Abstract
The aim of this study was to investigate the antifungal and antioxidant activities of Chamaecyparis obtusa (C. obtusa) extract (COE) against Candida albicans (C. albicans). Methods: The antioxidant activity was determined using three methods based on 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) scavenging, total polyphenol measurement, and total flavonoid measurement. In addition, the survival rate of human keratinocytes (HaCaT) was checked to confirm their stability. A one-way ANOVA also confirmed the antifungal activity of COE against C. albicans and its proliferative effect on HaCaT cells. In addition, to confirm the difference between each group, the Tukey HSD test was performed as a post hoc analysis, and the significance level was set at 0.05 as a two-tailed test. Results: COE was found to contain 424.150 mg GAE/g of polyphenols, 127.566 mg CE/g of flavonoids, and 83.594% of radical scavenging activity. Furthermore, 30 mg/mL of this extract exhibited complete antifungal activity. In addition, a noticeable killing effect on C. albicans was observed as the concentration of the extract increased. For instance, at a 2.5 mg/mL dosage of COE, HaCaT cells were safe, but C. albicans showed a distinct antifungal effect. Conclusions: COE has antioxidant and antifungal activity, so it has a high potential as an effective natural antifungal agent. Therefore, oral gargle containing COE is expected to be a safe and effective treatment in oral candidiasis in clinical practice.
1. Introduction
One of the most prevalent infections in humans, oral candidiasis, is caused by the overgrowth of Candida species [1]. In the 1980s, there was a clear surge of interest in oral candida infections largely due to the increased incidence of oral candida because of the escalation in the acquired immune deficiency syndrome (AIDS) epidemic, and, to date, oral candidiasis remains the most common oral opportunistic infection in human immunodeficiency virus (HIV)-positive individuals and in individuals with weakened immune systems [2]. These include Candida albicans (C. albicans), C. tropicalis, C. glabrata, C. pseudotropicalis, C. guilliermondii, C. krusei, C. lusitaniae, C. parapsilosis, and C. stellatoidea [3], and among them, C. albicans is the most common species [4]. With an organic relationship with S. aureus, C. albicans enhances bacterial colonization and biofilm formation, which resists antibiotic treatment and infects or destroys mucosal tissues [5]. The C. albicans biofilm matrix is largely composed of the polysaccharides β-1,3-glucan, β-1,6-glucan, and mannans, which form the mannan–glucan complex (MGCx) [6]. In the oral cavity, hyphae formation and the adherence to oral epithelial cells and other abiotic surfaces such as dentures promote the development of monomicrobial and polymicrobial biofilms [7]. Once a biofilm is established, the expression of Candida virulence factors increases, and susceptibility to antimicrobials and phagocytosis decreases drastically [8]. Importantly, in addition to Candida’s pathogenic factors and interactions with the host immune system, it is now acknowledged that the bacterial component of the oral microbiome plays an important role in the development and exacerbation of oral candidiasis [9]. Among the oral bacterial flora, streptococci are important for the colonization of C. albicans. Therefore, the interaction between C. albicans and streptococci is known to show a synergistic effect as an oral fungus–bacteria interaction. Specifically, interactions with Streptococcus oralis, Streptococcus mitis, Streptococcus gordonii, and Streptococcus mutans have been reported to adhere to the hyphae of C. albicans [10]. By using the lactic acid produced by streptococci, C. albicans lowers the partial pressure of oxygen to a level favorable to conditional streptococci [11]. In an experimental study, it was reported that C. albicans caused occlusal caries because, similar to S. mutans, C. albicans has the ability to produce and withstand acid. Therefore, the potential role of C. albicans in caries development through physical and metabolic interactions with S. mutans has been confirmed through several lines of evidence [12]. Additionally, exoenzyme glucosyltransferase B (gtfB) produced by S. mutans has been shown to be deposited on the surface of C. albicans hyphae, helping the fungus attach to oral surfaces [13]. In this respect, C. albicans can interact with oral bacteria and cause more serious diseases.
Oral candidiasis does not cause problems in healthy populations [1], but it primarily affects infants and the elderly and causes opportunistic infections in individuals with prolonged antibiotic use or immunocompromised status [14]. Therefore, antibiotics should not be used for a long period of time for the purpose of preventing oral infectious diseases or continuously suppressing oral pathogens. Accordingly, natural extracts, instead of synthetic chemical ingredients, are recommended in mouthwashes to control pathogens while maintaining oral microflora [15]. Thus, natural extracts have grown in popularity in recent years [16] as more research has been conducted.
Chamaecyparis obtusa (C. obtuse) is an evergreen conifer native to East Asia, including Japan and Taiwan, and a member of the Cupressaceae family. It produces a large amount of phytoncide with a distinct fragrance, which is known to be effective against pathogens in the oral cavity [17]. Phytoncides are volatile oils derived from the flowers, leaves, stems, roots, and resins of plants and are known to have antifungal effects against bacteria and fungi [18]. Specifically, phytoncide from cypress is known to be effective against Gram-positive bacteria (Staphylococcus epidermidis), Gram-negative bacteria (Vibrio parahaemolyticus, Pseudomonas aeruginosa, and Pseudomonas putida), yeast (C. albicans), and filamentous fungi (Aspergillus nidulans, Alternaria mali, and Fusarium oxysporum) [19]. Kang et al. [20] reported the antifungal effect of the phytoncide essential oil extracted from C. obtusa against C. albicans, which causes oral candidiasis. As a secondary photosynthetic metabolite, phytoncide is produced during stress responses in plants and microorganisms, including fungi [21]. This phenomenon, which directly and indirectly stresses the plants, is called allelopathy [22]. Allelochemicals are biochemical substances, and they can be refined in the form of essential oils through various methods such as steaming, compression, and extraction. The antimicrobial volatile (aromatic) organic chemicals contained in essential oils are called phytoncides. Representatively, the main components of phytoncides are terpenes (terpenoids), phenolics, alkaloids, phenylpropanes, and acetogenins [23]. Phytoncide essential oil from C. obtusa contains a complex mixture of compounds belonging to different groups with various functions, and the scent and flavor work individually or synergistically to have various effects such as calming effects in mind and body, blood circulation, anti-inflammatory, decongestive, immunomodulatory, antifungal, expectorant, antioxidant, psychotic, analgesic, and insecticidal effects. Toxicity and side effects, along with the purpose, dose, and route, need to be reviewed to confirm its safety and efficacy for clinical use [24]. For safety verification, human keratinocytes (HaCaT) extracted from adult skin are used due to their rapid cell proliferation and massive in vitro differentiation [25].
Therefore, in vitro study aimed to determine the antifungal and antioxidant effects of C. obtusa extract (COE) against oral C. albicans. In addition, the safety of COE as an oral candidiasis treatment to be used as basic data for clinical application was confirmed by observing the survival rate of HaCaT.
2. Materials and Methods
2.1. Sample Extraction
C. obtusa used in this study was grown in Gyeongsang, Gyeongsangbuk, South Korea, and was purchased from Cheongmyeong Co., Ltd. (Paju, Republic of Korea) COE was obtained using a rotary vacuum evaporator (N-1300E.V.S., Rikakikai Co., Ltd. (EYELA Co.), Tokyo, Japan) after filtration by adding 70% ethanol to 100 g of pulverized RVS at 60 °C for 12 h. C. obtusa was lyophilized using the freeze-drying process (Ilshin Lab Co., Ltd., Seoul, Republic of Korea). The COE was prepared in powder and stored in a sealed amber vial at −20 °C until used.
2.2. Measurement of Antioxidant Activity
2.2.1. Total Polyphenol Content
The Folin–Ciocalteau and Folin–Denis methods were used to measure the total polyphenol content [26]. CO was dissolved in distilled water to a concentration of 1 mg/mL; then, 0.15 mL of Folin–Ciocalteau phenol regent (Sigma-Aldrich, St. Louis, MO, USA) was mixed. After 0.3 mL of 20% Na2CO3 (Daejung Chemicals & Metals Co., Ltd., Gyeonggi, Republic of Korea) was added before leaving it for two hours in a dark room for the reaction, the absorbance of the supernatant was measured at 725 nm. Gallic acid (Sigma-Aldrich Co., St. Louis, MO, USA) was used as a standard, which was analyzed with the same method as the sample, and the polyphenol content was measured based on the calibration curve obtained: mg gallic acid equivalent (GAE; dry basis) per gram of the extract. Experiments were performed in triplicate.
2.2.2. Total Flavonoid Content
A modified Davis [27] method was used to measure total flavonoid content. Diethylene glycol (0.7 mL) was initially added to 0.1 mL of the sample, and then 0.1 mL of 1 N NaOH solution was further added. After a 1 h reaction in a dark room, the absorbance was measured at 420 nm. The calibration curve was measured with (+)-catechin hydrate (CE; dry basis) as a standard, and the total flavonoid content of the extract was shown as mg (+)-catechin hydrate (CE; dry basis). Experiments were performed in triplicate.
2.2.3. DPPH Scavenging Activity
The radical scavenging activity was measured using 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) (Sigma Chemical Co., St. Louis, MO, USA) [28]. COE (0.2 mL) was mixed with 0.2 mM DPPH solution (0.8 mL), and the absorbance was measured at 517 nm after incubation in a dark room at room temperature for 30 min. The antioxidant was measured by calculating the electron-donating ability (%) using the formula [1 − (absorbance of the sample with additives/absorbance of sample without additives)] × 100. Distilled water was added to the control group, and gallic acid was used as a positive control. The radical scavenging activity was expressed in percentage (%). Experiments were performed in triplicate.
2.3. Antifungal Assays
2.3.1. Bacterial Cultures
C. albicans (KCTC 7965/ATCC 10231) was incubated in the yeast mold (YM) broth (YM, Difco, Detroit, MI, USA) and cultivated at 37 °C under microaerophilic conditions for 24 h. The bacteria were diluted to a concentration of 1 × 105 colony-forming units per milliliter (CFU/mL).
2.3.2. Agar Disc Diffusion Assay
Paper discs (8.0 mm, Advantec, Toyo Roshi Kaisha, Ltd., Tokyo, Japan) were placed on top of YM agar plates, and 100 μL of different concentrations of each was dropped onto sterile 8 mm in diameter empty paper discs. The concentrations were used at concentrations of 1.25, 2.5, 5, 10, and 20 mg/mL and incubated anaerobically at 37 °C for 24 h. The size of the clear zone was measured around the soaked discs using a ruler.
2.3.3. Antimicrobial Test
To measure bacterial proliferation, COE was prepared at 1.25, 2.5, 5, 10, 20, and 30 mg/mL concentrations with C. albicans-containing medium into a 1.5 mL tube. After keeping them at a temperature of 37 °C for 24 h, the mixtures were diluted, and accurate amounts (1 mL) were uniformly spread onto YM agar plate. In order to check the antimicrobial activity of COE, the CFU were counted after a 24 h incubation. Experiments were performed in triplicate.
2.3.4. Minimum Inhibitory and Bactericidal Concentrations
The minimum inhibitory concentration (MIC) was determined via the broth dilution method [29]. The minimum bactericidal concentration (MBC) was determined by spreading from each test tube containing different concentrations on the plate. The MBC was described as the lowest concentration resulting in no bacterial growth [30]. The sample was prepared by dissolving COE in an aqueous solution containing dimethyl sulfoxide (DMSO). COE was made in 7 sterile test tubes containing 900 μL of different concentrations (1.25, 2.5, 5, 10, and 20 mg/mL) and 100 μL of C. albicans inoculum containing 1 × 105 CFU/mL to yield a final volume of 1 mL per each tube. After 24 h, the aliquots of 1mL from all the tubes were uniformly smeared in an YM agar medium and then cultured at 37 °C for 24 h to check the MIC and MBC. Experiments were repeated three times to observe the reproducibility of the measurements.
2.4. Cell Proliferation Assay
The quantification of the effect of COE on cell growth rate was measured using water-soluble tetrazolium salt-1 (WST-1) analysis [31]. HaCaT cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (USA) containing 10% (v/v) heat-inactivated fetal bovine serum (FBS; Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin (100 μL/mL; Gibco, Grand Island, NY, USA) at 37 °C and 5% CO2 in a humid chamber. Experiments were performed in 96-well plates containing a final volume of 100 μL of medium per well. HaCaT cells were seeded at an initial density of 1 × 105 cells/cm2 and incubated at 37 °C for 24 h. Then, the medium was changed to serum-free DMEM containing 1.25, 2.5, 5, 10, 20, and 30 mg/mL concentrations of COE. After 3 h, the incubation medium was removed, and the WST-1 solution was added. The plates were incubated at 37 °C for 2 h, and the optical density (OD) of the sample was measured at 450 nm using an ELISA reader (Multiskan FC, Thermo Fisher Scientific, Waltham, MA, USA). Cell viability was repeated independently at least three times.
2.5. Statistical Analysis
The antioxidant effect was confirmed using statistical software (SPSS v. 24.0, SPSS Inc., Chicago, IL, USA). In addition, ANOVA analysis was performed to confirm the bactericidal effect of C. albicans according to the dose of COE. To confirm the safety of COE, the viability of HaCaT cells was analyzed according to the dose, and the Tukey HSD test was performed as a post hoc analysis to confirm the difference according to each dose. The significance level was set to 0.05 in a two-tailed test.
3. Results
3.1. Antioxidant Activity
Table 1 displays the polyphenol content, flavonoid content, and DPPH radical scavenging activity of COE. The polyphenol content showed 424.150 mg GAE/g, the flavonoid content showed 127.566 mg CE/g, and the radical scavenging activity indicated 83.594%, indicating a high level of activity.

Table 1.
Effect of total phenolic and flavonoid contents and DPPH radical scavenging activity of the Chamaecyparis obtusa extract.
3.2. Clear Zone of Inhibition against C. albicans
The measured diameters by the disk diffusion method are presented in Figure 1. Growth inhibition showed a clear zone with diameters of 12, 14, and 15 mm at 5, 10, and 20 mg/mL, respectively. It was confirmed that the diameters increased as the concentrations of COE increased.
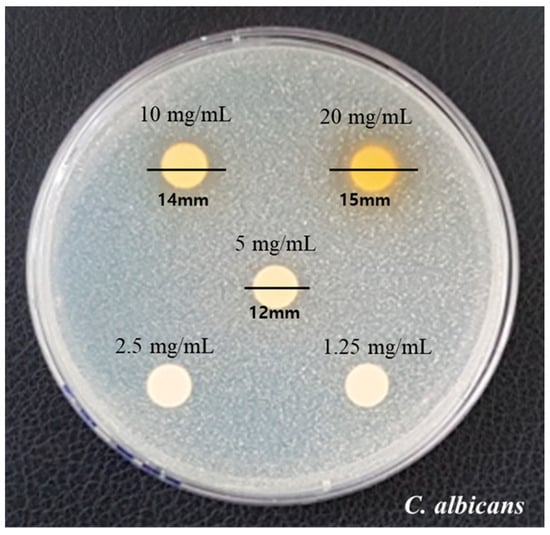
Figure 1.
Clear zone formation by different concentrates (1.25, 2.5, 5, 10, 20, and 30 mg/mL) of COE on C. albicans.
3.3. Antifungal Effects
Antifungal activity against C. albicans by COE was observed with no bacterial growth at 30 mg/mL (Figure 2). Table 2 shows the results of the statistical analysis of changes in CFU. As the concentration of COE increased, a clear inhibitory effect on C. albicans was observed. However, there was no statistically significant difference in the concentration of COE from 2.5 mg/mL to 30 mg/mL (p > 0.05).
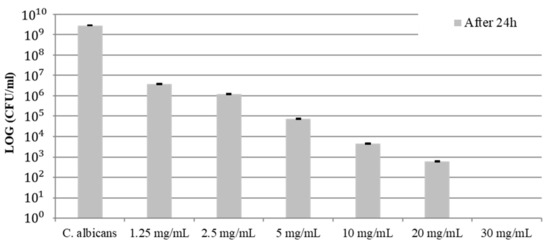
Figure 2.
C. albicans growth inhibition of COE at different concentrations.

Table 2.
Comparison of the CFU at different concentrations of COE.
3.4. MIC and MBC Determination
In concentrations of 10 mg/mL, the inhibition of bacterial growth was exhibited. The concentrations of 30 mg/mL were observed to show no growth of bacteria, hence confirming these concentrations as bactericidal. The MIC values of COE that prevented visible growth of C. albicans were 10 mg/mL, whereas the MBC values were 30 mg/mL for C. albicans, as presented in Figure 3.
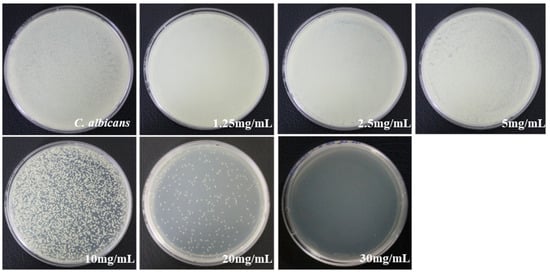
Figure 3.
MIC and MBC values of COE on C. albicans determined using the agar culture method.
3.5. Cell Viability Determination of HaCaT Cells
The cell viability was measured using the WST-1 assay at approximately 77%, 61%, 20%, 15%, 3%, and 2% after applying 1.25, 2.5, 5, 10, 20, and 30 mg/mL concentrations of COE, respectively, for 3 h (Figure 4). As shown in Figure 4, the half-maximal inhibitory concentration (IC50) was 2.5 g/mL, and the higher the COE concentration, the lower the survival rate, which indicated cytotoxicity.
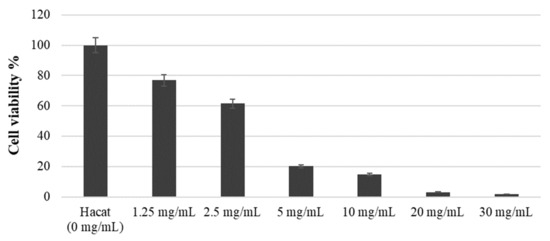
Figure 4.
Survival rate of HaCaT cell growth treated with COE.
4. Discussion
C. albicans colonizes mucous membranes and can act as an etiological factor in opportunistic infection, which can lead to life-threatening mucosal infections in immunosuppressed patients [32]. The local factors of oral candidiasis include oral cancer, dentures, decreased salivary gland function, and the use of steroids. The systemic factors include age, smoking, diabetes, Cushing syndrome, decreased immunity, malignant tumors, malnutrition, and antibiotics [19]. Topical local antifungal treatments, such as clotrimazole and nystatin, are used to treat C. albicans. However, clotrimazole has a risk of causing dental caries with its high sugar content, and in many cases with nystatin, the treatment is unsuccessful due to limitations such as oral structure (tongue, palate, gingiva, and teeth) and saliva dilution [33]. Among other things, the most important drawbacks of these antifungal agents are suboptimal selectivity, increased toxicity, and increased likelihood of developing resistance. In particular, although amphotericin B is considered the “standard” for antifungal therapy, it is intrinsically toxic because it lacks selectivity, given that fungi and mammalian cells are eukaryotic and share many similar biological processes [34]. The newest class of antifungals and the first fungi-specific antifungals are represented by echinocandins (caspofungins), which target key components of the fungal cell wall that are not present in mammalian cells. Unfortunately, the clinical use of echinocandins is limited to the treatment of systemic candidiasis, and the emergence of resistance in C. glabrata is of particular concern [35]. Therefore, natural mouthwash ingredients are actively researched to treat C. albicans. C. obtusa belongs to the Cupressaceae family and is native to Japan. It was introduced to South Korea in the early 20th century and is now naturalized in the central and southern regions of the country [36].
Antioxidant materials are extensively researched in various ways due to their safe and natural active-oxygen scavenging activity [37]. This study investigated the antioxidant activity of C. obtusa. As a result, the MIC value of COE preventing the visible growth of C. albicans was 10 mg/mL, while the MBC value was 30 mg/mL for C. albicans. According to Nagappan’s report, when 0.2% chlorhexidine mouthwash was used against C. albicans, the MIC was 12.5%, confirming that C. obtusa was more effective against C. albicans than chlorhexidine, which is widely used worldwide. Also, Arowash liquid mouthrinse containing three herbs (Cadila Pharmaceuticals, Cadila Pharmaceutical Ltd. Ahmedabad, Gujarat, India) also reported MBC at 50%, suggesting the possibility of using the COE-containing mouthwash used in this study [38].
Polyphenolic compounds are the secondary metabolites that are widely distributed in plants and contain two or more phenolic hydroxyl (–OH) groups in one molecule, which easily combine with various compounds. It has been reported that polyphenolic compounds have various physiological activities, including antioxidant, antimicrobial, anticancer, anti-inflammatory, and antidiabetic [39]. Furthermore, flavonoids are known to assist in antioxidant activity, platelet aggregation inhibition, and anti-inflammatory and anticancer activities [40]. The higher the total amounts of polyphenols and flavonoids, the higher the antioxidant activity, indicating a positive correlation. In this study, the polyphenol and flavonoid contents in COE were measured as 424.150 mg GAE/g and 127.566 mg CE/g, respectively. Compared to the polyphenol contained in the ethanol-extracted sea buckthorn leaf, which is reported to be 285.64 mg GAE/g [41], the total polyphenol amount from COE measured in this study was 1.49 times higher. Furthermore, the total flavonoid amount in this study was 2.13 times higher than the other research result, which reported 58.84 ± 0.47 mg CE/g in ethanol-extracted artichoke extract [42]. Furthermore, the free radical scavenging effect was measured with DPPH. The characteristic indigo color disappears when a relatively stable radical converts to a nonradical with electrons or hydrogen donated from an antioxidant. Using this characteristic, DPPH is widely used to measure the antioxidant activity of natural products due to its high correlation with actual antioxidant activity [43]. Therefore, 83.59% DPPH radical scavenging activity was observed at 1 mg/mL COE concentration, showing similar activity to a control group, gallic acid (89%). This confirms the high antioxidant power of COE with its high DPPH radical scavenging function.
When compared to the essential oils from Pinus densiflora, Pinus koraiensis, and Chamaecyparis pisifera, COE oil has one of the best antifungal properties. Specifically, it has been reported that yoshixol, isolated and purified from C. obtusa wood, has strong antifungal effects against Escherichia coli, Mycobacterium chelonae, Pseudomonas aeruginosa, and C. albicans [44]. Furthermore, COE oil inhibits mycelial growth of the two dermatophytes (Microsporum canis and Trichophyton mentagrophytes) at a concentration of 500 ppm and shows antifungal properties [36]. This study also confirmed the antifungal effects of COE on oral candidiasis. The higher the concentration of COE, the greater the diameter of the zone of inhibition, showing its devastating effect on C. albicans. However, no significant difference was observed between the concentrations ranging from 2.5 mg/mL to 30 mg/mL (p > 0.05). COE showed complete antifungal properties at a 30 mg/mL concentration. This result is consistent with the result of Lee et al. [19], which reported increased strain growth inhibition with increased COE concentration. Furthermore, Kang et al. [20] indicated the continuous growth inhibition of C. albicans when cultured with 0.25% phytoncide, decreasing the number of viable cells and resulting in the total elimination of C. albicans in 20 h. The growth-suppressing and lethal effects of phytoncide on C. albicans are consistent with the findings of this study. It has been reported that the antimicrobial mechanism of essential oils is due to phytoncide damaging the cell membranes of microorganisms and increasing their permeability to induce cytoplasmic liberation or affecting the bacterial respiratory metabolism [23,45].
Even though phytoncide shows antifungal effects against C. albicans and can kill cells through cell lysis, the level of cytotoxicity and cell viability depend on the concentration. After observing C. albicans with an electron microscope for 6 h after the phytoncide treatment, Kang et al. reported an increased number of atypical cell shapes, an increased electron density of the cytoplasm, increased high-density granules, and the division of intracellular organelles becoming more unclear in higher concentrations. Therefore, this study used HaCaT cells to confirm the safety of COE, which revealed a lower survival rate of HaCaT cells at higher concentrations, thus confirming its cytotoxicity. Overall, the COE concentration of 2.5 mg/mL indicated a clear antimicrobial effect without any observable cytotoxicity. Additional clinical studies are needed to actively utilize COE as a safe natural antibiotic. Therefore, it is necessary to confirm substantial changes in the patient’s oral condition by applying COE-containing gargles to humans in the clinical field. In addition to these functional confirmations, additional research will be needed to confirm the applicability of COE-containing gargles by examining satisfaction with the use of gargles.
For further research, through research on the quantification of phytochemical compounds of COE that cause antifungal effects, it is necessary to carry out chemical evaluation through component analysis. Above all, research should be conducted to confirm the continued harmlessness to the human body by analyzing the safety test through more specific and diverse experiments. In addition, the need to develop an antifungal agent class using various natural extracts should be emphasized by identifying the pathogenicity of C. albicans and the mechanism of the host immune response.
5. Conclusions
This study confirmed that COE at concentrations up to 2.5 mg/mL has the potential to be used as a safe and natural treatment for oral candidiasis due to its antioxidant and antifungal properties. Therefore, if oral gargles containing COE are applied in clinical settings, they will be used effectively and safely for the treatment of oral candidiasis.
Author Contributions
Conceptualization, S.-H.N. and G.-C.K.; data curation, Y.-R.K. and S.-H.N.; methodology, Y.-R.K. and S.-H.N.; resources, S.-H.N. and G.-C.K.; supervision, S.-H.N. and G.-C.K.; validation, Y.-R.K., S.-H.N. and G.-C.K.; writing the original draft, S.-H.N. and Y.-R.K.; writing review and editing, S.-H.N. and G.-C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ghannoum, M.A.; Radwan, S.S. Candida Adherence to Epithelial Cells, 1st ed.; CRC Press: Boca Raton, FL, USA, 1990; pp. 105–169. [Google Scholar] [CrossRef]
- Williams, D.W.; Jordan, R.P.; Wei, X.-Q.; Alves, C.T.; Wise, M.P.; Wilson, M.J.; Lewis, M.A. Interactions of Candida albicans with host epithelial surfaces. J. Oral Microbiol. 2013, 5, 22434. [Google Scholar] [CrossRef] [PubMed]
- Akpan, A.; Morgan, R. Oral candidiasis. Postgrad. Med. J. 2002, 78, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.K.; Garneau-Tsodikova, S. Synergistic combinations of azoles and antihistamines against Candida species in vitro. Med. Mycol. 2019, 57, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Andes, D.R.; Krysan, D.J. Candida-streptococcal interactions in biofilm-associated oral diseases. PLoS Pathog. 2018, 14, e1007342. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.F.; Zarnowskia, R.; Sancheza, H.; Edwarda, J.A.; Reinickea, E.L.; Netta, J.E.; Mitchell, A.P.; Andes, D.R. Community participation in biofilm matrix assembly and function. Proc. Natl. Acad. Sci. USA 2015, 112, 4092–4097. [Google Scholar] [CrossRef] [PubMed]
- Jabra-Rizk, M.A. Pathogenesis of polymicrobial biofilms. Open Mycol. J. 2011, 5, 39–43. [Google Scholar] [CrossRef]
- Jabra-Rizk, M.A.; Kong, E.; Tsui, C.; Nguyen, M.; Clancy, C.; Fidel, P.; Noverr, M. Candida albicans pathogenesis: Fitting within the host-microbe damage response framework. Infect. Immun. 2016, 84, 2724–2739. [Google Scholar] [CrossRef]
- Sultan, A.S.; Kong, E.F.; Rizk, A.M.; Jabra-Rizk, M.A. The oral microbiome: A lesson in co-existence. PLoS Pathog. 2018, 14, e1006719. [Google Scholar] [CrossRef]
- Montelongo-Jauregui, D.; Saville, S.P.; Lopez-Ribot, J.L. Contributions of Candida albicans dimorphism, adhesive interactions, and extracellular matrix to the formation of dual-species biofilms with Streptococcus gordonii. mBio 2019, 10, e01179-19. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Klein, M.I.; Colone, P.M.; Scott-Anne, K.; Gregoire, S.; Pai, C.-H.; Gonzalez, M.; Watson, G.; Krysan, D.J.; Bowen, W.H.; et al. Symbiotic relationship between Streptococcus mutans and Candida albicans synergizes the virulence of plaque-biofilms in vivo. Infect. Immun. 2014, 82, 1968–1981. [Google Scholar] [CrossRef]
- Lobo, C.I.V.; Rinaldi, T.B.; Christiano, C.M.S.; De Sales Leite, L.; Barbugli, P.A.; Klein, M.I. Dual-species biofilms of Streptococcus mutans and Candida albicans exhibit more biomass and are mutually beneficial compared with single-species biofilms. J. Oral Microbiol. 2019, 11, 1581520. [Google Scholar] [CrossRef] [PubMed]
- Hwang, G.; Liu, Y.; Kim, D.; Li, Y.; Krysan, D.J.; Koo, H. Candida albicans mannans mediate Streptococcus mutans exoenzyme GtfB binding to modulate cross-kingdom biofilm development in vivo. PLoS Pathog. 2017, 13, e1006407. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.B. Antifungal therapy in oropharyngeal mycotic infections. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Lis-Balchin, M. Essential oils and aromatherapy: Their modern role in healing. J. R. Soc. Health 1977, 117, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Eltayeb, L.M.H.; Yagi, S.; Mohamed, H.M.M.; Zengin, G.; Shariati, M.I.; Rebezov, M.; Uba, A.I.; Lorenzo, J.M. Essential Oils Composition and Biological Activity of Chamaecyparis obtusa, Chrysopogon nigritanus and Lavandula coronopifolia Grown Wild in Sudan. Molecules 2023, 28, 1005. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.T.; Lee, I.S.; Whang, K.; Yu, M.H. Antioxidant effects of Picrasma quassioides and Chamaecyparis obtuse (S. et Z.) ENDL extracts. J. Life Sci. 2012, 22, 354–359. [Google Scholar] [CrossRef]
- Hong, E.J.; Na, K.J.; Choi, I.G.; Choi, K.C.; Jeung, E.B. Antibacterial and antifungal effects of essential oils from Coniferous Trees. Biol. Pharm. Bull. 2004, 27, 863–866. [Google Scholar] [CrossRef]
- Lee, H.O.; Baek, S.H.; Han, D.M. Antimicrobial effects of Chamaecyparis obtuse essential oil. Korean J. Appl. Microbiol. Biotechnol. 2001, 29, 253–257. [Google Scholar]
- Kang, S.K.; Auh, Q.S.; Chun, Y.H.; Hong, J.P. Effect of Chamaecyparis obtusa tree Phytoncide on Candida albicans. J. Oral Med. Pain 2010, 35, 19–29. [Google Scholar]
- Lovett, J.V.; Ryuntyu, M.Y.; Liu, D.L. Allelopathy Chemical communication and plant defense. J. Chem. Ecol. 1989, 15, 1193–1202. [Google Scholar] [CrossRef]
- Patrick, Z.A. Allelopathy mechanism and their exploitation for biological control. Anandian J. Plant Pathol. 1986, 8, 225–228. [Google Scholar]
- Cowan, M.M. Plant products as antimcrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Pisseri, F.; Bertoli, A.; Pistelli, L. Essential oils in medicine: Principles of therapy. Parassitologia 2008, 50, 89–91. [Google Scholar] [PubMed]
- Schürer, N.; Köhne, A.; Schliep, V.; Barlag, K.; Goerz, G. Lipid composition and synthesis of HaCaT cells, an immortalized human keratinocyte line, in comparison with normal human adult keratinocytes. Exp. Dermatol. 1993, 2, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Folin, O.; Denis, W. On Phospaotungastic-phosohomdybdic Compounds as Color Reagents. J. Biol. Chem. 1912, 12, 239–249. [Google Scholar] [CrossRef]
- Davis, W.B. Determination of flavanones in citrus fruits. Anal. Chem. 1947, 19, 476–478. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1201–1205. [Google Scholar] [CrossRef]
- Mith, H.; Dure, R.; Delcenserie, V.; Zhiri, A.; Daube, G.; Clinquart, A. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2014, 2, 403–416. [Google Scholar] [CrossRef]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Lee, S.N. The antioxidant effect of rutin in human dermal fibroblasts damaged by reactive oxygen species. Korean J. Aesthet. Cosmetol. 2014, 12, 831–836. [Google Scholar]
- Odds, F.C. Activity of Cilofungin (Ly121019) against Candida species in vitro. J. Antimicrobial. Chem. 1988, 22, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Ellepola, A.N.; Samaranayake, L.P. Oral candida infections and antimycotics. Crit. Rev. Oral Biol. Med. 2000, 11, 172–198. [Google Scholar] [CrossRef]
- Pierce, C.G.; Lopez-Ribot, J.L. Candidiasis drug discovery and development: New approaches targeting virulence for discovering and identifying new drugs. Expert Opin. Drug Discov. 2013, 8, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Pristov, K.E.; Ghannoum, M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. 2019, 25, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Park, M.J.; Lee, S.M.; Gwak, K.S.; Jeung, E.B.; Chang, J.W.; Choi, I.G. Investigation of active antifungal compounds of essential oil from Chamaecyparis obtusa against Dermatophytes, Microsporum canis and Trichophyton. J. Korean Wood Sci. Technol. 2005, 33, 72–78. [Google Scholar]
- Masaki, H.; Sakaki, S.; Atsumi, T.; Sakurai, H. Active oxygen scavenging activity of plants extracts. Biol. Pharm. Bull. 1995, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, N.; Champakesan, B.; Tirupati, N.; D’cruz, T.M.; Ramasubramanian, P.P.; Premnath, P. Antimicrobial Efficacy of Two Mouthrinses Against Candida albicans: An in vitro study. J. Pharm. Bioallied Sci. 2019, 1, S293–S296. [Google Scholar] [CrossRef]
- Giovannini, C.; Scazzocchio, B.; Vari, R.; Santangelo, C.; D’Archivo, M.; Masella, R. Apoptosis in cancer and atherosclerosis: Polyphenol activities. Ann. Ist. Super. Sanita 2007, 43, 406. [Google Scholar]
- Craig, W.J. Phytochemicals: Guardians of our health. J. Am. Diet Assoc. 1997, 97, S199–S204. [Google Scholar] [CrossRef]
- Park, M.G.; Joo, S.Y. Comparison of antioxidant activities of sea buckthorn (Hippophae rhamnoides) leaf extracts at different ethanol ratios. Korean J. Food Sci. Technol. 2021, 53, 55–62. [Google Scholar] [CrossRef]
- Ahn, Y.H.; Gwon, M.J.; Lee, H.J.; Kwon, S.C. Antioxidant activity of artichoke extracts at different ethanol ratios. J. Biotechnol. Bioind. 2021, 9, 45–50. [Google Scholar] [CrossRef]
- Lee, J.M.; Chang, P.S.; Lee, J.H. Comparison of oxidative stability for the thermally-oxidized vegetable oils using a DPPH method. Korean J. Food Sci. Technol. 2007, 39, 133–137. [Google Scholar]
- Shozo, K.; Satoshi, T.; Yoshihiro, Y.; Jiro, M. Apoptosis like cell death induced by Yoshixol and wood oil of Chamaecyparis obtusa on Hela cell. Gen. Pharm. 1997, 28, 805–811. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).