Effectiveness of Immature Asian Pear Extract on Pulmonary Injury Caused by Particulate Matter in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Material
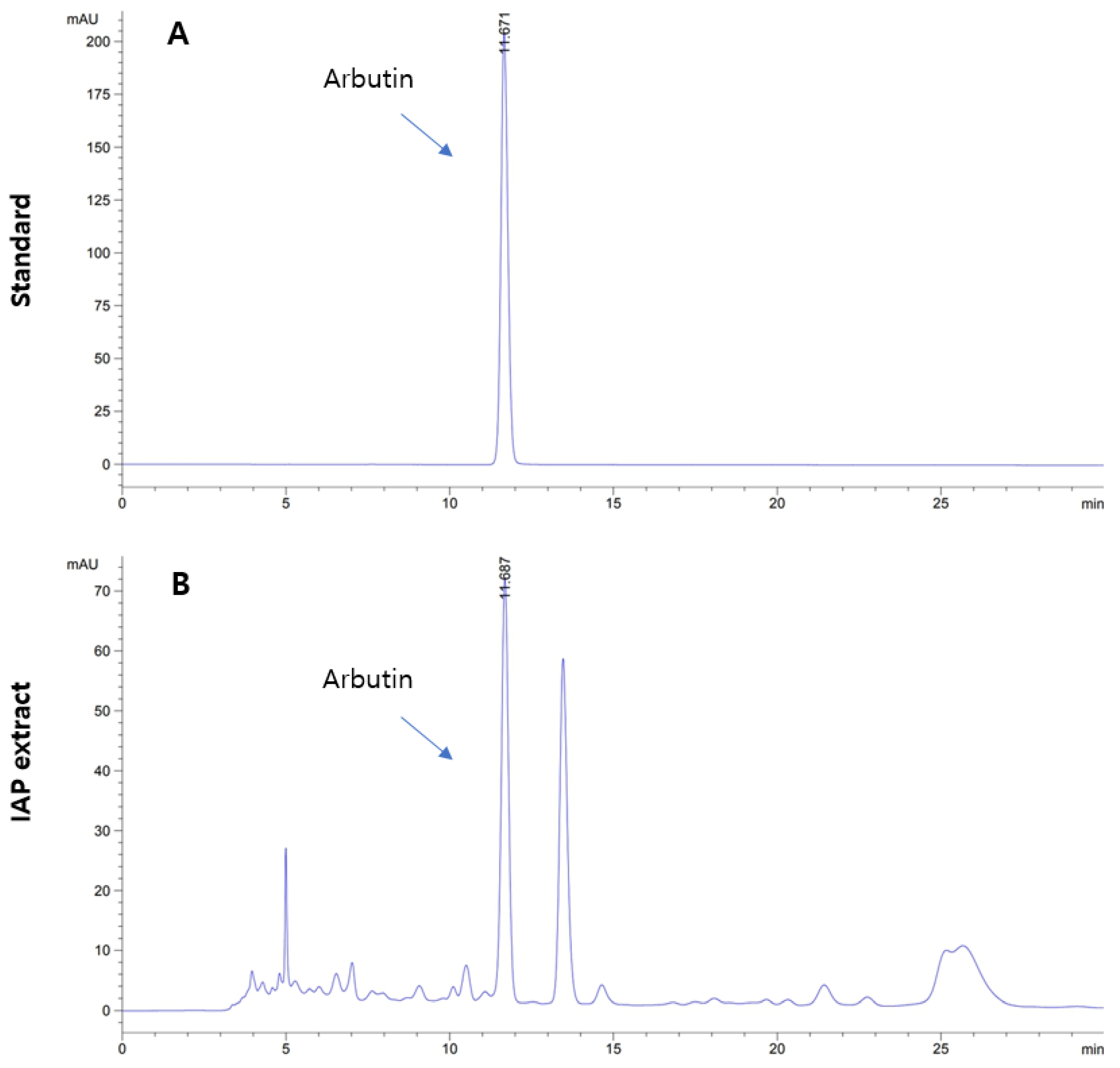
2.2. High-Performance Liquid Chromatography (HPLC)
2.3. Animals
- Intact (vehicle) control = D.W. (10 mL/kg) administered and intranasal saline instillation (0.1 mL/kg) mice.
- PM2.5 control = D.W. (10 mL/kg) administered and intranasal PM2.5 instillation (1 mg/kg) mice.
- DEXA = DEXA (0.75 mg/kg) administered and intranasal PM2.5 instillation (1 mg/kg) mice.
- IAP400 = IAP (400 mg/kg) administered and intranasal PM2.5 instillation (1 mg/kg) mice.
- IAP200 = IAP (200 mg/kg) administered and intranasal PM2.5 instillation (1 mg/kg) mice.
- IAP100 = IAP (100 mg/kg) administered and intranasal PM2.5 instillation (1 mg/kg) mice.
2.4. Induction of Lung Damage
2.5. Administration of Test Material
2.6. Removal of Lung Tissue
2.7. Observation Items
2.8. Lung Gross Necropsy Findings
2.9. BALF Leukocyte Fractionation
2.10. Lung Antioxidant Defense System
2.11. Histopathological Changes in Lung Tissue
2.12. mRNA Expression in Lung Tissue
2.13. Statistical Analysis
data of intact vehicle control mice)/data of intact vehicle control mice) × 100
treated mice − data of PM2.5 control mice)/data of PM2.5 control mice) × 100
3. Results
3.1. Level of Arbutin in IAP Extract
3.2. Changes on the Body Weight and Gains
3.3. Findings from the Macroscopic Examination of the Lungs and Changes in Weight
3.4. Changes in Total Cell Count, Total Leukocyte Count, and Leukocyte Differential Count in BALF
3.5. Changes in ALT and AST Levels in Serum
3.6. Changes in the Levels of Cytokines IL-6, TNF-α, CXCL1, and CXCL2 in Lung Tissue
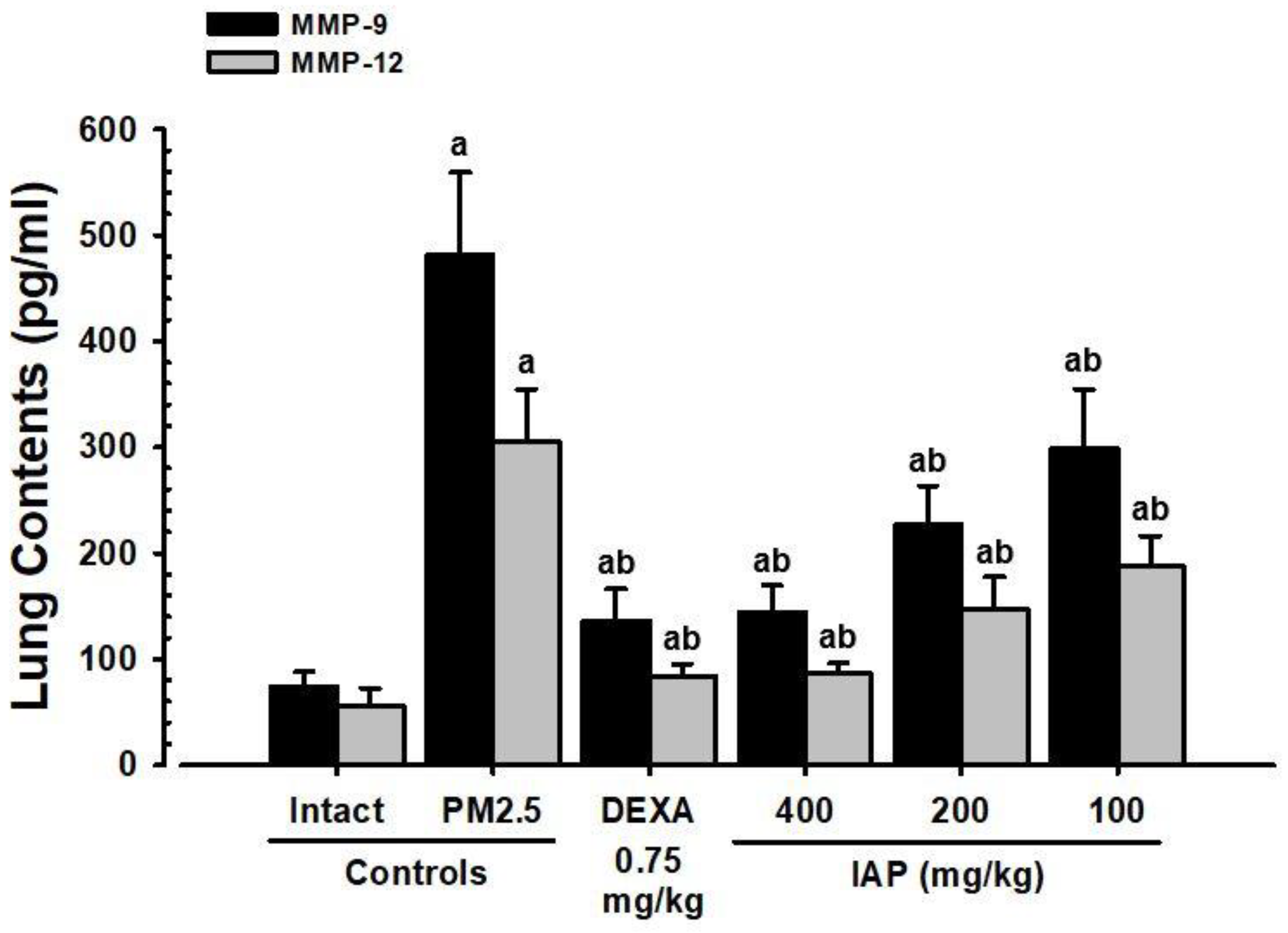
3.7. Changes in MMP-9 and MMP-12 Levels in Lung Tissue
3.8. Changes in Substance P and ACh Levels in Lung Tissue
3.9. Changes in Lipid Peroxidation and Antioxidant Defense System in Lung Tissue
3.10. Alterations in the mRNA Expression of MUC5B and MUC5AC, Which Are Related to Mucus Production in Lung Tissue
3.11. Alterations in the mRNA Expression of NF-κB, p38 MAPK, Akt, PI3K, and PTEN, Which Are Related to Oxidative Stress and Inflammation in Lung Tissue
3.12. Changes in Apoptosis-Related Bcl-2 and Bax mRNA Expression in Lung Tissue
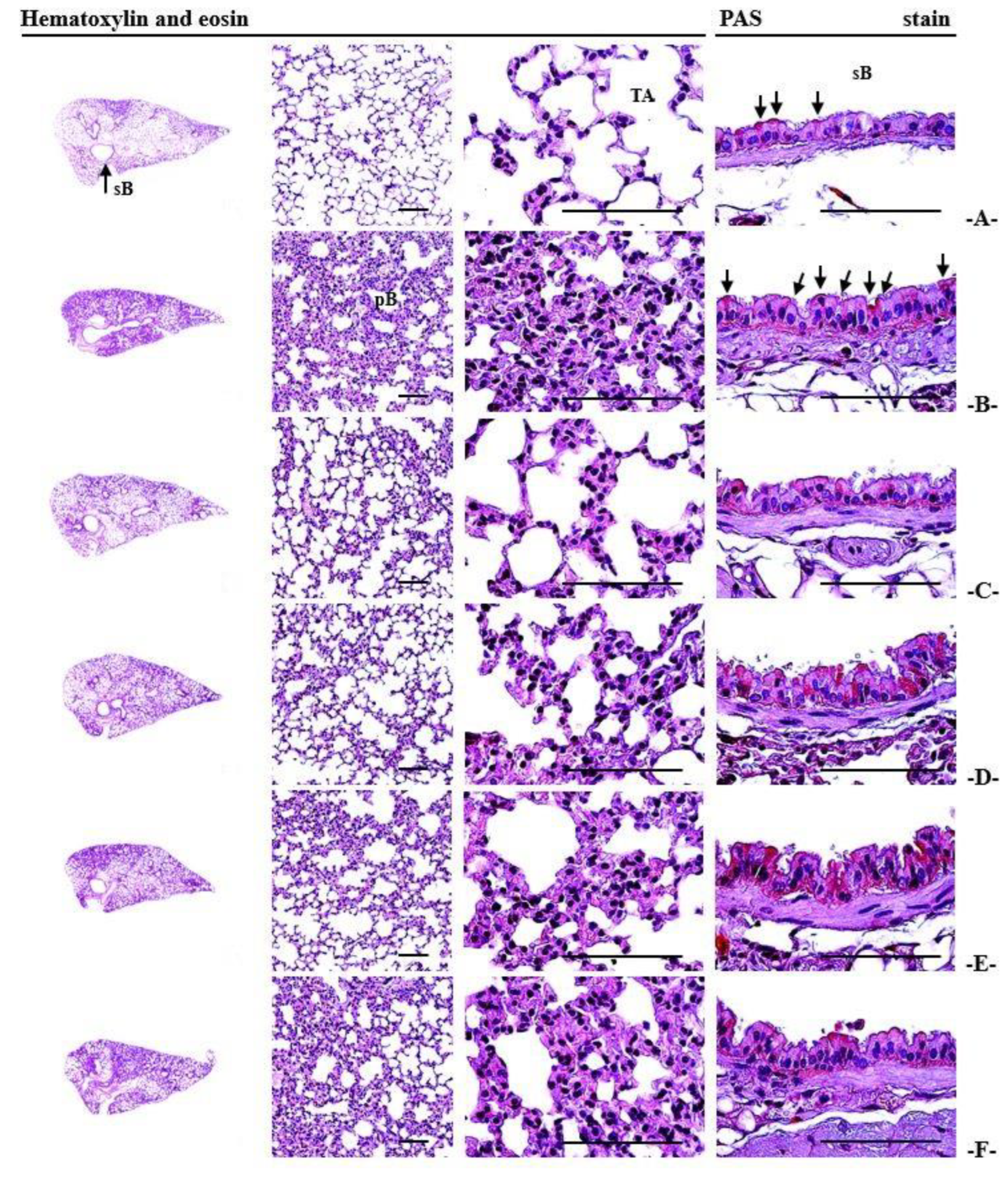
3.13. Histopathological Alterations Observed in the Lungs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.S.; Lee, W.W.; Vaas, A.P.J.P.; De Silva, H.I.C.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, S.H.; Song, G.Y.; Bae, J.S. Inhibitory effects of protopanaxatriol type ginsenoside fraction (Rgx365) on particulate matter-induced pulmonary injury. J. Toxicol. Environ. Health A 2019, 82, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ku, S.K.; Kim, J.E.; Cho, S.H.; Song, G.Y.; Bae, J.S. Inhibitory effects of black ginseng on particulate matter-induced pulmonary injury. Am. J. Chin. Med. 2019, 47, 1237–1251. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, G.; Guo, J.; Yuan, H.; Zhao, C. The compositions, sources, and size distribution of the dust storm from China in spring of 2000 and its impact on the global environment. Chin. Sci. Bull. 2001, 46, 895–900. [Google Scholar] [CrossRef]
- Wang, W.; Primbs, T.; Tao, S.; Simonich, S.L. Atmospheric particulate matter pollution during the 2008 Beijing Olympics. Environ. Sci. Technol. 2009, 43, 5314–5320. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.F.; He, L.Y.; Hu, M.; Zhang, Y.H. Annual variation of particulate organic compounds in PM2.5 in the urban atmosphere of Beijing. Atmos.Environ. 2006, 40, 2449–2458. [Google Scholar] [CrossRef]
- Lv, B.; Zhang, B.; Bai, Y. A systematic analysis of PM2.5 in Beijing and its sources from 2000 to 2012. Atmos. Environ. 2016, 124, 98–108. [Google Scholar] [CrossRef]
- Chiu, H.F.; Tsai, S.S.; Yang, C.Y. Short-term effects of fine particulate air pollution on hospital admissions for hypertension: A time-stratified case-crossover study in Taipei. J. Toxicol. Environ. Health A 2017, 80, 258–265. [Google Scholar] [CrossRef]
- WHO. Air Pollution. Available online: https://www.who.int/health-topics/air-pollution#tab=tab_1 (accessed on 15 December 2022).
- Nunes, C.; Pereira, A.M.; Morais-Almeida, M. Asthma costs and social impact. Asthma Res. Pract. 2017, 3, 1. [Google Scholar] [CrossRef]
- Wang, G.; Huang, L.; Gao, S.; Gao, S.; Wang, L. Measurements of PM10 and PM2.5 in urban area of Nanjing, China and the assessment of pulmonary deposition of particle mass. Chemosphere 2002, 48, 689–695. [Google Scholar] [CrossRef]
- Schaumann, F.; Borm, P.J.; Herbrich, A.; Knoch, J.; Pitz, M.; Schins, R.P.; Luettig, B.; Hohlfeld, J.M.; Heinrich, J.; Krug, N. Metal-rich ambient particles (particulate matter 2.5) cause airway inflammation in healthy subjects. Am. J. Respir. Crit. Care Med. 2004, 170, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, R.; Liu, H. The acute pulmonary toxicity in mice induced by Staphylococcus aureus, particulate matter, and their combination. Exp. Anim. 2019, 68, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, R.; De Berardis, B.; Paoletti, L.; Guastadisegni, C. Inflammatory mediators induced by coarse (PM2.5-10) and fine (PM2.5) urban air particles in RAW 264.7 cells. Toxicology 2003, 183, 243–254. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Jung, C.J.; Lee, D.G.; Choi, B.R.; Ku, T.H.; La, I.J.; Cho, I.J.; Ku, S.K. Adenophora stricta root extract protects lung injury from exposure to particulate matter 2.5 in mice. Antioxidants 2022, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Devipriya, D.; Gowri, S.; Nideesh, T.R. Hepatoprotective effect of Pterocarpus marsupium against carbon tetrachloride induced damage in albino rats. Anc. Sci. Life 2007, 27, 19–25. [Google Scholar] [PubMed]
- Kim, H.S.; Park, S.I.; Choi, S.H.; Song, C.H.; Park, S.J.; Shin, Y.K.; Han, C.H.; Lee, Y.J.; Ku, S.K. Single oral dose toxicity test of blue honeysuckle concentrate in mice. Toxicol. Res. 2015, 31, 61–68. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.; Fan, X.; Zhu, Z.; Zhu, Y. Effect of San’ao decoction on aggravated asthma mice model induced by PM2.5 and TRPA1/TRPV1 expressions. J. Ethnopharmacol. 2019, 236, 82–90. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Phenolic compounds and chromatographic profiles of pear skins (Pyrus spp.). J. Agric. Food Chem. 2008, 56, 9094–9101. [Google Scholar] [CrossRef]
- Tanrioven, D.; Eksi, A. Phenolic compounds in pear juice from different cultivars. Food Chem. 2005, 93, 89–93. [Google Scholar] [CrossRef]
- Lee, S.W.; Cho, J.Y.; Jeong, H.Y.; Na, T.W.; Lee, S.H.; Moon, J.H. Enhancement of antioxidative and antimicrobial activities of immature pear (Pyrus pyrifolia cv. Niitaka) fruits by fermentation with Leuconostoc mesenteroides. Food Sci. Biotechnol. 2016, 25, 1719–1726. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cho, J.Y.; Lee, H.J.; Ma, Y.K.; Kwon, J.; Park, S.H.; Lee, S.H.; Cho, J.A.; Kim, W.S.; Park, K.H.; et al. Hydroxycinnamoylmalic acids and their methyl esters from pear (Pyrus pyrifolia Nakai) fruit peel. J. Agric. Food Chem. 2011, 59, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Kim, C.H.; Lee, H.J.; Lee, S.H.; Cho, J.A.; Kim, W.S.; Park, K.H.; Moon, J.H. Caffeoyl triterpenes from pear (Pyrus pyrifolia Nakai) fruit peels and their antioxidative activities against oxidation of rat blood plasma. J. Agric. Food Chem. 2013, 61, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Cho, J.Y.; Lee, H.J.; Park, K.Y.; Ma, Y.K.; Lee, S.H.; Cho, J.A.; Kim, W.S.; Park, K.H.; Moon, J.H. Isolation and identification of phenolic compounds from an Asian pear (Pyrus pyrifolia Nakai) fruit peel. Food Sci. Biotechnol. 2011, 20, 1539–1545. [Google Scholar] [CrossRef]
- Lee, H.S.; Isse, T.; Kawamoto, T.; Baik, H.W.; Park, J.Y.; Yang, M. Effect of Korean pear (Pyrus pyrifolia cv. Shingo) juice on hangover severity following alcohol consumption. Food Chem. Toxicol. 2013, 58, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Cho, J.Y.; Kim, C.M.; Jeong, H.Y.; Lee, D.I.; Kim, S.R.; Lee, S.H.; Kim, W.S.; Park, K.H.; Moon, J.H. Isolation and identification of 3 low-molecular compounds from pear (Pyrus pyrifolia Nakai cv. Chuhwangbae) fruit peel. Korean J. Food Sci. Technol. 2013, 45, 174–179. [Google Scholar] [CrossRef][Green Version]
- Cho, J.Y.; Lee, Y.G.; Lee, S.H.; Kim, W.S.; Park, K.H.; Moon, J.H. An ether and three ester derivatives of phenylpropanoid from pear (Pyrus pyrifolia Nakai cv. Chuhwangbae) fruit and their radical-scavenging activity. Food Sci. Biotechnol. 2014, 23, 253–259. [Google Scholar] [CrossRef]
- Cho, J.Y.; Kim, E.H.; Yun, H.R.; Jeong, H.Y.; Lee, Y.G.; Kim, W.S.; Moon, J.H. Change in chemical constituents and free radical-scavenging activity during pear (Pyrus pyrifolia) cultivar fruit development. Biosci. Biotechnol. Biochem. 2015, 79, 260–270. [Google Scholar] [CrossRef]
- Lee, Y.G.; Cho, J.Y.; Park, J.; Lee, S.H.; Kim, W.S.; Park, K.H.; Moon, J.H. Large-scale isolation of highly pure malaxinic acid from immature pear (Pyrus pyrifolia Nakai) fruit. Food Sci. Biotechnol. 2013, 22, 1539–1545. [Google Scholar] [CrossRef]
- Lee, S.W.; Lee, Y.G.; Cho, J.Y.; Kim, Y.C.; Lee, S.H.; Kim, W.S.; Moon, J.H. Establishment of a simple method for purification of high purity chlorogenic acid from immature fruit of pear (Pyrus pyrifolia Nakai). Appl. Biol. Chem. 2015, 58, 335–341. [Google Scholar] [CrossRef]
- Cho, J.Y.; Park, K.Y.; Lee, K.H.; Lee, H.J.; Lee, S.H.; Cho, J.A.; Kim, W.S.; Shin, S.C.; Park, K.H.; Moon, J.H. Recovery of arbutin in high purity from fruit peels of pear (Pyrus pyrifolia Nakai). Food Sci. Biotechnol. 2011, 20, 801–807. [Google Scholar] [CrossRef]
- Hollander, J.L. Clinical use of dexamethasone: Role in treatment of patients with arthritis. J. Am. Med. Assoc. 1960, 172, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Min, B.G.; Park, S.M.; Choi, Y.W.; Ku, S.K.; Cho, I.J.; Kim, Y.W.; Byun, S.H.; Park, C.A.; Park, S.J.; Na, M.; et al. Effects of Pelargonium sidoides and Coptis Rhizoma 2 : 1 mixed formula (PS + CR) on ovalbumin-induced asthma in mice. Evid. Based Complement. Altern. Med. 2020, 2020, 9135637. [Google Scholar] [CrossRef] [PubMed]
- Piao, C.H.; Fan, Y.J.; Nguyen, T.V.; Song, C.H.; Chai, O.H. Mangiferin alleviates ovalbumin-induced allergic rhinitis via Nrf2/HO-1/NF-κB signaling pathways. Int. J. Mol. Sci. 2020, 21, 3415. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Cho, I.J.; Kim, J.W.; Lee, M.K.; Ku, S.K.; Choi, J.S.; Lee, H.J. Hepatoprotective effects of blue honeysuckle on CCl4-induced acute liver damaged mice. Food Sci. Nutr. 2019, 7, 322–338. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Li, D.; Ouyang, H.; Huang, J.; Long, Z.; Liang, Z.; Chen, Y.; Chen, Y.; Zheng, Q.; Kuang, M.; et al. Comparison and evaluation of two different methods to establish the cigarette smoke exposure mouse model of COPD. Sci. Rep. 2017, 7, 15454. [Google Scholar] [CrossRef] [PubMed]
- Glynos, C.; Bibli, S.I.; Katsaounou, P.; Pavlidou, A.; Magkou, C.; Karavana, V.; Topouzis, S.; Kalomenidis, I.; Zakynthinos, S.; Papapetropoulos, A. Comparison of the effects of e-cigarette vapor with cigarette smoke on lung function and inflammation in mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2018, 315, L662–L672. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase. In Methods in Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 673–686. [Google Scholar]
- Bannister, J.V.; Calabrese, L. Assays for superoxide dismutase. In Methods of Biochemical Analysis; Glick, D., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1987; Volume 32, pp. 279–312. [Google Scholar]
- Lebargy, F.; Lenormand, E.; Pariente, R.; Fournier, M. Morphological changes in rat tracheal mucosa immediately after antigen challenge. Bull. Eur. Physiopathol. Respir. 1987, 23, 417–421. [Google Scholar]
- Deng, X.; Rui, W.; Zhang, F.; Ding, W. PM2.5 induces Nrf2-mediated defense mechanisms against oxidative stress by activating PIK3/AKT signaling pathway in human lung alveolar epithelial A549 cells. Cell Biol. Toxicol. 2013, 29, 143–157. [Google Scholar] [CrossRef]
- Duong, C.; Seow, H.J.; Bozinovski, S.; Crack, P.J.; Anderson, G.P.; Vlahos, R. Glutathione peroxidase-1 protects against cigarette smoke-induced lung inflammation in mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2010, 299, L425–L433. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Ludbrook, J. Update: Microcomputer statistics packages. A personal view. Clin. Exp. Pharmacol. Physiol. 1997, 24, 294–296. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.K. What is the proper way to apply the multiple comparison test? Korean J. Anesthesiol. 2018, 71, 353–360. [Google Scholar] [CrossRef]
- Sauder, D.C.; DeMars, C.E. An updated recommendation for multiple comparisons. Adv. Methods Pract. Psychol. Sci. 2019, 2, 26–44. [Google Scholar] [CrossRef]
- Piper, S.L.; Laron, D.; Manzano, G.; Pattnaik, T.; Liu, X.; Kim, H.T.; Feeley, B.T. A comparison of lidocaine, ropivacaine and dexamethasone toxicity on bovine tenocytes in culture. J. Bone Jt. Surg. Br. 2012, 94, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.K.; Kim, J.W.; Cho, H.R.; Kim, K.Y.; Min, Y.H.; Park, J.H.; Kim, J.S.; Park, J.H.; Seo, B.I.; Roh, S.S. Effect of β-glucan originated from Aureobasidium pullulans on asthma induced by ovalbumin in mouse. Arch. Pharm. Res. 2012, 35, 1073–1081. [Google Scholar] [CrossRef]
- Fox, J.G.; Cohen, B.J.; Loew, F.M. Laboratory Animal Medicine; Academic Press. Inc.: Orlando, FL, USA, 1984. [Google Scholar]
- Tajima, Y. Biological Reference Data Book on Experimental Animals; Soft Science Inc.: Tokyo, Japan, 1989. [Google Scholar]
- Sodikoff, C.H. Laboratory Profiles of Small Animal Diseases, A Guide to Laboratory Diagnosis; Mosby: St. Louise, MO, USA, 1995; pp. 1–36. [Google Scholar]
- Tumes, D.J.; Cormie, J.; Calvert, M.G.; Stewart, K.; Nassenstein, C.; Braun, A.; Foster, P.S.; Dent, L.A. Strain-dependent resistance to allergen-induced lung pathophysiology in mice correlates with rate of apoptosis of lung-derived eosinophils. J. Leukoc. Biol. 2007, 81, 1362–1373. [Google Scholar] [CrossRef]
- Okada, S.; Kita, H.; George, T.J.; Gleich, G.J.; Leiferman, K.M. Migration of eosinophils through basement membrane components in vitro: Role of matrix metalloproteinase-9. Am. J. Respir. Cell Mol. Biol. 1997, 17, 519–528. [Google Scholar] [CrossRef]
- Tillie-Leblond, I.; Gosset, P.; Le Berre, R.; Janin, A.; Prangère, T.; Tonnel, A.B.; Guery, B.P. Keratinocyte growth factor improves alterations of lung permeability and bronchial epithelium in allergic rats. Eur. Respir. J. 2007, 30, 31–39. [Google Scholar] [CrossRef]
- Na, H.G.; Kim, Y.D.; Choi, Y.S.; Bae, C.H.; Song, S.Y. Diesel exhaust particles elevate MUC5AC and MUC5B expression via the TLR4-mediated activation of ERK1/2, p38 MAPK, and NF-κB signaling pathways in human airway epithelial cells. Biochem. Biophys. Res. Commun. 2019, 512, 53–59. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Zhao, P.; Tian, Y.; Liu, X.; He, H.; Jia, R.; Oliver, B.G.; Li, J. Exposure to air pollution exacerbates inflammation in rats with preexisting COPD. Mediators Inflamm. 2020, 2020, 4260204. [Google Scholar] [CrossRef] [PubMed]
- Rose, M.C.; Nickola, T.J.; Voynow, J.A. Airway mucus obstruction: Mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am. J. Respir. Cell Mol. Biol. 2001, 25, 533–537. [Google Scholar] [CrossRef]
- Groneberg, D.A.; Eynott, P.R.; Oates, T.; Lim, S.; Wu, R.; Carlstedt, I.; Nicholson, A.G.; Chung, K.F. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir. Med. 2002, 96, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Henke, M.O.; Renner, A.; Huber, R.M.; Seeds, M.C.; Rubin, B.K. MUC5AC and MUC5B mucins are decreased in cystic fibrosis airway secretions. Am. J. Respir. Cell Mol. Biol. 2004, 31, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chu, H.S.; Lee, J.Y.; Hwang, S.J.; Lee, S.H.; Lee, H.M. Up-regulation of MUC5AC and MUC5B mucin genes in chronic rhinosinusitis. Arch. Otolaryngol. Head Neck Surg. 2004, 30, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Ballard, S.T.; Spadafora, D. Fluid secretion by submucosal glands of the tracheobronchial airways. Respir. Physiol. Neurobiol. 2007, 159, 271–277. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ueki, I.; German, V.F.; Nadel, J.A. Micropipette measurement of airway submucosal gland secretion. Autonomic effects. Am. Rev. Respir. Dis. 1980, 121, 351–357. [Google Scholar]
- Haxhiu, M.A.; Haxhiu-Poskurica, B.; Moracic, V.; Carlo, W.A.; Martin, R.J. Reflex and chemical responses of trachea; submucosal glands in piglets. Respir. Physiol. 1990, 82, 267–278. [Google Scholar] [CrossRef]
- Trout, L.; Corboz, M.R.; Ballard, S.T. Mechanism of substance P-induced liquid secretion across porcine bronchial epithelium. Am. J. Physiol. 2001, 281, L639–L645. [Google Scholar]
- Phillips, J.E.; Hey, J.A.; Corboz, M.R. Tachykinin NK3 and NK1 receptor activation elicits secretion from porcine airway submucosal glands. Br. J. Pharmacol. 2003, 138, 254–260. [Google Scholar] [CrossRef][Green Version]
- Matsumoto, K.; Hosoya, T.; Tashima, K.; Namiki, T.; Murayama, T.; Horie, S. Distribution of trans ient receptor potential vanilloid 1 channel expressing nerve fibers in mouse rectal and colonic enteric nervous system: Relationship to peptidergic and nitrergic neurons. Neuroscience 2011, 172, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fan, X.; Wang, N.; Zhang, Y.; Yu, J. Exacerbating effects of PM2.5 in OVA-sensitized and challenged mice and the expression of TRPA1 and TRPV1 proteins in lungs. J. Asthma 2017, 54, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Wang, Z.; Chen, Z.; Jin, H.; Chen, C.; Chai, S.; Lv, H.; Yang, L.; Hu, Y.; Dong, R.; et al. Melatonin attenuates chronic cough mediated by oxidative stress via transient receptor potential melastatin-2 in guinea pigs exposed to particulate matter 2.5. Physiol. Res. 2018, 67, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Ren, Q.; Zhou, T.; Shang, L.; Ma, M.; Wang, B.; Xiao, C. Determination of endogenous substance change in PM2.5-induced rat plasma and lung samples by UPLC-MS/MS method to identify potential markers for lung impairment. Environ. Sci. Pollut. Res. Int. 2019, 26, 22040–22050. [Google Scholar] [CrossRef] [PubMed]
- Weldy, C.S.; Luttrell, I.P.; White, C.C.; Morgan-Stevenson, V.; Cox, D.P.; Carosino, C.M.; Larson, T.V.; Stewart, J.A.; Kaufman, J.D.; Kim, F.; et al. Glutathione (GSH) and the GSH synthesis gene Gclm modulate plasma redox and vascular responses to acute diesel exhaust inhalation in mice. Inhal. Toxicol. 2013, 25, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wu, W.; Zhang, W.; Zhao, Y.; Wu, Y.; Ge, G.; Ba, Y.; Guo, Q.; Gao, T.; Chi, X.; et al. Involvement of EGF receptor signaling and NLRP12 inflammasome in fine particulate matter-induced lung inflammation in mice. Environ. Toxicol. 2017, 32, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, H.J.; Heo, H.J. Ecklonia cava attenuates PM2.5-induced cognitive decline through mitochondrial activation and anti-inflammatory effect. Mar. Drugs 2021, 19, 131. [Google Scholar] [CrossRef] [PubMed]
- Videla, L.A. Energy metabolism, thyroid calorigenesis, and oxidative stress: Functional and cytotoxic consequences. Redox. Rep. 2000, 5, 265–275. [Google Scholar] [CrossRef]
- Subudhi, U.; Das, K.; Paital, B.; Bhanja, S.; Chainy, G.B. Alleviation of enhanced oxidative stress and oxygen consumption of L-thyroxine induced hyperthyroid rat liver mitochondria by vitamin E and curcumin. Chem. Biol. Interact. 2008, 173, 105–114. [Google Scholar] [CrossRef]
- Venditti, P.; Di Meo, S. Thyroid hormone-induced oxidative stress. Cell Mol. Life Sci. 2006, 63, 414–434. [Google Scholar] [CrossRef]
- Messarah, M.; Boumendjel, A.; Chouabia, A.; Klibet, F.; Abdennour, C.; Boulakoud, M.S.; Feki, A.E. Influence of thyroid dysfunction on liver lipid peroxidation and antioxidant status in experimental rats. Exp. Toxicol. Pathol. 2010, 62, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Chainy, G.B. Modulation of rat liver mitochondrial antioxidant defence system by thyroid hormone. Biochim. Biophys. Acta 2001, 1537, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Odabasoglu, F.; Cakir, A.; Suleyman, H.; Aslan, A.; Bayir, Y.; Halici, M.; Kazaz, C. Gastroprotective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J. Ethnopharmacol. 2006, 103, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Ribbons, K.A.; Thompson, J.H.; Liu, X.; Pennline, K.; Clark, D.A.; Miller, M.J. Anti-inflammatory properties of interleukin-10 administration in hapten-induced colitis. Eur. J. Pharmacol. 1997, 323, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Carini, M.; Aldini, G.; Piccone, M.; Facino, R.M. Fluorescent probes as markers of oxidative stress in keratinocyte cell lines following UVB exposure. Farmaco 2000, 55, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Zaki, M.H.; Man, S.M.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.D. Salmonella exploits NLRP12-dependent innate immune signaling to suppress host defenses during infection. Proc. Natl. Acad. Sci. USA 2014, 111, 385–390. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Dong, Y.L.; Kabir, S.M.; Lee, E.S.; Son, D.S. CXCR2-driven ovarian cancer progression involves upregulation of proinflammatory chemokines by potentiating NF-κB activation via EGFR-transactivated Akt signaling. PLoS ONE 2013, 8, e83789. [Google Scholar] [CrossRef]
- Zheng, H.; Ying, H.; Yan, H.; Kimmelman, A.C.; Hiller, D.J.; Chen, A.J.; Perry, S.R.; Tonon, G.; Chu, G.C.; Ding, Z.; et al. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature 2008, 455, 1129–1133. [Google Scholar] [CrossRef]
- Qi, Q.; Ling, Y.; Zhu, M.; Zhou, L.; Wan, M.; Bao, Y.; Liu, Y. Promoter region methylation and loss of protein expression of PTEN and significance in cervical cancer. Biomed. Rep. 2014, 2, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Li, L.; Kang, L.P.; Wang, Y. MicroRNA-92a promotes tumor growth and suppresses immune function through activation of MAPK/ERK signaling pathway by inhibiting PTEN in mice bearing U14 cervical cancer. Cancer Med. 2018, 7, 3118–3131. [Google Scholar] [CrossRef]
- Jiang, X.; Li, H. Overexpression of LRIG1 regulates PTEN via MAPK/MEK signaling pathway in esophageal squamous cell carcinoma. Exp. Ther. Med. 2016, 12, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Ebbesen, S.H.; Scaltriti, M.; Bialucha, C.U.; Morse, N.; Kastenhuber, E.R.; Wen, H.Y.; Dow, L.E.; Baselga, J.; Lowe, S.W. Pten loss promotes MAPK pathway dependency in HER2/neu breast carcinomas. Proc. Natl. Acad. Sci. USA 2016, 113, 3030–3035. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, D.; Zhang, H.; Yu, D.; Chen, R.; Zhang, B.; Tan, Y.; Niu, Y.; Duan, H.; Mai, B.; et al. The development of a cell-based model for the assessment of carcinogenic potential upon long-term PM2.5 exposure. Environ. Int. 2019, 131, 104943. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Yi, L.; Lan, L.L.; Wei, H.Y.; Wei, D. Long-term PM2.5 exposure increases the risk of non-small cell lung cancer (NSCLC) progression by enhancing interleukin-17a (IL-17a)-regulated proliferation and metastasis. Aging 2020, 12, 11579–11602. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.J.; Kim, E.J.; Oh, I.K.; Kim, Y.K.; Park, C.H.; Chung, J.H. Prevention of UV-induced skin damages by 11,14,17-eicosatrienoic acid in hairless mice in vivo. J. Korean Med. Sci. 2010, 25, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Kahari, V.M.; Saarialho-Kere, U. Matrix metalloproteinases in skin. Exp. Dermatol. 1997, 6, 199–213. [Google Scholar] [CrossRef]
- Woessner, J.F., Jr. Role of matrix proteases in processing enamel proteins. Connect. Tissue Res. 1998, 39, 69–73. [Google Scholar] [CrossRef]
- Nagase, H.; Woessne, J.F., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Guo, J.; Xiao, C. Effect of PM2.5 environmental pollution on rat lung. Environ. Sci. Pollut. Res. Int. 2018, 25, 36136–36146. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhao, Y.; Wang, Q.; Chen, H.; Zhou, X. Fine particulate matter exposure promotes M2 macrophage polarization through inhibiting histone deacetylase 2 in the pathogenesis of chronic obstructive pulmonary disease. Ann. Transl. Med. 2020, 8, 1303. [Google Scholar] [CrossRef] [PubMed]
- Monian, P.; Jiang, X. Clearing the final hurdles to mitochondrial apoptosis: Regulation post cytochrome C release. Exp. Oncol. 2012, 34, 185–191. [Google Scholar] [PubMed]
- Wang, A.S.; Xu, Y.; Zhang, Z.W.; Lu, B.B.; Yin, X.; Yao, A.J.; Han, L.Y.; Zou, Z.Q.; Li, Z.; Zhang, X.H. Sulforaphane protects MLE-12 lung epithelial cells against oxidative damage caused by ambient air particulate matter. Food Funct. 2017, 8, 4555–4562. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, Z.; Zhang, C.; Zhang, X.; Meng, Q.; Wu, S.; Wang, S.; Yin, L.; Pu, Y.; Chen, R. MicroRNA-1228(*) inhibit apoptosis in A549 cells exposed to fine particulate matter. Environ. Sci. Pollut. Res. Int. 2016, 23, 10103–10113. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell. 2014, 5, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Jung, T.Y.; Ku, S.K.; Yang, H.B.; Lee, H.S. Toxico-pathological study of p,p-DDE after experimental aerosol exposed to ICR Mouse. Toxicol. Res. 2005, 21, 151–160. [Google Scholar]
- Honda, H.; Fujimoto, M.; Miyamoto, S.; Ishikawa, N.; Serada, S.; Hattori, N.; Nomura, S.; Kohno, N.; Yokoyama, A.; Naka, T. Sputum Leucine-rich alpha-2 glycoprotein as a marker of airway inflammation in asthma. PLoS ONE 2016, 11, e0162672. [Google Scholar] [CrossRef]
- Hu, J.R.; Jung, C.J.; Ku, S.M.; Jung, D.H.; Ku, S.K.; Choi, J.S. Antitussive, expectorant, and anti-inflammatory effects of Adenophorae Radix powder in ICR mice. J. Ethnopharmacol. 2019, 239, 111915. [Google Scholar] [CrossRef]
- Wang, D.; Wang, S.; Chen, X.; Xu, X.; Zhu, J.; Nie, L.; Long, X. Antitussive, expectorant and anti-inflammatory activities of four alkaloids isolated from Bulbus of Fritillaria wabuensis. J. Ethnopharmacol. 2012, 139, 189–193. [Google Scholar] [CrossRef]
- Negara, B.F.S.P.; Kim, J.W.; Bashir, K.M.I.; Lee, J.H.; Ku, M.U.; Kim, K.Y.; Shin, S.; Hong, E.-J.; Ku, S.K.; Choi, J.-S. Expectorant effects of immature Asian pear extract on PM2.5-induced subacute pulmonary injury in mice. J. Food Biochem. 2023, 2023, 5671679. [Google Scholar] [CrossRef]
- Kim, M.R.; Bashir, K.M.I.; Lee, J.H.; Ku, M.U.; Kim, J.W.; Kim, K.Y.; Shin, S.; Hong, E.-J.; Ku, S.K.; Choi, J.-S. Efficacy confirm test of immature Asian pear extracts on the ovalbumin-induced asthma in mice. Appl. Sci. 2023, 13, 9342. [Google Scholar] [CrossRef]



| Items (Unit) Groups | Lung Weights | Congestional Regions (%)–Gross Findings | |
|---|---|---|---|
| Absolute (g) | Relative (%) | ||
| Controls | |||
| vehicle | 0.125 ± 0.005 | 0.640 ± 0.033 | 1.64 ± 1.58 |
| PM2.5 | 0.185 ± 0.008 a | 0.947 ± 0.061 a | 68.07 ± 12.46 a |
| DEXA | 0.136 ± 0.007 bc | 0.735 ± 0.036 ac | 8.72 ± 2.66 ac |
| Test substance–IAP | |||
| 400 mg/kg | 0.142 ± 0.015 c | 0.722 ± 0.080 c | 9.02 ± 2.57 ac |
| 200 mg/kg | 0.153 ± 0.009 ac | 0.771 ± 0.046 ac | 31.68 ± 11.46 ac |
| 100 mg/kg | 0.161 ± 0.010 ac | 0.829 ± 0.063 ac | 42.49 ± 11.09 ac |
| Items Groups | Total Cells | Total Leukocytes | Differential Counts | |||
|---|---|---|---|---|---|---|
| Lymphocytes | Neutrophils | Eosinophils | Monocytes | |||
| Controls | ||||||
| vehicle | 9.90 ± 2.02 | 6.70 ± 1.83 | 3.50 ± 1.35 | 1.25 ± 0.49 | 0.02 ± 0.02 | 1.28 ± 0.41 |
| PM2.5 | 79.70 ± 9.27 a | 60.30 ± 6.88 a | 35.70 ± 6.38 a | 13.72 ± 1.51 a | 1.85 ± 0.20 a | 7.45 ± 0.87 a |
| DEXA | 20.70 ± 3.86 ac | 13.30 ± 3.33 ac | 7.30 ± 3.27 c | 2.46 ± 0.81 bc | 0.28 ± 0.22 bc | 2.20 ± 0.40 ac |
| Test substance–IAP | ||||||
| 400 mg/kg | 21.40 ± 4.33 ac | 13.80 ± 2.82 ac | 7.40 ± 2.22 ac | 2.64 ± 0.86 ac | 0.32 ± 0.19 ac | 2.32 ± 0.75 bc |
| 200 mg/kg | 43.30 ± 4.88 ac | 32.20 ± 4.94 ac | 19.50 ± 3.87 ac | 6.77 ± 1.60 ac | 0.93 ± 0.36 ac | 3.70 ± 1.04 ac |
| 100 mg/kg | 54.40 ± 7.26 ac | 40.00 ± 5.03 ac | 23.50 ± 4.95 ac | 8.74 ± 1.48 ac | 1.21 ± 0.25 ac | 4.79 ± 1.03 ac |
| Items (Unit) Groups | Lung Contents (pg/mL) | |||
|---|---|---|---|---|
| TNF-α | IL-6 | CXCL1 | CXCL2 | |
| Controls | ||||
| vehicle | 30.55 ± 10.65 | 30.32 ± 12.46 | 32.54 ± 10.30 | 17.92 ± 5.80 |
| PM2.5 | 250.19 ± 43.99 a | 453.02 ± 86.77 a | 352.12 ± 80.52 a | 198.62 ± 25.01 a |
| DEXA | 68.92 ± 11.80 ab | 119.94 ± 47.36 ab | 97.70 ± 21.55 ab | 54.88 ± 16.88 ab |
| Test substance–IAP | ||||
| 400 mg/kg | 69.65 ± 12.87 ab | 122.59 ± 28.96 ab | 101.86 ± 29.95 ab | 56.88 ± 19.16 ab |
| 200 mg/kg | 115.42 ± 28.16 ab | 212.47 ± 53.70 ab | 162.32 ± 37.62 ab | 94.80 ± 32.39 ab |
| 100 mg/kg | 156.02 ± 26.86 ab | 289.37 ± 56.65 ab | 215.30 ± 27.37 ab | 124.52 ± 20.14 ab |
| Items (Unit) Groups | Lung Contents (nM/mg Protein) | Lung Enzyme Activity (U/mg Protein) | |||
|---|---|---|---|---|---|
| MDA | ROS | GSH | SOD | CAT | |
| Controls | |||||
| vehicle | 3.39 ± 0.97 | 23.29 ± 10.28 | 42.70 ± 10.63 | 337.60 ± 51.59 | 75.30 ± 14.03 |
| PM2.5 | 21.87 ± 4.56 c | 96.66 ± 11.71 a | 6.36 ± 2.05 c | 60.00 ± 18.68 cd | 9.40 ± 2.01 c |
| DEXA | 8.58 ± 1.44 cd | 43.15 ± 8.08 ab | 18.84 ± 4.56 cd | 189.60 ± 39.04 cd | 30.00 ± 7.32 cd |
| Test substance–IAP | |||||
| 400 mg/kg | 8.91 ± 2.46 cd | 44.30 ± 9.58 ab | 20.28 ± 3.86 cd | 197.40 ± 34.58 cd | 30.80 ± 12.45 cd |
| 200 mg/kg | 12.65 ± 2.97 cd | 57.72 ± 13.11 ab | 16.44 ± 3.03 cd | 157.00 ± 23.97 cd | 24.90 ± 7.17 cd |
| 100 mg/kg | 14.82 ± 1.69 cd | 66.53 ± 13.73 ab | 12.74 ± 3.28 cd | 129.30 ± 14.26 cd | 19.60 ± 3.10 cd |
| Groups Items | Controls | Test Substance–IAP | ||||
|---|---|---|---|---|---|---|
| Vehicle | PM2.5 | DEXA | 400 mg/kg | 200 mg/kg | 100 mg/kg | |
| MUC5AC | 1.00 ± 0.07 | 5.15 ± 0.57 c | 2.05 ± 0.43 ce | 2.10 ± 0.43 ce | 2.91 ± 0.46 ce | 3.61 ± 0.76 ce |
| MUC5B | 1.00 ± 0.06 | 2.95 ± 0.46 c | 1.61 ± 0.25 ce | 1.64 ± 0.22 ce | 1.93 ± 0.12 ce | 2.08 ± 0.19 ce |
| NF-κB | 1.00 ± 0.06 | 9.20 ± 1.59 c | 2.32 ± 0.83 ce | 2.57 ± 1.16 de | 4.08 ± 0.91 ce | 5.62 ± 1.03 ce |
| p38 MAPK | 1.00 ± 0.06 | 8.31 ± 0.88 c | 2.33 ± 0.53 ce | 2.39 ± 0.58 ce | 3.89 ± 1.17 ce | 5.38 ± 1.01 ce |
| PTEN | 1.00 ± 0.07 | 0.28 ± 0.10 a | 0.71 ± 0.12 ab | 0.70 ± 0.08 ab | 0.60 ± 0.10 ab | 0.52 ± 0.05 ab |
| PI3K | 1.00 ± 0.07 | 6.36 ± 0.72 c | 1.93 ± 0.36 ce | 1.99 ± 0.24 ce | 3.09 ± 0.81 ce | 4.33 ± 0.88 ce |
| Akt | 1.00 ± 0.06 | 5.45 ± 1.43 c | 1.83 ± 0.34 ce | 1.85 ± 0.19 ce | 2.65 ± 0.43 ce | 3.55 ± 0.47 cf |
| Bcl-2 | 1.00 ± 0.06 | 0.35 ± 0.07 a | 0.72 ± 0.11 ab | 0.71 ± 0.11 ab | 0.60 ± 0.10 ab | 0.52 ± 0.06 ab |
| Bax | 1.00 ± 0.06 | 7.18 ± 1.41 c | 2.70 ± 0.84 ce | 2.72 ± 1.23 de | 3.85 ± 0.82 ce | 4.75 ± 0.60 ce |
| Items Groups | Mean ASA (%/mm2) | Mean Alveolar Septal Thickness (μm) | Mean Thickness of SB (μm) | Mean IF Cell Numbers Infiltrated in AR (×10 cells/mm2) | PAS-Positive Cells on the SB (Cells/mm2) |
|---|---|---|---|---|---|
| Controls | |||||
| vehicle | 81.34 ± 8.03 | 7.52 ± 1.30 | 15.68 ± 1.81 | 62.00 ± 13.70 | 23.00 ± 3.16 |
| PM2.5 | 35.24 ± 7.72 a | 7.25 ± 10.07 a | 21.39 ± 1.34 a | 732.40 ± 140.45 a | 36.80 ± 4.54 a |
| DEXA | 71.16 ± 7.40 b | 21.77 ± 4.66 ab | 21.05 ± 2.04 a | 180.20 ± 64.34 ab | 34.00 ± 12.07 |
| Test substance–IAP | |||||
| 400 mg/kg | 57.25 ± 7.79 ab | 21.95 ± 5.39 ab | 31.60 ± 2.77 ab | 184.80 ± 30.01 ab | 86.20 ± 14.19 ab |
| 200 mg/kg | 60.39 ± 7.96 ab | 26.37 ± 5.00 ab | 28.82 ± 2.25 ab | 305.00 ± 59.75 ab | 69.60 ± 14.57 ab |
| 100 mg/kg | 50.54 ± 2.87 ab | 30.76 ± 4.50 ab | 26.59 ± 1.63 ab | 399.60 ± 90.98 ab | 53.60 ± 10.00 ac |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-R.; Lee, J.-H.; Ku, M.-U.; Kim, K.-Y.; Shin, S.; Hong, E.-J.; Ku, S.-K.; Choi, J.-S. Effectiveness of Immature Asian Pear Extract on Pulmonary Injury Caused by Particulate Matter in Mice. Appl. Sci. 2023, 13, 9578. https://doi.org/10.3390/app13179578
Kim M-R, Lee J-H, Ku M-U, Kim K-Y, Shin S, Hong E-J, Ku S-K, Choi J-S. Effectiveness of Immature Asian Pear Extract on Pulmonary Injury Caused by Particulate Matter in Mice. Applied Sciences. 2023; 13(17):9578. https://doi.org/10.3390/app13179578
Chicago/Turabian StyleKim, Mi-Ran, Jin-Hwa Lee, Mo-Un Ku, Ki-Young Kim, Su Shin, Eun-Jin Hong, Sae-Kwang Ku, and Jae-Suk Choi. 2023. "Effectiveness of Immature Asian Pear Extract on Pulmonary Injury Caused by Particulate Matter in Mice" Applied Sciences 13, no. 17: 9578. https://doi.org/10.3390/app13179578
APA StyleKim, M.-R., Lee, J.-H., Ku, M.-U., Kim, K.-Y., Shin, S., Hong, E.-J., Ku, S.-K., & Choi, J.-S. (2023). Effectiveness of Immature Asian Pear Extract on Pulmonary Injury Caused by Particulate Matter in Mice. Applied Sciences, 13(17), 9578. https://doi.org/10.3390/app13179578







