On the Arrays Distribution, Scan Sequence and Apodization in Coherent Dual-Array Ultrasound Imaging Systems
Abstract
:1. Introduction
2. Materials and Methods
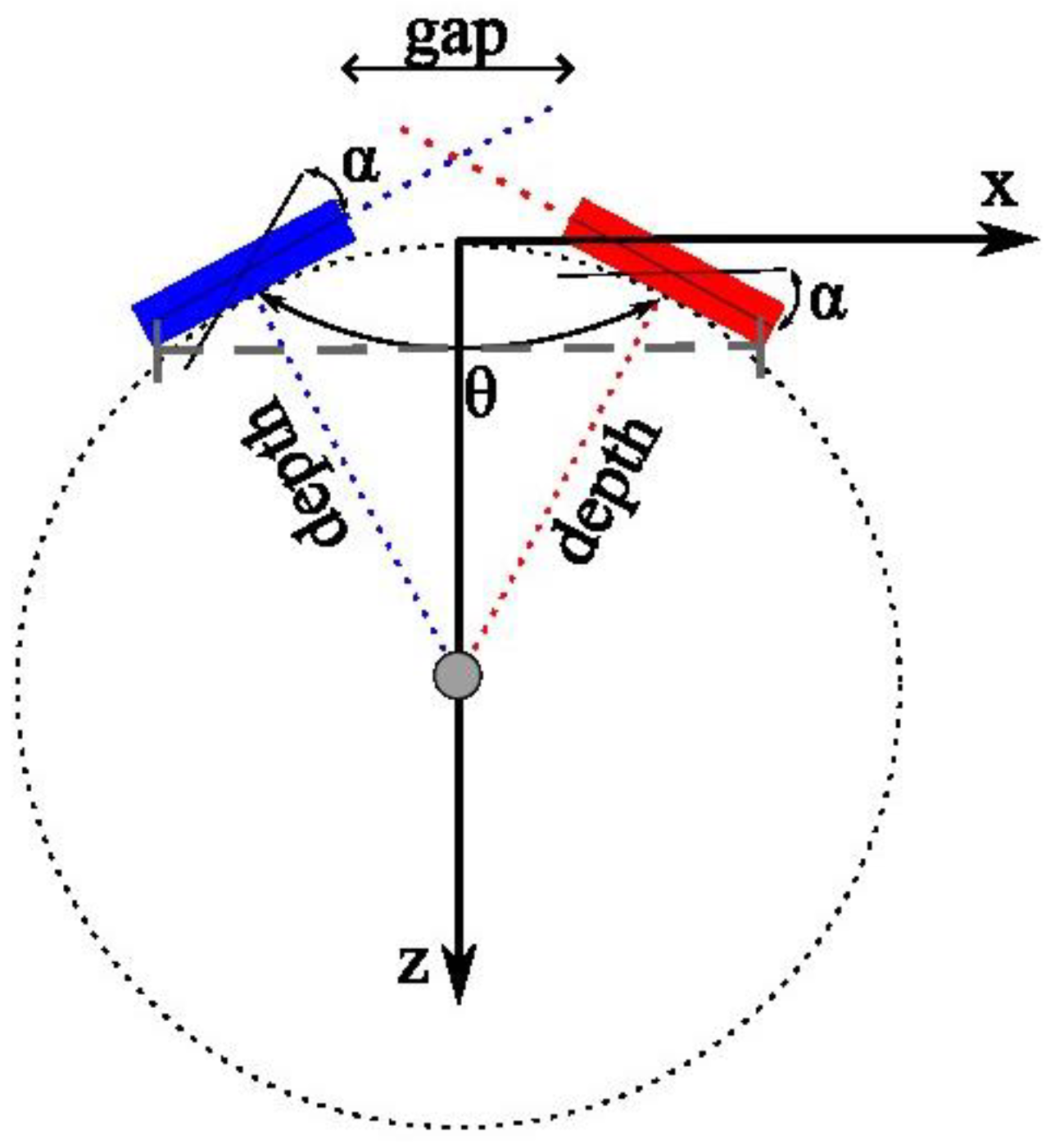
2.1. Coherent Dual-Array Imaging
2.2. Simulations
2.3. Experiments
2.4. Image Quality Metrics
3. Results
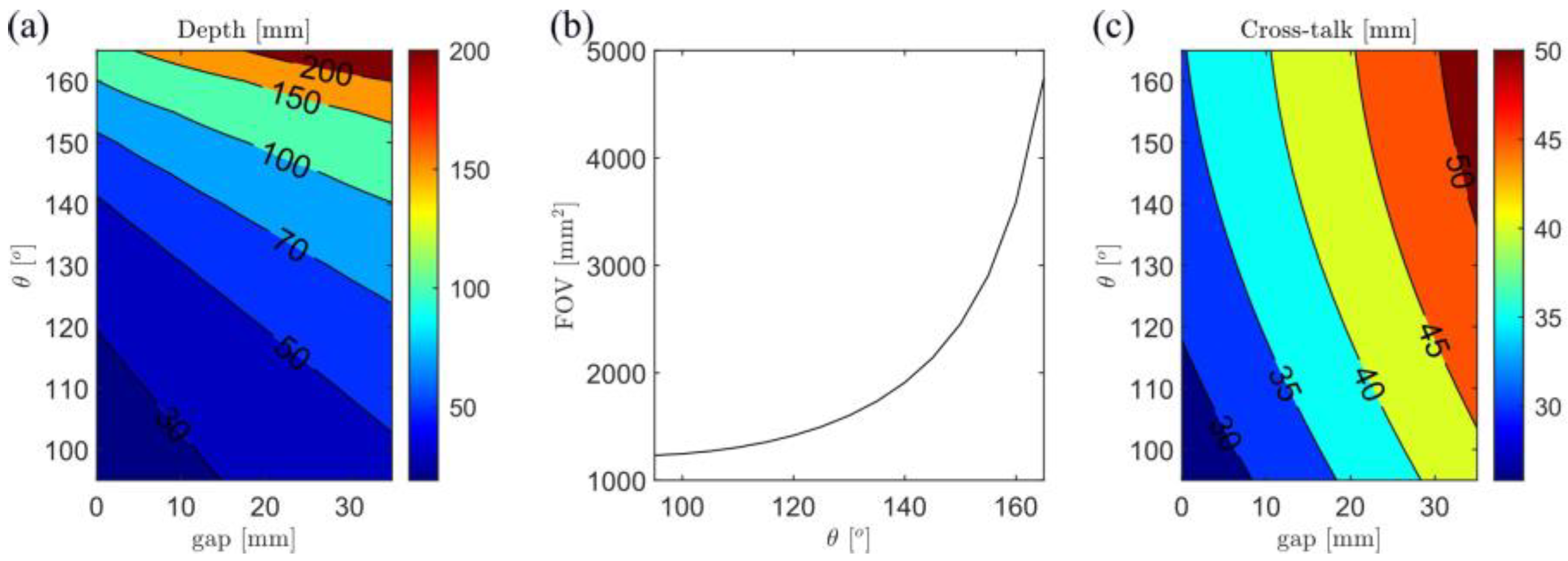
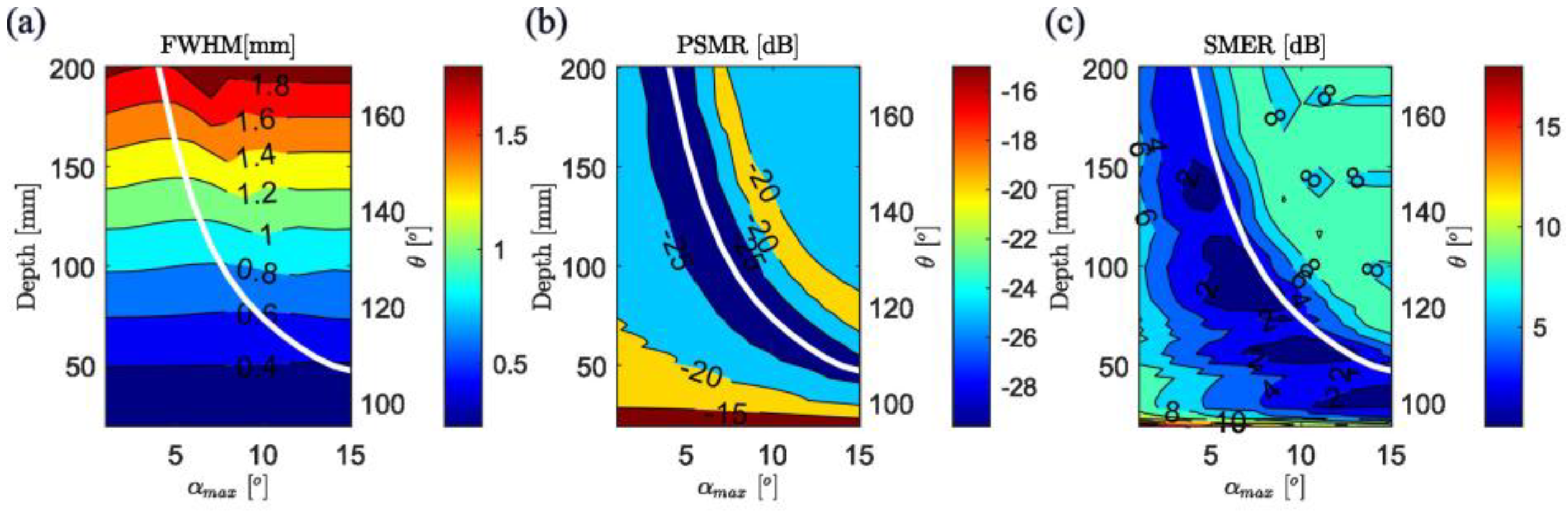
3.1. Simulation Results
3.2. Experimental Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraleigh, C.D.M.; Duff, E. Point-of-Care Ultrasound: An Emerging Clinical Tool to Enhance Physical Assessment. Nurse Pract. 2022, 47, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.J.S.; Loichinger, M.; Zalud, I. Obesity and the Challenges of Ultrasound Fetal Abnormality Diagnosis. Best Pract. Res. Clin. Obs. Gynaecol. 2015, 29, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Cobbold, R.S. Foundations of Biomedical Ultrasound; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- Jensen, J.A. Medical Ultrasound Imaging. Prog. Biophys. Mol. Biol. 2007, 93, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Bottenus, N.; Long, W.; Zhang, H.K.; Jakovljevic, M.; Bradway, D.P.; Boctor, E.M.; Trahey, G.E. Feasibility of Swept Synthetic Aperture Ultrasound Imaging. IEEE Trans. Med. Imaging 2016, 35, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Bottenus, N. Implementation of Constrained Swept Synthetic Aperture Using a Mechanical Fixture. Appl. Sci. 2023, 13, 4797. [Google Scholar] [CrossRef]
- Hunter, A.J.; Drinkwater, B.W.; Wilcox, P.D. Autofocusing Ultrasonic Imagery for Non-Destructive Testing and Evaluation of Specimens with Complicated Geometries. NDT E Int. 2010, 43, 78–85. [Google Scholar] [CrossRef]
- Lane, C.J.L. The Inspection of Curved Components Using Flexible Ultrasonic Arrays and Shape Sensing Fibres. Case Stud. Nondestruct. Test. Eval. 2014, 1, 13–18. [Google Scholar] [CrossRef]
- Noda, T.; Tomii, N.; Nakagawa, K.; Azuma, T.; Sakuma, I. Shape Estimation Algorithm for Ultrasound Imaging by Flexible Array Transducer. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 2345–2353. [Google Scholar] [CrossRef]
- Huang, X.; Lediju Bell, M.A.; Ding, K. Deep Learning for Ultrasound Beamforming in Flexible Array Transducer. IEEE Trans. Med. Imaging 2021, 40, 3178–3189. [Google Scholar] [CrossRef]
- Peralta, L.; Gomez, A.; Luan, Y.; Kim, B.-H.; Hajnal, J.V.; Eckersley, R.J. Coherent Multi-Transducer Ultrasound Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 66, 1316–1330. [Google Scholar] [CrossRef]
- van Hal, V.H.J.; De Hoop, H.; Muller, J.W.; van Sambeek, M.R.H.M.; Schwab, H.M.; Lopata, R.G.P. Multiperspective Bistatic Ultrasound Imaging and Elastography of the Ex Vivo Abdominal Aorta. IEEE Trans. Ultrason. Ferroelectr Freq. Control 2022, 69, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Foiret, J.; Cai, X.; Bendjador, H.; Park, E.-Y.; Kamaya, A.; Ferrara, K.W. Improving Plane Wave Ultrasound Imaging through Real-Time Beamformation across Multiple Arrays. Sci. Rep. 2022, 12, 13386. [Google Scholar] [CrossRef] [PubMed]
- de Hoop, H.; Petterson, N.J.; van de Vosse, F.N.; van Sambeek, M.R.H.M.; Schwab, H.M.; Lopata, R.G.P. Multiperspective Ultrasound Strain Imaging of the Abdominal Aorta. IEEE Trans. Med. Imaging 2020, 39, 3714–3724. [Google Scholar] [CrossRef] [PubMed]
- De Hoop, H.; Vermeulen, M.; Schwab, H.M.; Lopata, R.G.P. Coherent Bistatic 3-D Ultrasound Imaging Using Two Sparse Matrix Arrays. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2023, 70, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Peralta, L.; Ramalli, A.; Reinwald, M.; Eckersley, R.J.; Hajnal, J.V. Impact of Aperture, Depth, and Acoustic Clutter on the Performance of Coherent Multi-Transducer Ultrasound Imaging. Appl. Sci. 2020, 10, 7655. [Google Scholar] [CrossRef] [PubMed]
- Peralta, L.; Mazierli, D.; Gomez, A.; Hajnal, J.V.; Tortoli, P.; Ramalli, A. 3-D Coherent Multitransducer Ultrasound Imaging with Sparse Spiral Arrays. IEEE Trans Ultrason. Ferroelectr. Freq. Control 2023, 70, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Y.; Zou, H.; Greenleaf, J.F. Biomedical Ultrasound Beam Forming. Ultrasound Med. Biol. 1994, 20, 403–428. [Google Scholar] [CrossRef]
- Demi, L. Practical Guide to Ultrasound Beam Forming: Beam Pattern and Image Reconstruction Analysis. Appl. Sci. 2018, 8, 1544. [Google Scholar] [CrossRef]
- Montaldo, G.; Tanter, M.; Bercoff, J.; Benech, N.; Fink, M. Coherent Plane-Wave Compounding for Very High Frame Rate Ultrasonography and Transient Elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 489–506. [Google Scholar] [CrossRef]
- Ortiz, S.H.C.; Chiu, T.; Fox, M.D. Ultrasound Image Enhancement: A Review. Biomed. Signal Process. Control 2012, 7, 419–428. [Google Scholar] [CrossRef]
- Tong, L.; Gao, H.; D’Hooge, J. Multi-Transmit Beam Forming for Fast Cardiac Imaging-a Simulation Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1719–1731. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Ramalli, A.; Jasaityte, R.; Tortoli, P.; D’Hooge, J. Multi-Transmit Beam Forming for Fast Cardiac Imaging-Experimental Validation and in Vivo Application. IEEE Trans. Med. Imaging 2014, 33, 1205–1219. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-Y. 2D and 3D High Frame Rate Imaging with Limited Diffraction Beams. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1997, 44, 839–856. [Google Scholar] [CrossRef]
- Jensen, J.A.; Svendsen, N.B. Calculation of Pressure Fields from Arbitrarily Shaped, Apodized, and Excited Ultrasound Transducers. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1992, 39, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.A.; Jensen, J.A. FIELD: A Program for Simulating Ultrasound Systems. In Proceedings of the 10th Nordicbaltic Conference on Biomedical Imaging, Tampere, Finland, 9–13 June 1996; Volume 34, pp. 351–353. [Google Scholar]
- Boni, E.; Bassi, L.; Dallai, A.; Meacci, V.; Ramalli, A.; Scaringella, M.; Guidi, F.; Ricci, S.; Tortoli, P. Architecture of an Ultrasound System for Continuous Real-Time High Frame Rate Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2017, 64, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Mazierli, D.; Ramalli, A.; Boni, E.; Guidi, F. Tortoli Architecture for an Ultrasound Advanced Open Platform with an Arbitrary Number of Independent Channels. IEEE Trans. Biomed. Circuits Syst. 2021, 15, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Gao, H.; Choi, H.F.; D’Hooge, J. Comparison of Conventional Parallel Beamforming with Plane Wave and Diverging Wave Imaging for Cardiac Applications: A Simulation Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 1654–1663. [Google Scholar] [CrossRef]
- Rodriguez-Molares, A.; Rindal, O.M.H.; D’Hooge, J.; Masoy, S.E.; Austeng, A.; Lediju Bell, M.A.; Torp, H. The Generalized Contrast-to-Noise Ratio: A Formal Definition for Lesion Detectability. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 67, 745–759. [Google Scholar] [CrossRef]
- Denarie, B.; Tangen, T.A.; Ekroll, I.K.; Rolim, N.; Torp, H.; Bjastad, T.; Lovstakken, L. Coherent Plane Wave Compounding for Very High Frame Rate Ultrasonography of Rapidly Moving Targets. IEEE Trans. Med. Imaging 2013, 32, 1265–1276. [Google Scholar] [CrossRef]
- Karaman, M.; Li, P.C.; O’Donnell, M. Synthetic Aperture Imaging for Small Scale Systems. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1995, 42, 429–442. [Google Scholar] [CrossRef]
- Smith, S.W.; Wagner, R.F.; Sandrik, J.M.; Lopez, H. Low Contrast Detectability and Contrast/Detail Analysis in Medical Ultrasound. IEEE Trans. Sonics Ultrason. 1983, 30, 164–173. [Google Scholar] [CrossRef]
- Chau, G.; Lavarello, R.; Dahl, J. Short-Lag Spatial Coherence Weighted Minimum Variance Beamformer for Plane-Wave Images. In Proceedings of the IEEE International Ultrasonics Symposium, IUS, Tours, France, 18–21 September 2016. [Google Scholar] [CrossRef]
- Bell, M.A.L.; Dahl, J.J.; Trahey, G.E. Resolution and Brightness Characteristics of Short-Lag Spatial Coherence (SLSC) Images. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2015, 62, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Matrone, G.; Savoia, A.S.; Caliano, G.; Magenes, G. The Delay Multiply and Sum Beamforming Algorithm in Ultrasound B-Mode Medical Imaging. IEEE Trans. Med. Imaging 2015, 34, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-H.; Kumar, V.; Alizad, A.; Fatemi, M. Gap-Filling Method for Suppressing Grating Lobes in Ultrasound Imaging: Theory and Simulation Results. J. Acoust. Soc. Am. 2019, 145, EL236–EL242. [Google Scholar] [CrossRef] [PubMed]
- van Hal, V.H.J.; Muller, J.W.; van Sambeek, M.R.H.M.; Lopata, R.G.P.; Schwab, H.M. An Aberration Correction Approach for Single and Dual Aperture Ultrasound Imaging of the Abdomen. Ultrasonics 2023, 131, 106936. [Google Scholar] [CrossRef] [PubMed]
- Petterson, N.J.; van Sambeek, M.R.H.M.; van de Vosse, F.N.; Lopata, R.G.P. Enhancing Lateral Contrast Using Multi-Perspective Ultrasound Imaging of Abdominal Aortas. Ultrasound Med. Biol. 2021, 47, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Papadacci, C.; Pernot, M.; Couade, M.; Fink, M.; Tanter, M. High-Contrast Ultrafast Imaging of the Heart. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2014, 61, 288–301. [Google Scholar] [CrossRef]
- Moign, G.L.; Quaegebeur, N.; Masson, P.; Basset, O.; Robini, M.; Liebgott, H. Optimization of Virtual Sources Distribution in 3D Echography. In Proceedings of the IEEE International Ultrasonics Symposium, IUS, Glasgow, UK, 6–9 October 2019; pp. 904–907. [Google Scholar] [CrossRef]

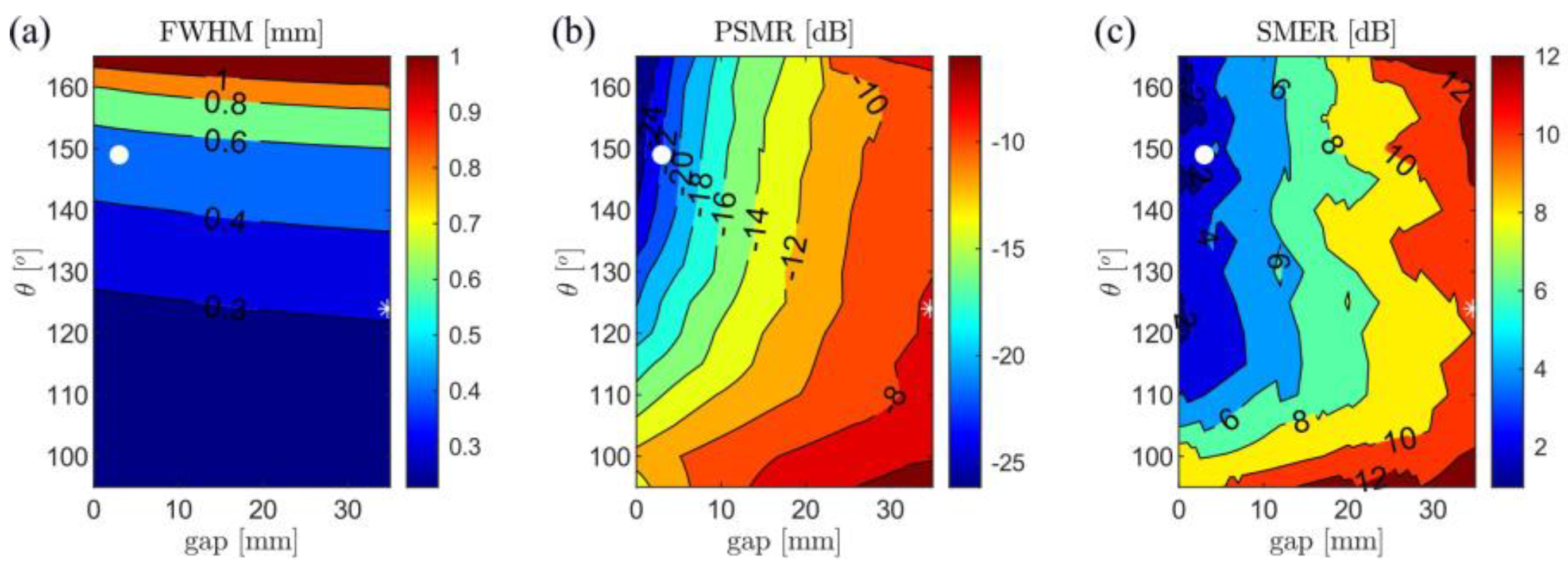
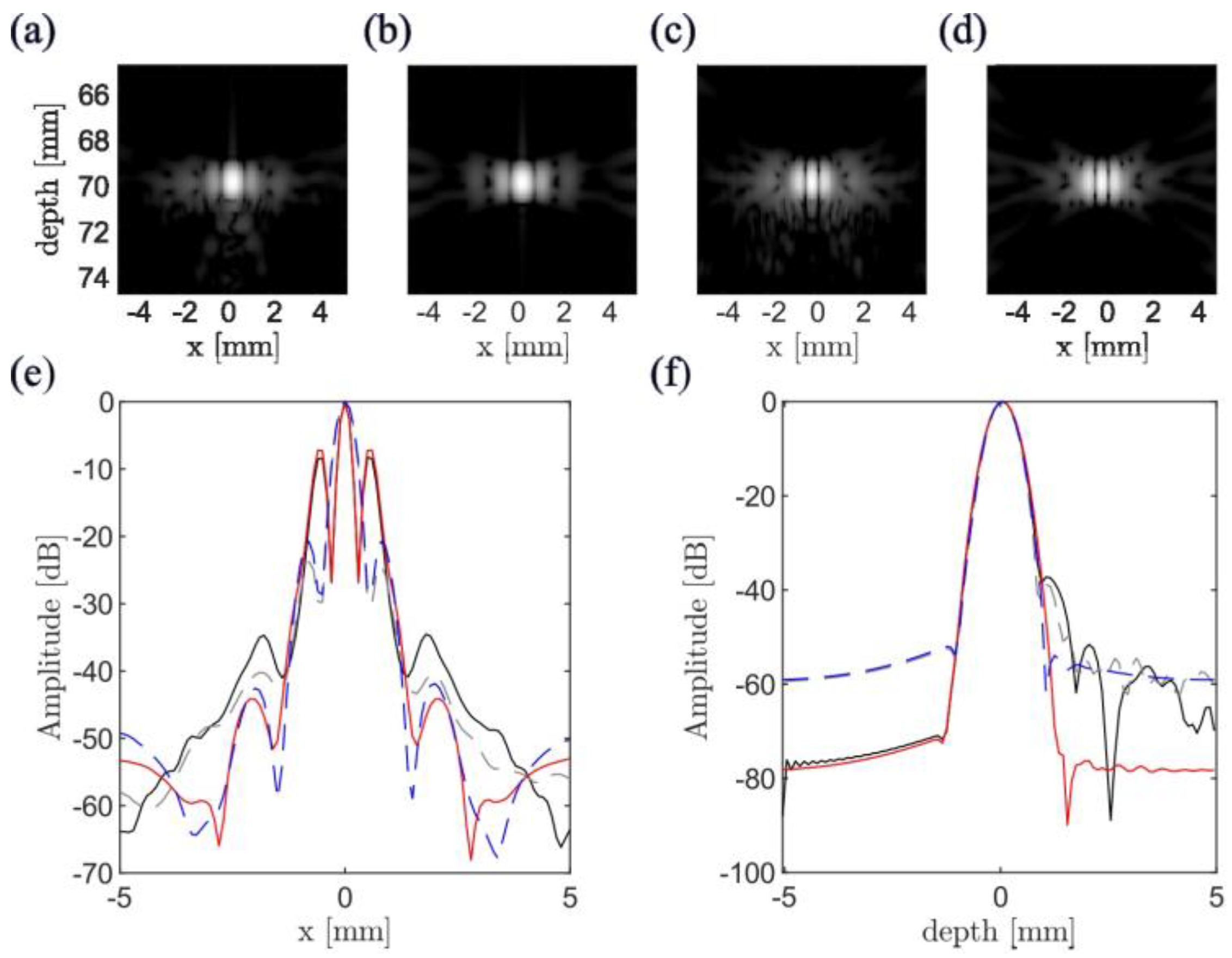
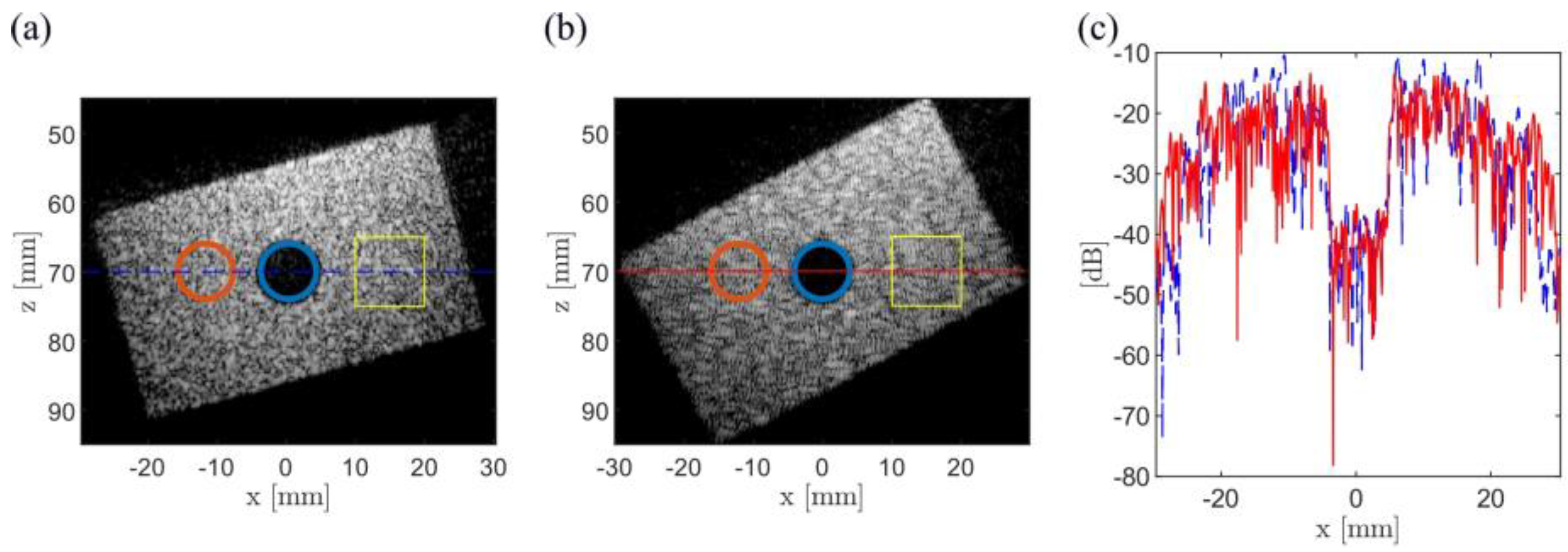
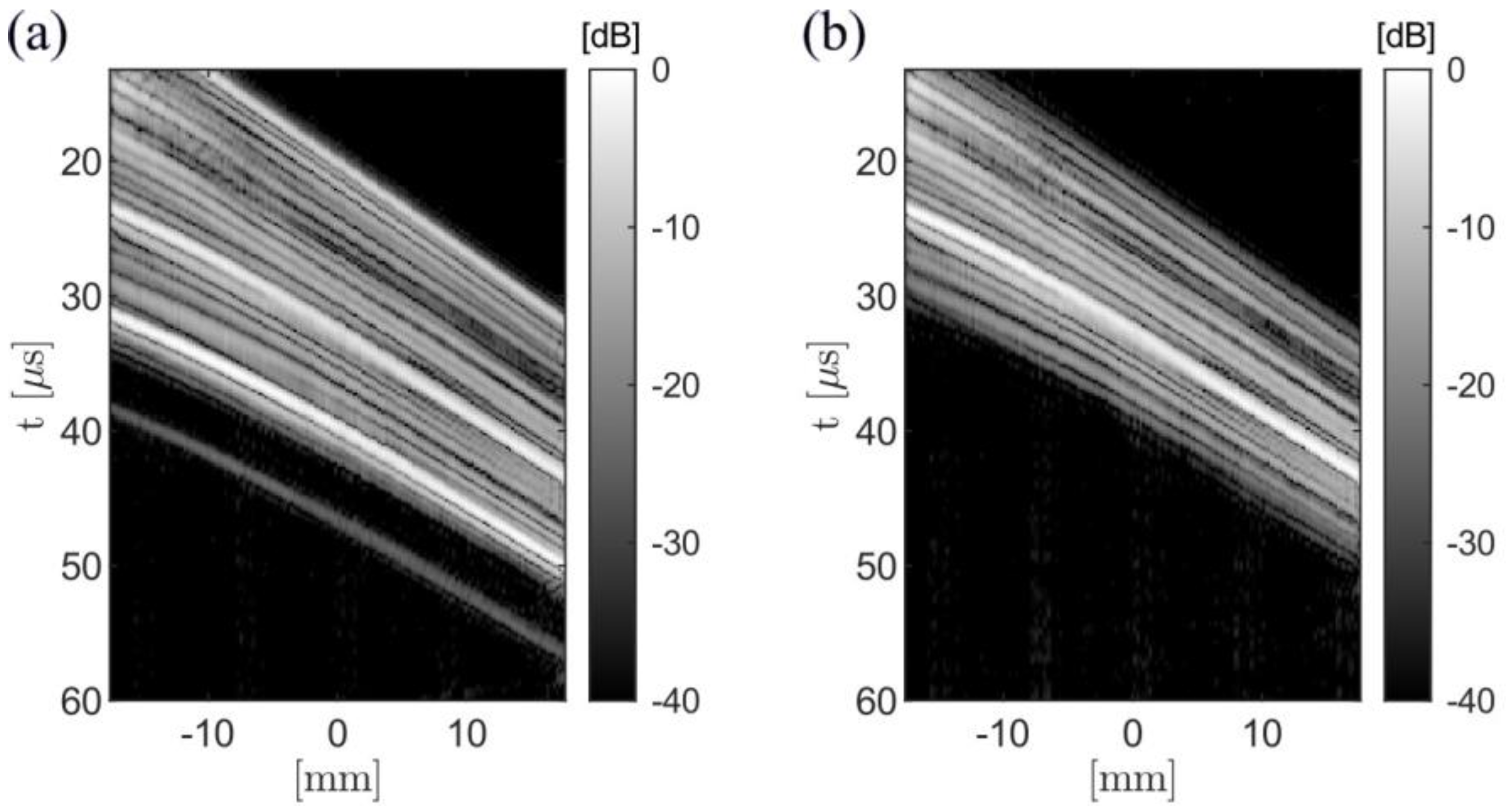


| Configuration | θ = 149°, gap = 3 mm (Minimum-Side Lobe Energy) | θ = 124°, gap = 35 mm (Minimum Main Lobe Width) | ||
|---|---|---|---|---|
| Tx. Apodization | Rectangular | Tukey | Rectangular | Tukey |
| FWHM [mm] | 0.49 | 0.49 | 0.29 | 0.29 |
| PSMR [dB] | −23.7 | −20.7 | −8.3 | −7.2 |
| SMER [dB] | 0.0 | 0.9 | 9.4 | 10.3 |
| CR [dB] | −22.7 | −22.6 | −23.4 | −23.6 |
| CNR [-] | 1.65 | 1.64 | 1.72 | 1.72 |
| gCNR [-] | 0.97 | 0.97 | 0.97 | 0.97 |
| Speckle resolution [mm] | 0.53 | 0.54 | 0.38 | 0.38 |
| Configuration | θ = 149°, gap = 3 mm (Minimum-Side Lobe Energy) | θ = 124°, gap = 35 mm (Minimum Main Lobe Width) | ||||
|---|---|---|---|---|---|---|
| Tx. Apodization | None | Tukey | Tukey | None | Tukey | Tukey |
| Weighted compounding | No | No | Yes | No | No | Yes |
| Speckle size [mm] | 0.40 | 0.40 | 0.37 | 0.28 | 0.30 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peralta, L.; Mazierli, D.; Christensen-Jeffries, K.; Ramalli, A.; Tortoli, P.; Hajnal, J.V. On the Arrays Distribution, Scan Sequence and Apodization in Coherent Dual-Array Ultrasound Imaging Systems. Appl. Sci. 2023, 13, 10924. https://doi.org/10.3390/app131910924
Peralta L, Mazierli D, Christensen-Jeffries K, Ramalli A, Tortoli P, Hajnal JV. On the Arrays Distribution, Scan Sequence and Apodization in Coherent Dual-Array Ultrasound Imaging Systems. Applied Sciences. 2023; 13(19):10924. https://doi.org/10.3390/app131910924
Chicago/Turabian StylePeralta, Laura, Daniele Mazierli, Kirsten Christensen-Jeffries, Alessandro Ramalli, Piero Tortoli, and Joseph V. Hajnal. 2023. "On the Arrays Distribution, Scan Sequence and Apodization in Coherent Dual-Array Ultrasound Imaging Systems" Applied Sciences 13, no. 19: 10924. https://doi.org/10.3390/app131910924
APA StylePeralta, L., Mazierli, D., Christensen-Jeffries, K., Ramalli, A., Tortoli, P., & Hajnal, J. V. (2023). On the Arrays Distribution, Scan Sequence and Apodization in Coherent Dual-Array Ultrasound Imaging Systems. Applied Sciences, 13(19), 10924. https://doi.org/10.3390/app131910924









