Abstract
In recent years, there has been significant attention toward the incorporation of alternative functional feed ingredients in monogastric diets. The objective is to improve sustainability and optimize animal performance both under normal conditions and in heat stress situations. Among these alternatives, Jerusalem artichoke (Helianthus tuberosus L.) has emerged as a promising candidate due to its nutritional composition and potential health benefits. This review aims to investigate the potential utilization of Jerusalem artichoke in monogastric diets and the impact on productive performance parameters. Moreover, the potential prebiotic effects of Jerusalem artichoke on the composition and activity of monogastric gut microbiota are revealed, showing its implications for gut health and reduction in pathogenic bacteria. The incorporation of Jerusalem artichoke in monogastric diets poses several challenges, such as limitation of the dietary inclusion rate. However, there are also future perspectives to consider, such as optimizing processing techniques, evaluating the effects of different cultivars, and exploring potential synergies with other dietary feed ingredients. In summary, this study provides a comprehensive overview of the key findings and unique perspectives on the utilization of Jerusalem artichoke in monogastric diets, highlighting its potential as a valuable feed ingredient.
1. Introduction
Jerusalem artichoke (Helianthus tuberosus L.), also known as sunroot, sunchoke, sunflower species, is a perennial plant (Asteraceae family, genus Helianthus) originating from North America, and it is used mostly for its edible tubers in human and animal nutrition. In a French translation dating from 1919, Lacaita [1] discovered extensive discussions about Jerusalem artichoke, referred to, in those times, as “Truffe du Canada”. Prior research, conducted on various farm animals, regarded Jerusalem artichoke (J.A.) as a potential feed ingredient substitute (both fresh/silage stems and leaves can be incorporated into the diet of dairy cows and pigs, including fresh/powder tubers for pigs or poultry diets) because of its distinct composition and the potential health advantages it offers, including for pigs [2,3,4,5,6,7], laying hens [8,9,10], broilers [11,12,13], Japanese quail [14], sheep [15,16,17], fish [18,19,20,21,22], rabbits [23,24,25], cattle [26], horses [27,28,29], goats [30,31,32], bees, and wasps [33]. Lindberg [34] suggests that innovative and sustainable feed sources can be derived from traditional food sources, agro-industrial byproducts, aquaculture, biotechnology, and novel technologies to supply the necessary nutrients for animal food production. In the poultry and swine farming sector, it is crucial to consider ensuring the essential amino acid availability of the novel proposed feed ingredients to be tested and introduced into diet formulations while simultaneously maintaining productive performances. This will enable the adoption of low- or reduced-protein diets, while guaranteeing that there are no negative impacts on the overall productivity [35]. The proximal analyses of J.A. showed that the plant presents almost all indispensable amino acids, including threonine and tryptophan; however, it is not recommended to be used as complete feed, but as a supplementary one [36], as a strategic approach to complement the diet, substituting more costly energy sources. Sawicka [37] considered J.A. an ideal raw material that is affordable, with lots of natural bioactive ingredients (inulin, vitamins B1, B2, B7, C, organic acids, polyphenols, flavonoids, anthocyanins, phenolic compounds, coumarins, terpenes, dietary fiber, enzymes, polysaccharides, minerals, and essential amino acids), and a highly versatile plant with minimal waste (e.g., tubers for ethanol production, human food and animal feed; leaves as soil fertilizer (nitrogen source); stalk mass as protein for animal feed). Shazoo [38] identified several primary utilizations of J.A.; among them, the most important ones include inulin, fructose syrup, yeast, and powder/capsules/tablets production for human pharmaceutical purposes. The potential applications of inulin, derived from both the tubers and aerial components of Helianthus tuberosus L., encompass various effects such as antidiabetic [39,40,41], antioxidant [42,43], anti-inflammatory [44,45], weight loss [46], anti-constipation [47], and increasing immunity benefits [48]. Manokhina [49] highlighted the importance of J.A. as a valuable bioenergetic crop due to its inulin, fructose, and protein content, global cultivation, and adaptability, emphasizing its utilization as a valuable feeding solution for farmers. Jerusalem artichoke is utilized for both its above-ground biomass and tubers, exhibiting high tolerance to water stress and remarkable adaptability to extreme temperatures (35–45 °C for the plants; −30 to −45 °C for the tubers). It has low soil, minimal fertilizer, water, and pesticide requirements, and can be cultivated on marginal lands [50]. Negosanu et al. [51], from the Vegetable Research Development Station (V.R.D.S.) in Buzău, Romania, conducted extensive research on Jerusalem artichoke, which is locally known as “nap-porcesc”, “morcov-porcesc”, “gulii”, “pere iernatice” and “cartoful săracilor” since 1996. They registered three varieties, namely Rareș, Dacic, and Flavius, in the Official Catalogue of Species and Varieties of Cultivated Crops [52]. Moreover, in 2018–2020, a study conducted by Dima et al. [53] at the Research and Development Station for Plant Culture on Sandy Soils (S.C.D.C.P.N.) Dăbuleni found that four Jerusalem artichoke genotypes (Dacic, Olimp, Rustic, and a local population) had an average inulin content of 12.93%, emphasizing its functional potential as a valuable fiber source. Agapie et al. [52] highlighted that J.A. exhibits a remarkable ability to adapt to challenging environmental conditions, able to resistant water scarcity and extreme temperature fluctuations, and showing resilience against diseases and pests. Consequently, it is suggested as a practical option for addressing climate change-related issues in Romania.
This review aims to disclose the potential advantages of incorporating Jerusalem artichoke into the diet of monogastric animals, highlighting potential benefits in terms of productivity and gastrointestinal health.
2. Literature Review Methodology
An extensive search across Medline, PubMed Central (PMC), Web of Science, Scopus, and Google Scholar was conducted without time or language constraints. For our database bibliography exploration, the keywords included were as follows: Jerusalem artichoke, inulin, co-products, waste, biofuel, bioethanol, diabetes, performances, monogastric, gut microbiota, immune function, pathogenic bacteria, lipid metabolism, and nutrient absorption.
3. Aspects Regarding the Nutritional Dynamics and Harvest Variability of Jerusalem Artichoke Tubers and Aerial Parts (Steams, Branches, and Leaves)
In order to unlock the full nutritional value of novel and underutilized feed sources, and optimize their ideal formulation for diets, it is crucial to comprehensively assess and compare their chemical and physical properties, and nutritional composition to other varieties or raw materials commonly used, especially in monogastric rations.
Kays and Nottingham [54] affirmed that the proximate analyses of J.A. tubers show a significant variation in data composition compared to other vegetables due to differences in cultivars, harvest period, production conditions, postharvest treatment, and preparation methods.
According to Ma [36], J.A. tubers are traditionally harvested in late autumn, especially during the period when carbohydrates are transferred from the plant’s aerial parts to underground tubers. Farzinmehr et al. [55] found that harvesting J.A. forage every 60 days optimally increased annual yields of dry matter, protein, and energy biomass in both tubers and forage, influenced by the maturity stage and harvesting frequency. According to Marien [56], the proximate analysis values for the average metabolizable energy (ME) content of J.A. tubers was 15.0 MJ/kg, with a CP content of 69 g/kg in dry matter. The crude protein content of J.A. leaves is about four times higher compared to tubers [57] and three times higher compared to stems [58], and is especially rich in lysine and methionine [59]. It is well known that ensuring an optimum dietary level of methionine and lysine is crucial for promoting growth, maximizing feed efficiency, and maintaining overall health in monogastric animals. These amino acids are often supplemented in animal feeds to meet their specific requirements and optimize their performance.
Table 1 presents the nutritional composition of Jerusalem artichoke’s fresh aerial parts and tubers.

Table 1.
Proximal composition of Jerusalem artichoke’s aerial parts and tubers (per 100 g/fresh weight).
The proximal composition between the two parts is highly significant when taking into consideration their utilization for different purposes or in farm animal feed, such as forage or raw/powder tuber consumption. Authors such as Keys and Nottingham [54] stated that information regarding the proximal composition of J.A. tubers is notably limited in comparison to other vegetables. They found that the CP content exhibits variability, ranging from 1.6 to 2.4 g per 100 g of fresh weight, but being constant during all developing stages [61]. Other authors, such as Okrouhlá et al. [62], reported the following composition for J.A. tubers: 90.00%DM, 8.57% CP, 0.33% EE, 3.51% CF, 7.47% ash, 70.12% N-free extract, 55.76% fructans, and 13.79 MJ/kg of metabolizable energy for pigs. In their study on the chemical composition of J.A. tubers concerning the effects of variety and harvest date, Florkiewicz et al. [63] recorded the following information regarding composition: 7.1–7.2 g protein/100 g dry matter, 21.5–25.6 g dry matter/100 g, 16.3–16.4 g dietary fiber/100 g dry matter, 7.1–8.1 mg vitamin C/100 g, 218–225.9 mg total polyphenols/100 g, 45.8–47.4 g fructans/100 g dry matter, 0.47–0.65 g glucose/100 g dry matter, and 13.4–14.5 g sucrose/100 g dry matter. The research unveiled significant variations in the content of the analyzed components, which were dependent on both the variety and harvest time of the tubers. Tubers harvested in spring exhibited higher amounts of glucose, fructose, sucrose, dietary fiber, phenolic compounds, and nitrate (V). Conversely, tubers harvested in autumn showed higher levels of fructans, vitamin C, and nitrate (III). The fiber content of J.A. tubers ranges from 11.4 to 20.8 g per 100 g of dry matter. This fiber mainly consists of cellulose, hemicellulose, pectin, and lignins, which are linked to cellulose but are composed of phenylpropan alcohol instead of monosaccharides [64]. Farzinmehr et al. [55] reported that the best harvesting interval for J.A. forage to achieve tubers with the highest yearly yield, water-soluble carbohydrates, and digestibility was every 120 days, while the highest nutritive value and yield of the forages were observed when harvesting J.A. every 60 days, with the DM biomass of 27.16 t/ha, CP of 98.6–145 g/kg DM, and OM digestibility between 0.607 and 0.691. The average value of the ME (MJ/kg DM) of J.A. tubers ranged from 12.1 to 12.6, depending on whether the tubers were harvested from plots with four, three, or two forage cuts per year. According to Kays and Nottingham [54], J.A. aerial parts exhibited higher total digestible nutrient levels while having lower protein concentrations compared to alfalfa (Medicago sativa L.). Thus far, the studies presented provide evidence that the properties of Jerusalem artichoke depend on various influencing factors, making these factors important to take into consideration (e.g., climate, cultivar [53], harvesting [55], technology, and growing conditions [65]) when assessing its nutritional value and potential applications. Furthermore, as demonstrated, the tubers and leaves of Jerusalem artichoke exhibit diverse nutrient compositions, and their utilization in farm animal diets can be optimized by taking into account both their nutritional content and growth development stage. Figure 1 presents the amino acid content of both the aerial parts and tubers of J.A. The literature regarding the amino acid content in J.A. is limited.
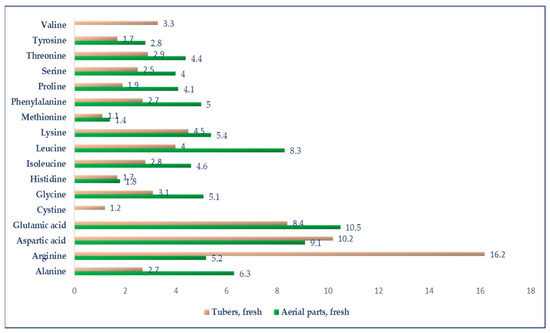
Figure 1.
The amino acid composition of fresh tubers and aerial parts of Jerusalem artichoke (% protein). Heuzé et al. [60] and Feedipedia, a program by INRAE, CIRAD, AFZ, and FAO, https://www.feedipedia.org/node/544 (accessed on 21 July 2023).
Cieslik et al. [64] analyzed red variety J.A. tubers, and observed variations in amino acid composition, influenced by cultivar and storage time. The significant presence of essential amino acids, such as methionine (72.6 mg/100 g), lysine (286.6 mg/100 g), leucine (255.2 mg/100 g), isoleucine (180.8 mg/100 g), threonine (182.6 mg/100 g), phenylalanine (173.9 mg/100 g), and valine (209.7 mg/100 g), suggests that these tubers could be a valuable source of high-quality protein. However, the absence of detectable tryptophan indicates a potential limitation regarding in this specific amino acid. These findings underscore the importance of considering the complete amino acid profile for a comprehensive understanding of the nutritional value of these tubers. Additionally, the tubers demonstrate a favorable EAA:TAA ratio of 47.5% and an EAA:NEAA ratio of 90.7%. This aligns with the recommended ideal amino acid pattern (EAA:TAA ratio of 40%; and EAA:NEAA ratio > 60%) by the Food and Agriculture Organization (FAO) and the World Health Organization (WHO) [66], indicating that J.A. tubers can be considered a valuable source of high-quality protein. Danilcenko et al. [67] stated that 55% of the total amino acids in J.A. tubers are represented by essential amino acids. Regardless of the cultivar and storage period, arginine was the predominant essential amino acid, while asparagine, glutamine, and alanine were dominant nonessential amino acids. Tyrosine and methionine made up the lowest content in tubers. When harvested, glutamine (9.18 mg kg−1 dry weight), glycine (2.31 mg kg−1 dry weight), and leucine (2.77 mg kg−1 dry weight) levels were the highest. Regarding the mineral content of J.A., some authors [68] registered that J.A. tubers had a high content of potassium (23.35 g/kg DM), phosphorus (3.89 g/kg DM), magnesium (1.89 g/kg DM), and calcium (1.86 g/kg DM), as well as a representative content of iron (39.22 mg/kg DM), zinc (13.56 g/kg DM), and copper (7.18 mg/kg DM).
4. Jerusalem Artichoke as an Important Source of Inulin for Monogastric Farm Animals
De Leenheer and Hoebregs [69] stated that the earliest-documented records of J.A. as a source of inulin dates back to approximately 1870. However, it was not until the 1950s that the complete structure of inulin was fully clarified. Van Loo et al. [70] mentioned that the inulin content of tubers varies from 7 to 30% by fresh weight and approximative 50% by dry weight. Its versatile compounds offer a range of positive effects and applications in both human and animal nutrition [37]. According to Long et al. [71], inulin constituted 80% of the carbohydrates found in tubers. Additionally, as stated by Somda et al. [72], inulin serves as the primary storage carbohydrate in J.A., with the majority of carbon in tubers being present in the form of inulin [73].
Jerusalem artichoke represents a significant source of inulin and oligofructose, providing high concentrations, at 16.0–22.0 g/100 g and 12.0–15.0 g/100 g, respectively. This makes it prominent among various food sources, surpassing many, including bananas (0.3–0.7 g/100 g), asparagus (2.0–3.0 g/100 g), garlic (9.0–16.0 g/100 g), and leeks (3.0–10.0 g/100 g). Moreover, J.A. competes favorably or exceeds others in this aspect, such as chicory root (35.7–47.6 g/100 g) and dandelion greens (12.0–15.0 g/100 g), showcasing it as a notable prebiotic fiber contributor [73]. These inulin-type fructans, functioning as both functional fructans and soluble dietary fibers, comprise a combination of inulin, oligofructose, and fructooligosaccharide with β-configuration, classified based on their chain length or degree of polymerization as follows: small (3–5), medium (6–10), and long chains (11–60). The length or degree of polymerization is influenced by various factors such as genotype, environmental conditions, harvest time, and storage processes [74]. Bhanja et al. [75] stated that inulin serves as a functional bioingredient in both food and feed applications, acting as a prebiotic agent that can promote the growth of beneficial gut microflora, contributing to the overall health benefits. The inulin content in the tubers was estimated based on the analysis of the sugar content, which was approximately 803.4 g/kg in dry matter, indicating a corresponding inulin content of 650 g/kg in dry matter [57]. The proven benefits linked to inulin were confirmed through a range of conducted experiments in monogastric and ruminant animals as well.
Figure 2 presents the advantages of incorporating J.A. in farm diets with positive results in alterations of intestinal microflora composition, which subsequently influences their metabolism and physiology. Extracts derived from J.A. tubers have the potential to enhance feed efficiency, improve digestion, and lower the occurrence of diarrhea in monogastric animals.
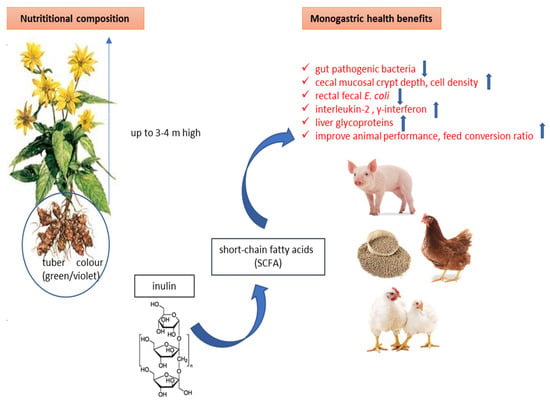
Figure 2.
The effects of incorporating J.A. into the diet of monogastric animals, and its benefits linked to inulin.
Flickinger [76] suggests that extracts derived from J.A. tubers hold potential as supplements for animal feed, primarily due to their potential to promote bifidogenic effects. Although inulin and fructooligosaccharides, typically sourced from chicory, are frequently incorporated into the diets of farm animals, their precise health advantages in animals are not as comprehensively studied as they are in humans. It is crucial to acknowledge that different animal species possess distinct digestive tract structures, meaning that a single fructooligosaccharide supplement may not consistently produce optimal results across all species. Depending on the species, such supplements might have suboptimal effects or even lead to digestive issues like loose stools or excessive flatulence. According to Vhile et al. [77], fructooligosacharides are resistant to enzyme hydrolysis in the small intestine, but they stimulate the growth of bifidobacteria in the hindgut, which facilitates inulin fermentation.
5. Jerusalem Artichoke Supplementation Effects in Monogastric Animals on Productive Performances
5.1. Effects of Jerusalem Artichoke Inclusion in Pig Diets
According to Kosaric [61], J.A. tubers are acknowledged to possess a high nutritional value for domestic animals. Historically, it was common practice to let pigs forage in fields where the aerial parts of J.A. plants had been cut, and the pigs would dig up and consume the tubers directly on the field [78]. Jerusalem artichoke tubers were considered a viable alternative feed for pigs during periods of potato scarcity [79]. In a study conducted by Kongsted et al. [7], the impact of free-range foraging on J.A. and the effects of feeding concentrates either restrictively (at 30% of energy recommendations) or ad libitum on pigs was investigated. The results revealed that pigs fed restrictively exhibited a significantly lower daily gain (560 vs. 1224 g per pig) and an improved feed conversion ratio (17.6 vs. 42.8 MJ ME concentrate per kg live weight gain). In their study, Farworth et al. [3] studied the impact of supplementing Jerusalem artichoke tuber flour (1.5%), which is rich in fructooligosaccharides, to 28-day-old weanling pigs and registered a decrease in daily feed intake, daily weight gain, and feed conversion ratio as a result of the supplementation. Again, Farnworth [5] studied raw tuber supplementation in 28-day-old weaned pigs and noticed significantly increased body weight gain, but a reduction in feed efficiency. In a second trial, the pigs were fed a diet containing dried J.A. (10 to 60 g/kg) and a significant increase in weight gain was observed, and although feed efficiency showed improvement, the increase was not statistically significant. Okrouhlá et al. [62] observed that the daily weight gain of the group fed with 12.2% J.A. showed significantly lower results (p = 0.042).
The digestion of intact J.A. tubers in pigs is clearly dependent on microbial activity in the digestive tract. Jerusalem artichoke tubers are very rich in fructan, and fructose liberated from fructans is 50% partially digested up to the ileum. A very low ileal digestibility of a diet containing fresh tubers of Jerusalem artichokes was obtained. By analyzing short fatty acids, it was observed that some of the resulting carbohydrates were subjected to microbial transformation in the fore-gut. Table 2 presents values of intestinal digestibility in pigs fed Jerusalem artichoke tubers in the study of Ly et al. [80].

Table 2.
The ileal and fecal digestibility of J.A. tubers in pigs.
A summary of the previous studies presented concludes that incorporating J.A. into pig diets presents opportunities as well as challenges. It served as a valuable alternative when potatoes were scarce; however, its impact on pig nutrition varies across studies. While some indicate enhanced feed efficiency and reduced undesirable compounds like skatole, others report fluctuations in weight gain and feed efficiency. Achieving the best results requires meticulous attention to diet composition and supplementation levels when using Jerusalem artichoke as a feed resource for pigs.
5.2. Effects of Jerusalem Artichoke in Reducing Skatole Levels
Feeding J.A. to male pigs resulted in a dose-dependent decrease in skatole levels in the hindgut and adipose tissue. This reduction in skatole levels may be attributed to the decrease in Clostridium perfringens and the increase in short-chain fatty acids (SCFAs), with a subsequent reduction in pH [77]. Adding 4% of dried Jerusalem artichoke tubers for 40 days to diet of piglets (28 days of age) decreased skatole levels in the colon and feces, as well as a decreased the dry matter content and pH in the colon [81]. Additionally, there was a reduction in enterobacteria levels in the colon with increasing levels of J.A. [79].
Okrouhlá et al. [62] supplemented different levels of J.A. (4.1%, 8.1%, or 12.2%) in pig diets for 13 days before slaughter to reduce skatole and indole levels responsible for boar taint, which can affect the taste and odor of pork. The group fed with 12.2% J.A. showed a decrease in skatole concentration in adipose tissue (p = 0.003) and reduced E. coli levels in feces (p ≤ 0.001). A significant correlation was found between J.A. concentration, E. coli levels, and skatole levels in adipose tissue.
The inclusion of J.A. in pig diets presents opportunities for reducing skatole levels and modifying the intestinal microbiota, but careful attention to diet composition and supplementation levels is necessary for achieving optimal results.
5.3. Effects of Jerusalem Artichoke Inclusion in Poultry’s Diets
Poultry studies also explored the effects of incorporating dietary Jerusalem artichoke on the productivity of poultry, as well as the quality characteristics of eggs and meat. Initially considered an alternative to synthetic antibiotics, Jerusalem artichoke was recognized for its natural, less toxic, and residue-free effects, making it an ideal feed additive for its utilization in poultry production [82]. Jawad and Al-Abboodi [83] indicated that J. A. supplementation in poultry diets can facilitate improved diet metabolism, which has a positive impact on the feed conversion ratio and other production parameters. In a 16-week feeding trial conducted by Yildiz et al. [84], the impact of dried J.A. (5%) in laying hen diets was examined, along with vetch (5%), and various combinations of J.A. (5% and 10%) and vetch (5% and 10%) to mitigate the diarrheal effects of inulin. The experimental group with a combination of 10% J.A. and 10% vetch showed a lower live weight by 4.36–10.09% compared to the other groups, but it exhibited improved feed efficiency. Notably, there were no adverse effects observed on egg quality of the hens across all groups. Sritiawthai et al. [10] studied the effects of supplementing dried J.A. (50 ppm and 100 ppm) as a dietary prebiotic in laying hen feed. The researchers examined the impact of J.A. on productive performance parameters, egg quality characteristics, and intestinal microflora. The results showed that there were no significant effects on feed intake, final body weight, egg production, egg weight, and egg mass with the inclusion of dried J.A. However, at the 100 ppm supplementation concentration, there was a significant increase in the albumen ratio, yolk–albumen ratio, and the albumen height of eggs. Additionally, the population of lactic acid bacteria in the caecum also significantly increased at this supplementation concentration.
Following the ban of antibiotics in broilers, researchers explored alternative methods for controlling gastrointestinal microorganisms. Kleessen et al. [11] conducted a study where they tested the use of 0.5% topinambur syrup in drinking water. The results showed a significant increase in the cecal counts of B. bacteriovorus, as well as significantly lower numbers of total aerobes, including Enterobacteriaceae and C. perfringens. Moreover, the study observed reduced levels of endotoxins in the blood and an increased body weight on day 35 of the trial period. Katiyanon et al. [85] researched the impact of supplementing J.A. (at 1%, 2%, 3%, or 4% concentrations) in broiler diet on production performances. The results revealed that supplementation at 1% and 4% concentrations led to an increased body weight gain and improved feed efficiency, along with a decrease in the mortality rate. Additionally, the serum cholesterol levels decreased significantly for all concentrations of J.A. supplementation. Notably, feeding J.A. at 3% in the broiler diet showed beneficial effects in terms of body weight gain, feed efficiency, and mortality. Al-Abboodi and Jawad [12] conducted a study to assess the efficiency of J.A. powder addition at different concentrations (0.5%, 1%, 1.5%, and 2%) in broiler diets (1 to 6 weeks of age). The supplementation of 2%, 0.5%, and 1.5% did not significantly influence the productive parameters. However, the experimental groups showed an overall improvement ranging from 2.5% to 4.2% in the final body weight, 5.1% to 18.5% in the production index, 0.1% to 0.2% in the growth rate, and 4.7% to 8.9% in the European production efficiency factor. The best results were obtained with 2% supplementation, which exhibited a higher improvement percentage for all production parameters of broiler chickens.
Therefore, including J.A. as a natural feed additive improved feed efficiency and overall production parameters. J.A. also has the potential to enhance egg quality and gut health. In broilers, it is connected to higher weight gain, better feed efficiency, and lower mortality rates, especially at certain supplementation concentrations.
6. Utilizing Jerusalem Artichoke as a Prebiotic to Enhance Gut Health in Monogastric Farm Animals
In modern intensive production systems, various feed solutions, such as prebiotics, have been employed to prevent intestinal disorders, enhance the gut ecosystem, and subsequently improve the health status and production performances of monogastric animals by modulating the intestinal microbiota [86]. The intestinal microbiota of animals is highly correlated with their physiological, developmental, nutritional, and immunological processes, directly impacting their health and productive performance [87].
Figure 3 presents various internal and external factors that influence animal microbiota, including the host genotype, diet, breeding environment, and exposure to antibiotics. According to Valdovska et al. [88], many environmental stressors in farm animals, including management practices and dietary changes, can disturb the intestinal ecosystem, increasing the risk of pathogen infections, and decreasing feed intake with negative impact on growth performances. As a result, the immune function and balance of intestinal microbiota may be negatively affected, causing gut disorders, infections, and diarrhea [89]. The weaning period for piglets highlights the vital role of gut microbiota as it involves separating them from sows and transitioning from milk to solid food [90]. Weaning is associated with reduced feed intake, growth, intestinal changes, diarrhea, and increased mortality. Piglets are particularly susceptible to E. coli overgrowth, including post-weaning colibacillosis [91]. According to Clavijo and Flórez [92], the dominant bacterial phyla found in chickens consist of Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes, and Cyanobacteria. The principal bacterial groups found in pigs’ gastrointestinal tract mostly consist of Streptococcus, Lactobacillus, Eubacterium, Fusobacterium, Bacteroides, Bifidobacterium, Clostridium, Escherichia, Prevotella, Proteobacteria, and Ruminococcus [93]. The Firmicutes and Bacteroidetes bacterial phyla represent the intestinal microbiota in all monogastric animals [94]. According to Bamigbade et al. [95], prebiotics are considered a group of biological nutrients that promote the growth and activity of Lactobacilli and Bifidobacteria in the gastrointestinal tract of monogastric animals.
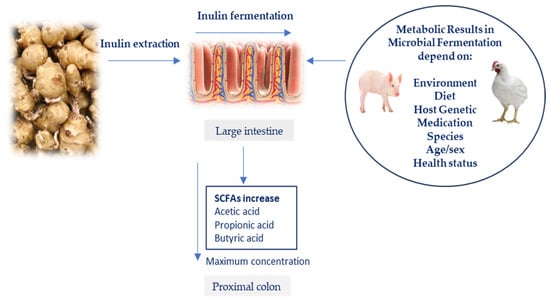
Figure 3.
Several key factors that influence the gastrointestinal microbiota of monogastric animals.
When these prebiotics are broken down by microflora in the gut, they produce short-chain fatty acids (SCFAs) that are released into the colon and subsequently absorbed into the bloodstream. The two primary groups of prebiotics extensively studied in this regard are fructo-oligosaccharides (FOSs) and galactooligosaccharides (GOSs). In their study, Barszcz et al. [81] incorporated either 2% inulin from chicory root or 4% dried J.A. tubers into pig diets. The researchers observed an increase in cecal valeric acid levels and a decreasing in isoacids concentration in the colon. Additionally, they found a reduction in β-glucosidase and β-glucuronidase activity in the middle colon and an increase in Bifidobacterium spp. populations in both the proximal and distal colon. Valdovska et al. [88] found that although J.A. can impact the concentration of short-chain fatty acids in pig manure and alter the microbiota composition in the large intestine, its prebiotic potential remains incompletely understood.
Using J.A. and probiotics (Lactobacillus reuteri and Pediococcus pentosaceus) as alternatives to ameliorate gut microbiota in weaned piglets (7 weeks old), the same authors [88] obtained a significant improvement of the microbial contents, defense mechanism, and regeneration processes in pigs’ intestines. In the study conducted by Farnworth et al. [3], the addition of J.A. tuber flour to the piglet diet did not result in any significant effect on the concentration of volatile fatty acids (VFAs). Despite observing an increase in propionic acid, isobutyric acid, butyric acid, iso-valeric acid, and valeric acid, along with a decrease in digesta acetic acid content, these experimental results were not statistically significant. Volatile fatty acids are the primary end products of carbohydrate digestion in ruminants, but they are also present in significant concentrations in pigs’ large intestines. The cecum and colon in pigs transport volatile fatty acids at rates similar to, or even greater than, horses’ large intestinal mucosa or cattle’s rumen epithelium [96].
Kleessen et al. [11] conducted a study to examine the effects of 0.5% topinambur syrup added to drinking water. The findings showed a decrease in bacterial endotoxin and Enterobacteriaceae levels. In their experiment, Yildiz et al. [84] incorporated 0%, 5%, and 10% J.A. in laying hen diets. However, the dietary treatments did not lead to any changes in fecal and intestinal pH values.
Despite having a short large intestine, poultry respond well to prebiotic administration. Fructooligosaccharides, with their high digestibility, have been observed to enhance Lactobacillus production. The type and solubility of fiber influence morphological and physiological changes in the digestive tract [97,98]. While initially seen as an antibiotic alternative, the utilization of prebiotics in the poultry industry remains limited.
Sevane et al. [99], utilized chicory (C. intybus L.) roots as an inulin source in the broiler diet, incorporating it at a concentration of 746 g kg−1. The results revealed that inulin-type fructans inclusion (5 g/kg diet) positively influenced the immune status, particularly by enhancing the production of long-chain fatty acids.
Other authors, such as Rebolé et al. [100], noticed that the addition of inulin-type fructans from dietary chicory roots (0, 10, or 20 g/kg) decreased serum cholesterol concentration and abdominal fat deposition in boilers. Both types of inulin showed beneficial prebiotic properties when experimented with on monogastric farm animals.
The wide variability in the results noted in studies involving the supplementation of monogastric animals with J.A. can be attributed to the differences in experimental variables such as the dietary level included, the period length of J.A. dietary supplementation, along with the specific animal species, age, and health status that were investigated.
7. Perspectives and Considerations regarding Jerusalem Artichoke Utilization
Despite the numerous favorable opinions in the literature regarding the utilization of J.A. in both human and animal nutrition, the cultivation of this crop currently holds a more important aspect from the standpoint of biofuel production. While this focus on biofuel is noteworthy, it is essential not to overlook the extensive benefits associated with J.A. incorporation proven by experimental studies. Its nutritional profile, including the presence of inulin, suggests potential improvements in gut health and overall animal well-being. Additionally, the crop’s resistance to drought makes it particularly suitable for cultivation in Romania, offering a sustainable and resilient alternative for feed production. It is imperative to reconsider and emphasize the multifaceted advantages of J.A. for animal nutrition. Exploring and highlighting these benefits can contribute to a more comprehensive understanding of the crop’s potential and encourage its broader adoption in various agricultural applications.
8. Conclusions
In summary, considerable evidence affirms the prebiotic advantages of Jerusalem artichoke for monogastric animals, demonstrating favorable effects on gastrointestinal microbiota and varied outcomes on production parameters. However, it is noteworthy that the majority of studies are either dated or limited in scope, particularly with regard to each specific monogastric farm animal. This underscores the necessity of additional research, emphasizing the need for a nuanced approach in integrating Jerusalem artichoke into animal diets. Addressing the raised concerns and undertaking further studies will significantly contribute to a more thorough comprehension of the role of Jerusalem artichoke in enhancing gut health and overall animal performance.
Author Contributions
Conceptualization, G.M.C. and T.D.P.; methodology, G.M.C.; validation, G.M.C., T.D.P. and C.S.; investigation, A.C. and C.C.M.; resources, G.M.C. and A.C.; data curation T.D.P. and C.S.; writing—original draft preparation, G.M.C.; writing—review and editing, G.M.C., T.D.P. and C.S.; visualization, C.S.; supervision, T.D.P.; project administration, T.D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Agriculture and Rural Development—ADER 8.1.6; Ministry of Research, Innovation and Digitization—Project PN 23 20 0101; National Research Development Project Projects to Finance Excellence: (PFE)-8/2021.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lacaita, C.C. The “Jerusalem Artichoke”(Helianthus tuberosus). Bull. Misc. Inform. 1919, 321–339. [Google Scholar] [CrossRef]
- Ruszczyc, Z.; Glaps, J. Silage of Jerusalem artichoke tubers for fattening pigs. Rocz. Nauk. Rol. Ser. B Zootech. 1958, 72, 565–571. [Google Scholar]
- Farnworth, E.R.; Modler, H.W.; Jones, J.D.; Cave, N.; Yamazaki, H.; Rao, A.V. Feeding Jerusalem artichoke flour rich in fructooligosaccharides to weanling pigs. Can. J. Anim. Sci. 1992, 72, 977–980. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Jones, J.D.; Modler, H.W.; Cave, N. The use of Jerusalem artichoke flour in pig and chicken diets. In Studies in Plant Science; Elsevier: Amsterdam, The Netherlands, 1993; Volume 3, pp. 385–389. [Google Scholar]
- Farnworth, E.R.; Modler, H.W.; Mackie, D.A. Adding Jerusalem artichoke (Helianthus tuberosus L.) to weanling pig diets and the effect on manure composition and characteristics. Anim. Feed Sci. Technol. 1995, 55, 153–160. [Google Scholar] [CrossRef]
- Ly, J. Nitrogen and energy balance in pigs fed Jerusalem artichokes (Helianthus tuberosus L.). RCPP 2000, 7, 32–37. [Google Scholar]
- Kongsted, A.G.; Horsted, K.; Hermansen, J.E. Free-range pigs foraging on Jerusalem artichokes (Helianthus tuberosus L.) Effect of feeding strategy on growth, feed conversion and animal behaviour. Acta Agric. Scand. A Anim. Sci. 2013, 63, 76–83. [Google Scholar]
- Yıldız, G.; Sacakli, P.; Gungor, T.; Uysal, H. The effect of jerusalem artichoke (Helianthus tuberasus L.) on blood parameters, liver enzymes and ıntestinal ph in laying hen. J. Anim. Vet. Adv. 2008, 7, 1297–1300. [Google Scholar]
- Park, S.O.; Park, B.S. Effect of feeding inulin oligosaccharides on cecum bacteria, egg quality and egg production in laying hens. Afr. J. Biotechnol. 2012, 11, 9516–9521. [Google Scholar]
- Sritiawthai, E.; Kaewtapee, C.; Bunchasak, C.; Poeikhampha, T. The effect of Jerusalem artichoke (Helianthus tuberosus L.) supplimentation on production performance, egg quality characteristics and intestinal microflora of laying hens. J. Appl. Sci. 2013, 13, 183–187. [Google Scholar] [CrossRef][Green Version]
- Kleessen, B.; Elsayed, N.A.A.E.; Loehren, U.; Schroedl, W.; Krueger, M. Jerusalem artichokes stimulate growth of broiler chickens and protect them against endotoxins and potential cecal pathogens. J. Food Prot. 2003, 66, 2171–2175. [Google Scholar] [CrossRef]
- Al-abboodi, A.A.; Jawad, H.S. Effect of supplementing different levels of Jerusalem artichoke (Helianthus tuberosus L.) on broiler production performance. Plant Arch. 2018, 18, 1570–1574. [Google Scholar]
- Saffah, S.F.; Jawad, H.S.; Maaeni, Y.M. Effect of supplementing two levels of jerusalem artichoke (Helianthus tuberosus L.) powder in broiler diets on histological parameters of small intestinal segments. Plant Arch. 2020, 20, 3543–3547. [Google Scholar]
- Abdel-Wahab, A.A.; Elnesr, S.S.; Abdel-Kader, I.A. Effect of dietary supplementation of Jerusalem Artichoke extract on performance, blood biochemistry, antioxidant parameters, and immune response of growing Japanese quail. J. Anim. Physiol. Anim. Nutr. 2023, 107, 920–927. [Google Scholar] [CrossRef] [PubMed]
- Razmkhah, M.; Rezaei, J.; Fazaeli, H. Use of Jerusalem artichoke tops silage to replace corn silage in sheep diet. Anim. Feed Sci. Technol. 2017, 228, 168–177. [Google Scholar] [CrossRef]
- Abd El-Mola, A.M.; Aboul-Fotouh, G.E. Effect of feeding Helianthus tuberosus (Jerusalem artichoke) on rations nutritive value and some blood parameters of Ossimi rams. Egypt. J. Nutr. Feed. 2018, 21, 365–371. [Google Scholar]
- Papi, N.; Kafilzadeh, F.; Fazaeli, H. Use of Jerusalem artichoke aerial parts as forage in fat-tailed sheep diet. Small Rumin. Res. 2019, 174, 1–6. [Google Scholar] [CrossRef]
- Tiengtam, N.; Khempaka, S.; Paengkoum, P.; Boonanuntanasarn, S. Effects of inulin and Jerusalem artichoke (Helianthus tuberosus) as prebiotic ingredients in the diet of juvenile Nile tilapia (Oreochromis niloticus). Anim. Feed Sci. Technol. 2015, 207, 120–129. [Google Scholar] [CrossRef]
- Goran, S.M.A.; Omar, S.S.; Anwer, A.Y. Water quality and physiological parameters of common carp fingerling fed on Jerusalem artichoke tubers. Polytechnic 2016, 6, 502–516. [Google Scholar]
- Boonanuntanasarn, S.; Tiengtam, N.; Pitaksong, T.; Piromyou, P.; Teaumroong, N. Effects of dietary inulin and Jerusalem artichoke (Helianthus tuberosus) on intestinal microbiota community and morphology of Nile tilapia (Oreochromis niloticus) fingerlings. Aquac. Nutr. 2018, 24, 712–722. [Google Scholar] [CrossRef]
- Sewaka, M.; Trullas, C.; Chotiko, A.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Pirarat, N. Efficacy of synbiotic Jerusalem artichoke and Lactobacillus rhamnosus GG-supplemented diets on growth performance, serum biochemical parameters, intestinal morphology, immune parameters and protection against Aeromonas veronii in juvenile red tilapia (Oreochromis spp.). Fish Shellfish Immunol. 2019, 86, 260–268. [Google Scholar]
- Trullàs, C.; Sewaka, M.; Rodkhum, C.; Chansue, N.; Boonanuntanasarn, S.; Kamble, M.T.; Pirarat, N. Effects of Jerusalem artichoke (Helianthus tuberosus) as a prebiotic supplement in the diet of red tilapia (Oreochromis spp.). Animal 2022, 12, 2882. [Google Scholar] [CrossRef] [PubMed]
- Dokoupilova, A.; Zita, L.; Kvaček, J.; Janda, K.; Hofmanova, B.; Masopustova, R. Jerusalem artichoke (Helinathus tuberosus) tops as a natural source of inulin in rabbit diet: Effect on growth performance and health status. J. Central Eur. Agric. 2019, 20, 796–801. [Google Scholar] [CrossRef]
- Kurchaeva, E.E.; Vostroilov, A.V.; Vysotskaya, E.A.; Artemov, E.S.; Maksimov, I.V. Efficiency of application of the probiotic “Enzymsporin” and grass flour of Jerusalem artichoke to increase the productivity of rabbits. IOP Conf. Ser. Earth Environ. Sci. 2020, 422, 012059. [Google Scholar] [CrossRef]
- Havlíček, Z.; Kvaček, J.; Dočkalová, H. Jerusalem artichoke (Helianthus tuberosus) in rabbit nutrition. J. Cent. Eur. Agric. 2023, 24, 95–103. [Google Scholar]
- Ārne, A.; Ilgaža, A. Jerusalem artichoke flour feeding effects on calf development in the first months of life. Res. Rural. Dev. 2014, 1, 169–175. [Google Scholar]
- Glatter, M.; Bochnia, M.; Goetz, F.; Gottschalk, J.; Koeller, G.; Mielenz, N.; Zeyner, A. Glycaemic and insulinaemic responses of adult healthy warm-blooded mares following feeding with Jerusalem artichoke meal. J. Anim. Physiol. Anim. Nutr. 2017, 101, 69–78. [Google Scholar] [CrossRef]
- Ersahince, A.; Kara, K. Nutrient composition and in vitro digestion parameters of Jerusalem artichoke (Helianthus tuberosus L.) herbage at different maturity stages in horse and ruminant. J. Anim. Feed Sci. 2017, 26, 213–225. [Google Scholar] [CrossRef]
- Glatter, M.; Borewicz, K.; van den Bogert, B.; Wensch-Dorendorf, M.; Bochnia, M.; Greef, J.M.; Zeyner, A. Modification of the equine gastrointestinal microbiota by Jerusalem artichoke meal supplementation. PLoS ONE 2019, 14, e0220553. [Google Scholar] [CrossRef]
- Stefler, J. Effect of feeding artichoke leaves on the milk production of lactating goats. Szaktanácsok 1990, 3–4, 22–27. [Google Scholar]
- Osman, A. Studies on the feeding value of green fodder from Jerusalem artichoke (Helianthus tuberosus L.). J. Anim. Poult. Prod. 2003, 28, 5277–5282. [Google Scholar] [CrossRef]
- Kanokwan, K.; Paengkoum, S.; Kongmun, P.; Yu, Z.; Paengkoum, P. Effect of Jerusalem artichoke supplementation on methanogenic achaea in dairy goats using real time PCR technique. In Proceedings of the First Asia Dairy Goat Conference, Kuala Lumpur, Malaysia, 9–12 April 2012; Volume 9, p. 82. [Google Scholar]
- Denisow, B.; Tymoszuk, K.; Dmitruk, M. Nectar and pollen production of Helianthus tuberosus L.—An exotic plant with invasiveness potential. Acta Bot. Croat. 2019, 78, 135–141. [Google Scholar] [CrossRef]
- Lindberg, J.E. Nutrient and energy supply in monogastric food producing animals with reduced environmental and climatic footprint and improved gut health. Animal 2023, 17, 100832. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.T.; Maynard, C.W.; Mullenix, G.J. Progress of amino acid nutrition for diet protein reduction in poultry. J. Anim. Sci. Biotechnol. 2021, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.Y.; Zhang, L.H.; Shao, H.B.; Xu, G.; Zhang, F.; Ni, F.T.; Brestic, M. Jerusalem artichoke (Helianthus tuberosus), a medicinal salt-resistant plan has high adaptability and multiple-use values. J. Med. Plants Res. 2011, 5, 1272–1279. [Google Scholar]
- Sawicka, B.; Skiba, D.; Pszczółkowski, P.; Aslan, I.; Sharifi-Rad, J.; Krochmal-Marczak, B. Jerusalem artichoke (Helianthus tuberosus L.) as a medicinal plant and its natural products. Cell Mol. Biol. 2020, 66, 160–177. [Google Scholar] [CrossRef] [PubMed]
- Shazzo, R.I.; Gish, R.A.; Ekutech, R.I.; Kornena, E.P.; Kajshev, V.G.; Krivorotov, S.B. Jerusalem Artichoke: Biology, Agricultural Cultivation, Place in the Ecosystem, Processing Technologies (Yesterday, Today, Tomorrow); KSAU, RAAS, Krasnodar Scientific Research Institute of Storage and Processing of Agricultural Products of the Russian Academy of Agricultural Sciences, Ministry of Agriculture of the Russian Federation: Krasnodar, Russia, 2013; 181p. (In Russian) [Google Scholar]
- Shao, T.; Yu, Q.; Zhu, T.; Liu, A.; Gao, X.; Long, X.; Liu, Z. Inulin from Jerusalem artichoke tubers alleviates hyperglycaemia in high-fat-diet-induced diabetes mice through the intestinal microflora improvement. Br. J. Nutr. 2020, 123, 308–318. [Google Scholar] [CrossRef]
- Ahn, H.Y.; Kim, M.; Seo, C.R.; Yoo, H.J.; Lee, S.H.; Lee, J.H. The effects of Jerusalem artichoke and fermented soybean powder mixture supplementation on blood glucose and oxidative stress in subjects with prediabetes or newly diagnosed type 2 diabetes. Nutr. Diabetes 2018, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Nakajima, A.; Matsumoto, Y.; Mori, H.; Inoue, K.; Yamanouchi, H.; Anzai, K. Administration of Jerusalem artichoke reduces the postprandial plasma glucose and glucose-dependent insulinotropic polypeptide (GIP) concentrations in humans. Food Nutr. Res. 2022, 4, 66. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Furman-Toczek, D.; Zagórska-Dziok, M. Antioxidant activity and cytotoxicity of Jerusalem artichoke tubers and leaves extract on HaCaT and BJ fibroblast cells. Lipids Health Dis. 2018, 17, 280. [Google Scholar] [CrossRef]
- Pan, L.; Sinden, M.R.; Kennedy, A.H.; Chai, H.; Watson, L.E.; Graham, T.L.; Kinghorn, A.D. Bioactive constituents of Helianthus tuberosus (Jerusalem artichoke). Phytochem. Lett. 2009, 2, 15–18. [Google Scholar] [CrossRef]
- Saiki, P.; Yoshihara, M.; Kawano, Y.; Miyazaki, H.; Miyazaki, K. Anti-Inflammatory Effects of Heliangin from Jerusalem artichoke (Helianthus tuberosus) Leaves Might Prevent Atherosclerosis. Biomolecule 2022, 12, 91. [Google Scholar] [CrossRef]
- Sasaki, H.; Lyu, Y.; Nakayama, Y.; Nakamura, F.; Watanabe, A.; Miyakawa, H.; Shibata, S. Combinatorial effects of soluble, insoluble, and organic extracts from Jerusalem artichokes on gut microbiota in mice. Microorganisms 2020, 8, 954. [Google Scholar] [CrossRef] [PubMed]
- Guess, N.D.; Dornhorst, A.; Oliver, N.; Bell, J.D.; Thomas, E.L.; Frost, G.S. A randomized control trial: The effect of inulin on weight management and ectopic fat in subjects with prediabetics. Nutr. Metab. 2015, 12, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Konikoff, T.; Gophna, U. Oscillospira: A central, enigmatic component of the human gut microbiota. Trends Microbiol. 2016, 24, 523–524. [Google Scholar] [CrossRef]
- Ali, S.S.R.; Ambasankar, K.; Musthafa, M.S.; Harikrishnan, R. Jerusalem artichoke enriched diet on growth performance, immuno-hematological changes and disease resistance against Aeromonas hydrophila in Asian seabass (Lates calcarifer). Fish Shellfish Immunol. 2007, 70, 335–342. [Google Scholar]
- Manokhina, A.A.; Dorokhov, A.S.; Kobozeva, T.P.; Fomina, T.N.; Starovoitov, V.I. Jerusalem artichoke as a strategic crop for solving food problems. Agronomy 2022, 12, 465. [Google Scholar] [CrossRef]
- Ciuciuc, E.; Drăghici, I.; Drăghici, R.; Croitoru, M.; Băjenaru, M.F. The behavior of varieties of Jerusalem artichoke on the sandy soils from south of Oltenia. Ann. Univ. Craiova-Agric. Mont. Cadastre Ser. 2020, 49, 52–57. [Google Scholar]
- Negoşanu, G.; Vînătoru, C.; Barcanu, E.; Agapie, O.L.; Tănase, B.; Gherase, I. Evaluation of the main phenotypic and physico-chemical characteristics in the new genotypes of Jerusalem artichoke (Helianthus tuberosus) obtained at VRDS Buzău. Sci. Pap.-Ser. B Hortic. 2020, 64, 442–447. [Google Scholar]
- Agapie, O.L.; Barcanu, E.; Gherase, I.; Tănase, B.; Dobre, G. Helianthus tuberosus L. as an Alternative to Climate Change in Romania. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Hortic. 2022, 79, 112–116. [Google Scholar]
- Dima, M.; Croitoru, M.; Drăghici, R.; Drăghici, I.; Ciuciuc, E.; Băjenaru, M.F. Researches on the behavior of Jerusalem artichoke varieties grown on sandy soils in terms of nutritional quality of tubers. Sci. Pap. Ser. Manag. Econom. Eng. Agric. Rural. Dev. 2021, 21, 309–316. [Google Scholar]
- Kays, S.J.; Nottingham, S.F. Biology and Chemistry of Jerusalem artichoke: Helianthus tuberosus L.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Farzinmehr, S.; Rezaei, J.; Fazaeli, H. Effect of harvesting frequency and maturity stage of Jerusalem artichoke forage on yield, chemical composition and in vitro fermentation of the tubers and forage. SJAR 2020, 18, e0602. [Google Scholar] [CrossRef]
- Marien, C. Effects of tubers of the Jerusalem artichoke (Helianthus tuberosus) and Potatoes (Solanum tuberosum) on the Intestinal Microbiota of Pigs and Evaluation of a Procedure for Quantification of Microbial Mass in Pig Faeces. Ph.D. Thesis, Kassel University, Kassel, Germany, 2011. [Google Scholar]
- Schweiger, P.; Stolzenburg, K. Mineral Stoffgehalte und Mineralstoffentzüge Verschiedener Topinambursorten; LAP: Forchheim, Germany, 2003. [Google Scholar]
- Malmberg, A.; Theander, O. Differences in chemical composition of leaves and stem in Jerusalem artichoke and changes in low-molecular sugar and fructan content with time of harvest. Wed. J. Agric. Res. 1986, 16, 7–12. [Google Scholar]
- Stauffer, M.D.; Chubey, B.B.; Dorrell, D.G. Growth, yield and compositional characteristics of Jerusalem artichoke as they relate to biomass production [Helianthus tuberosus]. Am. Chem. Soc. Div. Fuel Chem. Prepr. 1981, 25, CONF-800814-P3. [Google Scholar]
- Heuzé, V.; Tran, G.; Chapoutot, P.; Bastianelli, D.; Lebas, F. Jerusalem Artichoke (Helianthus tuberosus). Feedipedia, a Programme by INRAE, CIRAD, AFZ and FAO. 2015. Available online: https://www.feedipedia.org/node/544 (accessed on 21 July 2023).
- Kosaric, N.; Cosentino, G.P.; Wieczorek, A.; Duvnjak, Z. The Jerusalem artichoke as an agricultural crop. Biomass 1984, 5, 1–36. [Google Scholar] [CrossRef]
- Okrouhlá, M.; Čítek, J.; Švejstil, R.; Zadinová, K.; Pokorná, K.; Urbanová, D.; Stupka, R. The effect of dietary Helianthus tuberosus L. on the populations of pig faecal bacteria and the prevalence of skatole. Animals 2020, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Florkiewicz, A.; Cieslik, E.; Filipiak-Florkiewicz, A. Wpływ odmiany i terminu zbioru na skład chemiczny bulw topinamburu (Helianthus tuberosus L.). ŻNTJ 2007, 3, 71–81. [Google Scholar]
- Cieślik, E.; Gębusia, A.; Florkiewicz, A.; Mickowska, B. The content of protein and of amino acids in Jerusalem artichoke tubers (Helianthus tuberosus L.) of red variety Rote Zonenkugel. Acta Sci. Pol. Technol. Aliment. 2011, 10, 433–441. [Google Scholar]
- Sawicka, B.; Kalembasa, D. Fluctuations of selected microelements in Helianthus tuberosus L. tubers due to diverse nitrogen nutrition. Adv. Food Sci. 2011, 33, 166–173. [Google Scholar]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; Technical Report Series; World Health Organization: Geneva, Switzerland, 2007; 265p.
- Danilcenko, H.; Jariene, E.; Gajewski, M.; Sawicka, B.; Kulaitiene, J.; Cerniauskiene, J. Changes in amino acids content in tubers of Jerusalem artichoke (Helianthus tuberosus L.) Cultivars during storage. Acta Sci. Pol. Hortorum Cultus 2013, 12, 97–105. [Google Scholar]
- Harmankaya, M.; Juhaimi, F.A.; Özcan, M.M. Mineral contents of Jerusalem artichoke (Helianthus tuberosus L.) growing wild in Turkey. Anal. Lett. 2012, 45, 2269–2275. [Google Scholar] [CrossRef]
- De Leenheer, L. Production and use of inulin: Industrial reality with a promising future. In Carbohydrates as Organic Raw Materials III; VCH Verlagsgesellschaft mbH: Weinheim, Germany; VCH Publishers Inc.: New York, NY, USA, 1996; pp. 67–92. [Google Scholar]
- Van Loo, J.; Coussement, P.; De Leenheer, L.; Hoebregs, H.; Smits, G. On the presence of inulin and oligofructose as natural ingredients in the western diet. Crit. Rev. Food Sci. Nutr. 1995, 35, 525–552. [Google Scholar] [CrossRef] [PubMed]
- Long, X.H.; Mehta, S.K.; Liu, Z.P. Effect of NO3–N enrichment onseawater stress tolerance of Jerusalem artichoke (Helianthus tuberosus). Pedosphere 2008, 18, 113–123. [Google Scholar] [CrossRef]
- Somda, Z.C.; McLaurin, W.J.; Kays, S.J. Jerusalem artichoke growth, development, and field storage. II. Carbon and nutrient element allocation and redistribution. J. Plant Nutr. 1999, 22, 1315–1334. [Google Scholar] [CrossRef]
- Moshfegh, A.J.; Friday, J.E.; Goldman, J.P.; Ahuja, J.K.C. Presence of inulin and oligofructose in the diets of Americans. J. Nutr. 1999, 129, 1407S–1411S. [Google Scholar] [CrossRef]
- Du, G.; Sun, Z.; Bao, S.; Zhong, Q.; Yang, S. Diversity of bacterial community in Jerusalem artichoke (Helianthus tuberosus L.) during storage is associated with the genotype and carbohydrates. Front. Microbiol. 2022, 13, 986659. [Google Scholar] [CrossRef]
- Bhanja, A.; Sutar, P.P.; Mishra, M. Inulin—A polysaccharide: Review on its functional and prebiotic efficacy. J. Food Biochem. 2022, 46, e14386. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, E.A.; Van Loo, J.; Fahey, G.C. Nutritional responses to the presence of inulin and oligofructose in the diets of domesticated animals: A review. Crit. Rev. Food Sci. Nutr. 2003, 43, 19–60. [Google Scholar] [CrossRef]
- Vhile, S.G.; Kjos, N.P.; Sørum, H.; Øverland, M. Feeding Jerusalem artichoke reduced skatole level and changed intestinal microbiota in the gut of entire male pigs. Animal 2011, 6, 807–814. [Google Scholar] [CrossRef]
- Shoemaker, D.N. The Jerusalem Artichoke as a Crop Plant; US Department of Agriculture: Washington, DC, USA, 1927; p. 33.
- Scharrer, K.; Schreiber, R. The digestibility of fresh and silaged tubers of Helianthus tuberosus by pigs. Landw. Forsch. 1950, 2, 156–161. [Google Scholar]
- Ly, J.; Macias, M.; Reyes, J.L.; Figueroa, V. Ileal and faecal digestibility of Jerusalem artichokes (Helianthus tuberosus L.) in pigs. J. Anim. Feed Sci. 1995, 4, 195–205. [Google Scholar] [CrossRef]
- Barszcz, M.; Taciak, M.; Skomiał, J. The effects of inulin, dried Jerusalem artichoke tuber and a multispecies probiotic preparation on microbiota ecology and immune status of the large intestine in young pigs. Arch. Anim. Nutr. 2016, 70, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, R.; Kays, S.F. Biology and chemistry of Jerusalem artichoke (Helianthus tuberosus L.). Exper. Agric. 2008, 44, 439. [Google Scholar] [CrossRef]
- Jawad, H.S.; Al-Abboodi, A.A. Jerusalem artichoke (Helianthus tuberosus L.) use in the poultry farms. J. Nutraceutical Food Sci. 2017, 2, 12. [Google Scholar]
- Yildiz, G.; Sacakli, P.; Gungor, T. The effect of dietary Jerusalem artichoke (Helianthus tuberosus L.) on performance, egg quality characteristics and egg cholesterol content in laying hens. Czech J. Anim. Sci. 2006, 51, 349. [Google Scholar] [CrossRef]
- Katiyanon, P.; Khajarean, J.; Tengjaroenkul, B.; Pimpukdee, K. Effects of feeding Jerusalem artichoke (Helianthus tuberosus L.) on performance, carcass quality and health of broilers. Kaen Kaset 2006, 34, 199–204. [Google Scholar]
- Azad, M.A.; Gao, J.; Ma, J.; Li, T.; Tan, B.; Huang, X.; Yin, J. Opportunities of prebiotics for the intestinal health of monogastric animals. Anim. Nutr. 2020, 6, 379–388. [Google Scholar] [CrossRef]
- Gardiner, G.E.; Metzler-Zebeli, B.U.; Lawlor, P.G. Impact of intestinal microbiota on growth and feed efficiency in pigs: A review. Microorganisms 2020, 8, 1886. [Google Scholar] [CrossRef]
- Valdovska, A.; Jemeljanovs, A.; Pilmane, M.; Zitare, I.; Konosonoka, I.H.; Lazdins, M. Alternative for improving gut microbiota: Use of Jerusalem artichoke and probiotics in diet of weaned piglets. Pol. J. Vet. Sci. 2014, 17, 61–69. [Google Scholar] [CrossRef][Green Version]
- Modesto, M.; D’Aimmo, M.R.; Stefanini, I.; Trevisi, P.; De Filippi, S.; Casini, L.; Mazzoni, M.; Bosi, P.; Biavati, B. A novel strategy to select Bifidobacterium strains and prebiotics as natural growth promoters in newly weaned pigs. Livest. Sci. 2009, 122, 248–258. [Google Scholar] [CrossRef]
- Lallès, J.P.; Bosi, P.; Smidt, H.; Stokes, C.R. Nutritional management of gut health in pigs around weaning. Proc. Nutr. Soc. 2007, 66, 260–268. [Google Scholar] [CrossRef]
- Hopwood, D.E.; Hampson, D.J. Interactions between the intestinal microflora, diet and diarrhoea, and their influences on piglet health in the immediate post-weaning period. In Weaning the Pig: Concepts and Consequences; Wageningen Academic Publisher: Wageningen, The Netherlands, 2003; pp. 199–217. [Google Scholar]
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef] [PubMed]
- Knecht, D.; Cholewińska, P.; Jankowska-Mąkosa, A.; Czyż, K. Development of swine’s digestive tract microbiota and its relation to production indices—A review. Animals 2020, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Maltecca, C.; Tiezzi, F. Potential use of gut microbiota composition as a biomarker of heat stress in monogastric species: A review. Animals 2021, 11, 1833. [Google Scholar] [CrossRef] [PubMed]
- Bamigbade, G.B.; Subhash, A.J.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. An updated review on prebiotics: Insights on potentials of food seeds waste as source of potential prebiotics. Molecules 2022, 27, 5947. [Google Scholar] [CrossRef] [PubMed]
- Imoto, S.; Namioka, S. VFA production in the pig large intestine. J. Anim. Sci. 1978, 47, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Sieo, C.; Ramasamy, K.; Saad, W.; Wong, H.; Ho, Y. Performance, biochemical and haematological responses, and relative organ weights of laying hens fed diets supplemented with prebiotic, probiotic and synbiotic. BMC Vet. Res. 2017, 13, 248. [Google Scholar] [CrossRef]
- Karimian, R.; Rezaeipour, V. Effects of dietary mannan-oligosacharides an phytase supplementation alone or in combination on growth performance, serum metabolites, cecal microbiota activity and intestinal morphology in broiler chickens. Poult. Sci. 2020, 8, 27–32. [Google Scholar] [CrossRef]
- Sevane, N.; Bialade, F.; Velasco, S.; Rebolé, A.; Rodríguez, M.L.; Ortiz, L.T.; Dunner, S. Dietary inulin supplementation modifies significantly the liver transcriptomic profile of broiler chickens. PLoS ONE 2014, 9, e98942. [Google Scholar] [CrossRef]
- Rebolé, A.; Ortiz, L.T.; Rodríguez, M.; Alzueta, C.; Treviño, J.; Velasco, S. Effects of inulin and enzyme complex, indi-vidually or in combination, on growth performance, intestinal microflora, cecal fermentation characteristics, and jejunal histo-morphology in broiler chickens fed a wheat-and barley-based diet. Poult. Sci. 2010, 89, 276–286. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).