The Role of Ultrasound Guidance in Mini-Invasive Musculoskeletal Surgery—A Pictorial Essay
Abstract
1. Introduction
2. Materials and Methods
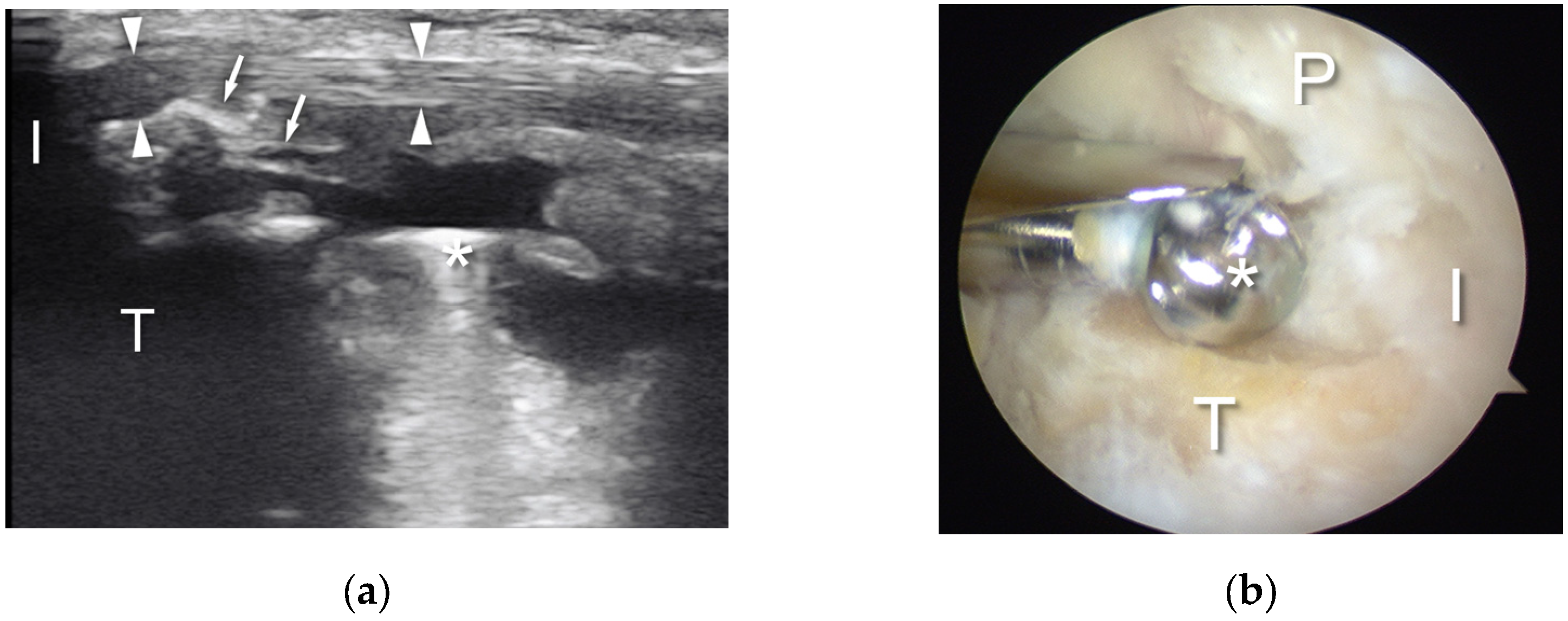
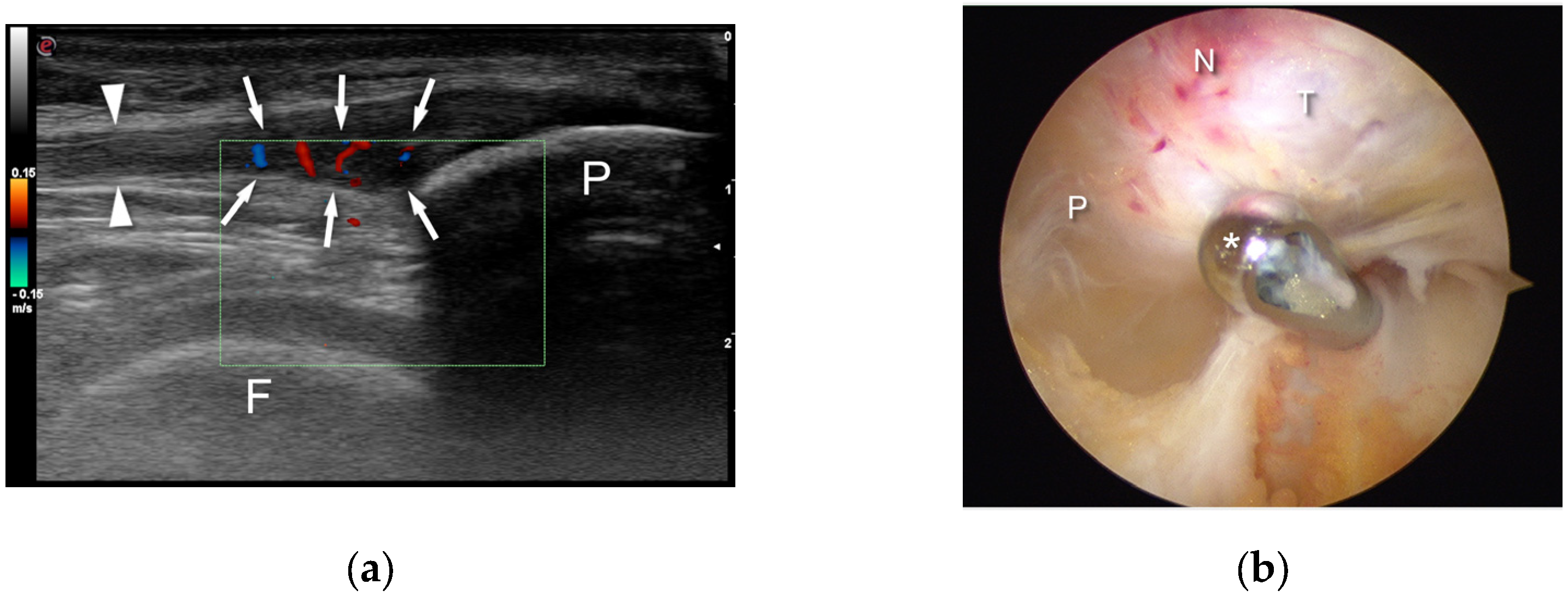
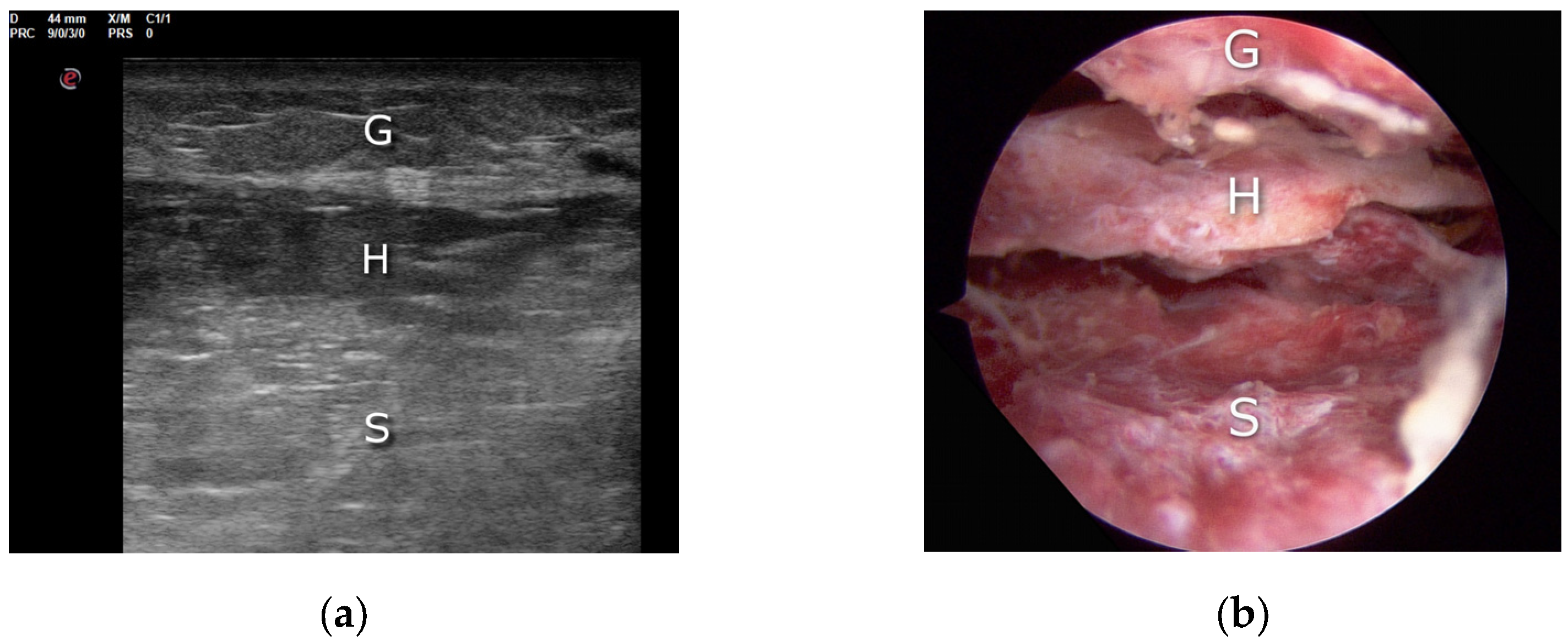
3. Technique Description
4. Possible Future Applications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hattori, S.; Onishi, K. Ultrasound-Guided Surgery in Musculoskeletal Medicine. J. Med. Ultrason. 2022, 49, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Tyryshkin, K.; Mousavi, P.; Pichora, D.R.; Abolmaesumi, P. Identification of Anatomical Landmarks for Registration of CT and Ultrasound Images in Computer-Assisted Shoulder Arthroscopy. In Proceedings of the 2006 International Conference of the IEEE Engineering in Medicine and Biology Society, New York, NY, USA, 30 August–3 September 2006; IEEE: New York, NY, USA, 2006; pp. 416–419. [Google Scholar]
- Ho, T.-F.; Lee, P.-Y.; Lan, H.H.-C.; Ku, M.-C. Establishing a Popliteal Portal Using the METRx System Under Ultrasound Guidance. Arthrosc. J. Arthrosc. Relat. Surg. 2006, 22, 1363.e1–1363.e4. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Takakura, Y.; Ishimura, M.; Habata, T.; Uematsu, K.; Ikeuch, K. Quantitative Arthroscopic Ultrasound Evaluation of Living Human Cartilage. Clin. Biomech. 2004, 19, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-P.; Zheng, Y.-P. Intravascular Ultrasound (IVUS): A Potential Arthroscopic Tool for Quantitative Assessment of Articular Cartilage. TOBEJ 2009, 3, 13–20. [Google Scholar] [CrossRef][Green Version]
- Huang, Y.P.; Zheng, Y.P. Development of an Arthroscopic Ultrasound Probe for Assessment of Articular Cartilage Degeneration. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; IEEE: Osaka, Japan, 2013; pp. 144–147. [Google Scholar]
- Liukkonen, J.; Hirvasniemi, J.; Joukainen, A.; Penttilä, P.; Virén, T.; Saarakkala, S.; Kröger, H.; Jurvelin, J.S.; Töyräs, J. Arthroscopic Ultrasound Technique for Simultaneous Quantitative Assessment of Articular Cartilage and Subchondral Bone: An In Vitro and In Vivo Feasibility Study. Ultrasound Med. Biol. 2013, 39, 1460–1468. [Google Scholar] [CrossRef]
- Kaleva, E.; Virén, T.; Saarakkala, S.; Sahlman, J.; Sirola, J.; Puhakka, J.; Paatela, T.; Kröger, H.; Kiviranta, I.; Jurvelin, J.S.; et al. Arthroscopic Ultrasound Assessment of Articular Cartilage in the Human Knee Joint: A Potential Diagnostic Method. Cartilage 2011, 2, 246–253. [Google Scholar] [CrossRef]
- Puhakka, J.; Afara, I.O.; Paatela, T.; Sormaala, M.J.; Timonen, M.A.; Virén, T.; Jurvelin, J.S.; Töyräs, J.; Kiviranta, I. In Vivo Evaluation of the Potential of High-Frequency Ultrasound for Arthroscopic Examination of the Shoulder Joint. Cartilage 2016, 7, 248–255. [Google Scholar] [CrossRef]
- Joukainen, A.; Virén, T.; Penttilä, P.; Liukkonen, J.; Puhakka, P.H.; Kröger, H.; Töyräs, J. Ultrasound Arthroscopy of Hip in Treatment of Osteochondritis Dissecans. Arthrosc. Tech. 2017, 6, e1063–e1068. [Google Scholar] [CrossRef]
- Bethune, R.; Bull, A.M.J.; Dickinson, R.J.; Emery, R.J.H. Removal of Calcific Deposits of the Rotator Cuff Tendon Using an Intra-Articular Ultrasound Probe. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 289–291. [Google Scholar] [CrossRef]
- Sabeti, M.; Schmidt, M.; Ziai, P.; Graf, A.; Nemecek, E.; Schueller-Weidekamm, C. The Intraoperative Use of Ultrasound Facilitates Significantly the Arthroscopic Debridement of Calcific Rotator Cuff Tendinitis. Arch. Orthop. Trauma. Surg. 2014, 134, 651–656. [Google Scholar] [CrossRef]
- Sabeti-Aschraf, M.; Gonano, C.; Nemecek, E.; Cichocki, L.; Schueller-Weidekamm, C. Intra-Operative Ultrasound Facilitates the Localization of the Calcific Deposit during Arthroscopic Treatment of Calcifying Tendinitis. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 1792–1794. [Google Scholar] [CrossRef]
- De Lucia, O.; Paresce, E.; Murgo, A.; Epis, O.; Pisoni, L.; Schito, E.; Valcamonica, E.; Piana, C.; Fantini, F. Simultaneous Ultrasonography and Arthroscopy for the Study of the Joint Environment: Indications and Limits. Reumatismo 2011, 59, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Paresce, E.; De Lucia, O.; Bruschi, E.; Giacomelli, L.; Epis, O.M. Use of Ultrasound-Assisted Arthroscopy in Rheumatology: An Experience in 11 Patients with Different Rheumatic Diseases. Expert. Opin. Med. Diagn. 2013, 7, 309–312. [Google Scholar] [CrossRef]
- Akatsu, Y.; Akagi, R.; Fukawa, T.; Yamaguchi, S.; Sasho, T. Ultrasound for Treating Meniscocapsular Separation Together With Arthroscopy. Arthrosc. Tech. 2016, 5, e1457–e1460. [Google Scholar] [CrossRef][Green Version]
- Willberg, L.; Sunding, K.; Forssblad, M.; Alfredson, H. Ultrasound- and Doppler-Guided Arthroscopic Shaving to Treat Jumper’s Knee: A Technical Note. Knee Surg. Sports Traumatol. Arthrosc. 2007, 15, 1400–1403. [Google Scholar] [CrossRef]
- Sunding, K.; Willberg, L.; Werner, S.; Alfredson, H.; Forssblad, M.; Fahlström, M. Sclerosing Injections and Ultrasound-Guided Arthroscopic Shaving for Patellar Tendinopathy: Good Clinical Results and Decreased Tendon Thickness after Surgery—A Medium-Term Follow-up Study. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Yang, Y.; Chen, S.; Wang, Y.; Li, Y.; Chen, J.; Li, H. Ultrasound-Guided Establishment of Hip Arthroscopy Portals. Arthrosc. J. Arthrosc. Relat. Surg. 2009, 25, 1491–1495. [Google Scholar] [CrossRef]
- Weinrauch, P.; Kermeci, S. Ultrasound-Assisted Hip Arthroscopy. Arthrosc. Tech. 2014, 3, e255–e259. [Google Scholar] [CrossRef]
- Keough, T.; Wilson, D.; Wong, I. Ultrasound-Guided Portal Placement for Hip Arthroscopy. Arthrosc. Tech. 2016, 5, e851–e856. [Google Scholar] [CrossRef]
- Ali, M.I.; Ravipati, A.P.T.; Wong, I. Hip Arthroscopy for Femoral Acetabular Impingement: A Bird’s-Eye/En Face Perspective With Ultrasound Guidance and 3-Dimensional Hip Printing. Arthrosc. Tech. 2019, 8, e1301–e1307. [Google Scholar] [CrossRef]
- Paczesny, L.; Kruczyński, J. Ultrasound-Guided Arthroscopic Management of Hallux Rigidus. Videosurgery Other Miniinvasive Tech. 2016, 3, 144–148. [Google Scholar] [CrossRef]
- Bartlett, D.H. Arthroscopic Management of Osteochondritis Dissecans of the First Metatarsal Head. Arthrosc. J. Arthrosc. Relat. Surg. 1988, 4, 51–54. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yamaguchi, S.; Sasho, T.; Fukawa, T.; Akatsu, Y.; Akagi, R.; Yamaguchi, T.; Takahashi, K.; Nagashima, K.; Takahashi, K. Quantitative US Elastography Can Be Used to Quantify Mechanical and Histologic Tendon Healing in a Rabbit Model of Achilles Tendon Transection. Radiology 2017, 283, 408–417. [Google Scholar] [CrossRef]
- Michalski, Ł.; Paczesny, Ł.; Zabrzyński, J.; Kruczyński, J. Ultrasound-Assisted Endoscopic Evacuation of Recurrent Calf Hematoma Following Anterior Cruciate Ligament Reconstruction. Case Study. Ortop. Traumatol. Rehabil. 2019, 21, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Longo, U.G.; Ronga, M.; Maffulli, N. Achilles Tendinopathy. Sports Med. Arthrosc. Rev. 2009, 17, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Kaux, J.-F.; Forthomme, B.; Goff, C.L.; Crielaard, J.-M.; Croisier, J.-L. Current Opinions on Tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253. [Google Scholar]
- Carmont, M.R.; Rossi, R.; Scheffler, S.; Mei-Dan, O.; Beaufils, P. Percutaneous & Mini Invasive Achilles Tendon Repair. BMC Sports Sci. Med. Rehabil. 2011, 3, 28. [Google Scholar] [CrossRef]
- Park, S.-H.; Lee, H.S.; Young, K.W.; Seo, S.G. Treatment of Acute Achilles Tendon Rupture. Clin. Orthop. Surg. 2020, 12, 1. [Google Scholar] [CrossRef]
- Maffulli, N. Minimally Invasive Surgery for Achilles Tendon Pathologies. Open Access J. Sports Med. 2010, 1, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Paczesny, Ł.; Zabrzyński, J.; Domżalski, M.; Gagat, M.; Termanowski, M.; Szwedowski, D.; Łapaj, Ł.; Kruczyński, J. Mini-Invasive, Ultrasound Guided Repair of the Achilles Tendon Rupture—A Pilot Study. J. Clin. Med. 2021, 10, 2370. [Google Scholar] [CrossRef]
- Giannetti, S.; Patricola, A.A.; Stancati, A.; Santucci, A. Intraoperative Ultrasound Assistance for Percutaneous Repair of the Acute Achilles Tendon Rupture. Orthopedics 2014, 37, 820–824. [Google Scholar] [CrossRef]
- Severyns, M.; Andriamananaivo, T.; Rollet, M.-E.; Kajetanek, C.; Lopes, R.; Renard, G.; Noailles, T.; Odri, G.A.; Rouvillain, J.-L. Acute Achilles Tendon Rupture: Ultrasonography and Endoscopy-Assisted Percutaneous Repair. Arthrosc. Tech. 2019, 8, e489–e493. [Google Scholar] [CrossRef]
- Villanueva, M.; Iborra, Á.; Rodríguez, G.; Sanz-Ruiz, P. Ultrasound-Guided Gastrocnemius Recession: A New Ultra–Minimally Invasive Surgical Technique. BMC Musculoskelet. Disord. 2016, 17, 409. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.M. Uniportal Endoscopic Gastrocnemius Recession for Treatment of Gastrocnemius Equinus with a Dedicated EGR System with Retractable Blade. J. Foot Ankle Surg. 2012, 51, 714–719. [Google Scholar] [CrossRef]
- Kamel, S.I.; Freid, B.; Pomeranz, C.; Halpern, E.J.; Nazarian, L.N. Minimally Invasive Ultrasound-Guided Carpal Tunnel Release Improves Long-Term Clinical Outcomes in Carpal Tunnel Syndrome. Am. J. Roentgenol. 2021, 217, 460–468. [Google Scholar] [CrossRef]
- Sommerfeldt, M.; Jack, E.; Playfair, L.; Satkunam, L.; Loh, E.; Rambaransingh, B.; Burnham, R. Ultrasound-Guided, Minimally Invasive Looped Thread Fasciotomy for Chronic Exertional Compartment Syndrome of the Lower Leg: A Cadaveric Feasibility Study. Interv. Pain. Med. 2022, 1, 100074. [Google Scholar] [CrossRef]
- Finnoff, J.T.; Fowler, S.P.; Lai, J.K.; Santrach, P.J.; Willis, E.A.; Sayeed, Y.A.; Smith, J. Treatment of Chronic Tendinopathy with Ultrasound-Guided Needle Tenotomy and Platelet-Rich Plasma Injection. PM&R 2011, 3, 900–911. [Google Scholar] [CrossRef]
- Roy, K.; Lee, J.E.-Y.; Lee, C. Thin-Film PMUTs: A Review of over 40 Years of Research. Microsyst. Nanoeng. 2023, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.; Ma, B.; Khuri-Yakub, B.T. Applications of Capacitive Micromachined Ultrasonic Transducers: A Comprehensive Review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2022, 69, 456–467. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paczesny, Ł.; Lorkowski, M.; Pielak, T.; Wójcicki, R.; Huri, G.; Zabrzyński, J. The Role of Ultrasound Guidance in Mini-Invasive Musculoskeletal Surgery—A Pictorial Essay. Appl. Sci. 2023, 13, 10900. https://doi.org/10.3390/app131910900
Paczesny Ł, Lorkowski M, Pielak T, Wójcicki R, Huri G, Zabrzyński J. The Role of Ultrasound Guidance in Mini-Invasive Musculoskeletal Surgery—A Pictorial Essay. Applied Sciences. 2023; 13(19):10900. https://doi.org/10.3390/app131910900
Chicago/Turabian StylePaczesny, Łukasz, Matthias Lorkowski, Tomasz Pielak, Rafał Wójcicki, Gazi Huri, and Jan Zabrzyński. 2023. "The Role of Ultrasound Guidance in Mini-Invasive Musculoskeletal Surgery—A Pictorial Essay" Applied Sciences 13, no. 19: 10900. https://doi.org/10.3390/app131910900
APA StylePaczesny, Ł., Lorkowski, M., Pielak, T., Wójcicki, R., Huri, G., & Zabrzyński, J. (2023). The Role of Ultrasound Guidance in Mini-Invasive Musculoskeletal Surgery—A Pictorial Essay. Applied Sciences, 13(19), 10900. https://doi.org/10.3390/app131910900







