Application of Extracellular Polymeric Substances during Cultivation of Microalgae Biomass
Abstract
:Featured Application
Abstract
1. Introduction
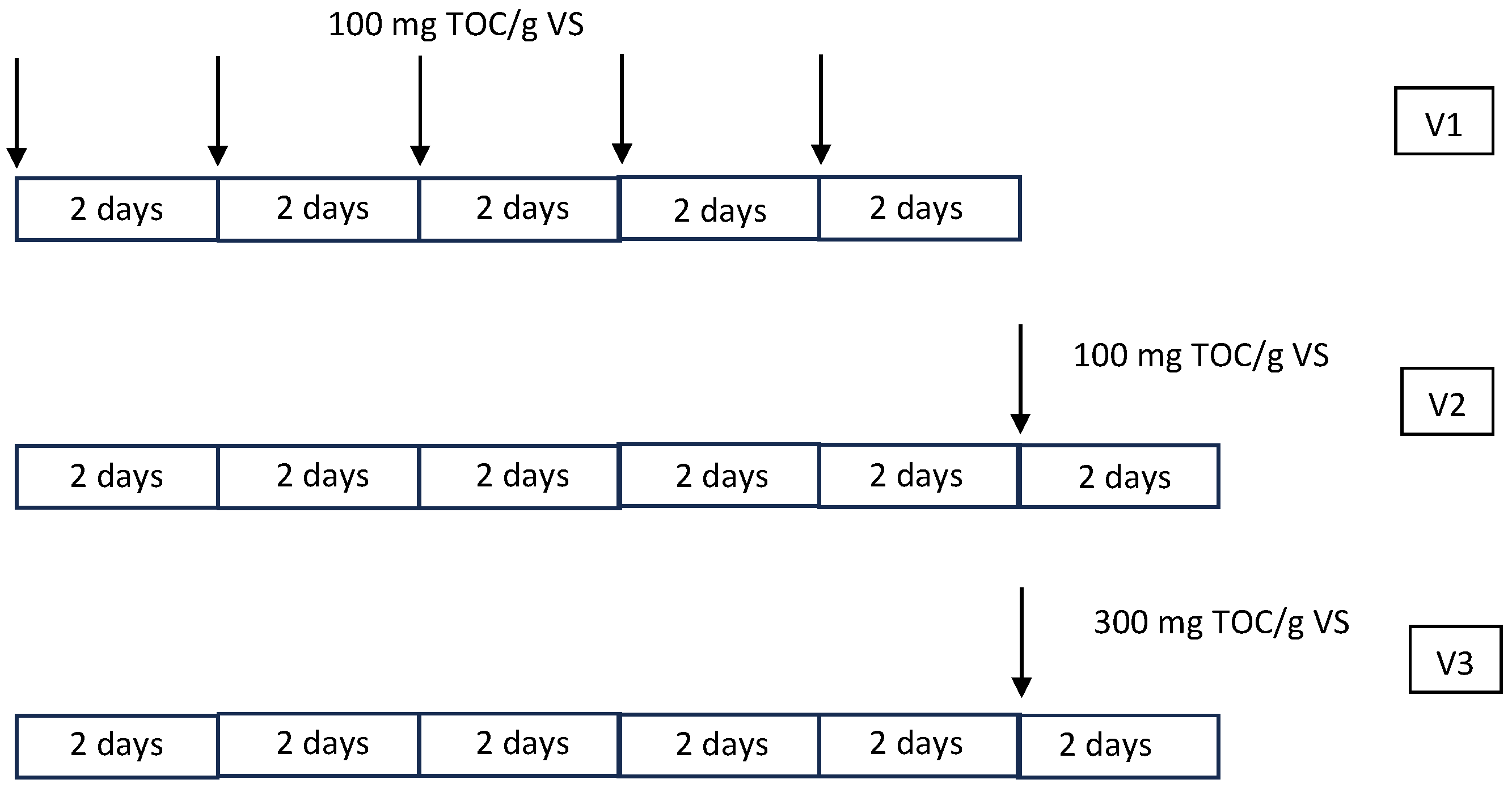
2. Materials and Methods
2.1. EPS Origin
2.2. EPS Extraction
2.3. Microalgae Cultivation
2.4. Bioflocculation
2.5. Analytical Methods
3. Results and Discussion
4. Conclusions
- -
- Extracellular polymeric substances from bacteria can be used as an external carbon source by microalgae/cyanobacteria;
- -
- Extracellular polymeric substances can be used for harvesting Tetraselmis subcordiformis and Arthrospira platensis;
- -
- The sugar content in Tetraselmis subcordiformis biomass was responsible for the higher sedimentation coefficient.
Author Contributions
Funding
Conflicts of Interest
References
- Felz, S.; Vermeulen, R.; van Loosdrecht, M.C.M.; Lin, Y.M. Chemical characterization methods for the analysis of structural extracellular polymeric substances (EPS). Water Res. 2019, 157, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Schambeck, C.M.; Magnus, B.S.; de Souza, L.C.R.; Leite, W.R.M.; Derlon, N.; Guimarães, L.B.; da Costa, R.H.R. Biopolymers recovery: Dynamics and characterization of alginate-like exopolymers in an aerobic granular sludge system treating municipal wastewater without sludge inoculum. J. Environ. Manag. 2020, 263, 110394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Zhang, X.Z. Microalgal harvesting using foam flotation: A critical review. Biomass Bioenergy 2019, 120, 176–188. [Google Scholar] [CrossRef]
- Dassey, A.J.; Theegala, C.S. Harvesting economics and strategies using centrifugation for cost effective separation of microalgae cells for biodiesel applicationsc. Bioresour. Technol. 2013, 128, 241–245. [Google Scholar] [CrossRef]
- Liu, Z.; Hao, N.; Hou, Y.; Wang, Q.; Liu, Q.; Yan, S.; Chen, F.; Zhao, L. Technologies for harvesting the microalgae for industrial applications: Current trends and perspectives. Bioresour. Technol. 2023, 387, 129631. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Ilyas, A.; Muylaert, K.; Vankelecom, I.F.J. Optimization of patterned polysulfone membranes for microalgae harvesting. Bioresour. Technol. 2020, 309, 123367. [Google Scholar] [CrossRef]
- Huang, K.X.; Vadiveloo, A.; Zhou, J.L.; Yang, L.; Chen, D.Z.; Gao, F. Integrated culture and harvest systems for improved microalgal biomass production and wastewater treatment. Bioresour. Technol. 2023, 376, 128941. [Google Scholar] [CrossRef]
- Wan, C.; Zhao, X.Q.; Guo, S.L.; Asraful Alam, M.; Bai, F.W. Bioflocculant production from Solibacillus silvestris W01 and its application in cost-effective harvest of marine microalga Nannochloropsis oceanica by flocculation. Bioresour. Technol. 2013, 135, 207–212. [Google Scholar] [CrossRef]
- Takahashi, E.; Ledauphin, J.; Goux, D.; Orvain, F. Optimising extraction of extracellular polymeric substances (EPS) from benthic diatoms: Comparison of the efficiency of six EPS extraction methods. Mar. Freshw. Res. 2009, 60, 1201–1210. [Google Scholar] [CrossRef]
- Frolund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- McSwain, B.S.; Irvine, R.L.; Hausner, M.; Wilderer, P.A. Composition and distribution of extracellular polymeric substances in aerobic flocs and granular sludge. Appl. Environ. Microbiol. 2005, 71, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Dudek, M.; Dębowski, M.; Zieliński, M.; Nowicka, A.; Rusanowska, P. Water from the Vistula Lagoon as a medium in mixotrophic growth and hydrogen production by Platymonas subcordiformis. Int. J. Hydrog. Energy 2018, 43, 9529–9534. [Google Scholar] [CrossRef]
- Aiba, S.; Ogawa, T. Assessment of growth yield of a blue-green alga: Spirulina platensis in axenic and continuous cultur. J. Gen. Microbiol. 1977, 102, 179–182. [Google Scholar] [CrossRef]
- Szwarc, K.; Szwarc, D.; Zieliński, M. Removal of biogenic compounds from the post-fermentation effluent in a culture of Chlorella vulgaris. Environ. Sci. Pollut. Res. 2020, 27, 111–117. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Gaudy, A.F. Colorimetric determination of protein and carbohydrate. Ind. Water Wastes 1962, 7, 17–22. [Google Scholar]
- Johan, F.; Jafri, M.Z.; Lim, H.S.; Wan Maznah, W.O. Laboratory measurement: Chlorophyll-a concentration measurement with acetone method using spectrophotometer. In Proceedings of the 2014 IEEE International Conference on Industrial Engineering and Engineering Management, Selangor, Malaysia, 9–12 December 2014; pp. 744–748. [Google Scholar]
- Sasadara, M.M.V.; Nayaka, N.M.D.M.; Yuda, P.E.S.K.; Dewi, N.K.L.A.; Cahyaningsih, N.; Wirawan, I.G.P.; Silalahi, D. Optimization of chlorophyll extraction solvent of bulung sangu (Gracilaria sp.) seaweed. IOP Conf. Ser. Earth Environ. Sci. 2021, 913, 012073. [Google Scholar] [CrossRef]
- Heredia-Arroyo, T.; Wei, W.; Ruan, R.; Hu, B. Mixotrophic cultivation of Chlorella vulgaris and its potential application for the oil accumulation from non-sugar materials. Biomass Bioenergy 2011, 35, 2245–2253. [Google Scholar] [CrossRef]
- Vidotti, A.D.S.; Riaño-Pachón, D.M.; Mattiello, L.; Giraldi, L.A.; Winck, F.V.; Franco, T.T. Analysis of autotrophic, mixotrophic and heterotrophic phenotypes in the microalgae Chlorella vulgaris using time-resolved proteomics and transcriptomics approaches. Algal Res. 2020, 51, 102060. [Google Scholar] [CrossRef]
- Liang, Y.; Sarkany, N.; Cui, Y. Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol. Lett. 2009, 31, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.S.; Gallaher, S.D.; Westcott, D.J.; Iwai, M.; Louie, K.B.; Mueller, M.; Walter, A.; Foflonker, F.; Bowen, B.P.; Ataii, N.N.; et al. Regulation of oxygenic photosynthesis during trophic transitions in the green alga Chromochloris zofingiensis. Plant Cell 2019, 31, 579–601. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Jinkerson, R.E.; Clowez, S.; Tran, C.; Krediet, C.J.; Onishi, M.; Cleves, P.A.; Pringle, J.R.; Grossman, A.R. Glucose-induced trophic shift in an endosymbiont dinoflagellate with physiological and molecular consequences. Plant Physiol. 2018, 176, 1793–1807. [Google Scholar] [CrossRef]
- Narayan, M.S.; Manoj, G.P.; Vatchravelu, K.; Bhagyalakshmi, N.; Mahadevaswamy, M. Utilization of glycerol as carbon source on the growth, pigment and lipid production in Spirulina platensis. Int. J. Food Sci. Nutr. 2005, 56, 521–528. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Y.; Li, Y.; Wang, Y. Mixotrophic cultivation of Platymonas subcordiformis. J. Appl. Phycol. 2001, 13, 343–347. [Google Scholar] [CrossRef]
- Schwarz, A.; Walther, J.; Geib, D.; Witthohn, M.; Strieth, D.; Ulber, R.; Muffler, K. Influence of heterotrophic and mixotrophic cultivation on growth behaviour of terrestrial cyanobacteria. Algal Res. 2020, 52, 102125. [Google Scholar] [CrossRef]
- Lacroux, J.; Seira, J.; Trably, E.; Bernet, N.; Steyer, J.; Van Lis, R. Mixotrophic Growth of Chlorella sorokiniana on Acetate and Butyrate: Interplay between Substrate, C:N Ratio and pH. Front. Microbiol. 2021, 12, 703614. [Google Scholar] [CrossRef]
- Tosuner, Z.V.; Ürek, R.Ö. Cultivation of Arthrospira platensis in heterotrophic and mixotrophic conditions with different concentrations of whey. Aquat. Res. 2022, 5, 146–153. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Haberkorn, I.; Böcker, L.; Mathys, A. Cultivation of Chlorella protothecoides under different growth modes and its utilisation in oil/water emulsions. Bioresour. Technol. 2019, 288, 121476. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Yao, J.; Cai, W. Effects of extracellular polymeric substances on aerobic granulation in sequencing batch reactors. Chemosphere 2006, 63, 1728–1735. [Google Scholar] [CrossRef] [PubMed]
- Campo, R.; Corsino, S.F.; Torregrossa, M.; Di Bella, G. The role of extracellular polymeric substances on aerobic granulation with stepwise increase of salinity. Sep. Purif. Technol. 2018, 195, 12–20. [Google Scholar] [CrossRef]
- Lutfi, M.; Nugroho, W.A.; Fridayestu, W.P.; Susilo, B.; Pulmar, C.; Sandra, S. Bioflocculation of Two Species of Microalgae by Exopolysaccharide of Bacillus subtilis. Nat. Environ. Pollut. Technol. 2019, 18, 167–173. [Google Scholar]
- Wang, H.; Qi, B.; Jiang, X.; Jiang, Y.; Yang, H.; Xiao, Y.; Jiang, N.; Deng, L.; Wang, W. Microalgal interstrains differences in algal-bacterial biofloc formation during liquid digestate treatment. Bioresour. Technol. 2019, 289, 121741. [Google Scholar] [CrossRef]
- Wang, Q.; Shen, Q.; Wang, J.; Zhao, J.; Zhang, Z.; Lei, Z.; Yuan, T.; Shimizu, K.; Liu, Y.; Lee, D.J. Insight into the rapid biogranulation for suspended single-cell microalgae harvesting in wastewater treatment systems: Focus on the role of extracellular polymeric substances. Chem. Eng. J. 2022, 430, 132631. [Google Scholar] [CrossRef]
- Manheim, D.; Nelson, Y. Settling and bioflocculation of two species of algae used in wastewater treatment and algae biomass production. Environ. Prog. Sustain. Energy 2013, 32, 946–954. [Google Scholar] [CrossRef]
- Hao, N.; Liu, Z.; Hou, Y.; Fan, Z.; Li, Y.; Chen, F.; Zhao, L. Small peptide glutathione-induced bioflocculation for enhancing the food application potential of Chlorella pyrenoidosa. Bioresour. Technol. 2022, 365, 128138. [Google Scholar] [CrossRef]
- Liber, J.A.; Bryson, A.E.; Bonito, G.; Du, Z.-Y. Harvesting Microalgae for Food and Energy Products. Small Methods 2020, 4, 2000349. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Frappart, M.; Jaouen, P.; Pruvost, J.; Bourseau, P. Harvesting Chlorella vulgaris by natural increase in pH: Effect of medium composition. Environ. Technol. 2014, 35, 1378–1388. [Google Scholar] [CrossRef]
- Gerken, H.G.; Donohoe, B.; Knoshaug, E.P. Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta 2013, 237, 239–253. [Google Scholar] [CrossRef]
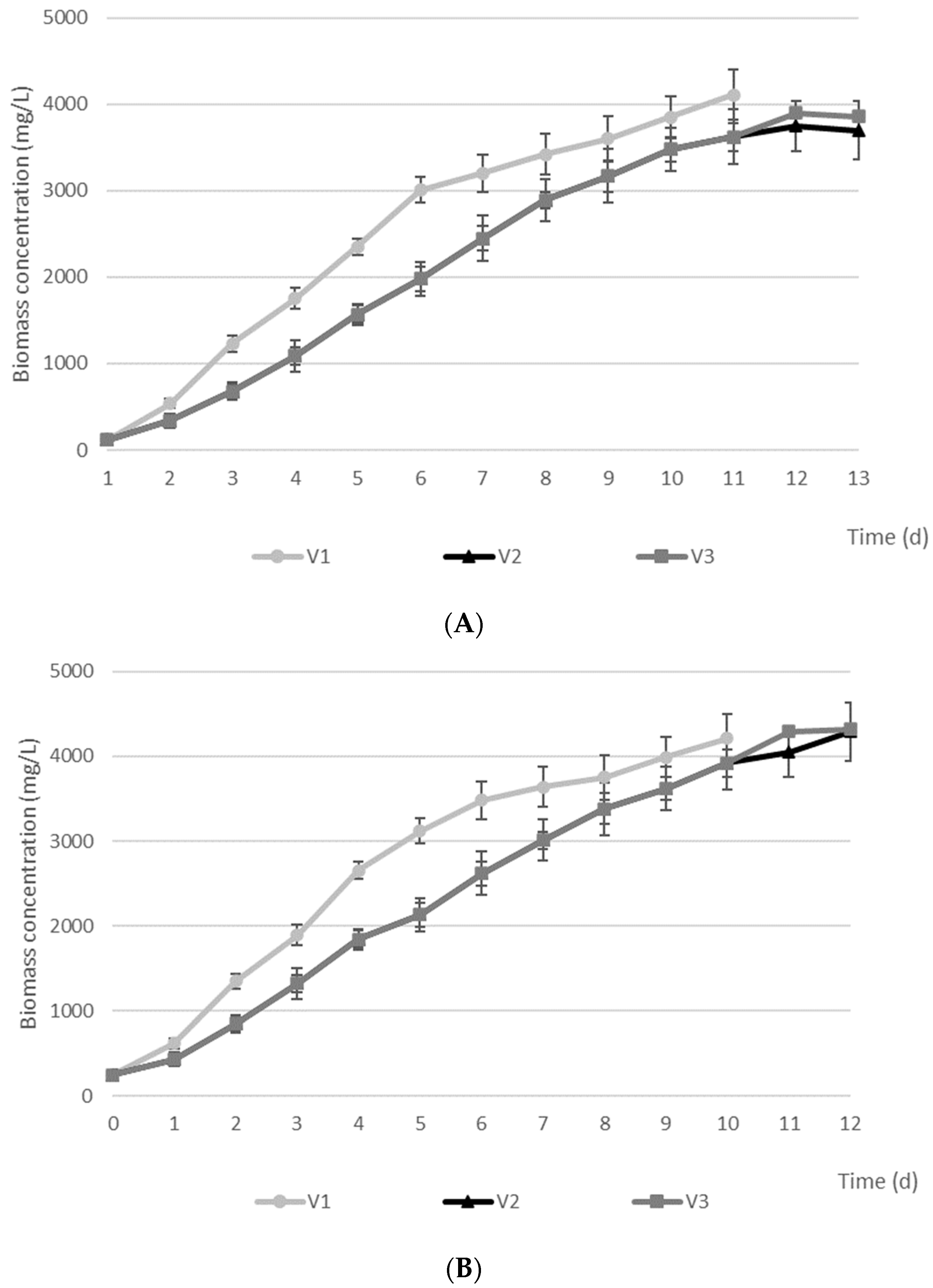

| Variant of Experiment | Biomass Concentration (mg/L) | Chlorophyll Content (mg/g VS) | EPS Content (mg TOC/g VS) | Sedimentation Efficiency (%) | |
|---|---|---|---|---|---|
| Tetraselmis subcordiformis | C | 3621 ± 174 | 40 ± 11 | 95 ± 11 | 40 |
| V1 | 4109 ± 160 | 11 ± 4 | 97 ± 7 | 42 | |
| V2 | 3698 ± 102 | 32 ± 9 | 97 ± 25 | 55 | |
| V3 | 3856 ± 111 | 28 ± 12 | 97 ± 10 | 66 | |
| Chlorella sp. | C | 3920 ± 289 | 28 ± 8 | 60 ± 20 | 32 |
| V1 | 4210 ± 320 | 8 ± 6 | 61 ± 9 | 31 | |
| V2 | 4290 ± 255 | 13 ± 5 | 62 ± 12 | 34 | |
| V3 | 4321 ± 262 | 10 ± 2 | 61 ± 19 | 36 | |
| Arthrospira platensis | C | 1213 ± 182 | 16 ± 4 | 81 ± 6 | 49 |
| V1 | 1512 ± 191 | 8 ± 4 | 83 ± 9 | 54 | |
| V2 | 1760 ± 195 | 11 ± 6 | 91 ± 12 | 68 | |
| V3 | 1912 ± 150 | 9 ± 4 | 96 ± 2 | 72 |
| Variant of Experiment | Protein Content (mg/g VS) | Sugar Content (mg/g VS) | Carbohydrates | ||||
|---|---|---|---|---|---|---|---|
| Glucose (mg/g VS) | Mannose (mg/g VS) | Rhamnose (mg/g VS) | Galactose (mg/g VS) | ||||
| Tetraselmis subcordiformis | C | 27.4 ± 6.7 | 21.5 ± 4.5 | 15.2 ± 5.4 | - | - | 1.3 ± 0.5 |
| V1 | 28 ± 4.8 | 25.3 ± 5.6 | 21 ± 1.1 | - | - | 1.9 ± 0.4 | |
| V2 | 27.9 ± 3.5 | 32 ± 3 | 24.3 ± 2.7 | - | - | 1.9 ± 1.5 | |
| V3 | 28.3 ± 9.2 | 41 ± 5 | 22.5 ± 3.2 | - | - | 2.1 ± 0.8 | |
| Chlorella sp. | C | 32.9 ± 4.7 | 41.1 ± 4 | 17.8 ± 3.2 | 12.9 ± 5.2 | - | 1.1 ± 0.2 |
| V1 | 33.1 ± 8.8 | 50.3 ± 8.2 | 23.8 ± 2.5 | 19.4 ± 3.8 | - | 1.7 ± 0.1 | |
| V2 | 33.7 ± 5.3 | 53.7 ± 9.1 | 24.1 ± 5.3 | 20.1 ± 2.7 | - | 1.7 ± 0.5 | |
| V3 | 35.6 ± 4.9 | 56.3 ± 7.7 | 23.9 ± 4.5 | 21.1 ± 3.2 | - | 2.2 ± 1.1 | |
| Arthrospira platensis | C | 45 ± 10.3 | 37.8 ± 6.4 | 11.4 ± 1.2 | - | 25.1 ± 3.2 | 10.9 ± 1.4 |
| V1 | 59.1 ± 9.1 | 42.8 ± 9.8 | 10.5 ± 5 | - | 26.7 ± 1.9 | 8.6 ± 3.1 | |
| V2 | 57 ± 13.2 | 45.2 ± 8.1 | 11.9 ± 2.1 | - | 29.6 ± 0.5 | 9.1 ± 4 | |
| V3 | 60.1 ± 11 | 48 ± 10.1 | 12.4 ± 2.9 | - | 30.4 ± 3 | 9.0 ± 3.2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusanowska, P.; Zieliński, M.; Dudek, M.; Dębowski, M. Application of Extracellular Polymeric Substances during Cultivation of Microalgae Biomass. Appl. Sci. 2023, 13, 10796. https://doi.org/10.3390/app131910796
Rusanowska P, Zieliński M, Dudek M, Dębowski M. Application of Extracellular Polymeric Substances during Cultivation of Microalgae Biomass. Applied Sciences. 2023; 13(19):10796. https://doi.org/10.3390/app131910796
Chicago/Turabian StyleRusanowska, Paulina, Marcin Zieliński, Magda Dudek, and Marcin Dębowski. 2023. "Application of Extracellular Polymeric Substances during Cultivation of Microalgae Biomass" Applied Sciences 13, no. 19: 10796. https://doi.org/10.3390/app131910796
APA StyleRusanowska, P., Zieliński, M., Dudek, M., & Dębowski, M. (2023). Application of Extracellular Polymeric Substances during Cultivation of Microalgae Biomass. Applied Sciences, 13(19), 10796. https://doi.org/10.3390/app131910796










