2.1. The Collection of Biomass
The yeast cell biomass of Rhodotorula glutinis var. rubescens LOCKR13 samples were taken every 24 h during cultivation in a bioreactor, centrifuged (6400× g for 20 min at 20 °C (Eppendorf 5810 Centrifuge, Hamburg, Germany), washed twice with sterile saline solution, dried at 80 °C, and finally crushed into a powder in a pulse mill (Pulverisette, Fritsch, Idar-Oberstein, Germany) for 5 min using a low range of pulsation. The parameters of cultivation in the BioFlo 3000 bioreactor (New Brunswick Scientific Co., Edison, NJ, USA) were as follows: working volume; 3 L; temperature, 20 °C; stirring, 200 rpm; and air rate, 2 v/v/min. As previously described, a medium with deproteinated potato wastewater and glucose was prepared [10]. As antifoaming agent silicone (Antifoam 204, Sigma-Aldrich, Burlington, MA, USA) was used after 72 h of daily operation, and a sterile glucose solution (1:1) was added to the total concentration of glucose, which was 50 g L−1. The inoculum (10 v/v) was cultured for two days containing cells grown previously on a reciprocating shaker (SM-30 Control, Buechler, Germany) in YPD medium at 28 °C.
2.2. The Lipid Content and Fatty Acids Analysis
The lipid content (% d.w.) in powdered biomass determination was performed using the gravimetrical method of Bligh and Dyer [13] with Zhang et al.’s modification [14] by taking 600 ± 30 mg of sample for lipid extraction. Gas chromatography (YL6100, Young Lin Bldg., Anyang, Hogye-dong, Republic of Korea) with a flame ionization detector was used to determine the fatty acid content in the extracted lipid samples according to the method evaluated by Górska et al. [12]. Measurements were performed in triplicate. The identification of fatty acids was carried out based on the retention time using a lipid standard purchased from Sigma Aldrich (SupelcoTM 37 Component Mix) and quantified as a percentage of the total FAMES content.
The data from fatty acid composition analysis were used to determine the nutritional quality of the lipid fraction:
- (1)
- Polyunsaturated fatty acid/saturated fatty (Σ PUFA/Σ SFA) acid ratio
- (2)
- n-6/n-3 ratio
- (3)
- Saturation index (SI) using the formula:SI = (C 14:0 + C 16:0 + C 18:0)/(MUFA cis + PUFA)
- (4)
- Unsaturation index (UI) using the formula:UI = 1 × (% monoenoics) + 2 × (% dienoics) + 3 × (% trienoics) + 4 × (% tetraenoics) + 5 × (% pentaenoics) + 6 × (% hexaenoics)
- (5)
- Nutritional value (NV)NV = (C 12:0 + C 14:0 + C 16:0)/(C 18:1 cis9 + C 18:2 n-6)
- (6)
- Atherogenicity index (IA) [15]where UFA means unsaturated fatty acidsIA = [C 12:0 + (4 × C 14:0) + C 16:0]/Σ UFA
- (7)
- Thrombogenicity index (IT) [15]IT = (C14:0 + C16:0 + C18:0)/
[(0.5 × Σ MUFA) + (0.5 × Σ n-6 PUFA) + (3 × Σ n-3 PUFA) + (n-3/n-6)] - (8)
- Fatty acids hypocholesterolemic/hypercholesterolemic ratio (hH) [16]hH = (C 18:1 cis9 + C 18:2 × 6 + C 20:4 × 6 + C 18:3 × 3+ C 20:5 × 3 + C 22:5 × 3 + C 22:6 × 3)/(C 14:0 + C 16:0)
- (9)
- Health-promoting index (HPI)HPI = Σ UFA/[C 12:0+ (4 × C 14:0) + C 16:0],
- (10)
- Linoleic acid/α-linolenic acid (LA/ALA) ratio
2.3. The Protein Content and Amino Acids Analysis
The total nitrogen content was determined using the Kjeldahl method where 200 mg of dried biomass sample was used for mineralization [17], and then the KjeFlex apparatus was used for distillation with an automatic titrator system, and the result of nitrogen was multiplied by a factor of 6.25 to obtain protein content. The recovery of the l-method nitrogen analysis (with ammonium sulfate) was >99.2%. The amino acid composition was determined by analysing the content of amino acids in the hydrolysates by ion exchange chromatography with post-column derivatization with ninhydrin with the use of an automatic amino acid analyser with a sodium column (AAA 400 INGOS, Prague, the Czech Republic) [18]. Albumin (Sigma-Aldrich) was used as a reference sample. The biomass samples were subjected to acid hydrolysis with 6 M HCl at 110 °C for 23 h and sulphur amino acids were hydrolyzed separately at 125 °C for 23 h after oxidation (9:1 formic acid: hydrogen peroxide, 16 h at 4 °C). The HCl was then removed under reduced pressure and the residual amino acids were dissolved in a pH 2.2 buffer. The ninhydrin amino acid derivatives were detected at 570 nm for primary amino acids and 440 nm for proline [19], according to the standard protocol of the manufacturer [20].
Based on the amino acid composition, selected parameters characterizing the nutritional value of the protein present in the biomass were calculated as follows:
- (1)
- The chemical score (CS) [21]where aa is amino acids in the protein (mg g−1) with the same amino acid content in the reference protein (AA).CS % = (aa/AA) × 100
- (2)
- The essential amino acid index (EEAI) [22]where EAAI is the n root of the essential amino acids in the yeast biomass (aa) in the content of each of those amino acids in the reference protein (AA) and n is the total number of amino acids evaluated [23].EAAI = n/[(aa1/AA1)·(aa2/AA2) … (aan/AAn)]
2.4. The Thermal Analysis of Powdered Biomass
The red yeast biomass powders (dried and crushed biomass) were studied via differential scanning calorimetry (DSC, TA Instruments Q 200, New Castle, DE, USA) in a normal pressure cell using the methodology described by Ostrowska-Ligęza et al. [24]. The sample for DSC analysis was 10–15 mg hermetically sealed in aluminum pans and then heated from −60 °C to 300 °C with a heating rate of 5 °C/min. All analyses were completed in triplicate.
2.5. The Statistical Analysis
The data’s mean and SD were calculated. The results were evaluated using one-way analysis of variance (ANOVA), with p < 0.050, using Statistica ver. 13.3 software (TIBCO company software, Palo Alto, CA, USA). The homogenous groups were analyzed with the use of Tukey’s HSD test.
3. Results and Discussion
3.1. The Rhodotorula glutinis var. rubescens Cell Composition during Fed-Batch Cultivation
The content of the main components of the biomass obtained from the fed-batch bioreactor culture varied with the time of cultivation (Figure 1). The cells collected after 48 h were characterized by the highest share of protein and it was over 51% d.w. The synthesis of proteins is directly related to the growth of yeast cells due to the number of performed functions. After the third day of incubation, the protein amount decreased significantly to just over 42% d.w. and gradually decreased to about 33% d.w. on the final day of incubation. When the culture was fed with glucose, the start of the lipogenic phase was observed, and the fat content increased significantly as a consequence of the excess of glucose in the medium. The phenomenon that simultaneous excess carbon and nitrogen depletion promotes fat by de novo biosynthesis in oleaginous yeast cells is well described in the literature [25,26]. The biomass obtained in the later days of cultivation (5 and 6) was characterized by the highest lipid content (>28% of CDW). At the end, the approx. ratio of lipid:protein:sugar in red yeast cells was 24:34:27.
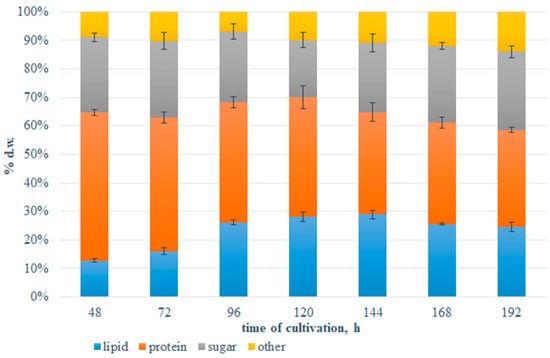
Figure 1.
The content of lipid (blue), protein (red), sugar (grey), and other components (orange colour) in the biomass of Rhodotorula glutinis var. rubescens during the glucose fed-batch cultivation at 20 °C in potato wastewater medium. The content of other components was calculated as 100% d.w.—sum of lipid, protein, and sugar content (% d.w.).
3.2. The Thermal Analysis of Powdered Biomass of Rhodotorula glutinis var. rubescens
In the DSC diagrams of the red yeast biomass after cultivation for 48, 72, 96, 120, and 144 h, two small and mild peaks were identified (Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7 and Figure 8). The peaks were endothermic. The course of third endothermic peaks were observed as distinct and sharp shapes, as shown in Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6. The maximum temperature of the first endothermic peaks was observed from about −1.10 to −3.26 °C for all the samples. The low peak maximum temperature indicated the presence of fat in yeast biomass. It is perplexing to compare the findings obtained in this study due to the scarcity of published data on the thermal analysis of powdered yeast biomass. The DSC curves of cocoa powders were obtained with mild, similarly endothermic peaks at temperatures of about 0 °C, regardless of the heat treatment type used for beans [27]. However, the thermal analysis of extracted lipid (not powdered biomass) from Rhodotorula mucilaginosa obtained crystallization DSC curves with two exothermic peaks [28], and the second peak was characterized by a temperature of about −17 °C. Red yeast biomass contains unsaturated fatty acids, and its characterized melting temperature is below 0 °C. The next sharp peaks, which were observed at temperatures ranging from 157.70 to 168.04 °C, were associated with the presence of low molecular weight carbohydrates. The third sharp, distinct peaks, (maximum temperatures were noticed within the range from 199.21 to 213.69 °C), were associated with the presence of crystalline sugars, which may indicate the accumulation of trehalose by the yeast. Li and Mansour [29] investigated dehydrated trehalose and observed three sharp endothermic peaks in the DSC curve. The third peak was characterized by the highest maximum temperature of about 210 °C, which was found to be associated with the transition of the β form of trehalose.
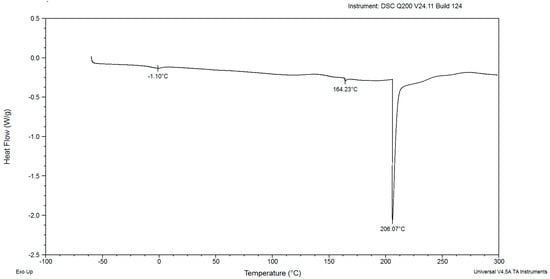
Figure 2.
The DSC curves of powdered biomass of Rhodotorula glutinis var. rubescens after 48 h cultivation in glucose fed-batch in potato wastewater.
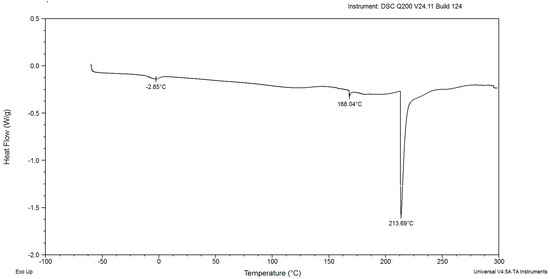
Figure 3.
The DSC curves of powdered biomass of Rhodotorula glutinis var. rubescens after 72 h cultivation in glucose fed-batch in potato wastewater.
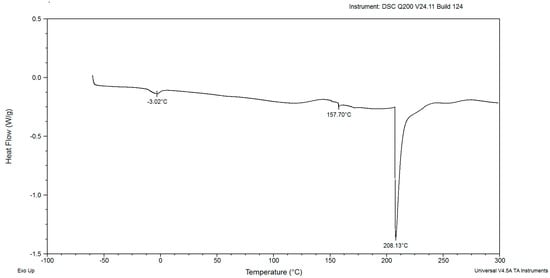
Figure 4.
The DSC curves of powdered biomass of Rhodotorula glutinis var. rubescens after 96 h cultivation in glucose fed-batch in potato wastewater.
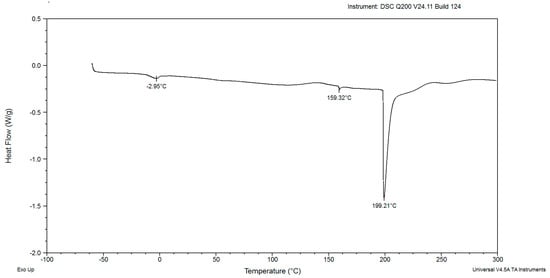
Figure 5.
The DSC curves of powdered biomass of Rhodotorula glutinis var. rubescens after 120 h cultivation in glucose fed-batch in potato wastewater.
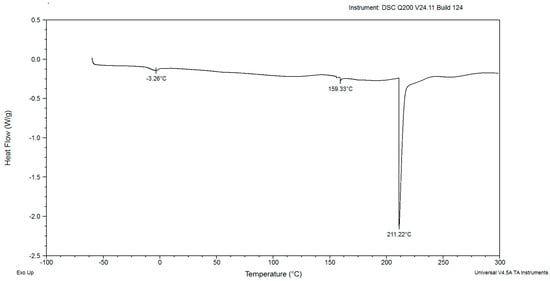
Figure 6.
The DSC curves of powdered biomass of Rhodotorula glutinis var. rubescens after 144 h cultivation in glucose fed-batch in potato wastewater.
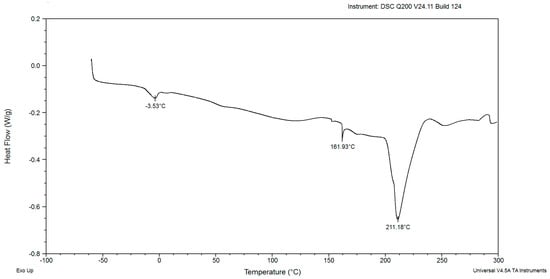
Figure 7.
The DSC curves of powdered biomass of Rhodotorula glutinis var. rubescens after 168 h cultivation in glucose fed-batch in potato wastewater.
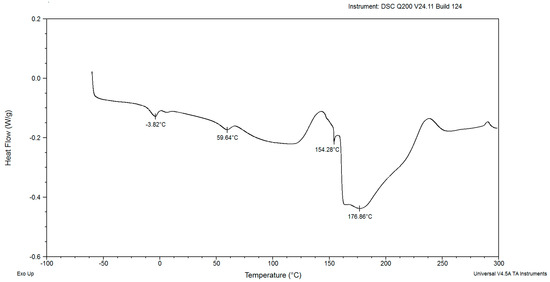
Figure 8.
The DSC curves of powdered biomass of Rhodotorula glutinis var. rubescens after 192 h cultivation in glucose fed-batch in potato wastewater.
The DSC diagrams of red yeast biomass after 168 and 192 h of cultivation in Figure 7 and Figure 8 were presented and characterized in a different course than the previous ones. The first endothermic peaks were observed at −3.53 and −3.82 °C in Figure 7 and Figure 8, respectively. These results indicate the presence of fat in the cells of red yeast. The second endothermic peak was characterized by a mild course at maximum temperatures of about 59.46 °C in dry biomass from the 7th day of incubation and 59.64 °C on the 8th day. The thermal properties of Saccharomyces cerevisiae and Kluyveromyces fragilis biomass revealed temperature peaks of 66.66 and 63.67 °C, respectively, suggesting the denaturation of proteins [30]. The difference between the temperatures for S. cerevisiae and K. fragilis and this study strain was insignificant. The third peak indicating the presence of saccharides in powdered biomass samples was characterized by a similar shape and maximum temperatures of 161.93 °C after 168 h and 154.28 °C after 192 h, respectively, as shown in Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6. The shape of the fourth endothermic peak (Figure 7) was different from the previous figures. The course of this peak indicated the presence of a mixture of saccharides. The maximum temperature of the peak was 211.18 °C. The maximum temperature value of the peak was similar to the maximum temperatures of the peaks that indicated saccharide decomposition in yeast from earlier days of cultivation. The analysis of the thermal properties of Tremella fuciformis polysaccharides illustrated a similar shape of the DSC curves [31], as shown in Figure 7. The fourth peak in Figure 8 is characterized by a different course and shape. The endothermic peak was characterized by a mild shape and course. The obtained maximum temperature was about 176.86 °C. The course and shape of the peaks indicated the presence of polysaccharides. DSC curves of Saccharomyces cerevisiae cells had a similar shape when the temperature increased from 100 to 250 °C [32].
3.3. The Composition and Nutritional Suitability of Rhodotorula glutinis var. rubescens Yeast Biomass Lipid
The cells with the highest lipid content as well as satisfactory biomass yield (41.55 g d.w./L) were selected for further analysis. Accordingly, biomass samples were obtained on the 5th and 6th day of cultivation. The high content of oleic acid (over 73% on average), followed by palmitic acid (>13%), stearic acid, and linoleic acid in approximate proportions characterized the lipids biosynthesized by Rhodotorula cells (Table 1). C 18:0 and C 18:2 were dependent on the length of time of cultivation. The dominant share of oleic acid, and consequently the sum of MUFA content, influenced the general characteristics of Rhodotorula lipids.

Table 1.
Fatty acids composition of lipid from Rhodotorula glutinis var. rubescens biomass obtained in 120 and 144 h of glucose fed-batch cultivation.
The composition of fatty acids is important for the prevention and treatment of diseases, which affects human health. It also determines the nutritional and technological suitability of fat. Based on fatty acids, many indices have been calculated to assess the usefulness of lipids from red yeast biomass in nutrition. The purpose of calculating the indicators (Table 2) was to assess the nutritional and/or pharmaceutical value of the lipids extracted from Rhodotorula cells.

Table 2.
Nutritional values of oleaginous yeast Rhodotorula glutinis var. rubescens lipids obtained in 120 and 144 h of glucose-fed-batch cultivation.
The sum of PUFA to the sum of SFA is one of the indicators characterizing the nutritional and health value of fats. a well-known hypothesis is that all SFAs increase the levels of serum cholesterol [33]. Consequently, a higher ratio of PUFA/SFA might result in a more positive effect. This index for fat extracted from the biomass of red yeast was similar to some green (Ulva reticulata), red (Gelidium micropterum), or brown seaweeds (Sargassum pallidum) [34]. However, for some seaweeds (Gracilaria changii), this ratio can be higher than 2 or even 6 [35], as well as for sunflower oils (>4). On the other hand, the PUFA/SFA ratio indicator of lipids in red yeast is higher than that in cow milk [36], sheep milk [37], or lamb meat [38]. The ∑PUFA to ∑SFA index can be insufficient when n-3 PUFA and n-6 PUFA show a variety of effects on cardiovascular health. The consumption of fats rich in n-6 acids with a very high proportion of n-6 to n-3 acids is considered to contribute to the development of many cardiovascular diseases, inflammatory diseases, and even cancer. Increasing the level of n-3 PUFA (while maintaining a low n-6/n-3 ratio) has the opposite effect. A low n-6/n-3 PUFA ratio (1:1 and 5:1) in the diet is recommendable by showing anti-inflammatory and anti-oxidative stress effects and improving endothelial function. A high n-6/n-3 PUFA ratio (20:1) has adverse effects [39]. Examples of fat-rich food or oils with a recommended n-6/n-3 ratio are canola oil 2:1 [40] or walnut oil (5:1) [41]. The red yeast lipid n-6/n-3 ratio was lower than 3,3:1, which is adequate for recommendation. The average value of this ratio of skeletal muscles of Polish Holstein-Friesian bulls (beef) was 2.40, full-fat milk and minced beef ratio was 2:1, and turkey and chicken breast were 12:1 and 11:1 [42].
Certain fatty acids, such as C12:0, C14:0, and C16:0, may support lipid adhesion to cells of the immune and circulatory system [43,44]. According to the high content of C 16:0 in Rhodotorula lipids, we calculated the values of the index of atherogenicity (AI), which were 0.223 and 0.204 (for lipids extracted on the 5th and 6th day, respectively). For comparison, higher values range from 1.4 to 5.1 for milk products [36], and lower values for poultry [45] or pork meat [46] were described. The same SFAs (C 12:0, C 14:0, and C 16:0) are pro-thrombogenic. So, it is preferable for cardiovascular health food and oils to have a low value of index of thrombogenicity (IT). The IT of lipids extracted from red yeast biomass was in a range of 0.438–0.416, which is similar to sea fruits such as sardines Hyporhamphus unifasciatus [47], largemouth bass Micropterus salmoides [48], or freshwater mullet Mugil cephalus [49]. The value of IT is naturally higher for milk and cheese [50], and some kinds of meat, such as veal, beef, lamb, and chicken [45,51,52]. On the other hand, some oils such as Camelina oil (Camelina sativa) [53], and rapeseed oil [54] are anti-thrombogenic, resulting in low SFA content. In some opinions, AI and IT provide clear evidence [55] and are the most commonly used.
Other indices identify the quality of oil-rich food products, such as the h/H (hypocholesterolemic and hypercholesterolemic) index, and the value of this is better when it is higher. It is characterized by the relationship between the sum of PUFA and cis-C18 and the sum of C 12:0, C 14:0, and C 16:0 [16]. The h/H ratios of the Rhodotorula yeast oils (Table 2) were quite high: 5.252–5.547 in comparison to food products such as fish, shellfish, meat, and milk products [55]. However, the plant oils rich in PUFA, such as Camelina oil, can have a three times higher h/H value.
The impact of highly unsaturated FA, as well as FAs that have a low degree of unsaturation, can be expressed by the unsaturation index (UI). However, it does not differentiate between n-6 and n-3 fatty acids. The values of UI red yeast’s lipids acquired from different sources were 86.465 and 87.320, which were rather low compared to the extraordinarily rich in PUFA in some algae [56] or seaweed oils [57].
The nutritional value of dietary fat can also be described by HPI (the health-promoting index) first proposed in 2004 [58]. This index is an inverse of IA. Generally, food products, characterized by high values of HPI should be eaten more often as they are assumed to be more beneficial. This index was mainly used for dairy product research [58,59,60], which did not exceed the value of 0.7. It is worth stating that the values of HPI for oil extracted from red biomass were a few times higher (4.6–5) compared to dairy products.
3.4. The Amino Acid Content and Nutritional Value of Protein of Rhodotorula glutinis var. rubescens Yeast Biomass
The growth and reproduction of yeast cells are directly related to protein biosynthesis due to the number of their structural, enzymatic, and signalling functions. With the time of cultivation under the glucose limitation, the level of protein accumulation decreases gradually until the yeast cells enter the stationary phase and after entering the phase of stationary growth [61]. Similarly, under excess glucose, which promotes lipid synthesis, the content of protein decreases with time (Figure 1). However, the biomass of the red oleaginous yeast after 5 and 6 days of incubation directed for lipid biosynthesis still contained a large amount of protein (av. > 35% d.w.).
The presence and appropriate content of all essential amino acids (EAA) determine the nutritional value of proteins in the diet. They cannot be synthesized from metabolic intermediates by humans or other vertebrates. Because the body cells lack the biochemical pathways synthesizing EAA (His, Ile, Leu, Lys, Met, Phe, Tre, Thr, Trp, and Val) must be supplied from a diet [62]. For the very first time, the content of individual amino acids including EAA in the biomass of the red yeast Rhodotorula glutinis was determined, and the results are shown in Figure 9.
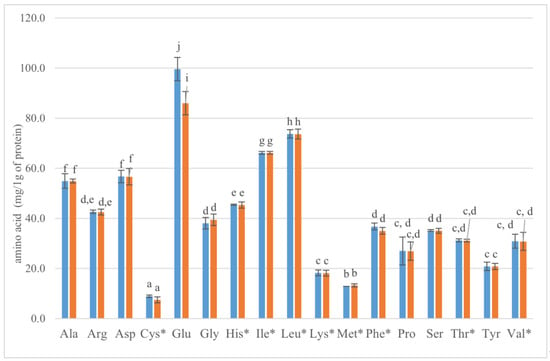
Figure 9.
The content of individual amino acids (mg/1 g of protein) in biomass of Rhodotorula glutinis var. rubescens yeast after 120 h (blue) and 144 h (orange) of cultivation. The superscripts * go for essential amino acids. Ala—alanine, Arg—arginine, Asp—aspartic acid, Cys—cysteine, Glu—glutamic acid, Gly—glycine, His—histidine, Ile—isoleucine, Lys—lysine, Met -methionine, Phe—phenylalanine, Pro—proline, Ser—serine, Thr—threonine, Val—valine. a–j were added only for significantly different values (ANOVA).
A high content of glutamic acid (99 mg/1 g of protein), leucine (>77 mg/1 g of protein), and isoleucine (>66 mg/1 g of protein), followed by Ala and Asp, was observed in Rhodotorula yeast protein. We did not observe significant differences between the samples dependent on the incubation time, except for glutamic acid. The determination of amino acids in the microbial protein was performed earlier, mainly because yeast cells can be used as SCP (single-cell protein). For instance, S. cerevisiae biomass contained a high percentage of protein (55.19%), characterized by the high content of Lys, Leu, Asp, Glu, and Ile [63]. The major component of biomass S. cerevisiae enriched in Cu, Fe, and Mn were glutamic acid (>45 g/kg), aspartic acid (av. 37 g/kg), and lysine (31–34 g/kg). On the contrary, the lowest concentration was observed for cysteine and methionine (<5 g/kg) [64]. The amino acid composition of dried yeast biomass after beer production [65] also showed the highest content of glutamic acid (5457 mg/100 g), lysine (4541 mg/100 g), and aspartic acid (4150 mg/100 g). The analysis of amino acid content in the biomass of Candida utilis [66] showed that glutamic acid (15.5 g/100 g) and aspartic acid (8 g/100 g) leucine (7.12 g/100 g) accounted for the largest share in cell protein. The following, alanine, valine, and lysine should be mentioned, as these exceeded 5 mg/100 g. The smallest amount was observed for methionine 1.58.
Lysine limits the value and quality of protein in animal feeding considering the physiology of digestion, so protein rich in Lys may prove to be excellent in the development of feed. The content of lysine in the protein of crustaceans such as M. macrocopa and Simocephalus vétulus was successfully increased by adding red yeast biomass to zooplankton [67]. However, the authors did not reveal the amino acid content of the biomass used. Our research showed that 1 g of protein of Rhodotorula glutins yeast contains 18 mg of Lys (Figure 9).
Block and Mitchel introduced a chemical score index in the forties of the past century [21]. It is a useful factor for the compartment of any protein with the reference pattern, according to the essential amino acid content. The limiting amino acid is the one that occurs in the smallest amount relative to the reference protein. The FAO/WHO developed a reference protein, a complete protein containing all EAA, for the first time in 1973 [68], and changed some recommendations later. The latest testimonials were identified in 2007, reducing the requirements for Phe [69], and in our study, we used this recommendation to calculate the chemical score. The examined red yeast biomass was distinguished by a high value of the chemical score for His, Ile, Leu, the sum of Phe, Tyr, and Thr (Table 3).

Table 3.
The comparison of Rhodotorula glutinis var. rubescens biomass to standard protein.
Proteins haveh a high nutritional value when they haveh a balanced amino acid composition expressed by a high value of the essential amino acid index. A promising value of EAAI (Table 3) was achieved for the protein obtained from the biomass of oleaginous red yeast. This suggests that the amino acid composition is superior to that of the FAO/WHO standard. However, despite higher EAAI, the projected diet based on yeast protein would not be satisfactory in terms of the content of some essential amino acids. Disadvantageously, they were all limiting in Lys and Met, with chemical scores lower than the standard. Notwithstanding, the amount of lysine is higher (1.8%) than that of cereal proteins, where the content is 0.31% (maize), 0.21–0.28% (millet), or 0.24% (sorghum) [70]. The essential amino acid composition of Saccharomyces cerevisiae shared from the largest to the smallest of the individual EAA was Leu, Lys, Arg, Val, Thr, Ile, Phe, His, Tyr, Met, and Cys. The EAAI of S. cerevisiae was approx. 67% for both strains [71]. The oleaginous yeast strain Yarrowia lipolytica can also be a valuable source of protein. For example, when cells are bred on raw glycerol, the EAA profile is characterized by a higher content of aromatic amino acids (Phe/Tyr), valine, threonine, and lysine compared to the whole egg [72].
4. Conclusions
The thermal as well as quantitative and qualitative characteristics of Rhodotorula yeast cell biomass after cultivation in lipid-promoting biosynthesis conditions were determined. The differential scanning calorimetry diagrams of red yeast biomass revealed two small and mild endothermic peaks where the first indicated the presence of fat in yeast biomass and the melting temperature indicated its composition of unsaturated fatty acids. The next small peaks were associated with the presence of low molecular weight carbohydrates, and the third distinct peaks were associated with the presence of crystalline sugars, indicating the presence of trehalose. However, the thermal characteristic of biomass originating from the last days of cultivation revealed additional peaks, suggesting the denaturation of protein, and the shape of the last peak indicated the presence of a mixture of saccharides rather than one crystalline sugar. DSC was successfully used to determine the melting behavior of the dried biomass of Rhodotorula sp. and indicated the presence of lipids, sugars, and proteins.
The oleic, palmitic, and stearic acids were predominant, resulting in a high MUFA concentration in the biomass. According to the fatty acid composition as well as amino acid content, the nutritional properties and healthy factors were calculated. Similarly to other yeast strains, Rhodotorula yeast protein was characterized by a high content of glutamic acid. However, the order of amino acid contributions appears to be species-specific and depends on the carbon source. The value of the essential amino acid index of the proteins present in the red yeast biomass suggested a superior amino acid composition compared to the FAO/WHO standard. Despite the high EAAI, yeast protein is characterized by a small content of Lys or Met. However, the amount of lysine was higher than that in some cereals, such as maize, millet, or sorghum. The nutritional characteristics of lipids extracted from Rhodotorula glutinis var. rubescens revealed better quantity compared to dairy products when atherogenicity, thrombogenicity, and health-promoting index, as well as the PUFA/SFA ratio, were taken into account. The ratio n-6/n-3 of the studied fat was adequate for recommendation. However, the lack of long PUFA does not entitle us to conclude that lipids obtained from red yeast without modification can be an alternative to fish oil.
After the extraction of the microbial lipids, post-extraction waste remains. Post-extraction wastes contain protein, sugar (e.g., polysaccharides of the cell wall and trehalose), and other components, e.g., vitamins, that are not extracted into solvents along with lipids. We considered the usefulness of the protein residue after lipid extraction theoretically (based only on calculations). Nevertheless, during the extraction procedure, unfavourable changes may occur, affecting the amino acid composition and nutritional value of the protein, such as digestibility or bioavailability. In further work, the potential of post-extraction residues as food or feed additives usage should be tested in practice.
The circular production model in the food sector is concerned with limiting the inflow of raw materials and waste production. From a broader perspective, this study’s results allow us to conclude that the use of waste nitrogen as a media component may be an important element in the implementation of a circular economy in the starch industry. The producers of potato starch might valorize the deproteinated wastewater and obtain red yeast biomass rich in valuable lipids and proteins.
Author Contributions
Conceptualization, I.G.; methodology and formal analysis, I.G., A.S., M.W.-W. and E.O.-L.; writing—original draft preparation, I.G. and E.O.-L. writing—review and editing, I.G., E.O.-L. and M.W.-W.; visualization, I.G.; project administration, I.G.; funding acquisition, I.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Polish National Science Centre (NCN), within the project (No. 2019/03/X/NZ9/00148) titled “How feeding with carbon source and temperature influence the metabolism of oleaginous yeast during cultivation in media with a waste nitrogen source?”. Research equipment was purchased as part of the “Food and Nutrition Centre—modernisation of the WULS campus to create a Food and Nutrition Research and Development Centre (CŻiŻ)” co-financed by the European Union from the European Regional Development Fund under the Regional Operational Programme of the Mazowieckie Voivodeship for 2014-2020 (Project No. RPMA.01.01.00-14-8276/17).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
SCP—single cell protein, SCO—single cell oils, DSC—differential scanning calorimetry, FA—fatty acid, AA—amino acids, SFA—saturated fatty acid, UFA—unsaturated fatty acid, MUFA—monosaturated fatty acid, PUFA—polyunsaturated fatty acid, SI—saturation index, UI—unsaturation index, NV—nutritional value, IA—atherogenicity index, IT—thrombogenicity index, hH—hypocholesterolemic/hypercholesterolemic ratio, HPI—health-promoting index, EAA—essential amino acid, EEAI—essential amino acid index, and CS—chemical score.
References
- Thiviya, P.; Gamage, A.; Kapilan, R.; Merah, O.; Madhujith, T. Single Cell Protein Production Using Different Fruit Waste: A Review. Separations 2022, 9, 178. [Google Scholar] [CrossRef]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein—State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 2009. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of Oleaginous Yeasts. Part II: Technology and Potential Applications. Eur. J. Lipid Sci. Technol. 2011, 113, 1052–1073. [Google Scholar] [CrossRef]
- Parsons, S.; Allen, M.J.; Chuck, C.J. Coproducts of algae and yeast-derived single cell oils: A critical review of their role in improving biorefinery sustainability. Bioresour. Technol. 2020, 303, 122862. [Google Scholar] [CrossRef]
- Vasilakis, G.; Karayannis, D.; Massouras, T.; Politis, I.; Papanikolaou, S. Biotechnological Conversions of Mizithra Second Cheese Whey by Wild-Type Non-Conventional Yeast Strains: Production of Yeast Cell Biomass, Single-Cell Oil and Polysaccharides. Appl. Sci. 2022, 12, 11471. [Google Scholar] [CrossRef]
- Janssen, M.; Wijffels, R.H.; Barbosa, M.J. Microalgae based production of single-cell protein. Curr. Opin. Biotechnol. 2022, 75, 102705. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily Yeasts as Oleaginous Cell Factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef]
- Gientka, I.; Duda, M.; Bzducha-Wróbel, A.; Błażejak, S. Deproteinated potato wastewater as a low-cost nitrogen substrate for very high yeast biomass quantities: Starting point for scaled-up applications. Eur. Food Res. Technol. 2019, 245, 919–928. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Garay, L.A.; Sestric, R.; Levin, D.; Block, D.E.; German, J.B.; Boundy-Mills, K.L. Oleaginous Yeasts for Biodiesel: Current and Future Trends in Biology and Production. Biotechnol. Adv. 2014, 32, 1336–1360. [Google Scholar] [CrossRef]
- Gientka, I.; Wirkowska-Wojdyła, M.; Ostrowska-Ligęza, E.; Janowicz, M.; Reczek, L.; Synowiec, A.; Błażejak, S. Enhancing Red Yeast Biomass Yield and Lipid Biosynthesis by Using Waste Nitrogen Source by Glucose Fed-Batch at Low Temperature. Microorganisms 2022, 10, 1253. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula glutinis—Potential Source of Lipids, Carotenoids, and Enzymes for Use in Industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [CrossRef] [PubMed]
- Górska, A.; Brzezińska, R.; Wirkowska-Wojdyła, M.; Bryś, J.; Domian, E.; Ostrowska-Ligęza, E. Application of Thermal Methods to Analyze the Properties of Coffee Silverskin and Oil Extracted from the Studied Roasting By-Product. Appl. Sci. 2020, 10, 8790. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.C.; French, W.T.; Hernandez, R.; Alley, E.; Paraschivescu, M. Effects of Furfural and Acetic Acid on Growth and Lipid Production from Glucose and Xylose by Rhodotorula glutinis. Biomass Bioenergy 2011, 35, 734–740. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Santos-Silva, J.; Bessa, R.J.B.; Santos-Silva, F. Effect of Genotype, Feeding System and Slaughter Weight on the Quality of Light Lambs: II. Fatty Acid Composition of Meat. Livest. Prod. Sci. 2002, 77, 187–194. [Google Scholar] [CrossRef]
- Kirk, P.L. Kjeldahl Method for Total Nitrogen. Anal. Chem. 1950, 22, 354–358. [Google Scholar] [CrossRef]
- Jaworska, G.; Bernaś, E. Comparison of Amino Acid Content in Canned Pleurotus Ostreatus and Agaricus Bisporus Mushrooms. J. Fruit Ornam. Plant Res. 2011, 74, 107–115. [Google Scholar] [CrossRef]
- Buňka, F.; Hrabě, J.; Kráčmar, S. The Effect of Sterilisation on Amino Acid Contents in Processed Cheese. Int. Dairy J. 2004, 14, 829–831. [Google Scholar] [CrossRef]
- INGOS. Amino Acid Analyzer AAA 500 Clarity EN User Manual; INGOS: Praha, Czech Republic, 2021. [Google Scholar]
- Block, R.J.; Mitchell, H.H. The Correlation of the Amino Acid Composition of Proteins with Their Nutritive Value. Nutr. Abstr. Rev. 1946, 16, 249–278. [Google Scholar]
- Oser, B.L. An Integrated Essential Amino Acid Index for Predicting the Biological Value of Proteins. In Protein and Amino Acid Nutrition; Elsevier: Amsterdam, The Netherlands, 1959; pp. 281–295. [Google Scholar] [CrossRef]
- Tidwell, J.H.; Webster, C.D.; Yancey, D.H.; D’Abramo, L.R. Partial and Total Replacement of Fish Meal with Soybean Meal and Distillers’ by-Products in Diets for Pond Culture of the Freshwater Prawn (Macrobrachium rosenbergii). Aquaculture 1993, 118, 119–130. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Górska, A.; Wirkowska, M.; Koczoń, P. An Assessment of Various Powdered Baby Formulas by Conventional Methods (DSC) or FT-IR Spectroscopy. J. Therm. Anal. Calorim. 2012, 110, 465–471. [Google Scholar] [CrossRef]
- Fakas, S. Lipid Biosynthesis in Yeasts: A Comparison of the Lipid Biosynthetic Pathway between the Model Nonoleaginous Yeast Saccharomyces cerevisiae and the Model Oleaginous Yeast Yarrowia lipolytica. Eng. Life Sci. 2016, 17, 292–302. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of Oleaginous Yeasts. Part I: Biochemistry of Single Cell Oil Production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar] [CrossRef]
- Ostrowska-Ligęza, E.; Dolatowska-Żebrowska, K.; Wirkowska-Wojdyła, M.; Bryś, J.; Górska, A. Comparison of Thermal Characteristics and Fatty Acids Composition in Raw and Roasted Cocoa Beans from Peru (Criollo) and Ecuador (Forastero). Appl. Sci. 2021, 11, 2698. [Google Scholar] [CrossRef]
- Tsai, S.-Y.; Yu, H.-T.; Lin, C.-P. The Potential of the Oil-Producing Oleaginous Yeast Rhodotorula mucilaginosa for Sustainable Production of Bio-Oil Energy. Processes 2022, 10, 336. [Google Scholar] [CrossRef]
- Li, X.; Mansour, H.M. Physicochemical Characterization and Water Vapor Sorption of Organic Solution Advanced Spray-Dried Inhalable Trehalose Microparticles and Nanoparticles for Targeted Dry Powder Pulmonary Inhalation Delivery. AAPS PharmSciTech 2011, 12, 1420–1430. [Google Scholar] [CrossRef]
- Otero, M.A.; Wagner, J.R.; Vasallo, M.C.; García, L.; Añón, M.C. Thermal Behavior and Hydration Properties of Yeast Proteins from Saccharomyces cerevisiae and Kluyveromyces fragilis. Food Chem. 2000, 69, 161–165. [Google Scholar] [CrossRef]
- Lin, C.-P.; Tsai, S.-Y. Differences in the Moisture Capacity and Thermal Stability of Tremella fuciformis Polysaccharides Obtained by Various Drying Processes. Molecules 2019, 24, 2856. [Google Scholar] [CrossRef]
- Paramera, E.I.; Konteles, S.J.; Karathanos, V.T. Microencapsulation of Curcumin in Cells of Saccharomyces cerevisia. Food Chem. 2011, 125, 892–902. [Google Scholar] [CrossRef]
- Bansal, N.; Dasgupta, D.; Hazra, S.; Bhaskar, T.; Ray, A.; Ghosh, D. Effect of utilization of crude glycerol as substrate on fatty acid composition of an oleaginous yeast Rhodotorula mucilagenosa IIPL32: Assessment of nutritional indices. Bioresour. Technol. 2020, 309, 123330. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, P.; Trivedi, N.; Shukla, M.K.; Gupta, V.; Reddy, C.R.K.; Jha, B. Minerals, PUFAs and Antioxidant Properties of Some Tropical Seaweeds from Saurashtra Coast of India. J. Appl. Phycol. 2011, 23, 797–810. [Google Scholar] [CrossRef]
- Chan, P.T.; Matanjun, P. Chemical Composition and Physicochemical Properties of Tropical Red Seaweed, Gracilaria changii. Food Chem. 2017, 221, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Nantapo, C.T.W.; Muchenje, V.; Hugo, A. Atherogenicity Index and Health-Related Fatty Acids in Different Stages of Lactation from Friesian, Jersey and Friesian × Jersey Cross Cow Milk under a Pasture-Based Dairy System. Food Chem. 2014, 146, 127–133. [Google Scholar] [CrossRef]
- Sinanoglou, V.J.; Koutsouli, P.; Fotakis, C.; Sotiropoulou, G.; Cavouras, D.; Bizelis, I. Assessment of Lactation Stage and Breed Effect on Sheep Milk Fatty Acid Profile and Lipid Quality Indices. Dairy Sci. Technol. 2015, 95, 509–531. [Google Scholar] [CrossRef]
- Brogna, D.M.R.; Nasri, S.; Salem, H.B.; Mele, M.; Serra, A.; Bella, M.; Priolo, A.; Makkar, H.P.S.; Vasta, V. Effect of Dietary Saponins from Quillaja saponaria L. on Fatty Acid Composition and Cholesterol Content in Muscle Longissimus dorsi of Lambs. Animal 2011, 5, 1124–1130. [Google Scholar] [CrossRef]
- Yang, L.G.; Song, Z.X.; Yin, H.; Wang, Y.Y.; Shu, G.F.; Lu, H.X.; Wang, S.K.; Sun, G.J. Low N-6/n-3 PUFA Ratio Improves Lipid Metabolism, Inflammation, Oxidative Stress and Endothelial Function in Rats Using Plant Oils as n-3 Fatty Acid Source. Lipids 2016, 51, 49–59. [Google Scholar] [CrossRef]
- da Costa, C.A.S.; Carlos, A.S.; de Paula Lopes Gonzalez, P.; Reis, R.P.G.; Ribeiro, M.D.S.; Dos Santos, A.d.S.; Monteiro, A.M.V.; de Moura, E.G.; do Nascimento-Saba, C.C.A. Diet Containing Low N-6/n-3 Polyunsaturated Fatty Acids Ratio, Provided by Canola Oil, Alters Body Composition and Bone Quality in Young Rats. Eur. J. Nutr. 2012, 51, 191–198. [Google Scholar] [CrossRef]
- FoodData Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/171030/nutrients (accessed on 13 August 2023).
- Michaelsen, K.F.; Dewey, K.G.; Perez-Exposito, A.B.; Nurhasan, M.; Lauritzen, L.; Roos, N. Food Sources and Intake of N-6 and n-3 Fatty Acids in Low-Income Countries with Emphasis on Infants, Young Children (6–24 Months), and Pregnant and Lactating Women. Matern. Child Nutr. 2011, 7, 124–140. [Google Scholar] [CrossRef]
- Omri, B.; Chalghoumi, R.; Izzo, L.; Ritieni, A.; Lucarini, M.; Durazzo, A.; Abdouli, H.; Santini, A. Effect of Dietary Incorporation of Linseed Alone or Together with Tomato-Red Pepper Mix on Laying Hens’ Egg Yolk Fatty Acids Profile and Health Lipid Indexes. Nutrients 2019, 11, 813. [Google Scholar] [CrossRef]
- Monteiro, M.; Matos, E.; Ramos, R.; Campos, I.; Valente, L.M.P. A Blend of Land Animal Fats Can Replace up to 75% Fish Oil without Affecting Growth and Nutrient Utilization of European Seabass. Aquaculture 2018, 487, 22–31. [Google Scholar] [CrossRef]
- Mir, N.A.; Tyagi, P.K.; Biswas, A.K.; Tyagi, P.K.; Mandal, A.B.; Kumar, F.; Sharma, D.; Biswas, A.; Verma, A.K. Inclusion of Flaxseed, Broken Rice, and Distillers Dried Grains with Solubles (DDGS) in Broiler Chicken Ration Alters the Fatty Acid Profile, Oxidative Stability, and Other Functional Properties of Meat. Eur. J. Lipid Sci. Technol. 2018, 120, 1700470. [Google Scholar] [CrossRef]
- Alvarenga, A.L.N.; Sousa, R.V.; Parreira, G.G.; Chiarini-Garcia, H.; Almeida, F.R.C.L. Fatty Acid Profile, Oxidative Stability of Pork Lipids and Meat Quality Indicators Are Not Affected by Birth Weight. Animal 2014, 8, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.E.; da Silva Vasconcelos, M.A.; de Almeida Ribeiro, M.; Sarubbo, L.A.; Andrade SA, C.; de Melo Filho, A.B. Nutritional and Lipid Profiles in Marine Fish Species from Brazil. Food Chem. 2014, 160, 67–71. [Google Scholar] [CrossRef]
- Subhadra, B.; Lochmann, R.; Rawles, S.; Chen, R. Effect of Dietary Lipid Source on the Growth, Tissue Composition and Hematological Parameters of Largemouth Bass (Micropterus salmoides). Aquaculture 2006, 255, 210–222. [Google Scholar] [CrossRef]
- Bouzgarrou, O.; El Mzougui, N.; Sadok, S. Smoking and Polyphenols’ Addition to Improve Freshwater Mullet (Mugil cephalus) Fillets’ Quality Attributes during Refrigerated Storage. Int. J. Food Sci. Technol. 2016, 51, 268–277. [Google Scholar] [CrossRef]
- Vargas-Bello-Pérez, E.; Íñiguez-González, G.; Fehrmann-Cartes, K.; Toro-Mujica, P.; Garnsworthy, P.C. Influence of Fish Oil Alone or in Combination with Hydrogenated Palm Oil on Sensory Characteristics and Fatty Acid Composition of Bovine Cheese. Anim. Feed. Sci. Technol. 2015, 205, 60–68. [Google Scholar] [CrossRef]
- Wójciak, K.M.; Stasiak, D.M.; Ferysiuk, K.; Solska, E. The Influence of Sonication on the Oxidative Stability and Nutritional Value of Organic Dry-Fermented Beef. Meat Sci. 2019, 148, 113–119. [Google Scholar] [CrossRef]
- Majdoub-Mathlouthi, L.; Saïd, B.; Kraiem, K. Carcass Traits and Meat Fatty Acid Composition of Barbarine Lambs Reared on Rangelands or Indoors on Hay and Concentrate. Animal 2015, 9, 2065–2071. [Google Scholar] [CrossRef]
- Ratusz, K.; Symoniuk, E.; Wroniak, M.; Rudzińska, M. Bioactive Compounds, Nutritional Quality and Oxidative Stability of Cold-Pressed Camelina (Camelina sativa L.) Oils. Appl. Sci. 2018, 8, 2606. [Google Scholar] [CrossRef]
- Okrouhlá, M.; Stupka, R.; Čítek, J.; Lebedová, N.; Zadinová, K. Effect of Duration of Dietary Rapeseed and Soybean Oil Feeding on Physical Characteristics, Fatty Acid Profile, and Oxidative Stability of Pig Backfat. Animals 2018, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, H. Nutritional Indices for Assessing Fatty Acids: A Mini-Review. Int. J. Mol. Sci. 2020, 21, 5695. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, Y.; Liu, T.; Zhang, L.; Liu, H.; Guan, H. Comparative Studies on the Characteristic Fatty Acid Profiles of Four Different Chinese Medicinal Sargassum Seaweeds by GC-MS and Chemometrics. Mar. Drugs 2016, 14, 68. [Google Scholar] [CrossRef] [PubMed]
- Dellatorre, F.G.; Avaro, M.G.; Commendatore, M.G.; Arce, L.; Díaz de Vivar, M.E. The Macroalgal Ensemble of Golfo Nuevo (Patagonia, Argentina) as a Potential Source of Valuable Fatty Acids for Nutritional and Nutraceutical Purposes. Algal Res. 2020, 45, 101726. [Google Scholar] [CrossRef]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and Sensory Properties of Dairy Products from Cows with Various Milk Fatty Acid Compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef]
- Bobe, G.; Zimmerman, S.; Hammond, E.G.; Freeman, A.E.; Porter, P.A.; Luhman, C.M.; Beitz, D.C. Butter Composition and Texture from Cows with Different Milk Fatty Acid Compositions Fed Fish Oil or Roasted Soybeans. J. Dairy Sci. 2007, 90, 2596–2603. [Google Scholar] [CrossRef]
- Bonanno, A.; Grigoli, A.D.; Mazza, F.; Pasquale, C.D.; Giosuè, C.; Vitale, F.; Alabiso, M. Effects of Ewes Grazing Sulla or Ryegrass Pasture for Different Daily Durations on Forage Intake, Milk Production and Fatty Acid Composition of Cheese. Animal 2016, 10, 2074–2082. [Google Scholar] [CrossRef]
- Boucherie, H. Protein Synthesis during Transition and Stationary Phases under Glucose Limitation in Saccharomyces Cerevisiae. J. Bacteriol. 1985, 161, 385–392. [Google Scholar] [CrossRef]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Abdel-Hafez, A.M.; Mahmoud, S.A.Z.; El-Sawy, M.; Ramadan, E.M. Studies on Protein Production by Yeasts: II. Protein, Non-Protein Nitrogen, and Amino Acid Content of Yeast Strains. Zentralblatt für Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Zweite Naturwissenschaftliche Abt. Allg. Landwirtsch. Und Tech. Mikrobiol. 1977, 132, 631–640. [Google Scholar] [CrossRef]
- Dobrzański, Z.; Opaliński, S.; Dolińska, B.; Chojnacka, K.; Kołacz, R. The nutritive value of yeast Saccharomyces cerevisiae enriched in copper, iron, and manganes. In Proceedings of the ISAH-2007 Tartu, XIIIth International Congress in Animal Hygiene, Estonian University of Life Sciences, Tartu, Estonia, 17–21 June 2007. [Google Scholar]
- Yalçın, S.; Erol, H.; Özsoy, B.; Onbaşılar, I.; Yalçın, S. Effects of the Usage of Dried Brewing Yeast in the Diets on the Performance, Egg Traits and Blood Parameters in Quails. Animal 2008, 2, 1780–1785. [Google Scholar] [CrossRef]
- Nicolas, O.; Aly, S.; Marius, K.S.; François, T.; Cheikna, Z.; Alfred, S.T. Effect of Mineral Salts and Nitrogen Source on Yeast (Candida Utilis NOY1) Biomass Production Using Tubers Wastes. Afr. J. Biotechnol. 2017, 16, 359–365. [Google Scholar] [CrossRef]
- Khudyi, O.; Kushniryk, O.; Khuda, L.; Marchenko, M. Differences in Nutritional Value and Amino Acid Composition of Moina Macrocopa (Straus) Using Yeast Saccharomyces cerevisiae and Rhodotorula glutinis as Fodder Substrates. ILNS 2018, 68, 27–34. [Google Scholar] [CrossRef]
- FAO; WHO. Energy and Protein Requirements; Report of a Joint FAO/WHO Ad hoc Expert committee World Health Organization Technical Report Series No. 522/FAO Nutrition Meetings Report Series No. 52; World Health Organization: Geneva, Switzerland, 1973.
- WHO; FAO; UNU. Protein and Amino Acid Requirements in Human Nutrition; Weltgesundheitsorganisation, FAO, Vereinte Nationen, Eds.; Report of a Joint WHO/FAO/UNU Expert Consultation; WHO: Geneva, Switzerland, 2007.
- Vasan, P.; Mandal, A.B.; Dutta, N.; Maiti, S.K.; Sharma, K. Digestibility of Amino Acids of Maize, Low Tannin Sorghum, Pearl Millet and Finger Millet in Caecectomized Roosters. Asian-Australas. J. Anim. Sci. 2008, 21, 701–706. [Google Scholar] [CrossRef]
- Michalik, B.; Biel, W.; Lubowicki, R.; Jacyno, E. Chemical Composition and Biological Value of Proteins of the Yeast Yarrowia lipolytica Growing on Industrial Glycerol. Can. J. Anim. Sci. 2014, 94, 99–104. [Google Scholar] [CrossRef][Green Version]
- Juszczyk, P.; Tomaszewska, L.; Kita, A.; Rymowicz, W. Biomass Production by Novel Strains of Yarrowia lipolytica Using Raw Glycerol, Derived from Biodiesel Production. Bioresour. Technol. 2013, 137, 124–131. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).