Microalgae (Chlorella vulgaris and Spirulina platensis) as a Protein Alternative and Their Effects on Productive Performances, Blood Parameters, Protein Digestibility, and Nutritional Value of Laying Hens’ Egg
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Microalgae Purchase and Chemical Analyses
2.3. Animals, Housing, and Experimental Diets
2.4. Laying Hens Performance
2.5. Nutrient Digestibility Trial
2.6. Blood Collection and Analysis
2.7. Egg Quality Measurement
2.8. Chemical Analysis of Samples
2.8.1. Determination of In Vitro Digestibility of Nutrients
2.8.2. Pigment Extraction from Spirulina platensis and Chlorella
2.8.3. Measurement of Some Antioxidant Enzyme Activity and GSH in Blood Serum
2.8.4. Egg Yolk β-Carotene and Antioxidant Activity Determination
2.8.5. Egg Yolk Cholesterol Content and Fatty Acids Profile
2.8.6. Color Measurement of Fresh and Boiled Eggs
2.9. Statistical Analysis
3. Results
3.1. Nutritional Value of Chlorella and Spirulina Powder
3.2. Nutritional Value of the Experimental Diets
3.3. Production Performances
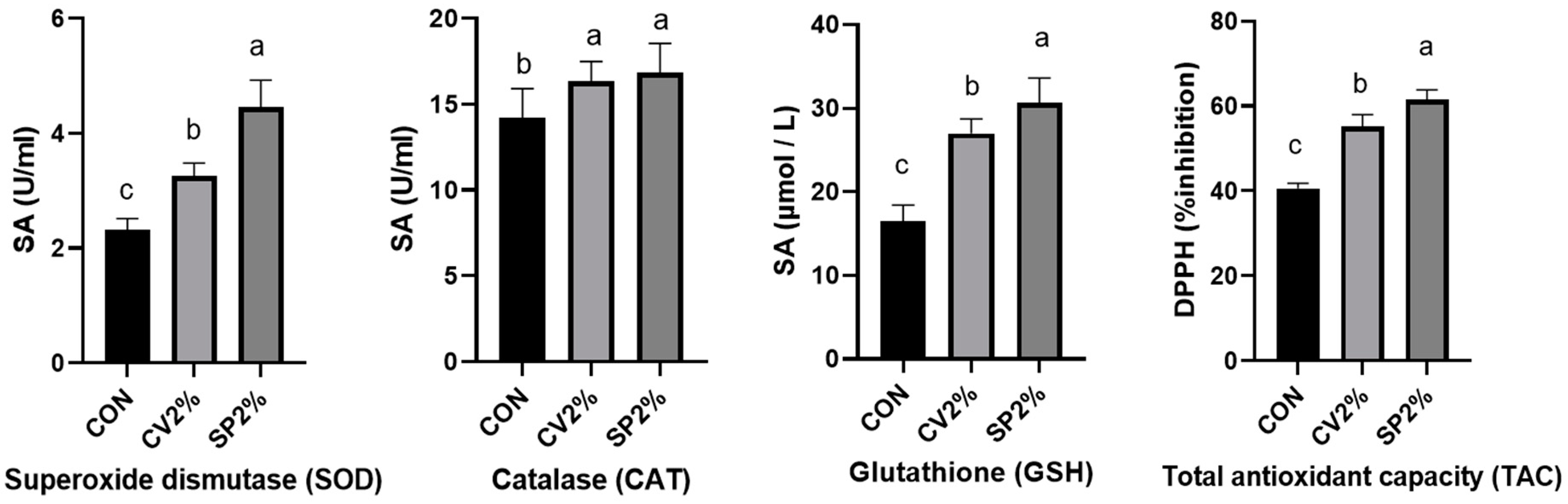
3.4. Serum Antioxidant Status
3.5. Digestibility Trial
3.6. Nutritional Egg Quality Parameters
3.7. Yolk Cholesterol Content and Fatty Acid Profile
3.8. The Effect of Dietary Chlorella and Spirulina Powder on Egg Yolk Color in Fresh and Boiled Eggs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ∑ PUFA n-3 | sum of polyunsaturated fatty acids with omega-3 double bond |
| ∑ PUFA n-6 | sum of polyunsaturated fatty acids with omega-6 double bond |
| a* | red-green intensity (in egg color determination) |
| ALA | alpha-linolenic acid |
| b* | yellow-blue intensity (in egg color determination) |
| Ca | chlorophyll a |
| CAT | catalase |
| Cb | chlorophyll b |
| Cc | total carotenoids |
| CF | crude fiber |
| CON | conventional diet |
| CP | crude protein |
| CV2% | diet with 2% Chlorella vulgaris |
| DCP | in vitro digestibility of protein |
| DDM | in vitro digestibility of dry matter |
| DEE | in vitro digestibility of ether extract |
| DF | dilution factor |
| DFI | daily feed intake (g/day/layer) |
| DHA | docosahexaenoic acid |
| DM | dry matter |
| DNFE | in vitro digestibility of non-fermentable extractive substance |
| DOM | in vitro digestibility of organic matter |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DTNB | 5,5’-dithio-bis-[2-nitrobenzoic acid] (Ellman’s reagent) |
| EDTA | ethylendiaminotetraacetic acid |
| EE | ether extract |
| EPA | eicosapentaenoic acid |
| EW | egg weight (g) |
| FAME | fatty acid methyl esters (for fatty acid chromatography) |
| FCR | feed conversion ratio (g feed/g egg) |
| GAE | gallic acid equivalents (for polyphenols) |
| GLA | gamma-linolenic acid |
| GSH | reduced glutathione (peroxidase) |
| HDEP | hen day egg production (%) |
| L* | lightness (in egg color determination) |
| ME | metabolizable energy |
| MUFA | total monounsaturated fatty acids |
| NBT | nitro blue tetrazolium |
| NFE | non-fermentable extractive substance |
| OM | organic matter |
| PUFA | total polyunsaturated fatty acids |
| SFA | total saturated fatty acids |
| SOD | superoxide dismutase |
| SP2% | diet with 2% Spirulina platensis |
| TAC | total antioxidant capacity |
| TNB | 5-thio-2-nitrobenzoic acid |
References
- Bryden, W.L.; Li, X.; Ruhnke, I.; Zhang, D.; Shini, S. Nutrition, feeding and laying hen welfare. Anim. Prod. Sci. 2021, 61, 893–914. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Wang, H.; Wang, J.; Wu, S.; Qi, G. Effect of dietary protein sources on production performance, egg quality, and plasma parameters of laying hens. Asian-Australas. J. Anim. Sci. 2017, 30, 400–409. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kudełka, W.; Kowalska, M.; Popis, M. Quality of Soybean Products in Terms of Essential Amino Acids Composition. Molecules 2021, 26, 5071. [Google Scholar] [CrossRef]
- Qin, P.; Wang, T.; Luo, Y. A review on plant-based proteins from soybean: Health benefits and soy product development. J. Agric. Food Res. 2022, 7, 100265. [Google Scholar] [CrossRef]
- Alshelmani, M.I.; Abdalla, E.A.; Kaka, U.; Basit, M.A. Nontraditional feedstuffs as an alternative in poultry feed. In Advances in Poultry Nutrition Research; IntechOpen: London, UK, 2021. [Google Scholar]
- Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, R.; Arora, A. Health promoting functional lipids from microalgae pool: A review. Algal Res. 2020, 46, 101800. [Google Scholar] [CrossRef]
- Herrera, A.; D’imporzano, G.; Fernandez, F.G.A.; Adani, F. Sustainable production of microalgae in raceways: Nutrients and water management as key factors influencing environmental impacts. J. Clean. Prod. 2021, 287, 125005. [Google Scholar] [CrossRef]
- Zhou, L.; Li, K.; Duan, X.; Hill, D.; Barrow, C.; Dunshea, F.; Martin, G.; Suleria, H. Bioactive compounds in microalgae and their potential health benefits. Food Biosci. 2022, 49, 101932. [Google Scholar] [CrossRef]
- Riccio, G.; Lauritano, C. Microalgae with Immunomodulatory Activities. Mar. Drugs 2019, 18, 2. [Google Scholar] [CrossRef]
- Coelho, D.F.M.; Alfaia, C.M.R.P.M.; Assunção, J.M.P.; Costa, M.; Pinto, R.M.A.; de Andrade Fontes, C.M.G.; Lordelo, M.M.; Prates, J.A.M. Impact of dietary Chlorella vulgaris and carbohydrate-active enzymes incorporation on plasma metabolites and liver lipid composition of broilers. BMC Veter-Res. 2021, 17, 229. [Google Scholar] [CrossRef]
- Zheng, L.; Oh, S.T.; Jeon, J.Y.; Moon, B.H.; Kwon, H.S.; Lim, S.U.; Kang, C.W. The dietary effects of fermented Chlorella vulgaris (CBT®) on production performance, liver lipids and intestinal microflora in laying hens. Asian-Australas. J. Anim. Sci. 2012, 25, 261. [Google Scholar] [CrossRef] [PubMed]
- Hyrslova, I.; Krausova, G.; Smolova, J.; Stankova, B.; Branyik, T.; Malinska, H.; Huttl, M.; Kana, A.; Doskocil, I.; Curda, L. Prebiotic and Immunomodulatory Properties of the Microalga Chlorella vulgaris and Its Synergistic Triglyceride-Lowering Effect with Bifidobacteria. Fermentation 2021, 7, 125. [Google Scholar] [CrossRef]
- Matos, J.; Cardoso, C.L.; Falé, P.; Afonso, C.M.; Bandarra, N.M. Investigation of nutraceutical potential of the microalgae Chlorella vulgaris and Arthrospira platensis. Int. J. Food Sci. Technol. 2020, 55, 303–312. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Jiang, S.; Farag, M.R.; Azzam, M.; Al-Abdullatif, A.A.; Alhotan, R.; Dhama, K.; Hassan, F.-U.; Alagawany, M. Potential of Spirulina platensis as a feed supplement for poultry to enhance growth performance and immune modulation. Front. Immunol. 2023, 14, 1072787. [Google Scholar] [CrossRef]
- Altmann, B.A.; Rosenau, S. Spirulina as Animal Feed: Opportunities and Challenges. Foods 2022, 11, 965. [Google Scholar] [CrossRef]
- Smetana, S.; Sandmann, M.; Rohn, S.; Pleissner, D.; Heinz, V. Autotrophic and heterotrophic microalgae and cyanobacteria cultivation for food and feed: Life cycle assessment. Bioresour. Technol. 2017, 245, 162–170. [Google Scholar] [CrossRef]
- Carpenter, K.J.; Clegg, K.M. The metabolizable energy of poultry feeding stuffs in relation to their chemical composition. J. Sci. Food Agric. 1956, 7, 45–51. [Google Scholar] [CrossRef]
- Mhlongo, G.; Mnisi, C. Effect of seaweed (Ecklonia maxima) on apparent nutrient digestibility, growth performance, and physiological and meat quality parameters in Boschveld cockerels. Poult. Sci. 2023, 102, 102361. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Poultry, 9th ed.; National Academic Press: Washington, DC, USA, 1994.
- Panaite, T.D.; Nour, V.; Saracila, M.; Turcu, R.P.; Untea, A.E.; Vlaicu, P.A. Effects of linseed meal and carotenoids from different sources on egg characteristics, yolk fatty acid and carotenoid profile and lipid peroxidation. Foods 2021, 10, 1246. [Google Scholar] [CrossRef]
- Boisen, S.; Fernandez, J.A. Prediction of the apparent ileal digestibility of protein and amino acids in feedstuffs and feed mix-tures for pigs by in vitro analyses. Anim. Feed Sci. Technol. 1995, 51, 29–43. [Google Scholar] [CrossRef]
- Panaite, T.D.; Vlaicu, P.A.; Saracila, M.; Cismileanu, A.; Varzaru, I.; Voicu, S.N.; Hermenean, A. Impact of watermelon rind and sea buckthorn meal on performance, blood parameters, and gut microbiota and morphology in laying hens. Agriculture 2022, 12, 177. [Google Scholar] [CrossRef]
- Arslan, R.; Eroglu, E.C.; Aksay, S. Determination of bioactive properties of protein and pigments obtained from Spirulina platensis. J. Food Process. Preserv. 2021, 45, e15150. [Google Scholar] [CrossRef]
- Beyer, W.F., Jr.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef]
- Chrzczanowicz, J.; Gawron, A.; Zwolinska, A.; de Graft-Johnson, J.; Krajewski, W.; Krol, M.; Markowski, J.; Kostka, T.; Nowak, D. Simple method for determining human serum 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity—Possible application in clinical studies on dietary antioxidants. Clin. Chem. Lab. Med. 2008, 46, 342–349. [Google Scholar] [CrossRef]
- AOAC. AOAC final action method 17.002, colour of eggs. J. Assoc. Off. Anal. Chem. 1973, 56, 272. [Google Scholar]
- Untea, A.; Lupu, A.; Saracila, M.; Panaite, T. Comparison of ABTS, DPPH, Phosphomolybdenum Assays for Estimating An-tioxidant Activity and Phenolic Compounds in Five Different Plant Extracts. Bull. UASVM Anim. Sci. Biotech-Nologies 2018, 75, 2. [Google Scholar]
- Untea, A.E.; Varzaru, I.; Panaite, T.D.; Gavris, T.; Lupu, A.; Ropota, M. The Effects of Dietary Inclusion of Bilberry and Walnut Leaves in Laying Hens’ Diets on the Antioxidant Properties of Eggs. Animals 2020, 10, 191. [Google Scholar] [CrossRef]
- Panaite, T.D.; Mironeasa, S.; Iuga, M.; Vlaicu, P.A. Liquid egg products characterization during storage as a response of novel phyto-additives added in hens diet. Emir. J. Food Agric. 2019, 31, 304–314. [Google Scholar] [CrossRef]
- Kotrbáček, V.; Skřivan, M.; Kopecký, J.; Pěnkava, O.; Hudečková, P.; Uhríková, I.; Doubek, J. Retention of carotenoids in egg yolks of laying hens supplemented with heterotrophic Chlorella. Czech J. Anim. Sci. 2013, 58, 193–200. [Google Scholar] [CrossRef]
- An, B.K.; Kim, K.E.; Jeon, J.Y.; Lee, K.W. Effect of dried Chlorella vulgaris and Chlorella growth factor on growth perfor-mance, meat qualities and humoral immune responses in broiler chickens. Springerplus 2016, 5, 718. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.O.; Mendes, M.A. Chlorella and spirulina microalgae as sources of functional foods, nutraceuticals, and food supplements; an overview. MOJ Food Process. Technol. 2018, 6, 00144. [Google Scholar] [CrossRef]
- Kang, H.K.; Salim, H.M.; Akter, N.; Kim, D.W.; Kim, J.H.; Bang, H.T.; Kim, M.J.; Na, J.C.; Hwangbo, J.; Choi, H.C.; et al. Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult. Res. 2013, 22, 100–108. [Google Scholar] [CrossRef]
- Abdul-Adel, E.; Saleh, M.M.; Salman, J.M. Biodiesel production by Spirulina platensis under different NaCl concen-trations. Biochem. Cell. Arch. 2020, 20, 1745–1749. [Google Scholar]
- Abou-El-Souod, G.W.; Hassan, L.H.; Morsy, E.M. Comparison of different media formulations and the optimal growing conditions on growth, morphology and chlorophyll content of green alga, chlorella vulgaris. J. Am. Sci. 2016, 12, 86–95. [Google Scholar]
- Khan, F.; Shuvo, A.A.S.; Islam, K.M.S. Effects of dietary inclusion of Spirulina platensis on egg yolk pigmentation. Live Res. Rural. Dev. 2021, 33, 101. [Google Scholar]
- Stunda-Zujeva, A.; Berele, M.; Lece, A.; Šķesters, A. Comparison of antioxidant activity in various spirulina containing products and factors affecting it. Sci. Rep. 2023, 13, 4529. [Google Scholar] [CrossRef]
- Chen, B.; Wan, C.; Mehmood, M.A.; Chang, J.-S.; Bai, F.; Zhao, X. Manipulating environmental stresses and stress tolerance of microalgae for enhanced production of lipids and value-added products—A review. Bioresour. Technol. 2017, 244, 1198–1206. [Google Scholar] [CrossRef]
- Torres-Durán, P.V.; Ferreira-Hermosillo, A.; Ramos-Jiménez, A.; Hernández-Torres, R.P.; Juárez-Oropeza, M.A. Effect of Spirulina maxima on postprandial lipemia in young runners: A preliminary report. J. Med. Food 2012, 15, 753–757. [Google Scholar] [CrossRef]
- Mariey, Y.A.; Samak, H.R.; Ibrahem, M.A. Effect of using Spirulina platensis algae as a feed additive for poultry diets: 1-productive and reproductive performances oflocal spirulina algae- organic selenium-egg production-heat stress- laying hens. Egypt. Poult. Sci. 2015, 32, 201–215. [Google Scholar]
- Shanmugapriya, B.; Saravanababu, S. Supplementary effect of Spirulinaplatensison performance, hematology andcarcass yield of broiler chickens. Indian Streams Res. J. 2014, 4, 1–7. [Google Scholar]
- Abd El Wahab, E.; Abaza, I.M.; Saad, A. Effect of dietary supplementation of Spirulina Platensis and organic selenium on some biological traits of local laying hen under heat stress conditions. Egypt. Poult. Sci. J. 2019, 39, 207–217. [Google Scholar] [CrossRef][Green Version]
- Halle, P.; Janczyk; Freyer, G.; Souffrant, W.B. Effect of microalgae chlorella vulgaris on laying hen performance. Arch. Zootech. 2009, 12, 5–13. [Google Scholar]
- Omri, B.; Amraoui, M.; Tarek, A.; Lucarini, M.; Durazzo, A.; Cicero, N.; Santini, A.; Kamoun, M. Arthrospira platensis (spirulina) supplementation on laying hens’ performance: Eggs physical, chemical, and sensorial qualities. Foods 2019, 8, 386. [Google Scholar] [CrossRef]
- Mirzaie, S.; Sharifi, S.D.; Zirak-Khattab, F. The effect of a Chlorella by-product dietary supplement on immune response, antioxidant status, and intestinal mucosal morphology of broiler chickens. J. Appl. Phycol. 2020, 32, 1771–1777. [Google Scholar] [CrossRef]
- Hosseini-Vashan, S.J.; Golian, A.; Yaghobfar, A.; Zarban, A.; Afzali, N.; Esmaeilinasab, P. Antioxidant status, immune system, blood metabolites and carcass characteristic of broiler chickens fed turmeric rhizome powder under heat stress. Afr. J. Biotechnol. 2012, 11, 16118–16125. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090 . [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.I.; Kim, I.H. Effect of dietary Spirulina (Arthrospira) platensis on the growth performance, antioxidant enzyme activity, nutrient digestibility, cecal microflora, excreta noxious gas emission, and breast meat quality of broiler chickens. Poult. Sci. 2018, 97, 2451–2459. [Google Scholar] [CrossRef]
- Wu, L.C.; Ho, J.A.A.; Shieh, M.C.; Lu, I.W. Antioxidant and antiproliferative activities of Spirulina and Chlorella water ex-tracts. J. Agric. Food Chem. 2005, 53, 4207–4212. [Google Scholar] [CrossRef]
- Nhlane, L.T.; Mnisi, C.M.; Madibana, M.J.; Mlambo, V. Nutrient digestibility, growth performance and blood indices of Boschveld chickens fed seaweed-containing diets. Animals 2020, 10, 1296. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-Y.; Kim, K.-E.; Im, H.-J.; Oh, S.-T.; Lim, S.-U.; Kwon, H.-S.; Moon, B.-H.; Kim, J.-M.; An, B.-K.; Kang, C.-W. The production of lutein-enriched eggs with dietary chlorella. Korean J. Food Sci. Anim. Resour. 2012, 32, 13–17. [Google Scholar] [CrossRef]
- Englmaierová, M.; Skřivan, M.; Bubancová, I. A comparison of lutein, spray-dried Chlorella, and synthetic carotenoids effects on yolk colour, oxidative stability, and reproductive performance of laying hens. Czech J. Anim. Sci. 2013, 58, 412–419. [Google Scholar] [CrossRef]
- Llave, Y.; Fukuda, S.; Fukuoka, M.; Shibata-Ishiwatari, N.; Sakai, N. Analysis of color changes in chicken egg yolks and whites based on degree of thermal protein denaturation during ohmic heating and water bath treatment. J. Food Eng. 2017, 222, 151–161. [Google Scholar] [CrossRef]
- Muñoz-Miranda, L.A.; Iñiguez-Moreno, M. An extensive review of marine pigments: Sources, biotechnological applications, and sustainability. Aquat. Sci. 2023, 85, 68. [Google Scholar] [CrossRef]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, old sustainable food and fashion nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef]
- Rey, A.I.; De-Cara, A.; Rebolé, A.; Arija, I. Short-Term Spirulina (Spirulina platensis) Supplementation and Laying Hen Strain Effects on Eggs’ Lipid Profile and Stability. Animals 2021, 29, 1944. [Google Scholar] [CrossRef]
- Yunitasari, F.; Jayanegara, A.; Ulupi, N. Performance, Egg Quality, and Immunity of Laying Hens due to Natural Carotenoid Supplementation: A Meta-Analysis. Food Sci. Anim. Resour. 2023, 43, 282–304. [Google Scholar] [CrossRef]
- Zahroojian, N.; Moravej, H.; Shivazad, M. Effects of dietary marine algae (Spirulina platensis) on egg quality and production performance of laying hens. J. Agr. Sci. Technol. 2013, 15, 1353–1360. [Google Scholar]
- Kumar, R.S.P.; Sibi, G. Spirulina as poultry feed supplement to enhance nutritional value of chicken meat and eggs: A review. Int. J. Microbiol. Res. 2020, 11, 67–71. [Google Scholar]
- Bruneel, C.; Lemahieu, C.; Fraeye, I.; Ryckebosch, E.; Muylaert, K.; Buyse, J.; Foubert, I. Impact of microalgal feed supplemen-tation on omega-3 fatty acid enrichment of hen eggs. J. Funct. Foods 2013, 5, 897–904. [Google Scholar] [CrossRef]
- Neijat, M.; Ojekudo, O.; House, J. Effect of flaxseed oil and microalgae DHA on the production performance, fatty acids and total lipids of egg yolk and plasma in laying hens. Prostaglandins Leukot. Essent. Fat. Acids 2016, 115, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Mens, A.J.W.; van Krimpen, M.M.; Kar, S.K.; Guiscafre, F.J.; Sijtsma, L. Enriching table eggs with n-3 polyunsaturated fatty acids through dietary supplementation with the phototrophically grown green algae Nannochloropsislimnetica: Effects of microalgae on nutrient retention, performance, egg characteristics and health parameters. Poult Sci. 2022, 101, 101869. [Google Scholar] [PubMed]
| Ingredients, % | Experimental Diets | ||
|---|---|---|---|
| Control (CON) | Chlorella Powder (CV2%) | Spirulina Powder (SP2%) | |
| Corn | 40.00 | 40.00 | 40.00 |
| Wheat bran | 22.49 | 23.72 | 23.60 |
| Chlorella vulgaris | - | 2.00 | - |
| Spirulina platensis | - | - | 2.00 |
| Soybean meal | 24.36 | 21.58 | 21.63 |
| Vegetable oil | 1.48 | 0.97 | 1.04 |
| L-lysine HCl | - | 0.01 | 0.01 |
| DL–methionine | 0.16 | 0.17 | 0.17 |
| Calcium carbonate | 8.83 | 8.84 | 8.84 |
| Monocalcium phosphate | 1.32 | 1.33 | 1.33 |
| Salt | 0.33 | 0.33 | 0.33 |
| Choline premix | 0.04 | 0.04 | 0.04 |
| Vitamin–mineral premix * | 1.00 | 1.00 | 1.00 |
| Total | 100.00 | 100.00 | 100.00 |
| Calculated analysis (%) ** | |||
| Metabolizable energy (Kcal/kg) | 2.750.00 | 2.750.00 | 2.750.00 |
| Crude protein | 17.00 | 17.09 | 17.00 |
| Lysine | 0.87 | 0.80 | 0.80 |
| Methionine+Cystine | 0.73 | 0.71 | 0.71 |
| Threonine | 0.64 | 0.59 | 0.59 |
| Calcium | 3.90 | 3.90 | 3.90 |
| Phosphorus | 0.63 | 0.62 | 0.62 |
| Parameters | Chlorella Powder | Spirulina Powder |
|---|---|---|
| Proximate composition * | ||
| Calculated metabolizable energy (ME), MJ/kg | 9.77 ± 0.41 | 9.68 ± 0.25 |
| Crude protein (CP), % | 51.06 ± 0.35 | 67.02 ± 0.04 |
| Dry matter (DM), % | 94.68 ± 0.80 | 92.70 ± 0.62 |
| Organic matter (OM), % | 88.33 ± 0.75 | 87.75 ± 0.50 |
| Ether extract (EE), % | 3.56 ± 0.50 | 0.48 ± 0.03 |
| Crude fiber (CF), % | 0.49 ± 0.09 | 0.19 ± 0.11 |
| Non-fermentable extractive substance (NFE), % | 33.22 ± 0.39 | 20.06 ± 0.07 |
| Ash, % | 6.35 ± 0.25 | 4.95 ± 0.18 |
| In vitro nutrient digestibility ** | ||
| Digestible crude protein (DCP), % | 96.59 | 96.71 |
| Digestible dry matter (DDM), % | 99.56 | 99.05 |
| Digestible organic matter (DOM), % | 99.59 | 99.12 |
| Antioxidant activity * | ||
| Chlorophyll a, mg/g | 5.56 ± 1.08 | 9.06 ± 0.79 |
| Chlorophyll b, mg/g | 0.88 ± 0.20 | 1.34 ± 0.34 |
| Carotenes, mg/g | 1.52 ± 0.19 | 1.68 ± 0.31 |
| Total polyphenols, mg/g GAE | 1.16 ± 0.16 | 1.35 ± 0.05 |
| Antioxidant capacity (DPPH % inhibition) | 73.29 ± 2.93 | 81.27 ± 1.60 |
| Antioxidant capacity (µM Trolox) | 15.49 ± 3.87 | 16.78 ± 2.47 |
| Fatty acids (g FAME/100 g Total FAME) * | ||
| Caproic (C 6:0) | 0.65 ± 0.05 | 0.16 ± 0.02 |
| Caprilic (C 8:0) | 0.25 ± 0.02 | 7.73 ± 0.65 |
| Capric (C 10:0) | 1.20 ± 0.10 | - |
| Lauric (C 12:0) | 0.07 ± 0.001 | 0.68 ± 0.06 |
| Miristic (C 14:0) | 0.96 ± 0.08 | 0.88 ± 0.07 |
| Pentadecanoic (C 15:0) | 0.11 ± 0.001 | - |
| Palmitic (C 16:0) | 27.25 ± 2.32 | 34.71 ± 3.12 |
| Stearic (C 18:0) | 2.51 ± 0.21 | 6.87 ± 0.55 |
| Heneicosanoic (C 21:0) | 0.17 ± 0.01 | - |
| Behenic (C 22:0) | 0.60 ± 0.05 | - |
| ∑ SFA | 33.77 ± 3.00 | 51.03 ± 4.34 |
| Pentadecenoic (C 15:1) | 0.05 ± 0.004 | - |
| Palmitoleic (C 16:1) | 0.19 ± 0.02 | 4.87 ± 0.46 |
| Heptadecenoic (C 17:1) | 0.08 ± 0.007 | - |
| Oleic cis (C 18:1) | 8.73 ± 0.72 | 22.25 ± 2.14 |
| ∑ MUFA | 9.05 ± 0.19 | 27.12 ± 1.3 |
| Linoleic cis (C 18:2n6) | 14.13 ± 1.20 | 16.84 ± 1.43 |
| Linolenic γ (C 18:3n6) | 0.16 ± 0.01 | - |
| Linolenic α (C 18:3n3) | 37.37 ± 3.18 | 4.41 ± 0.39 |
| Octadecatetraenoic (C18:4n3) | 0.73 ± 0.06 | - |
| Eicosadienoic (C20:2n6) | 0.33 ± 0.03 | - |
| Eicosatrienoic (C20:3n6) | 3.61 ± 0.31 | - |
| Arachidonic (C20:4n6) | 0.21 ± 0.01 | 0.60 ± 0.05 |
| ∑ PUFA | 56.56 ± 4.80 | 21.85 ± 1.94 |
| ∑ PUFA n-3 | 38.10 ± 3.65 | 4.41 ± 0.41 |
| ∑ PUFA n-6 | 18.45 ± 1.77 | 17.44 ± 1.48 |
| ∑ PUFA n-6/∑ PUFA n-3 | 0.48 ± 0.05 | 3.95 ± 0.35 |
| Other fatty acids | 0.62 ± 0.05 | - |
| Parameters | Dietary Treatments | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | CV2% | SP2% | |||
| Antioxidant activity | |||||
| Chlorophyll a, µg/g | 10.31 c | 344.76 b | 383.22 a | 5.41 | <0.0001 |
| Chlorophyll b, µg/g | 2.77 b | 40.93 a | 38.92 a | 1.44 | <0.0001 |
| Carotenes, µg/g | 31.45 b | 84.92 a | 81.70 a | 1.08 | <0.0001 |
| Total polyphenols, mg/g GAE | 1.49 | 1.67 | 1.64 | 0.177 | 0.933 |
| Antioxidant capacity (DPPH % inhibition) | 32.00 c | 57.57 b | 67.23 a | 1.23 | <0.0001 |
| Antioxidant capacity (µM Trolox) | 5.19 | 6.25 | 5.79 | 0.403 | 0.620 |
| Fatty acid composition (% of total fat) | |||||
| ΣSFA | 29.03 a | 19.72 b | 22.44 b | 0.893 | 0.001 |
| ΣMUFA | 47.42 a | 37.06 b | 40.12 ab | 2.06 | 0.030 |
| ∑ PUFA, from which: | 23.16 b | 42.95 a | 37.11 a | 2.36 | 0.003 |
| Σ n-3 PUFA | 1.03 b | 1.43 a | 1.21 ab | 0.06 | 0.007 |
| ∑ n-6 PUFA | 22.14 b | 41.52 a | 35.90 a | 2.32 | 0.003 |
| ∑ n-6/∑ n-3 | 21.58 b | 29.18 a | 29.47 a | 1.22 | 0.006 |
| Parameters | Dietary Treatments | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | CV (2%) | SP (2%) | |||
| Initial body weight (g/layer) | 1561.30 | 1599.17 | 1556.88 | 23.23 | 0.580 |
| Final body weight (g/layer) | 1670.68 | 1681.52 | 1643.96 | 23.63 | 0.688 |
| Daily feed intake (g/day/layer) | 112.78 | 112.40 | 113.46 | 0.441 | 0.608 |
| Feed conversion ratio (g feed/g egg) | 2.04 b | 2.17 a | 1.92 c | 0.024 | <0.0001 |
| Egg weight (g) | 59.78 b | 62.42 a | 62.44 a | 0.110 | <0.0001 |
| Hen day egg production (%) | 94.61 ab | 93.32 b | 95.56 a | 0.412 | 0.046 |
| Egg classification *, % | |||||
| “XL” (>73 g), extra large | 0.15 b | 2.99 a | 2.08 a | 0.350 | <0.0001 |
| “L” (63–73 g), large | 22.87 b | 38.50 a | 39.65 a | 1.01 | <0.0001 |
| “M” (53–63 g), medium | 71.22 a | 57.321 b | 56.50 b | 1.03 | <0.0001 |
| “S” (<53 g), small | 5.76 a | 1.19 b | 1.77 b | 0.346 | <0.0001 |
| Parameters | Dietary Treatments | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | CV2% | SP2% | |||
| Digestible dry matter (DDM),% | 73.17 | 71.29 | 72.61 | 0.835 | 0.288 |
| Digestible organic matter (DOM), % | 73.05 | 70.52 | 71.62 | 0.806 | 0.112 |
| Digestible crude protein (DCP), % | 85.81 | 85.20 | 84.85 | 0.607 | 0.539 |
| Digestible crude fat (DCF), % | 90.58 a | 87.82 b | 89.07 ab | 0.740 | 0.052 |
| Digestible non-fermentable extractive substance (DNFE), % | 68.60 | 66.27 | 67.59 | 0.768 | 0.128 |
| Parameters | Dietary Treatments | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | CV2% | SP2% | |||
| Nutrition quality of egg yolk | |||||
| β-carotene, (µg/g) | 30.77 b | 38.82 a | 38.25 a | 0.432 | <0.0001 |
| Total polyphenols (mg/g GAE) | 0.534 | 0.598 | 0.574 | 0.027 | 0.271 |
| Antioxidant capacity (DPPH% inhibition) | 16.84 b | 25.14 a | 28.15 a | 0.869 | <0.0001 |
| Antioxidant capacity (µM Trolox) | 0.74 b | 0.79 a | 0.82 a | 0.019 | 0.033 |
| External and internal egg quality parameters | |||||
| Egg weight (g), of which: | 61.28 | 61.53 | 61.82 | 0.389 | 0.617 |
| albumen white (g) | 37.00 | 37.76 | 37.38 | 0.352 | 0.320 |
| egg yolk (g) | 16.37 | 15.65 | 16.26 | 0.242 | 0.087 |
| eggshell (g) | 7.91 | 8.12 | 8.17 | 0.137 | 0.358 |
| Albumen pH (value) | 8.62 ab | 8.44 b | 8.80 a | 0.069 | 0.002 |
| Yolk pH (value) | 6.51 | 6.38 | 6.46 | 0.093 | 0.625 |
| t° albumen (°C) | 19.10 a | 18.57 ab | 17.79 b | 0.258 | 0.003 |
| t° yolk (°C) | 19.99 a | 19.12 b | 19.92 a | 0.134 | 0.011 |
| White height, mm | 10.96 | 11.74 | 11.48 | 0.292 | 0.168 |
| Haugh units (value) | 102.92 | 106.04 | 104.82 | 1.150 | 0.163 |
| Yolk color, (value) | 4.06 c | 8.11 b | 11.06 a | 0.113 | <0.0001 |
| Experimental Groups | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | CON | CV2% | SP2% | SEM | p-Value | CON | CV2% | SP2% | SEM | p-Value | |
| Yolk fat (% DM) | 29.454 | 29.570 | 30.412 | 0.771 | 0.640 | ||||||
| Cholesterol (mg col/egg) | 224.00 | 173.00 | 192.00 | 0.013 | 0.061 | ||||||
| Fatty acid content | g FAME/100 g Total FAME | mg fatty acid/egg | |||||||||
| Miristic C14:0 | 0.308 b | 0.346 a | 0.346 a | 0.008 | 0.005 | 4.716 | 5.063 | 5.266 | 0.196 | 0.167 | |
| Pentadecanoic C15:0 | 0.060 | 0.140 | 0.060 | 0.052 | 0.399 | 0.932 | 2.182 | 0.918 | 0.760 | 0.423 | |
| Palmitic C16:0 | 25.44 | 26.14 | 26.05 | 0.242 | 0.121 | 389.9 | 380.2 | 395.0 | 13.500 | 0.738 | |
| Heptadecanoic C17:0 | 0.128 b | 0.128 b | 0.145 a | 0.003 | 0.001 | 1.974 ab | 1.869 b | 2.227 a | 0.093 | 0.043 | |
| Stearic C18:0 | 10.09 | 10.11 | 9.61 | 0.232 | 0.256 | 154.71 | 146.93 | 145.79 | 5.990 | 0.533 | |
| ∑SFA | 36.03 | 36.87 | 36.22 | 0.409 | 0.339 | 552.2 | 536.3 | 549.2 | 19.20 | 0.825 | |
| Miristioleic C14:1 | 0.079 c | 0.092 b | 0.108 a | 0.003 | <0.0001 | 1.217 b | 1.345 b | 1.645 a | 0.066 | 0.001 | |
| Pentadecenoic C15:1 | 0.100 | 0.101 | 0.417 | 0.184 | 0.394 | 1.53 | 1.46 | 6.35 | 2.810 | 0.392 | |
| Palmitoleic C16:1 | 4.37 | 3.98 | 4.34 | 0.421 | 0.765 | 65.58 | 57.95 | 65.86 | 5.170 | 0.488 | |
| Heptadecenoic C17:1 | 0.069 b | 0.075 b | 0.089 c | 0.003 | 0.002 | 1.056 b | 1.095 b | 1.358 a | 0.070 | 0.016 | |
| Oleic C18:1 | 36.09 b | 36.80 ab | 37.59 a | 0.406 | 0.050 | 552.8 | 535.8 | 570.1 | 20.100 | 0.499 | |
| Erucic C22 (1n9) | 0.121 a | 0.120 a | 0.100 b | 0.004 | 0.002 | 1.854 a | 1.456 b | 1.819 a | 0.077 | 0.004 | |
| Nervonic C24 (1n9) | 0.277 | 0.279 | 0.272 | 0.006 | 0.640 | 4.246 | 4.063 | 4.128 | 0.161 | 0.724 | |
| ∑MUFA | 41.11 | 41.43 | 42.94 | 0.508 | 0.050 | 628.3 | 603.2 | 651.2 | 20.4 | 0.280 | |
| Linoleic C18:2 | 15.66 a | 14.27 b | 13.97 b | 0.108 | <0.0001 | 239.94 a | 207.67 b | 211.94 ab | 8.320 | 0.031 | |
| Linolenic γ C18:3n6 | 0.126 b | 0.134 ab | 0.138 a | 0.002 | 0.0007 | 1.931 | 1.944 | 2.089 | 0.080 | 0.326 | |
| Linolenic α C18:3n3 | 0.218 b | 0.319 a | 0.233 b | 0.017 | 0.001 | 3.343 b | 4.651 a | 3.541 b | 0.283 | 0.011 | |
| Eicosadienoic C20 (2n6) | 0.229 b | 0.232 b | 0.269 a | 0.007 | 0.002 | 3.515 | 3.389 | 4.084 | 0.190 | 0.046 | |
| Eicosatrienoic C20 (3n6) | 0.290 a | 0.257 b | 0.289 a | 0.005 | <0.0001 | 4.451 a | 3.743 b | 4.393 ab | 0.178 | 0.023 | |
| Eicosatrienoic C20 (3n3) | 0.252 b | 0.234 b | 0.274 a | 0.006 | 0.001 | 3.866 ab | 3.401 b | 4.154 a | 0.172 | 0.023 | |
| Arachidonic C20 (4n6) | 3.613 a | 3.548 a | 3.178 b | 0.093 | 0.010 | 55.420 | 51.570 | 48.230 | 2.34 | 0.129 | |
| Docosatetraenoic C22 (4n6) | 1.501 a | 1.318 b | 1.537 a | 0.043 | 0.005 | 23.01 a | 19.16 b | 23.32 a | 1.01 | 0.019 | |
| Docosapentaenoic C22 (5n3) | 0.071 b | 0.109 a | 0.071 b | 0.003 | <0.0001 | 1.087 b | 1.593 a | 1.073 b | 0.054 | <0.0001 | |
| Docosahexaenoic C22 (6n3) | 0.691 a | 1.056 b | 0.632 b | 0.021 | <0.0001 | 10.591 b | 15.357 a | 9.595 b | 0.483 | <0.0001 | |
| ∑PUFA | 22.65 a | 21.45 b | 20.59 c | 0.183 | <0.0001 | 347.2 | 312.5 | 312.4 | 12.30 | 0.102 | |
| ∑Ω3 | 1.23 b | 1.72 a | 1.21 b | 0.024 | <0.0001 | 18.887 b | 25.001 a | 18.364 b | 0.784 | <0.0001 | |
| ∑Ω6 | 21.42 a | 19.76 b | 19.39 b | 0.168 | <0.0001 | 328.270 | 294.059 | 287.475 | 11.5 | 0.053 | |
| ∑Ω6/Ω3 | 17.40 a | 11.51 c | 16.06 b | 0.228 | <0.0001 | 266.27 a | 167.44 b | 243.36 a | 8.59 | <0.0001 | |
| Other fatty acids | 0.207 | 0.214 | 0.245 | 0.025 | 0.517 | 3.164 | 3.108 | 3.749 | 0.393 | 0.460 | |
| Yolk Color Parameter | L* | a* | b* | |
|---|---|---|---|---|
| CON | fresh yolk | 45.76 f | 1.321 e | 14.03 f |
| 10 min boiling time | 79.94 e | −1.389 g | 19.32 ef | |
| 20 min boiling time | 104.33 c | −3.836 h | 23.46 de | |
| 30 min boiling time | 125.97 a | −5.514 i | 25.65 d | |
| CV2% | fresh yolk | 43.82 f | 3.156 d | 17.74 ef |
| 10 min boiling time | 76.56 e | 0.883 e | 33.14 c | |
| 20 min boiling time | 100.05 cd | 0.073 ef | 51.60 a | |
| 30 min boiling time | 122.15 a | −1.070 fg | 48.21 a | |
| SP2% | fresh yolk | 40.49 f | 4.924 c | 14.18 f |
| 10 min boiling time | 74.86 e | 6.414 ab | 32.16 c | |
| 20 min boiling time | 95.11 d | 7.601 a | 39.83 b | |
| 30 min boiling time | 116.46 b | 5.591 bc | 49.31 a | |
| Main effect | ||||
| Treatment | CON | 88.99 a | −2.354 c | 20.61 c |
| CV2% | 85.65 b | 0.761 b | 37.68 a | |
| SP2% | 81.73 c | 6.132 a | 33.87 b | |
| SEMtreatment | 0.583 | 0.151 | 0.653 | |
| Boiling time | fresh yolk | 43.36 d | 3.134 a | 15.318 d |
| 10 min boiling time | 77.12 c | 1.969 b | 28.205 c | |
| 20 min boiling time | 99.83 b | 1.279 c | 37.897 b | |
| 30 min boiling time | 121.53 a | −0.331 d | 41.457 a | |
| SEMboiling time | 0.682 | 0.177 | 0.764 | |
| p-Value | ||||
| Treatment | <0.0001 | <0.0001 | <0.0001 | |
| Boiling time | <0.0001 | <0.0001 | <0.0001 | |
| Treatment × Boiling time | 0.293 | <0.0001 | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panaite, T.D.; Cornescu, G.M.; Predescu, N.C.; Cismileanu, A.; Turcu, R.P.; Saracila, M.; Soica, C. Microalgae (Chlorella vulgaris and Spirulina platensis) as a Protein Alternative and Their Effects on Productive Performances, Blood Parameters, Protein Digestibility, and Nutritional Value of Laying Hens’ Egg. Appl. Sci. 2023, 13, 10451. https://doi.org/10.3390/app131810451
Panaite TD, Cornescu GM, Predescu NC, Cismileanu A, Turcu RP, Saracila M, Soica C. Microalgae (Chlorella vulgaris and Spirulina platensis) as a Protein Alternative and Their Effects on Productive Performances, Blood Parameters, Protein Digestibility, and Nutritional Value of Laying Hens’ Egg. Applied Sciences. 2023; 13(18):10451. https://doi.org/10.3390/app131810451
Chicago/Turabian StylePanaite, Tatiana Dumitra, Gabriela Maria Cornescu, Nicoleta Corina Predescu, Ana Cismileanu, Raluca Paula Turcu, Mihaela Saracila, and Cristina Soica. 2023. "Microalgae (Chlorella vulgaris and Spirulina platensis) as a Protein Alternative and Their Effects on Productive Performances, Blood Parameters, Protein Digestibility, and Nutritional Value of Laying Hens’ Egg" Applied Sciences 13, no. 18: 10451. https://doi.org/10.3390/app131810451
APA StylePanaite, T. D., Cornescu, G. M., Predescu, N. C., Cismileanu, A., Turcu, R. P., Saracila, M., & Soica, C. (2023). Microalgae (Chlorella vulgaris and Spirulina platensis) as a Protein Alternative and Their Effects on Productive Performances, Blood Parameters, Protein Digestibility, and Nutritional Value of Laying Hens’ Egg. Applied Sciences, 13(18), 10451. https://doi.org/10.3390/app131810451










