1. Introduction
Porous polymers have very diverse applications, such as absorption, separation, decontamination, microelectromechanical systems, optical interference coatings, tissue engineering, and (bio)sensing [
1,
2]. The generation of pores in polymers may be performed by “top-down” approaches, such as nanoimprinting or etching, or by “bottom-up” approaches, which rely on phase separation and block copolymer self-assembly. Methods for producing pores involve the use of porogens in the form of gas, liquid, or solid dispersed phases and combinations of various templates, which leave voids of different sizes after being removed. Other technologies are also used for pore generation, such as electrospinning, 3D printing, freeze drying, or injecting, sometimes combined with template-based ones, aiming to generate hierarchical porous materials.
Silicones, with their most-known representative, polydimethylsiloxane (PDMS), have unique properties, dictated by the particularities of the siloxane bond [
3]. PDMS is an insulator and has a low viscosity, a low glass transition temperature (around −120 °C), very low surface tension and pronounced hydrophobicity, good thermal stability, optical transparency, stability to UV and corona discharge, and water vapor permeability. In addition, silicones are chemically stable, resistant to weathering, have good blood compatibility, and are considered biologically inert. Their chemical modification is possible by various methods and confers special properties and utility to the resultant silicone materials, spanning from liquid crystals and surfactants to electroactive dielectrics [
4].
Creating pores in silicones is another way to expand their use, since silicone rubber foams bring together the properties of silicone elastomers and those of foams in materials with low density, heat and cold resistance, good electrical insulation, and biocompatibility [
5]. Porous silicones are proposed for applications as sensors [
6], sorbents [
7], acoustic metamaterials [
8], and cell-growth scaffolds [
9]. The methods for pore generation in silicones are diverse. The most-known ones rely on crystalline materials with a narrow particle size distribution as sacrificial direct templates [
9,
10]; employ the emulsion template method, where water droplets act as the porogen [
11,
12,
13]; or use various foaming agents. The latter can be, for example, supercritical CO
2 [
14] or sodium bicarbonate as a CO
2 source [
6], alkane by-products in Piers–Rubinsztajn reactions [
15], or hydrogen gas. The in situ evolution of hydrogen in dehydrocoupling reactions occurring between Si-H and OH groups in the presence of a platinum catalyst specific for hydrosilylation curing was explored long ago [
16]. Additives, such as water, ethanol, ethylene glycol, and 2-methoxyethanol, have been used in order to obtain pores within the membranes, which are possibly useful in ultrafiltration. It was reported that the pore size depends on the additive’s reactivity, which controls the rate of H
2 evolution (foam formation). When poly(methylhydrogen)siloxane (PMHS) is crosslinked with a vinyl silicone, pores form due to dehydrocoupling side reactions, and the hydrophilic–hydrophobic properties can be modified [
17].
Adjusting the surface properties of polymeric materials is a key aspect for their practical use. For example, foaming issues in the petroleum industry are in close connection to the surface tension and surfactant–polymer interactions [
18]. In the biomedical field, the hydrophobic surfaces increase the protein adsorption, which may be undesired in a biological environment, but useful in other applications, like protein separation [
17]. Superhydrophobic surfaces have contact angles (CAs) larger than 150°, but an essential parameter that defines their behavior against water is the contact angle hysteresis (CAH) [
19]. When the CAH is very low, the surfaces mimic lotus leaves, where water drops roll off [
20], and are of interest in self-cleaning coatings, for example. When CAH values are very high, the water drops are pinned on the surface, like in the case of rose petals [
21].
Silicones are inherently hydrophobic materials, providing low energy surfaces. Superhydrophobicity can be achieved by chemical modification of silica films or by creating micro-/nanostructures from PDMS using lithography, templates, or chemical reactions, as recently reviewed [
22]. For example, a superhydrophobic and oleophobic silicone sponge with hierarchical structures was obtained by introducing a secondary structure on the pore walls of a hydrophobic and oleophilic silicone sponge [
23]. Superhydrophobic and superoleophilic behavior was reported for PDMS-MWCNT porous composites, based on the combination of methyl groups on the surface of PDMS and increased surface roughness due to MWCNTs [
24]. However, apart from a few exceptions, the literature on porous silicones rarely addresses the surface properties in correlation with chemical structure. On the one hand, assessing the surface properties of porous materials may be challenging, and on the other hand, most porous silicones are designed for specific applications and emphasis is given to their performance. Also, in most articles, the basic materials are commercially available silicone kits, and parameters other than composition or chemical structure are followed.
In our previous report, the emulsion template method was adapted based on a pair of siloxane surfactants soluble in water and in toluene, respectively, and used to obtain porous silicones [
13]. In this method, the continuous phase is a solution of silicones in toluene, and the disperse phase is water. The pores are formed by rapid UV-initiated thiol-ene photoaddition reaction, which ensures the cross-linking of the vinyl-containing silicone precursor, followed by the solvent’s evaporation.
Herein, the emulsion template method was combined with the dehydrocoupling reaction and chemical modification in order to obtain silicone materials with tunable morphology and surface properties. First, PMHS was cross-linked in emulsion with a Pt catalyst without other additions. Then, the composition of the reaction precursors was varied in order to introduce PDMS segments and lower the cross-linking density. Next, the PMHS was chemically modified in situ with n-hexene or allyltrimethylsilane, aiming to obtain porous materials with increased hydrophobic behavior. In another series, a dimethyl-methylvinyl siloxane copolymer was cross-linked by thiol-ene photoaddition and chemically modified in situ with mercaptoethanol to induce hydrophilic behavior. The resultant materials were investigated in powder state by performing tensiometry wetting measurements, and the obtained results were correlated with qualitative observations and contact angle measurements. Depending on the chemical structure and morphology, the wetting behavior of these materials can span from hydrophilic to superhydrophobic, with “lotus” or “petal” effects. As a consequence, a large diversity of related materials can be obtained by very simple and mild methods, with applications spanning from the sorption of organic compounds to self-cleaning. In particular, the petal effect, observed on three of our samples, and which has rarely been reported in the case of porous silicones, is considered interesting for applications, such as single-molecule spectroscopy and controlled transport of small volumes of liquid in open microfluidic devices [
25].
3. Results and Discussion
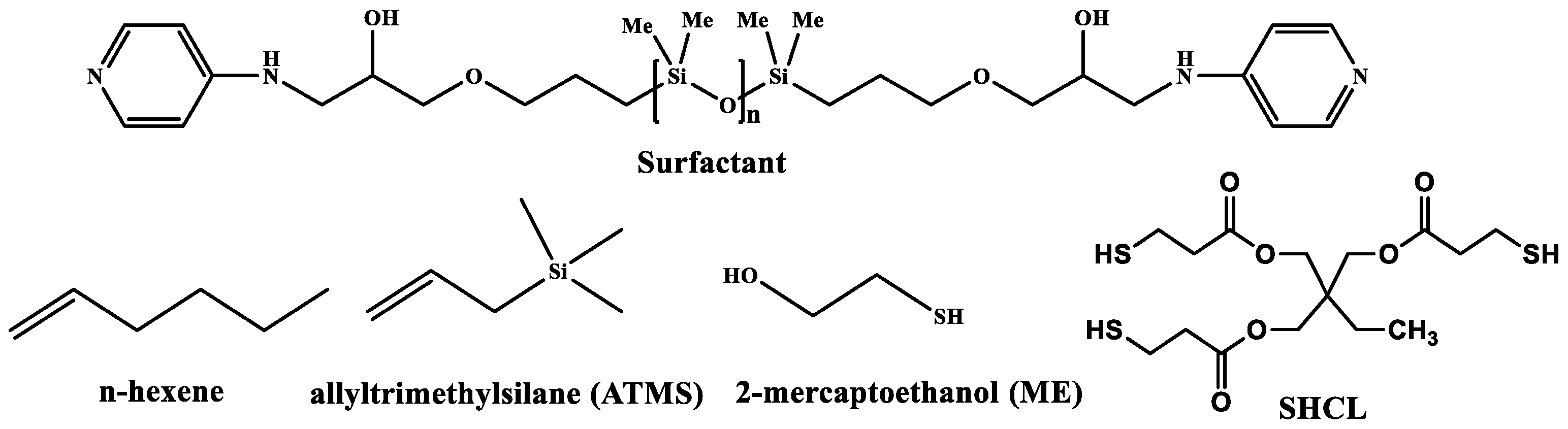
The emulsion stabilizer was an in-house prepared telechelic siloxane oligomer with aminopyridyl end groups (
Scheme 1, n = 15, HLB 4.4), which is soluble in toluene. The medium internal phase emulsion (f = 0.5) of water in toluene was stable from one day to another, when partial demulsification was observed and re-emulsification was very easily performed with an ultrasonic cleaning bath. The stability of the emulsion at 50 °C was also very good within the timeframe of the reaction, since its appearance remained unchanged (
Figure S1). The surface tension of the emulsion was 25.27 ± 0.07 mN/m at room temperature and 24.43 ± 0.1 mN/m at 50 °C (by the Wilhelmy plate method).
The dehydrocoupling reaction of PMHS was used to generate pores in combination with the emulsion template method. This reaction, occurring very fast in the presence of Karstedt catalyst at room temperature in bulk, is accompanied by the evolution of hydrogen gas, due to atmospheric moisture. The first step is the partial hydrolysis of Si-H groups in the precursor PHMS [
17], followed by the condensation of Si-H and Si-OH groups. In our case, PMHS was formulated as emulsion, and important volume expansion due to the evolution of hydrogen was instantaneously observed once the Pt catalyst was added. The tight cross-linking by Si-O-Si bridges (
Scheme 1A) generated a rigid, rather frail foam, in which unreacted Si-H and Si-OH groups were still present, due to the high reaction rate leading to a high cross-linking density and thus rapidly reduced mobility. These reminiscent functional groups can be observed in the FT-IR spectrum (
Figure S2), at 3440 cm
−1 (OH), 2174 cm
−1 (Si-H), shifted from 2170 cm
−1 in PMHS, and around 910 cm
−1 (Si(H)CH
3). The wide polysiloxane (Si-O-Si) band is located at 1030–1140 cm
−1 in PMHS and DH100. Other bands at 1410 cm
−1 (asymmetric deformation vibration of the CH
3 group), 1261 cm
−1 (symmetric deformation vibration of the CH
3 group), 845, and 781 cm
−1 (methyl rocking vibration and Si-C stretching vibration) are characteristic of siloxane compounds [
30].
The same reaction was used for preparing several porous silicones (
Table 1), varying the amount of Si-H groups, either by using a poly(dimethyl-methylhydrogen)siloxane with 30% Si-H groups (DH30) or by combining PHMS with a copolymer with 10% (DH70) or 30% Si-H groups (DH44). In this way, polydimethylsiloxane (PDMS) chains were introduced, which are known as hydrophobic and ensure a more elastic framework, which is useful for certain applications. The presence of PDMS segments was seen in FT-IR by a narrower polysiloxane band, with peaks at 1024 and 1094 cm
−1, a stronger band at 2968 cm
−1 (CH
3 asymmetric stretching vibration), and shifted Si-C stretching vibration bands, the strongest one at 800 cm
−1 (
Figure S2). In
Table 2, the theoretical cross-linking degree (CLD) is the proportion of Si-H groups in the reaction mixture. However, since the dehydrocoupling reaction cannot be total, the CLD was calculated from the FT-IR spectrum, by the ratio between the absorption of Si-H and Si-CH
3 groups, reported to the same ratio of intensities in PMHS, which was practically 1. So, the calculated CLD in DH100 was 77%. Small Si-H signals were observed in the copolymers, too, and the CLD was calculated for DH30, taking as a reference the CoSiH30 precursor. For the remaining DH materials, the CLD cannot be calculated in the same manner, since in these cases the proportion of CH
3 groups is not constant.
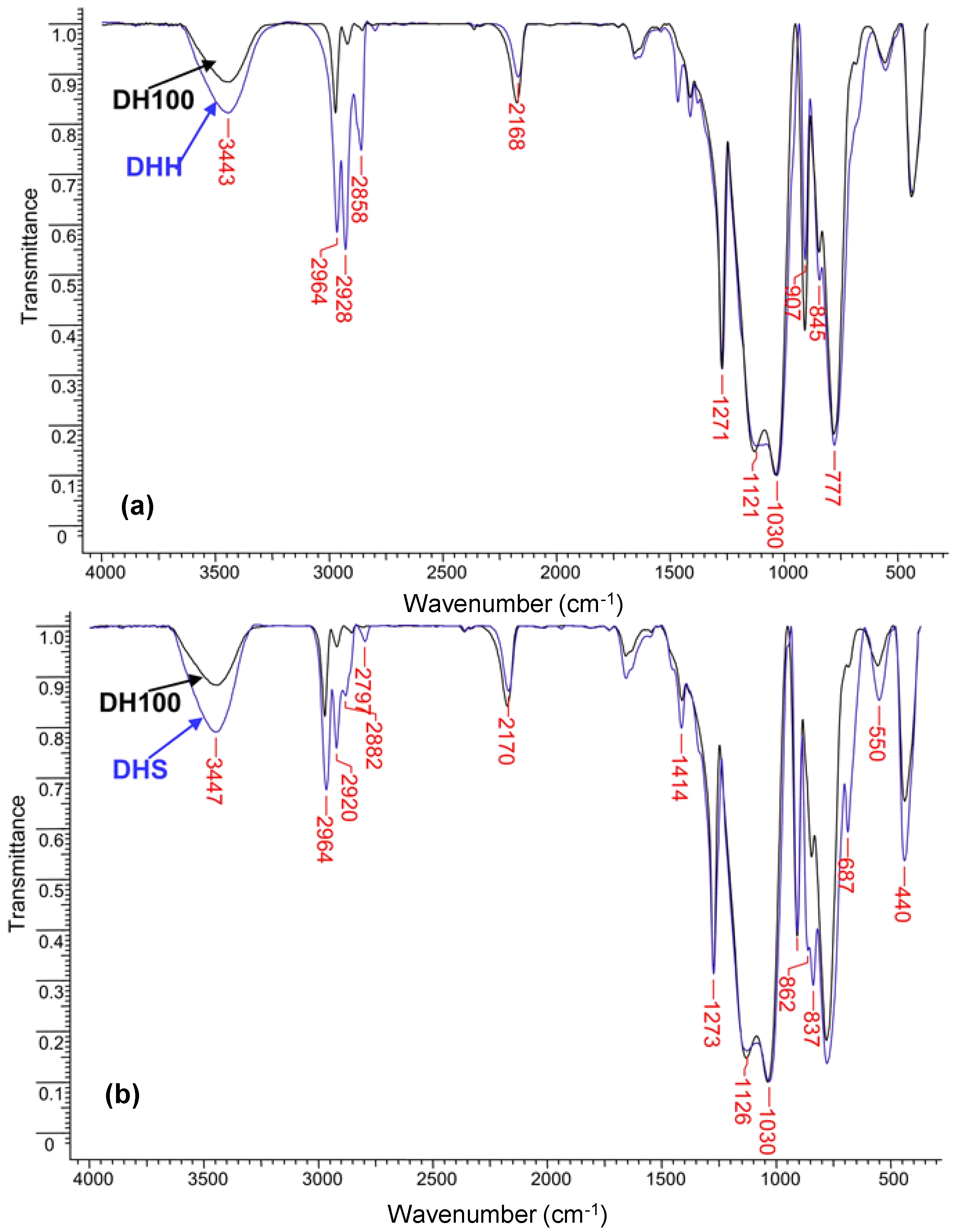
PHMS was chemically modified with hexene (DHH) or with allyltrimethylsilane (DHS), both hydrosilylation reactions occurring in the presence of Karstedt catalyst, simultaneously with the dehydrocoupling reaction. The long hexyl and the trimethylsilyl groups were intended to increase the hydrophobic character of the porous materials. The FT-IR spectra are shown in
Figure 1, in both cases compared with DH100 as a reference. The presence of aliphatic CH
2 groups in DHH is proved by the adsorption bands at 2928 cm
−1 (asymmetric stretching vibration) and 2858 cm
−1 (symmetric stretching vibration) (
Figure 1a). In DHS, the CH
2 asymmetric stretching can be seen at 2920 cm
−1, while the band at 2960 (CH
3 asymmetric stretching vibration) is more pronounced than in DH100. In addition, two bands at 862 and 837 cm
−1 are assignable to Si-C stretching vibrations, one of them being due to trimethylsilyl groups [
30]. The absence of a double bond absorption band, which would have appeared at around 3050 cm
−1, implies that the conversion of hexene and allyltrimethylsilane was high and that the unreacted reagent was removed in the purification step. Like in the other cases, reminiscent Si-H bands still exist in both chemically modified samples. Taking PMHS as a reference, the conversion of the Si-H groups is higher than in DH100. However, two concurrent reactions occur, and part of the reacted Si-H groups is involved in cross-linking. Assuming the same conversion of Si-H groups in the cross-linking reaction, calculations indicate 28.5% modification with n-hexene (theoretical 28%) and 24% modification with ATMS (vs. 26% theoretical). Thus, we can conclude that the hexyl and propylene-trimethylsilyl groups are chemically attached to the polysiloxane backbone, and the modification reactions occurred in high yield.
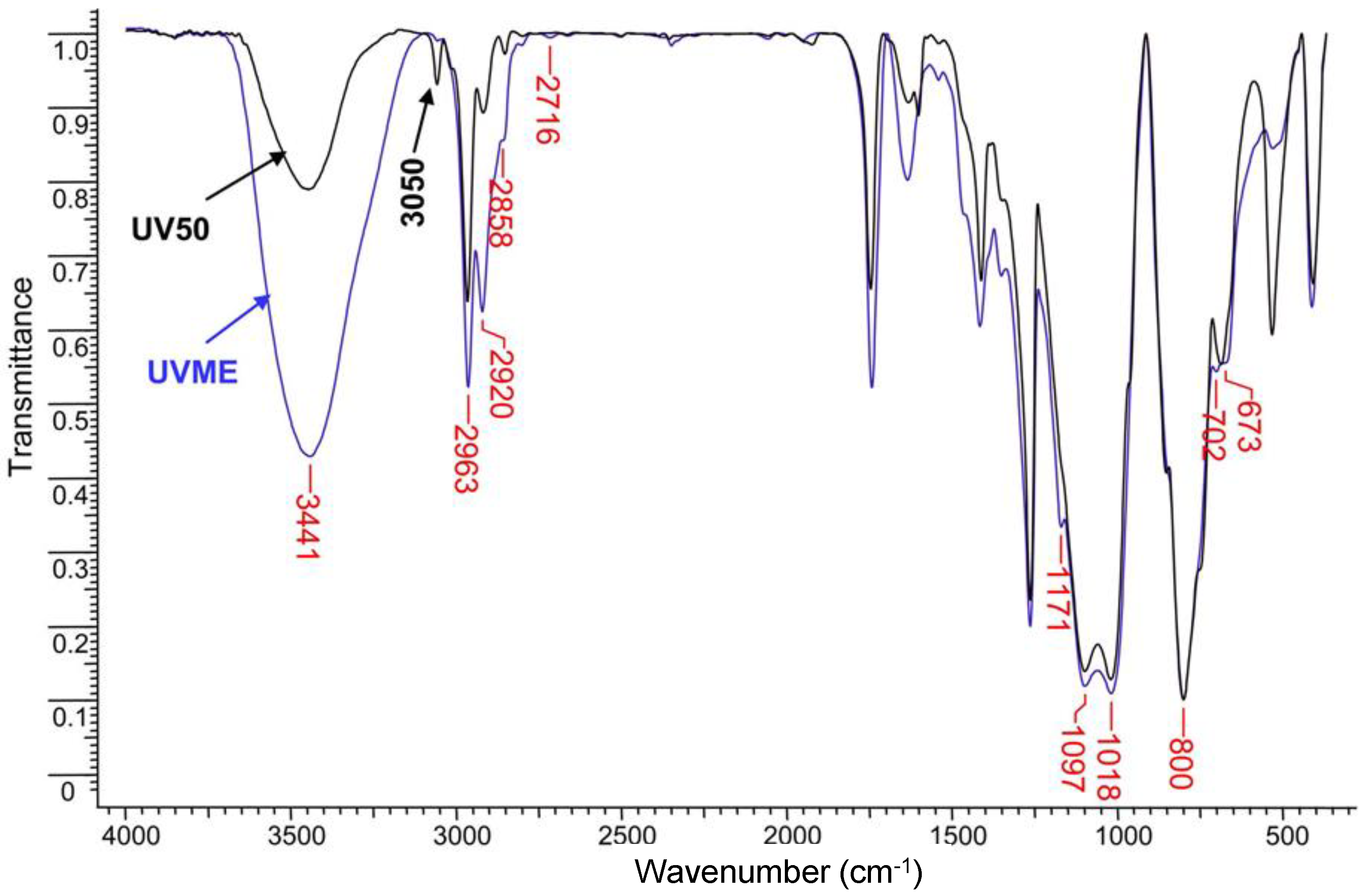
An alternative set of samples was obtained by UV-photoinitiated thiol-ene addition (
Scheme 1B), using a poly(dimethyl-methylvinyl)siloxane copolymer with ~50% vinyl groups, trimethylolpropane tris(3-mercaptopropionate) (SHCL) as a cross-linking agent, and 2,2-dimethoxy-2-phenylacetophenone (DMPA) as a photoinitiator (UV50). Modification of this copolymer by the addition of mercaptoethanol, occurring simultaneously with the cross-linking process, was designed to provide hydrophilicity in the resultant porous silicone (UVME). The FT-IR spectrum of UV50 showed incomplete conversion of the vinyl groups, since absorption bands at 3057 cm
−1 (CH stretching) and 532 cm
−1 (twisting vibrations) were still present. The chemical modification with mercaptoethanol resulted in the nearly complete disappearance of these bands, along with an increase in the CH
2 band at 2920 cm
−1 and in the OH band and the presence of thioether bands at 700 and 673 cm
−1 (C-S-C stretching) [
30] (
Figure 2). The conversion and experimental CLD values in
Table 2 were calculated by taking the spectrum of the initial Vi50 precursor as a reference.
The premises of the chemical modification were that, taking the polysiloxane chain as a reference, the OH groups would provide hydrophilic behavior, while dimethyl units and hexyl and trimethylsilyl groups would increase the hydrophobic behavior. Powder wetting experiments were performed in order to obtain contact angle values, but the Washburn method which was applied here has several limitations and is not suitable for hydrophobic materials [
31,
32]; thus, it could not be fully used as initially intended. Nevertheless, the water sorption isotherms shown in
Figure 3 can be used to compare the samples. It is worth noting that all samples were packed in the same way: the same amount of powder was placed between identical pieces of filter paper. The isotherms are depicted as they were registered, without extracting the effect of the filter paper, since they are used only for comparison. The wetting experiments revealed some unexpected outcomes, and it turned out that the morphology of the materials had a significant influence on their surface properties.
In
Figure 3, the samples can be roughly divided into three categories, according to the amount of water adsorbed: below 0.12 g, around 0.2 g, and above 0.3 g. In the dehydrocoupling sample series, very little difference was registered for DH100, DH70, and DH44, although their dimethylsiloxane content increased. DH100 set the trend, being hydrophobic, in spite of some Si-OH groups being present. The other two samples containing copolymers had similar water sorption and slightly lower hexane sorption capacities (
Figure S3). The sample modified with hexene (DHH) exhibited the lowest water sorption and a slightly higher affinity for hexane (
Figure S3).
Unexpectedly, the water sorption capacities of the DH30 sample, with the highest content of PDMS, and the DHS modified with trimethylsilyl were significantly higher than that of the unmodified DH100, placing these in the middle range of the graph. Their sorption curves had different aspects, showing a stepwise increase before leveling. Sample UV50, which is also placed in the “middle” zone of the wetting graph, showed a very slow increase in adsorbed water after a small initial “burst”. In the attempt to verify this apparently peculiar behavior and to measure the contact angle, drops of water (ca. 10 µL) were placed on the porous surfaces of the initial monoliths. In all these three cases, the “petal” effect was observed, i.e., a water drop did not fall off when the piece of material was tilted or even turned upside down (
Supplementary Materials S2; Figure S4). This behavior is assigned to superhydrophobic materials with very high adhesion (high contact angle hysteresis), in the so-called Cassie impregnating state [
21]. In the wetting measurements, water was first adsorbed on the bottom paper layer, then it adhered to the material, filling gradually the pores. This can be seen in the slowly increasing region of the graph, which seems to characterize only the materials showing the petal effect in our experiments. The contact angle hysteresis (calculated as the difference between the CA values on the two sides of the droplet) at a 90° tilt angle (
Figure S4) [
19] had values of 45–60°.
It is worth mentioning that, since the samples were crushed, the observed behavior characterizes the bulk material and not necessarily the surface of the monoliths or films; thus, it could be best correlated with the morphology of the “inner” part of the materials, which will be discussed further. The static water contact angle (CA) was measured for broken samples in order to correlate the data. As for the surfaces, contact angle values could be misleading in such cases due to several effects: the migration/orientation of PDMS chains to the surface, the presence of pores larger than the water droplets, different morphologies on the two surfaces due to stratification during cross-linking [
13], and so on. The apparent contact angles are summarized in
Table 2. The samples exhibiting the petal effect were highly hydrophobic, with apparent CAs of around 130°, while the sample modified with ME had a CA value of 87°. Sample DHH, modified with hexyl groups, had a higher contact angle than DH100.
The CA of sample DH70 was 151°, which is at the limit of superhydrophobic materials. Sample DH44 exhibited the “lotus” effect, assigned to superhydrophobic materials with low contact angle hysteresis [
21], which manifests by very low adhesion, the water droplets rolling very quickly off the surface (
Supplementary Materials S3). The CA for this sample was evaluated from video captures, since the droplets rolled too quickly from the surface (estimated CAH close to 0°). This situation completes the wetting observations in correlation with the chemical structure, confirming that by introducing PDMS segments in large amounts, the hydrophobicity of the materials in fact increased compared with DH100, in spite of an apparently similar water uptake registered for the powder state. In this case, the water absorbed by the filter paper in the first seconds is repelled by the porous powder and cannot protrude between the particles to reach the second filter paper. Indeed, after the measurement, it was found out that the top layer of filter paper was dry to the touch in the case of all the samples in the lower region of the adsorption graph.
UVME showed no surprise, having the highest water adsorption capacity, since it contains hydroxyethyl groups, which are hydrophilic. In this case, the contact angle could be calculated from the powder wettability data with the Washburn method, and the obtained value was 86.8°, which is very close to that measured with the water drops.
Superhydrophobic porous silicones with CAs over 150° and low sliding angles (hysteresis) have been reported [
23]. The respective materials, chemically modified at the surface with perfluoroalkyl groups, were also oleophobic (CA against n-decane ~121°). On the other hand, superhydrophobic and superoleophilic PDMS–MWNT sponges with very good performance in selective oil absorption have been reported [
24]. The authors explained their results as a consequence of very large pores and the key role of MWCNTs being oleophilic and hydrophobic with a large surface area and a rougher surface. To our knowledge, the petal effect for highly hydrophobic porous silicones has not been reported.
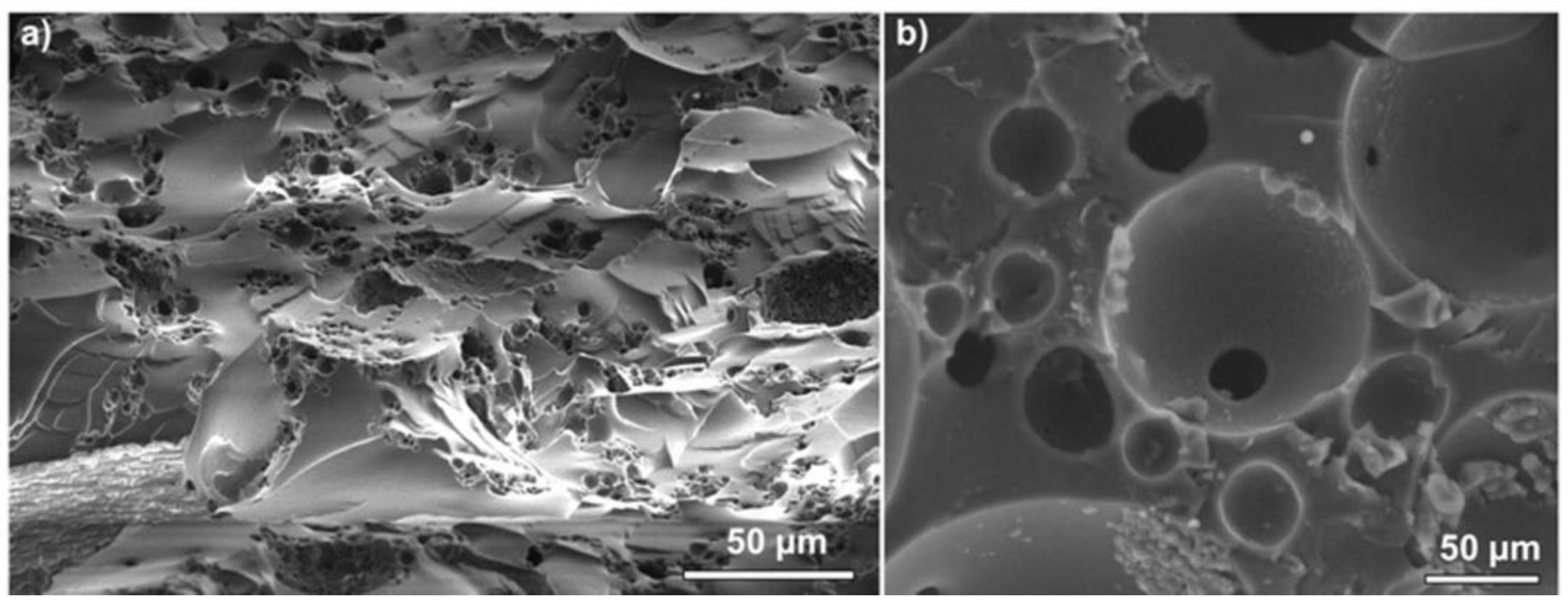
The morphology of the porous samples was analyzed by SEM in cross section. In most cases, open-cell pores were observed, with particularities depending on the process and composition. In the dehydrocoupling/hydrosilylation series, the morphology was in close dependency with the composition of the reaction mixture. The material obtained from neat PMHS (DH100) had large pores due to rapid H
2 evolution and smaller ones which are characteristic for the materials obtained by emulsion template [
13] (
Figure 4a). With increasing PDMS content in the DH series, the proportion of large pores (tens of micrometers) diminished at the same observation scale, along with the decrease in the cross-linking degree of the copolymers, and practically disappeared in DH30 without the PMHS precursor (
Figure 4). This trend is in agreement with experimental observations of diminished foaming and prolonged cross-linking time when the proportion of PMHS diminished. Thus, the emulsion template had an increasing role in tuning the pore size. The generation of large pores in dehydrocoupling increased the interconnectivity of the structures, since the small pores are practically windows in the open cells; thus, hierarchical structures formed.
When the PMHS was modified with ATMS, the morphology looked like a fine lace: very small pores formed almost exclusively (
Figure 4e). Due to the small size, the interconnectivity of the pores could not be clearly observed in all samples. Thus, the open-cell structure was verified by immersing the samples in an acetone solution of Disperse Red 1 (DR1) for a few minutes. The pieces of porous materials were removed on a filter paper, then gently cut (or crushed). The inner parts of the materials were colored (
Figure S5), which is an indication of pore interconnection [
33]. When hexene was used as a modifying agent, the formation of a latex was noticed, which can be observed in the final morphology (
Figure 4f) showing pores and particles.
In the absence of hydrogen evolution, the morphology of the samples obtained by photoaddition was dictated only by the emulsion template and the surface energy of the reaction mixture (
Figure 5). UV50 showed fewer and interconnected pores and apparently smooth regions. A combination of particles and holes characterizes sample UVME, due to the water solubility of ME, which probably induced a combination of w/o and o/w emulsion within the reaction mixture, generating the latex.
When trying to correlate the wetting behavior with the morphology, it appears that the samples that manifested highly hydrophobic behavior with petal or lotus effects had uniformly small pores and fewer very large pores (tens of micrometers). It is well documented that such effects are generated by combinations of micro- and nanostructures; more precisely, the microstructure pitch and nanostructure density are the key parameters, but the surface energy is an important element as well [
25]. It was observed that the sizes of hierarchical micro- and nanostructures are both larger in the petal than in the lotus leaf [
21]. Petals from two rose species with microstructures of around 10 µm could show different effects, depending on fine details [
34]: the one with a smaller pitch value (spacing) and a larger P-B height presented a superhydrophobic surface with low adhesion. When similar microstructures and different densities of nanostructures are considered, the lower nanostructure density generates high adhesion (the petal effect) [
25].
Compared with the reported examples, in our case, the effect was observed on pores and not on protuberant micro-/nanostructures, not to mention that the surface energy was modified due to changes in the chemical structure of the porous materials. When samples with similar structures (and thus surface energies) were compared at a larger scale (
Figure S6), it appeared that in the case of our porous materials, the trend was somehow reversed: DH44, with larger pores, exhibited low adhesion, as compared with sample DH30, with smaller pores, which showed high adhesion. On the other hand, taking into account that the emulsion template dictated the distribution (density) of small (nano-) pores, this should be considered approximately constant in the bulk samples. When micropores are generated as a supplementary element, it results that the share of nanostructures on a microstructure is larger in materials with larger pores. Thus, in fact, when there are larger pores, there should be a higher density of nanopores on a microstructure, giving low adhesion (the case of DH44). This dual-scale porosity tunes the surface properties, similar to the dual-scale roughness [
25].
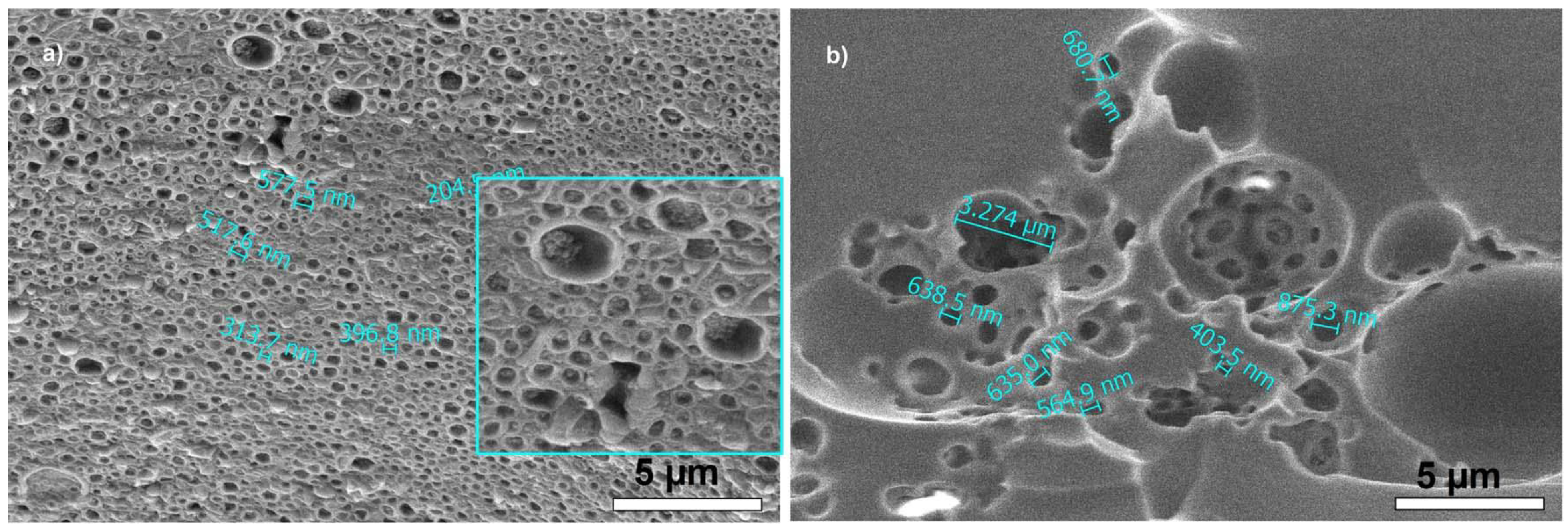
Fine morphological details can be observed in
Figure 6 for samples DH44 and UV50. In DH44 (showing the lotus effect), besides large pores, as seen in
Figure 4c, a very high density of small (“nano”) pores of 200–500 nm was observed, with dimensions similar to the droplet sizes measured by DLS for the staring emulsion (
Figure S7). These nanopores were distributed inside the larger ones, forming “windows” in the open cells. In UV50 (showing the petal effect), hierarchical, dual-scale pores were seen, of a few micrometers and around 500 nm. These areas with interconnected pores were spaced by relatively large regions without pores (
Figure 5a), which could be assimilated with large pitch (spacing between microstructures).
Other factors have an important influence on these macroscopic effects, for example, the droplets’ size [
21] or the regularity and order of hierarchical elements. In the porous materials investigated herein, the topography is rather irregular, as compared with a real petal or leaf, and the microscopic elements have roughly one order smaller dimensions. The water droplet size was ca. 10 µL, similar as in other studies [
21], in order to prevent the evaporation effect, since it was established that the use of a drop volume of up to 10 microliters has no influence on water static contact angle values [
35]. One should also consider the hydrophobic nature of the bulk materials, in addition to the induced porosity.
The as-obtained DH materials did not show good mechanical properties, due to the large pores and thin walls, and some of them were crushed during manipulation. In the case of photoaddition reaction, UV50 was a more robust material, with a certain flexibility. This correlates with the morphology of this sample, which showed large smooth areas (very thick walls of the pores). Selected samples were measured in compression stress–strain tests and gave the following Young’s modulus values: 45.5 kPa for DH100, 561 kPa for DH70, and 395 kPa for UV50.