Alkaloid and Nitrogenated Compounds from Different Sections of Coryphantha macromeris Plants and Callus Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Germination, Greenhouse Culture, and Callus Induction of C. macromeris
2.2. Media Preparation and Callus Culture Conditions
2.3. Sample Preparation for Untargeted Metabolomics Analysis
2.4. Untargeted Metabolomics Analysis Using UHPLC-PDA-HESI-Orbitrap-MS/MS
2.4.1. MS Parameters
2.4.2. Metabolite Identification Using Fragmentation Pattern Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tiwari, R.; Rana, C.S. Plant secondary metabolites: A review. Int. J. Eng. Res. Gen. Sci. 2015, 3, 661–670. [Google Scholar]
- Yeshi, K.; Crayn, D.; Ritmejerytė, E.; Wangchuk, P. Plant secondary metabolites produced in response to abiotic stresses has potential application in pharmaceutical product development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.-L.; Li, J.; Li, J.-X.; Liu, S.-J.; Huang, L.-Q.; Gao, W.-Y. Production of active compounds in medicinal plants: From plant tissue culture to biosynthesis. Chin. Herb. Med. 2017, 9, 115–125. [Google Scholar] [CrossRef]
- Sulaiman, C.T.; Nasiya, K.K.; Balachandran, I. Isolation and mass spectroscopic characterization of phytochemicals from the bark of Acacia leucophloea (Roxb.) Willd. Spectrosc. Lett. 2016, 49, 391–395. [Google Scholar] [CrossRef]
- Santos Díaz, M.d.S.; Barba de la Rosa, A.-P.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia spp.: Characterization and benefits in chronic diseases. Oxidative Med. Cell. Longev. 2017, 2017, 8634249. [Google Scholar] [CrossRef]
- Honma, A.; Koyama, T.; Yazawa, K. Anti-hyperglycaemic effects of the Japanese red maple Acer pycnanthum and its constituents the ginnalins B and C. J. Enzym. Inhib. Med. Chem. 2011, 26, 176–180. [Google Scholar] [CrossRef]
- Cornejo, A.; Salgado, F.; Caballero, J.; Vargas, R.; Simirgiotis, M.; Areche, C. Secondary metabolites in Ramalina terebrata detected by UHPLC/ESI/MS/MS and identification of parietin as Tau protein inhibitor. Int. J. Mol. Sci. 2016, 17, E1303. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, R.; Fernández-Galleguillos, C.; Pastene, E.; Simirgiotis, M.; Romero-Parra, J.; Ahmed, S.; Echeverría, J. Metabolomic Analysis, Fast Isolation of Phenolic Compounds, and Evaluation of Biological Activities of the Bark from Weinmannia trichosperma Cav. (Cunoniaceae). Front. Pharmacol. 2020, 11, 780. [Google Scholar] [CrossRef]
- Debnath, B.; Singh, W.S.; Das, M.; Goswami, S.; Singh, M.K.; Maiti, D.; Manna, K. Role of plant alkaloids on human health: A review of biological activities. Mater. Today Chem. 2018, 9, 56–72. [Google Scholar] [CrossRef]
- Vecino, X.; Devesa-Rey, R.; de Lima Stebbins, D.M.; Moldes, A.B.; Cruz, J.M.; Alcantar, N.A. Evaluation of a cactus mucilage biocomposite to remove total arsenic from water. Environ. Technol. Innov. 2016, 6, 69–79. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Saenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia spp. mucilage’s: A functional component with industrial perspectives. J. Arid. Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Rivera-Corona, J.L.; Rodríguez-González, F.; Rendón-Villalobos, R.; García-Hernández, E.; Solorza-Feria, J. Thermal, structural and rheological properties of sorghum starch with cactus mucilage addition. LWT—Food Sci. Technol. 2014, 59, 806–812. [Google Scholar] [CrossRef]
- Ramírez-Moreno, E.; Cariño-Cortés, R.; Cruz-Cansino, N.d.S.; Delgado-Olivares, L.; Ariza-Ortega, J.A.; Montañez-Izquierdo, V.Y.; Hernández-Herrero, M.M.; Filardo-Kerstupp, T. Antioxidant and antimicrobial properties of cactus pear (Opuntia) seed oils. J. Food Qual. 2017, 2017, 8. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilage and maltodextrin as encapsulating agents. Food Chem. 2015, 187, 174–181. [Google Scholar] [CrossRef]
- Mata, A.; Ferreira, J.P.; Semedo, C.; Serra, T.; Duarte, C.M.M.; Bronze, M.R. Contribution to the characterization of Opuntia spp. juices by LC–DAD–ESI-MS/MS. Food Chem. 2016, 210, 558–565. [Google Scholar] [CrossRef]
- Kuti, J.O. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Brugh, J.G.; Agurell, S. O-methylpellotine, a new peyote alkaloid from Lophophora diffusa. Phytochemistry 1975, 14, 1442–1443. [Google Scholar] [CrossRef]
- Ogunbodede, O.; McCombs, D.; Trout, K.; Daley, P.; Terry, M. New mescaline concentrations from 14 taxa/cultivars of Echinopsis spp. (Cactaceae) (“San Pedro”) and their relevance to shamanic practice. J. Ethnopharmacol. 2010, 131, 356–362. [Google Scholar] [CrossRef]
- Baldera-Aguayo, P.A.; Reyna Pinedo, V.M. Phytochemical study of Echinopsis Peruviana. Rev. Soc. Química Perú 2014, 80, 202–210. [Google Scholar]
- El-Seedi, H.R.; Smet, P.A.G.M.D.; Beck, O.; Possnert, G.; Bruhn, J.G. Prehistoric peyote use: Alkaloid analysis and radiocarbon dating of archaeological specimens of Lophophora from Texas. J. Ethnopharmacol. 2005, 101, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Massingill, J.L.; Hodgkins, J.E. Cactus alkaloids. Phytochemistry 1968, 7, 2031–2036. [Google Scholar] [CrossRef]
- Starha, R. Alkaloids from the cactus genus Gymnocalycium (Cactaceae). Biochem. Syst. Ecol. 1996, 24, 85–86. [Google Scholar] [CrossRef]
- Starha, R.; Urbánková, K.; Kuchyňa, J. Alkaloids from the genus Gymnocalycium (Cactaceae)—II. Biochem. Syst. Ecol. 1997, 25, 363–364. [Google Scholar] [CrossRef]
- Starha, R.; Chybidziurová, A.; Lance, Z. Alkaloids of the genus Turbinicarpus (Cactaceae). Biochem. Syst. Ecol. 1999, 27, 839–841. [Google Scholar] [CrossRef]
- Follas, W.D.; Cassady, J.M.; McLaughlin, J.L. β-Phenethylamines from the cactus genus Lobivia. Phytochemistry 1977, 16, 1459–1460. [Google Scholar] [CrossRef]
- Song, K.; Kang, H.; Ak, G.; Zengin, G.; Cziáky, Z.; Jekő, J.; Kim, D.H.; Lee, O.N.; Sivanesan, I. Micropropagation, phytochemistry and biological activity of the critically endangered Mammillaria herrerae Werdermann. S. Afr. J. Bot. 2021, 143, 312–321. [Google Scholar] [CrossRef]
- Ruvalcaba-Ruiz, D.; Rojas-Bravo, D.; Valencia-Botín, A.J. Propagación in vitro de Coryphantha retusa (Britton & Rose) un cactus endémico y amenazado. Trop. Subtrop. Agroecosystems 2010, 12, 139–143. [Google Scholar]
- Patel, A.K.; Agarwal, T.; Phulwaria, M.; Kataria, V.; Shekhawat, N.S. An efficient in vitro plant regeneration system from leaf of mature plant of Leptadenia reticulata (Jeewanti): A life giving endangered woody climber. Ind. Crops Prod. 2014, 52, 499–505. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crops Prod. 2016, 82, 9–22. [Google Scholar] [CrossRef]
- Qin, L.; Markham, K.R.; Paré, P.W.; Dixon, R.A.; Mabry, T.J. Flavonoids from elicitor-treated cell suspension cultures of Cephalocereus senilis. Phytochemistry 1993, 32, 925–928. [Google Scholar] [CrossRef]
- Cabañas-García, E.; Areche, C.; Jáuregui-Rincón, J.; Cruz-Sosa, F.; Pérez-Molphe Balch, E. Phytochemical profiling of Coryphantha macromeris (Cactaceae) growing in greenhouse conditions using ultra-high-performance liquid chromatography–tandem mass spectrometry. Molecules 2019, 24, 705. [Google Scholar] [CrossRef]
- Cabañas-García, E.; Areche, C.; Gomez-aguirre, Y.A.; Jáuregui-Rincón, J.; Cruz-Sosa, F.; Pérez Molphe Balch, E. Phytochemical profile of Coryphantha macromeris (Engelm.) Britton & Rose (Cactaceae) obtained from in vitro cultures. Rev. Mex. Ing. Química 2020, 19, 239–249. [Google Scholar]
- Cabañas-García, E.; Areche, C.; Gómez-Aguirre, Y.A.; Borquez, J.; Muñoz, R.; Cruz-Sosa, F.; Pérez-Molphe Balch, E. Biomass production and secondary metabolite identification in callus cultures of Coryphantha macromeris (Engelm.) Britton & Rose (Cactaceae), a traditional medicinal plant. S. Afr. J. Bot. 2021, 137, 1–9. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Cabañas-García, E.; Areche, C.; Bórquez-Ramírez, J.; Muñoz-Miranda, R.; Rosales- Lopez, K.M.; Pérez-Molphe Balch, E.; Gómez-Aguirre, Y.A.; Cruz-Sosa, F. Coryphantha macromeris Cell Suspension Cultures: Phytochemical Profiling and Agitation Velocity Effect on Cell Morphology and Viability. In Recent Research Advances in Biology, 1st ed.; BP International: New York, NY, USA, 2021; Volume 8, pp. 115–131. [Google Scholar]
- Luo, Z.; Yu, G.; Han, X.; Liu, Y.; Wang, G.; Li, X.; Yang, H.; Sun, W. Exploring the active components of Simotang oral liquid and their potential mechanism of action on gastrointestinal disorders by integrating Ultrahigh-Pressure Liquid Chromatography coupled with Linear Ion Trap-Orbitrap analysis and network pharmacology. ACS Omega 2021, 6, 2354–2366. [Google Scholar] [CrossRef]
- Zou, J.; Wu, S.; Sheng, B.; An, J.; Meng, J.; Xiong, W.; Tao, J.; Han, W.; Zhao, L.; Xu, H.; et al. UPLC-Q-TOF-MS/MS analysis on the chemical composition of malts under different germination cycles and prepared with different processing methods. Fitoterapia 2023, 165, 105313. [Google Scholar] [CrossRef]
- Pawar, R.S.; Sagi, S.; Leontyev, D. Analysis of bitter orange dietary supplements for natural and synthetic phenethylamines by LC-MS/MS. Drug Test. Anal. 2020, 12, 1241–1251. [Google Scholar] [CrossRef]
- Ni, J.; Guo, Y.; Chang, N.; Cheng, D.; Yan, M.; Jiang, M.; Bai, G. Effect of N-methyltyramine on the regulation of adrenergic receptors via enzymatic epinephrine synthesis for the treatment of gastrointestinal disorders. Biomed. Pharmacother. 2019, 111, 1393–1398. [Google Scholar] [CrossRef]
- Kikuchi, H.; Uchiyama, N.; Ogata, J.; Kikura-Hanajiri, R.; Goda, Y. Chemical constituents and DNA sequence analysis of a psychotropic herbal product. Forensic Toxicol. 2010, 28, 77–83. [Google Scholar] [CrossRef]
- McFarlane, I.J.; Slaytor, M. Alkaloid biosynthesis in Echinocereus merkeri. Phytochemistry 1972, 11, 235–238. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Khair-Ul-Bariyah, S.; Arshad, M.; Ali, M.; Din, M.I.; Sharif, A.; Ahmed, E. Benzocaine: Review on a Drug with Unfold Potential. Mini-Rev. Med. Chem. 2020, 20, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Slothower, J.D.; Wiegand, T.J. Mescaline. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 221–222. [Google Scholar]
- Vamvakopoulou, I.A.; Narine, K.A.D.; Campbell, I.; Dyck, J.R.B.; Nutt, D.J. Mescaline: The forgotten psychedelic. Neuropharmacology 2023, 222, 109294. [Google Scholar] [CrossRef] [PubMed]
- Keller, W.J.; McLaughlin, J.L. Cactus alkaloids XIII: Isolation of (−)-normacromerine from Coryphantha macromeris var. runyonii. J. Pharm. Sci. 1972, 61, 147–148. [Google Scholar] [CrossRef] [PubMed]
- Keller, W.J.; McLaughlin, J.L.; Brady, L.R. Cactus Alkaloids XV: β-Phenethylamine Derivatives from Coryphantha macromeris var. runyonii. J. Pharm. Sci. 1973, 62, 408–411. [Google Scholar] [CrossRef]
- Keller, W.J. Macromerine and Normacromerine Biosynthesis in Coryphantha macromeris var. runyonii. J. Pharm. Sci. 1979, 68, 85–87. [Google Scholar] [CrossRef]
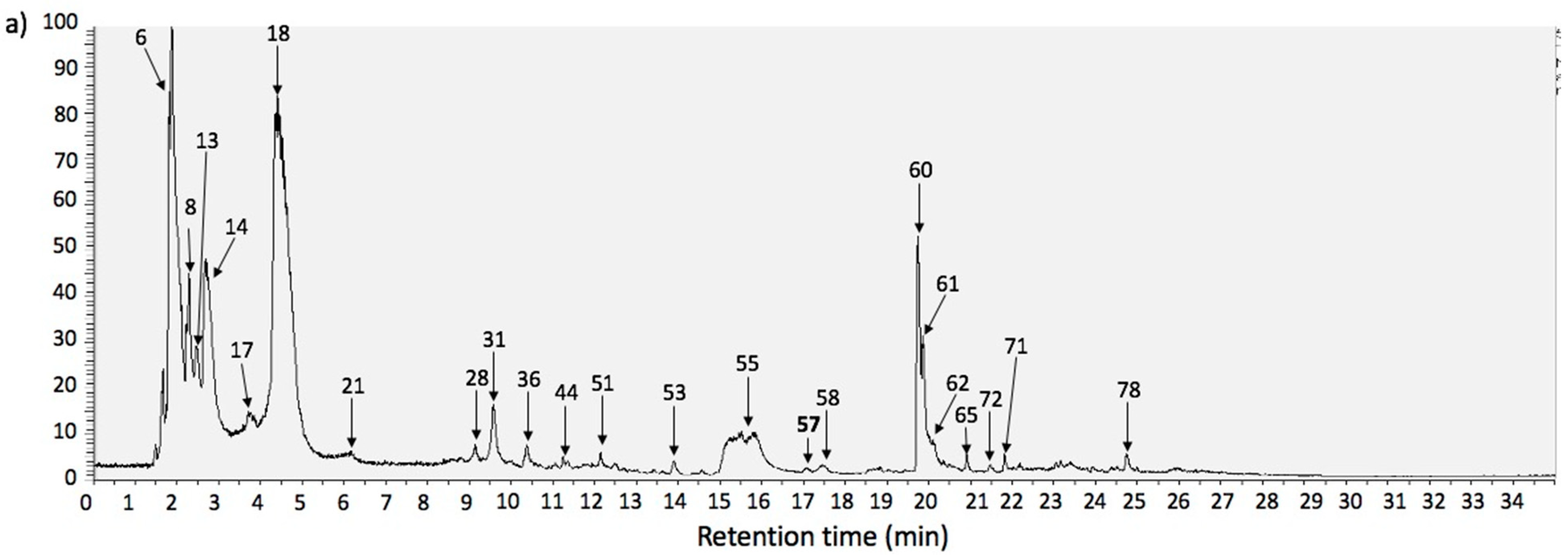


| Peak | Retention Time (min) | UV Max | Tentative Identification | Elemental Composition [M + H]+ | Theoretical Mass (m/z) | Measured Mass (m/z) | Accuracy (ppm) | MSn Ions | Tissue |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.35 | 196, 210 | Unknown | C20H15NO7+ | 381.0848 | 381.0839 | 2.36 | 203.1038, 112.8961, 104.1078 | c |
| 2 | 1.51 | 221, 274 | N-methyltiramine derivative | C9H12NO+ | - | 150.0917 | - | 121.0651, 103.0546 | ri, rg |
| 3 | 1.52 | 210 | (3-ethenylphenol) N-methyltiramine derivative | C8H9O+ | 121.0647 | 121.0653 | 4.21 | 103.0545 | c |
| 4 | 1.67 | 225, 277 | Salsoline isomer I | C11H16NO2+ | 194.1175 | 194.1179 | 2 | 148.0759, 121.0651, 118.0654, 104.0497 | ag |
| 5 | 1.83 | 204 | Benzocaine isomer I | C9H12NO2+ | 166.0862 | 166.0868 | 3.61 | 107.0495, 123.0446 | c |
| 6 | 1.87 | 220, 276 | N-methyltiramine | C9H14NO+ | 152.1069 | 152.1072 | 1.90 | 121.0652, 103.0546 | ai |
| 7 | 1.88 | 225, 274 | N-methyltiramine derivative | C9H12NO+ | - | 150.0918 | - | 121.0651, 103.0545 | rg |
| 8 | 1.97 | 220, 277 | N-methylphenylalanine | C10H14NO2+ | 180.1019 | 180.1025 | 3.33 | 148.0761, 120.0812, 107.0496 | ai, ag |
| 9 | 2.05 | 215 | (3-ethenylphenol) N-methyltiramine derivative isomer | C8H9O+ | 121.0647 | 121.0652 | 3.38 | 103.0545 | c |
| 10 | 2.10 | 220 | N-methyltiramine derivative isomer | C9H12NO+ | - | 150.0918 | - | 121.0651, 103.0547, | ri |
| 11 | 2.31 | 220 | N-methyltiramine isomer | C9H14NO+ | 152.1069 | 152.1074 | 2.69 | 103.0545 | c, rg |
| 12 | 2.32 | 220, 280 | (3-ethenylphenol) N-methyltiramine derivative isomer | C8H9O+ | 121.0647 | 121.0652 | 3.38 | 103.0546 | ri |
| 13 | 2.45 | 220, 276 | Salsoline isomer II | C11H16NO2+ | 194.1175 | 194.1178 | 1.59 | 148.0760, 121.0650, 118.0657 | ai, ag |
| 14 | 2.60 | 223, 275 | N-methyltiramine isomer | C9H14NO+ | 152.1069 | 152.1073 | 2.62 | 121.0652, 103.0546 | ai, ri |
| 15 | 3.16 | 226, 276 | Salsoline isomer III | C11H16NO2+ | 194.1175 | 194.1182 | 3.34 | 148.0761, 121.0651, 118.0866 | ag, rg |
| 16 | 3.35 | 210 | Benzocaine isomer II | C9H12NO2+ | 166.0862 | 166.0868 | 3.61 | 107.0495, 123.0446 | c |
| 17 | 3.73 | 220, 276 | Salsoline isomer IV | C11H16NO2+ | 194.1175 | 194.1181 | 3.24 | 148.0760, 121.0652, 118.0655 | ai, ri, ag, rg |
| 18 | 4.41 | 205, 228, 278 | Salsoline isomer V | C11H16NO2+ | 194.1175 | 194.1181 | 3.09 | 148.0761, 121.0651, 118.0867 | ai, ri |
| 19 | 5.34 | 220, 277 | Salsoline isomer VI | C11H16NO2+ | 194.1175 | 194.1182 | 3.50 | 148.0761, 121.0652, 118.0655 | ag |
| 20 | 5.62 | 225 | N-methylphenylalanine derivative | C10H14NO+ | - | 164.1075 | - | 148.0759, 120.0810, 107.0495 | ri |
| 21 | 6.16 | 226, 278 | Salsoline isomer VII | C11H16NO2+ | 194.1175 | 194.1181 | 3.19 | 148.0761, 121.0651, 118.0867 | ai |
| 22 | 7.76 | 225, 279 | 3-amino-2-naphthoic acid | C11H10NO2+ | 188.0706 | 188.0713 | - | 143.0734, 118.0655, 107.0494 | rg |
| 23 | 8.39 | 218, 285 | 3-amino-2-naphthoic acid | C11H10NO2+ | 188.0706 | 188.0712 | 3.34 | 143.0734, 118.0655, 107.0494 | ri |
| 24 | 8.50 | 230, 279 | 1,2-ethanediol, 1-(3-ethenylphenyl) | C10H13O2+ | 165.0910 | 165.0916 | 3.63 | 135.0444, 121.0651, 107.0495, 103.0546 | ag, rg |
| 25 | 8.69 | 224, 280 | Unknown | C13H25NO3+ | - | 243.1841 | - | 149.0238, 123.0445, 107.0495, 102.0918 | c |
| 26 | 8.90 | 220, 275 | Salsoline isomer VIII | C11H16NO2+ | 194.1175 | 194.1181 | 3.24 | 148.0761, 121.0650, 118.0654 | ag, rg |
| 27 | 9.04 | 230, 282 | Unknown isomer | C13H25NO3+ | - | 243.1841 | - | 149.0238, 107.0494, 102.0917 | c |
| 28 | 9.14 | 222, 275 | 1,2-ethanediol, 1-(3-ethenylphenyl) | C10H13O2+ | 165.0910 | 165.0915 | 3.02 | 135.0444, 121.0651, 107.0495, 103.0546 | ai |
| 29 | 9.35 | 221, 279 | 3-amino-2-naphthoic acid | C11H10NO2+ | 188.0706 | 188.0713 | 3.82 | 143.0734, 118.0655, 107.0496 | rg |
| 30 | 9.35 | 226, 280 | Unknown | C13H24NO3+ | - | 243.1841 | - | 166.1232, 135.0809, 103.0546, 102.0917 | ri |
| 31 | 9.59 | 224, 276 | Hordenine | C10H15NO+ | 166.1226 | 166.1231 | 2.88 | 121.0655, 105.0702 | ai |
| 32 | 9.62 | 236, 285 | 3,4,5-triethoxyphenethylamine | C14H24NO3+ | 254.1751 | 254.1764 | 5.11 | 138.0918 | c |
| 33 | 9.73 | 222, 284 | 3-amino-2-naphthoic acid | C11H10NO2+ | 188.0706 | 188.0712 | 3.5 | 143.0734, 118.0655, 107.0495 | ri |
| 34 | 10.10 | 223, 280 | N-methylphenylalanine derivative | C10H10N+ | 144.0807 | 144.0812 | 3.05 | 120.0811, 107.0496 | ri |
| 35 | 10.19 | 240, 285 | Unknown | C21H32N2O12+ | - | 504.1962 | - | 177.0551, 145.0288, 117.0339 | c |
| 36 | 10.38 | 223, 275 | Maclurin | C13H11O6+ | 263.0550 | 263.0542 | 2.85 | 121.0647, 107.0491 | ai |
| 37 | 10.52 | 234, 277 | 3,4-dihydro-8-hydroxy-6,7- dimethoxy-1-methylisoquinoline | C12H15NO3+ | 222.1124 | 222.1134 | 4.05 | 162.0917, 148.0761 | ag, rg |
| 38 | 10.55 | 238, 282 | 3,4-didehydrochromen-2-one derivative | C22H18N2O2+ | - | 342.1367 | - | 145.0280, 117.0339 | c |
| 39 | 10.67 | 236, 326 | Unknown | C12H9N2O2+ | - | 213.0667 | - | 167.0608, 145.0287, 140.0498, 115.0546, 105.0339 | rg |
| 40 | 10.77 | 235, 327 | Unknown | C21H17NO7+ | - | 395.1000 | - | 194.1180, 167.0705, 145.0284, 105.0339 | ag |
| 41 | 10.83 | 233, 298, 320 | 3,4-didehydrochromen-2-one derivative | C23H32N2O13+ | - | 544.1908 | - | 342.1368, 145.0280, 117.0339 | c |
| 42 | 10.91 | 225, 277 | Salsoline | C11H16NO2+ | 194.1175 | 194.1179 | 2 | 148.0759, 121.0651, 118.0654 | ag |
| 43 | 11.01 | 236, 285 | Unknown | C14H17O4+ | - | 249.1133 | - | 177.0551, 145.0287, 128.0624, 115.0545 | c |
| 44 | 11.26 | 237, 327 | Unknown | C10H9O3+ | - | 177.0553 | - | 145.0288, 117.0339, 103.0546, | ai |
| 45 | 11.37 | 238, 285 | Toluic acid derivative | C16H30N2O4+ | - | 314.2168 | - | 137.0601 | c |
| 46 | 11.38 | 228, 283 | Unknown | C29H36N2O9+ | - | 556.2421 | - | 256.1010, 144.0813, 130.0556, 115.0547 | rg |
| 47 | 11.54 | 236, 285, 319 | 3,4-didehydrochromen-2-one derivative | C21H16N2O+ | - | 312.1255 | 1.06 | 145.0288, 117.0339 | c |
| 48 | 11.72 | 227, 282 | Unknown | C12H26N2O10+ | - | 358.1588 | - | 172.0762, 144.0881, 130.0655 | rg |
| 49 | 11.92 | 238, 282 | 3,4-didehydrochromen-2-one derivative | C22H18N2O2+ | - | 342.1368 | - | 145.0280, 117.0339 | c |
| 50 | 12.00 | 234, 277 | 3,4-didehydrochromen-2-one derivative | C22H18N2O2+ | - | 342.1368 | - | 145.0280, 117.0339 | ri, ag, rg |
| 51 | 12.15 | 238, 324 | 3,4-didehydrochromen-2-one derivative | C21H16N2O+ | - | 312.1255 | 1.12 | 145.0288, 117.0339 | ai |
| 52 | 12.85 | 259 | Unknown | C16H15NO2+ | - | 253.1083 | - | 145.0288, 133.0651, 118.0416, 103.0547 | ag, rg |
| 53 | 13.80 | 285 | 3,4-didehydrochromen-2-one derivative | - | - | 314.1412 | - | 145.0287, 117.0339, 109.1015 | ai, ri |
| 54 | 14.75 | 230, 290 | Toluic acid | C8H9O2+ | 137.0597 | 137.0601 | 2.84 | 107.0495, 121.0652, 109.0651 | ri |
| 55 | 14.74 | 221, 283 | Toluic acid | C8H9O2+ | 137.0597 | 137.0601 | 2.84 | 107.0495, 121.0651, 109.0651 | c, ai, ri, ag, rg |
| 56 | 16.36 | 283 | Picein | C14H19O7+ | 299.1125 | 299.1123 | 0.63 | 137.0601, 121.0652, 107.0495 | c, ag, rg |
| 57 | 17.08 | 221, 286 | Toluic acid | C8H9O2+ | 137.0597 | 137.0601 | 3.21 | 107.0496, 121.0651, 109.0651 | ai |
| 58 | 15.27 | 285 | Picein isomer | C14H19O7+ | 299.1125 | 299.1123 | 0.53 | 137.0601, 121.0653, 107.0491 | ai, ri |
| 59 | 18.70 | 290 | Toluic acid derivative | - | - | 353.2334 | - | 137.0601, 121.0654 | ri |
| 60 | 19.50 | 283 | Lauryldiethanolamine | C16H36NO2+ | 274.2740 | 274.2755 | 5.5 | 256.2643, 230.2486, 102.0916 | c, ai, ri, ag, rg |
| 61 | 19.70 | 282 | Toluic acid derivative | - | - | 230.2487 | - | 137.0601, 121.0649 | c, ai, ri, ag, rg |
| 62 | 19.90 | 276 | 4-amino-2-dodecyltetrahydro-3-furanol | C16H34NO2+ | 272.2584 | 272.2600 | 5.98 | 254.2491 | ai, ri |
| 63 | 20.25 | 282 | Lauryldiethanolamine isomer | C16H36NO2+ | 274.2740 | 274.2755 | 5.5 | 256.2640, 230.2489 | c, ri |
| 64 | 20.39 | 243, 291 | M-toluic acid derivative | - | - | 289.0990 | - | 137.0600, 121.0651 | rg |
| 65 | 20.56 | 283 | M-toluic acid derivative | - | - | 387.1849 | - | 137.0600, 121.0651 | c, ai, ag |
| 66 | 20.60 | 284 | Toluic acid derivative | - | - | 289.0992 | - | 137.0600, 121.0651, | ri, ag |
| 67 | 20.86 | 283 | 1-oxo-2-phenyl-phenalen-3-yl benzoate | C26H17O3+ | 377.1172 | 377.1178 | 1.53 | 123.0407 | ag |
| 68 | 20.90 | 282 | Alibendol | C13H18NO4+ | 252.1230 | 252.1244 | 5.43 | 196.0610, 178.0504, 122.0600 | c, rg |
| 69 | 21.03 | 266 | Alibendol | C13H18NO4+ | 252.1230 | 252.1243 | 5.39 | 196.0610, 178.0504, 122.0600 | ag |
| 70 | 21.33 | 290 | Alibendol | C13H18NO4+ | 252.1230 | 252.1243 | 5.31 | 196.0610, 178.0504, 122.0600 | ri |
| 71 | 21.50 | 284 | Unknown | C17H35O11+ | - | 415.2175 | - | 149.0236, 119.0859, 105.0702 | c, ai, ag, rg |
| 72 | 21.48 | 284 | Alibendol | C13H18NO4+ | 252.1230 | 252.1243 | 5.15 | 196.0610, 178.0504, 122.0600 | ai |
| 73 | 21.70 | 295 | Unknown | C16H36NO+ | - | 258.2804 | - | 119.0859 | ri |
| 74 | 21.92 | 285 | Unknown | C10H23NO11+ | - | 333.1264 | - | 167.0609, 140.0499, 113.0390 | ag, rg |
| 75 | 22.54 | 284 | Unknown | C21H45NO11+ | - | 487.2968 | - | 133.1016, 119.0848, 105.0703 | ag |
| 76 | 23.11 | 282 | Unknown | C27H60N5O6+ | - | 550.4589 | - | 256.2649, 165.0073, 135.0444, 105.0703, 102.0918 | c, ri, rg |
| 77 | 23.94 | 283 | 2-[dodecyl(methyl)amino] acetic acid | C15H32NO2+ | 258.2427 | 258.2442 | 5.8 | 240.2334 | c |
| 78 | 24.45 | 284 | Unknown | C28H33NO+ | - | 399.2558 | - | 149.0236, 129.0700, 108.9046, 105.0702 | c, ai, ri, ag, rg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viera-Escareño, V.; Perez-Molphe Balch, E.; Gómez-Aguirre, Y.A.; Ramos-Herrera, O.J.; Abdi, G.; Cruz-Sosa, F.; Cabañas-García, E. Alkaloid and Nitrogenated Compounds from Different Sections of Coryphantha macromeris Plants and Callus Cultures. Appl. Sci. 2023, 13, 9947. https://doi.org/10.3390/app13179947
Viera-Escareño V, Perez-Molphe Balch E, Gómez-Aguirre YA, Ramos-Herrera OJ, Abdi G, Cruz-Sosa F, Cabañas-García E. Alkaloid and Nitrogenated Compounds from Different Sections of Coryphantha macromeris Plants and Callus Cultures. Applied Sciences. 2023; 13(17):9947. https://doi.org/10.3390/app13179947
Chicago/Turabian StyleViera-Escareño, Valeria, Eugenio Perez-Molphe Balch, Yenny Adriana Gómez-Aguirre, Oscar Javier Ramos-Herrera, Gholamreza Abdi, Francisco Cruz-Sosa, and Emmanuel Cabañas-García. 2023. "Alkaloid and Nitrogenated Compounds from Different Sections of Coryphantha macromeris Plants and Callus Cultures" Applied Sciences 13, no. 17: 9947. https://doi.org/10.3390/app13179947
APA StyleViera-Escareño, V., Perez-Molphe Balch, E., Gómez-Aguirre, Y. A., Ramos-Herrera, O. J., Abdi, G., Cruz-Sosa, F., & Cabañas-García, E. (2023). Alkaloid and Nitrogenated Compounds from Different Sections of Coryphantha macromeris Plants and Callus Cultures. Applied Sciences, 13(17), 9947. https://doi.org/10.3390/app13179947







