Continuous-Flow Microwave Heating Inactivation Kinetics of α-Amylase from Bacillus subtilis and a Comparison with Conventional Heating Conditions
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzyme and Different Buffers
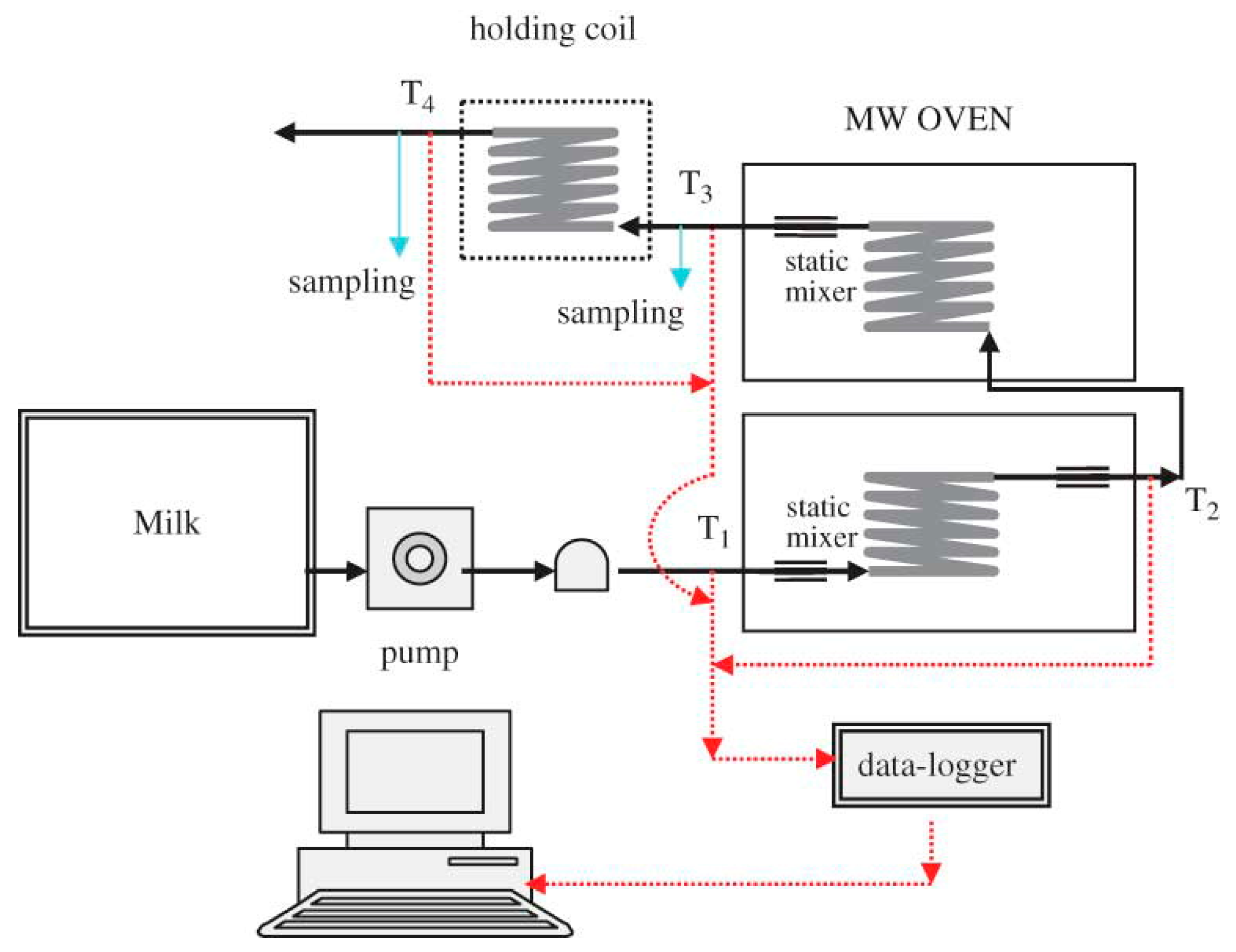
2.2. Microwave Heating System
2.3. Kinetic Data Analysis
3. Results
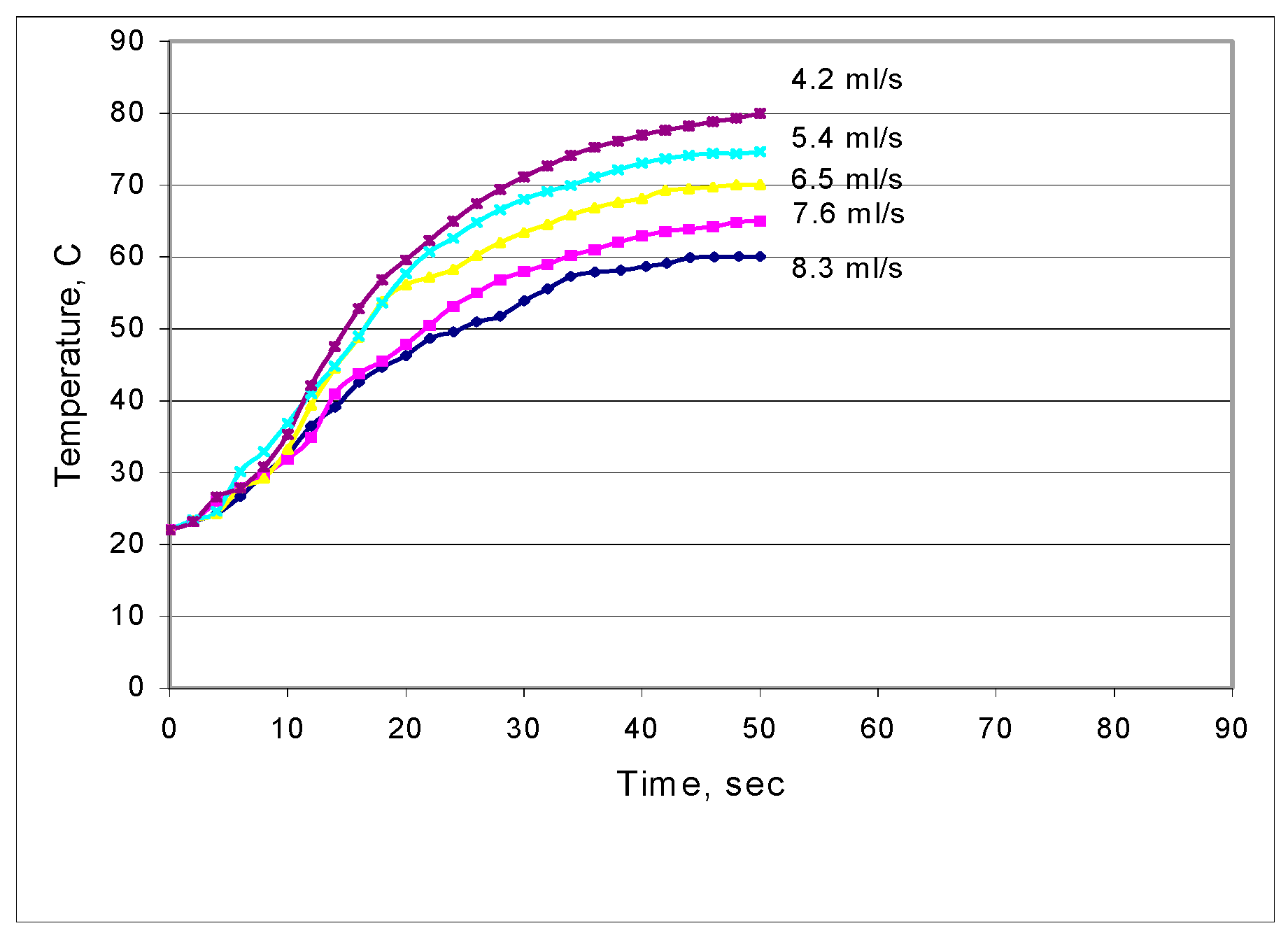
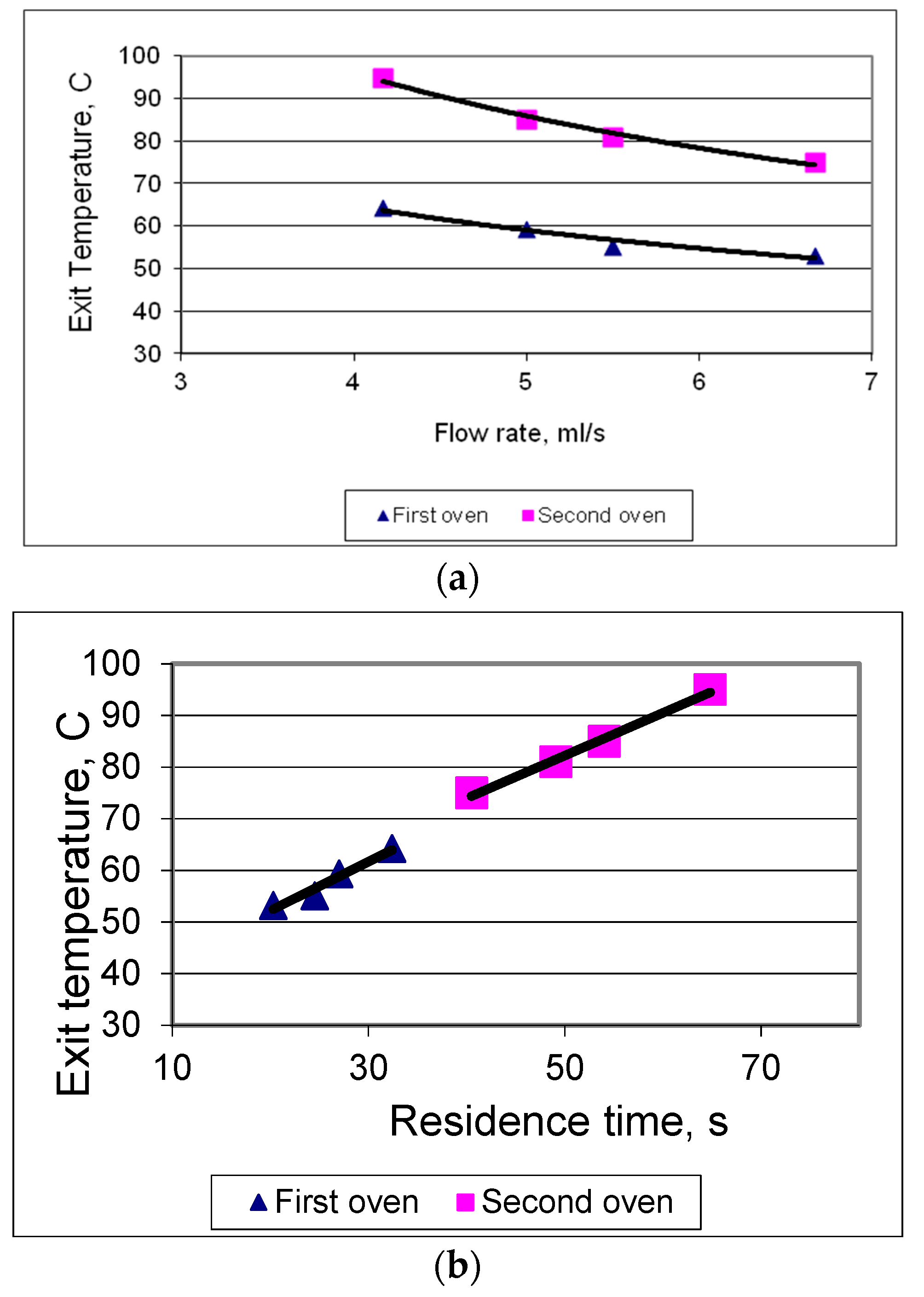
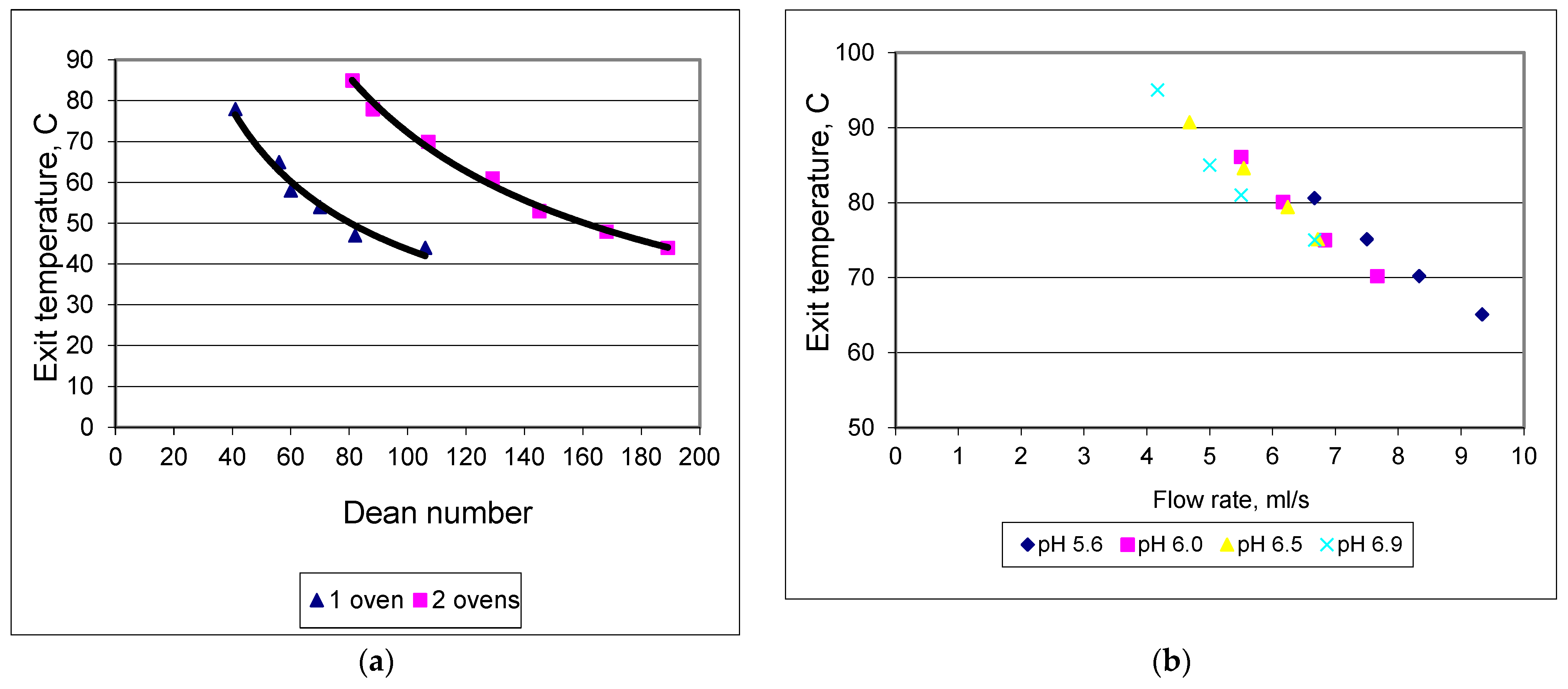
3.1. Microwave Heating Characteristics
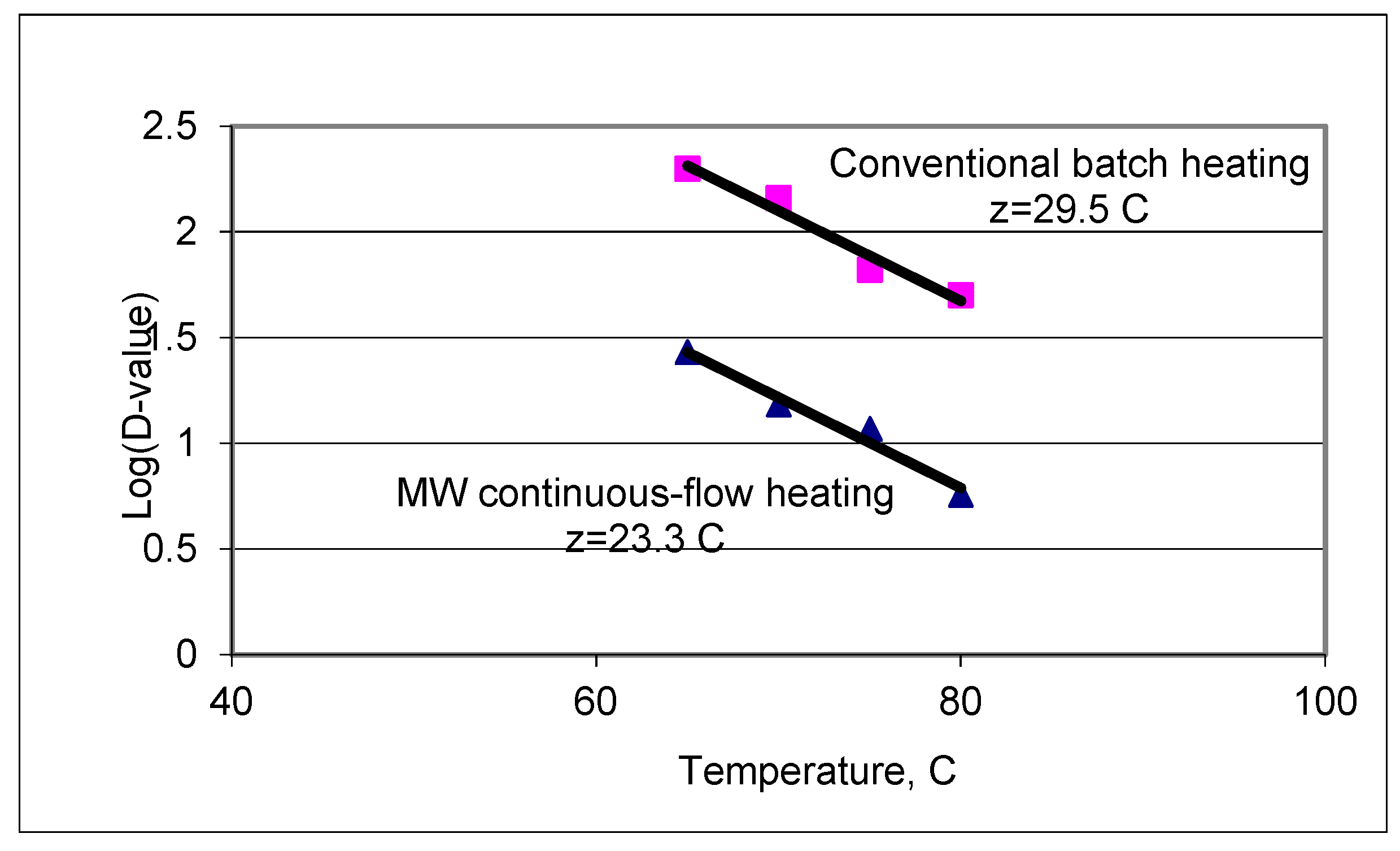
3.2. Decimal Reduction Time Comparisons
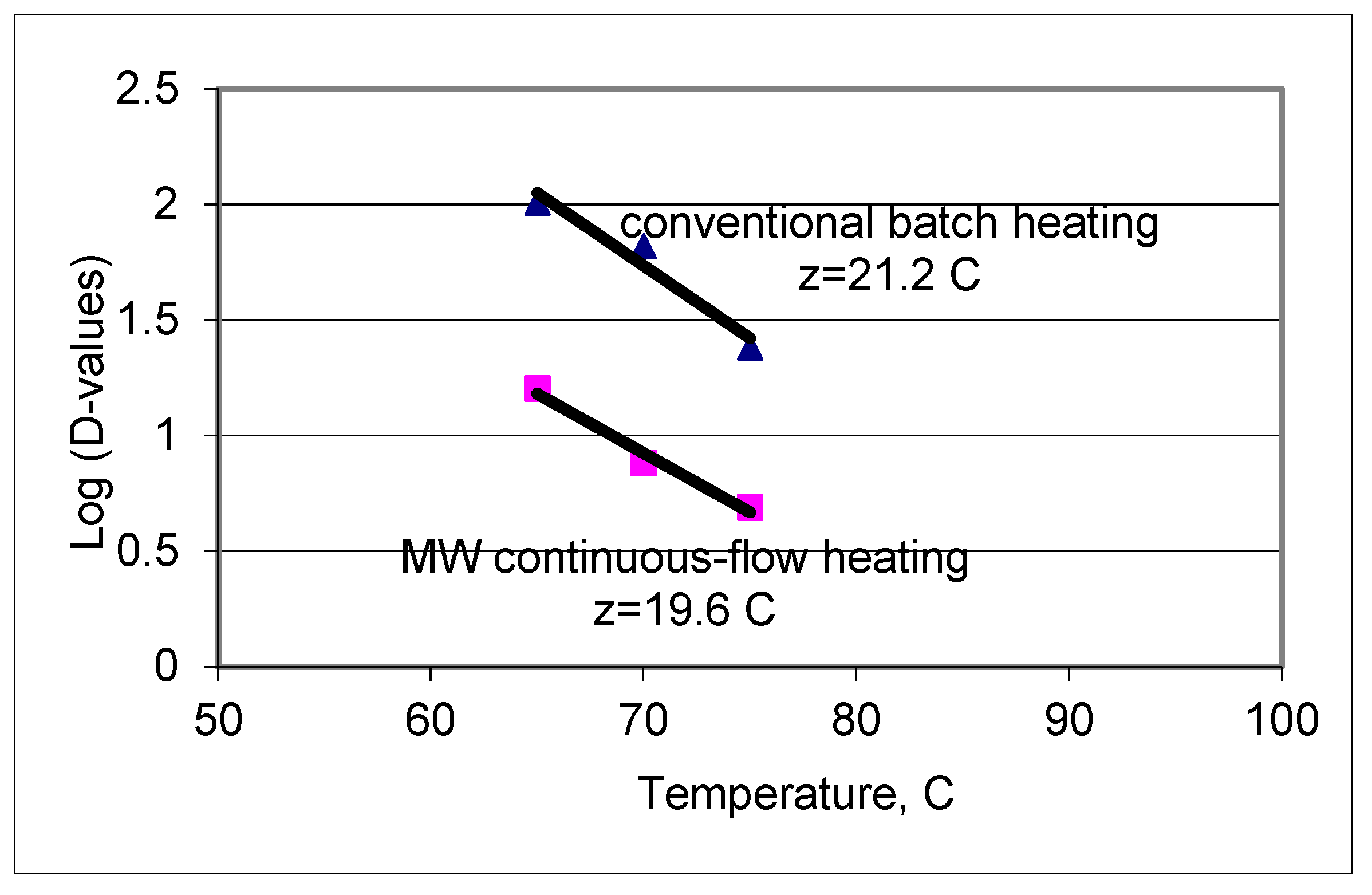
3.3. Enzyme Inactivation Profiles under Continuous-flow Thermal Hold Section
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mudgett, R.E. Microwave properties and heating characteristics of foods. Food Technol. 1986, 40, 84–93. [Google Scholar]
- Mudgett, R.E. Microwave food processing. A Scientific Status Summary by the lFT Expert Panel on Food Safety and Nutrition. Food Technol. 1989, 43, 117–126. [Google Scholar]
- Knutson, K.M.; Marth, E.H.; Wagner, M.K. Use of microwave ovens to pasteurize milk. J. Food Prot. 1988, 51, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Kudra, T.; Van de Voort, F.R.; Raghavan, G.S.v.; Ramaswamy, H.S. Heating characteristics of milk constituents in a microwave pasteurization system. J. Food Sci. 1991, 56, 931–937. [Google Scholar] [CrossRef]
- Nikdel, S.; Mackellar, D.G. A microwave system for continuous pasteurization of orange juice. Proc. Fla. State Hort. Soc. 1992, 105, 108–110. [Google Scholar]
- Tajchakavit, S.; Rarnaswamy, H.S. Continuous-Flow Microwave Heating of Orange Juice: Evidence of Non-Thermal Effects. J. Microw. Power Electromagn. Energy 1995, 30, 141–148. [Google Scholar]
- Tajchakavit, S.; Rarnaswamy, H.S. Continuous-flow microwave inactivation kinetics of pectin rnethyl esterase in orange juice. J. Food Process. Preserv. 1997, 21, 365–378. [Google Scholar] [CrossRef]
- Tajchakavit, S.; Rarnaswamy, H.S. Thermal vs. Microwave Inactivation Kinetics of Pectin Methylesterase in Orange Juice under Batch Mode Heating Condition. Lebensm.-Wiss. Technol. 1997, 30, 85–93. [Google Scholar] [CrossRef]
- Tajchakavit, S.; Rarnaswamy, H.S.; Fustier, P. Enhanced destruction of spoilage microorganisrns in apple juice during continuous flow microwave heating. Food Res. Int. 1998, 31, 713–722. [Google Scholar]
- Fujikawa, H. Patterns of bacterial destruction in solutions by microwave irradiation. J. Appl. Bacteriol. 1994, 76, 389–394. [Google Scholar]
- Welt, B.A.; Tong, C.H.; Rossen, J.L.; Lund, D.B. Effect of microwave radiation on inactivation of Clostridium sporogenes (PA) spores. Appl. Environ. Microbiol. 1994, 60, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.H. Effect of Microwaves on Biological and Chemical Systems. Microw. World 1996, 7, 14–23. [Google Scholar]
- Kermasha, S.; Bisakowski, B.; Ramaswamy, H.S.; Van de Voort, F.R. Comparison of microwave, conventional and combination treatment inactivation on wheat germ lipase activity. Int. J. Food Sci. Technol. 1993, 28, 617–623. [Google Scholar] [CrossRef]
- Kermasha, S.; Bisakowski, B.; Ramaswarny, H.S.; Van de Voort, F.R. Thermal and microwave inactivation of soybean lipoxygenase. Lebensm.-Wiss. Technol. 1993, 26, 215–219. [Google Scholar]
- Kozempel, M.; Annous, B.A.; Cook, R.; Scullen, O.J.; Whiting, R. Inactivation of microorganisms with microwaves at reduced temperature. J. Food Prot. 1998, 61, 582–585. [Google Scholar]
- Chen, Z.; Li, Y.; Wang, L.; Liu, S.; KWang, K.; Sun, J.; Xu, B. Evaluation of the possible non-thermal effect of microwave radiation on the inactivation of wheat germ lipase. J. Food Process Eng. 2016, 40, e12506. [Google Scholar] [CrossRef]
- Xu, B.; Wang, L.K.; Miao, W.J.; Wu, Q.F.; Liu, Y.X.; Sun, Y.; Gao, C. Thermal versus microwave inactivation kinetics of lipase and lipoxygenase from wheat germ. J. Food Process Eng. 2016, 39, 247–255. [Google Scholar]
- Siguemoto, E.S.; Pereira, L.J.; Gut, J.A.W. Inactivation kinetics of pectin methylesterase, polyphenol oxidase, and peroxidase in cloudy apple juice under microwave and conventional heating to evaluate non-thermal microwave effects. Innov. Food Sci. Emerg. Technol. 2018, 45, 84–91. [Google Scholar]
- Cavalcante, T.A.B.B.; Funcia, E.D.S.; Gut, J.A.W. Inactivation of polyphenol oxidase by microwave and conventional heating: Investigation of thermal and non-thermal effects of focused microwaves. Food Chem. 2021, 340, 127911. [Google Scholar]
- Kubo, M.T.K.; Siguemoto, E.S.; Funica, E.S.; Augusto, P.E.D.; Curet, S.; Boillereaux, L.; Sastry, S.; Gut, J.A.W. Non-thermal effects of microwave and ohmic processing on microbial and enzyme inactivation: A critical review. Curr. Opin. Food Sci. 2020, 35, 36–48. [Google Scholar]
- Gençdağ, E.; Görgüç, A.; Anakiz, S.; Yılmaz, F.M. Processing of verjuice by ultrasound-assisted microwave heating: An assessment on the enzyme activity retention, technological parameters, and bioactive properties. Food Sci. Technol. Int. 2023. [Google Scholar] [CrossRef]
- Ceci, L.; Lozano, J. Amylase for Apple Juice Processing: Effects of pH, Heat, and Ca2+ Ions. Food Technol. Biotechnol. 2002, 40, 33–38. [Google Scholar]
- LeBail, A.; Koutchma, T.; Ramaswamy, H.S. Modeling of temperature profiles under continuous tube-flow microwave and steam heating conditions. J. Food Process Eng. 2000, 23, 1–24. [Google Scholar]
- Dravid, A.N.; Smith, K.A.; Merrill, E.W.; Brian, P.L.T. Effect of secondary fluid motion on laminar flow heat transfer in helically coiled tubes. Am. Inst. Chem. Eng. J. 1971, 17, 1114–1122. [Google Scholar]
- Ramaswamy, H.S.; Koutchma, T.; Tajchakavit, S. Enhanced Thermal Effects Under Microwave Heating Conditions. In Engineering and Food for the 21st Century; Welti-Chanes, J., Barbosa-Cánovas, G.V., Aguilera, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2002; Chapter 45; pp. 739–762. [Google Scholar]
- Riva, M.; Lucisano, M.; GaUi, M.; Armatori, A. Comparative microbial lethality and thermal damage during microwave and conventional heating in mussels (Mytilus edulis). Ann. Microbiol. 1991, 41, 147–160. [Google Scholar]
- Aktas, N.; Ozligel1, M. Injury of E. coli and degradation ofriboflavin during pasteurizaion with microwaves in a tubular flow reactor. Lebensm. Wiss. Technol. 1992, 25, 422–425. [Google Scholar]
- van Boekel, M.A. On the use of the Weibull model to describe thermal inactivation of microbial vegetative cells. Int. J. Food Microbiol. 2002, 74, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Stumbo, C.R. Thermobacteriology in Food Processing; Academic Press: New York, NY, USA, 1973. [Google Scholar]
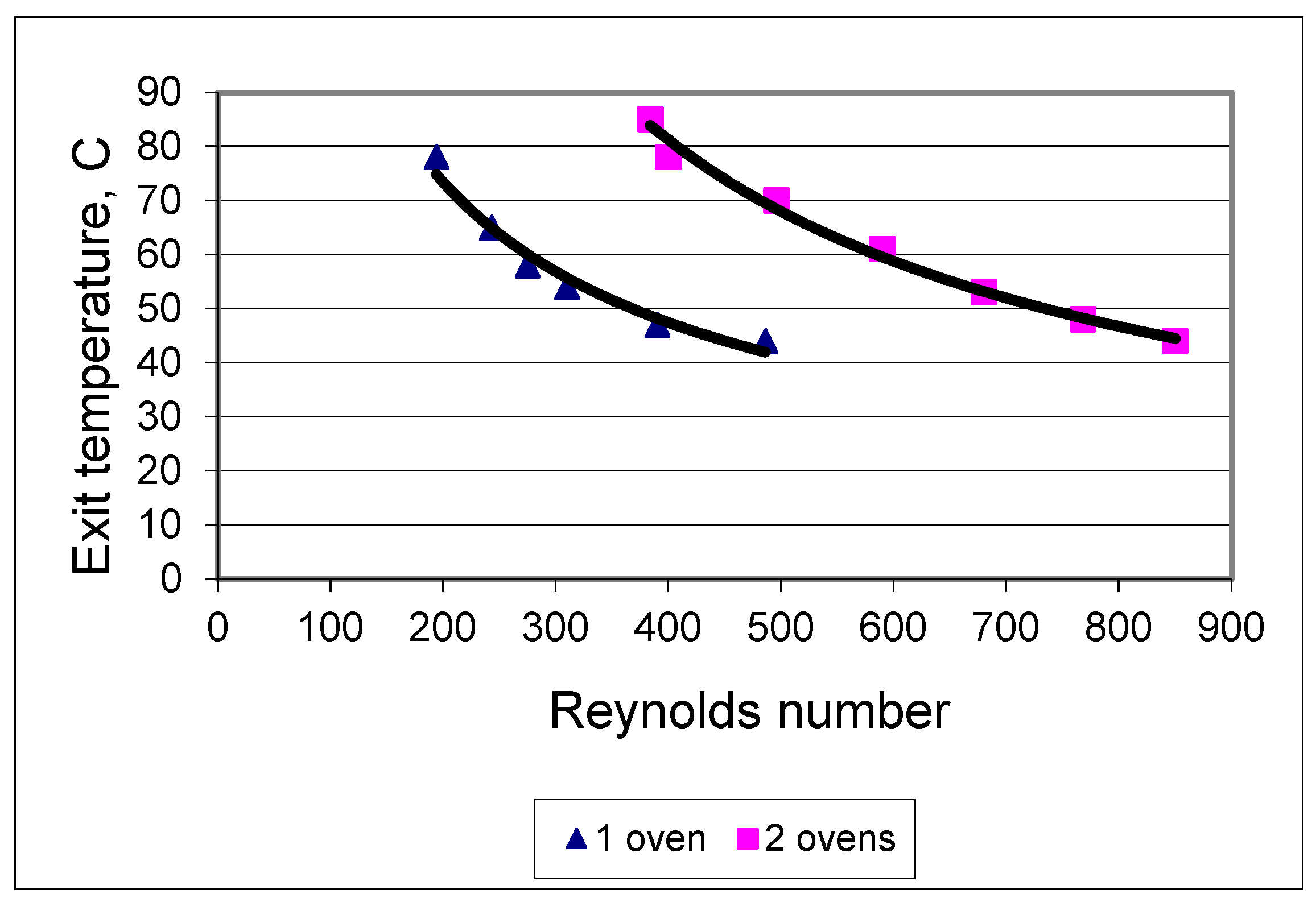
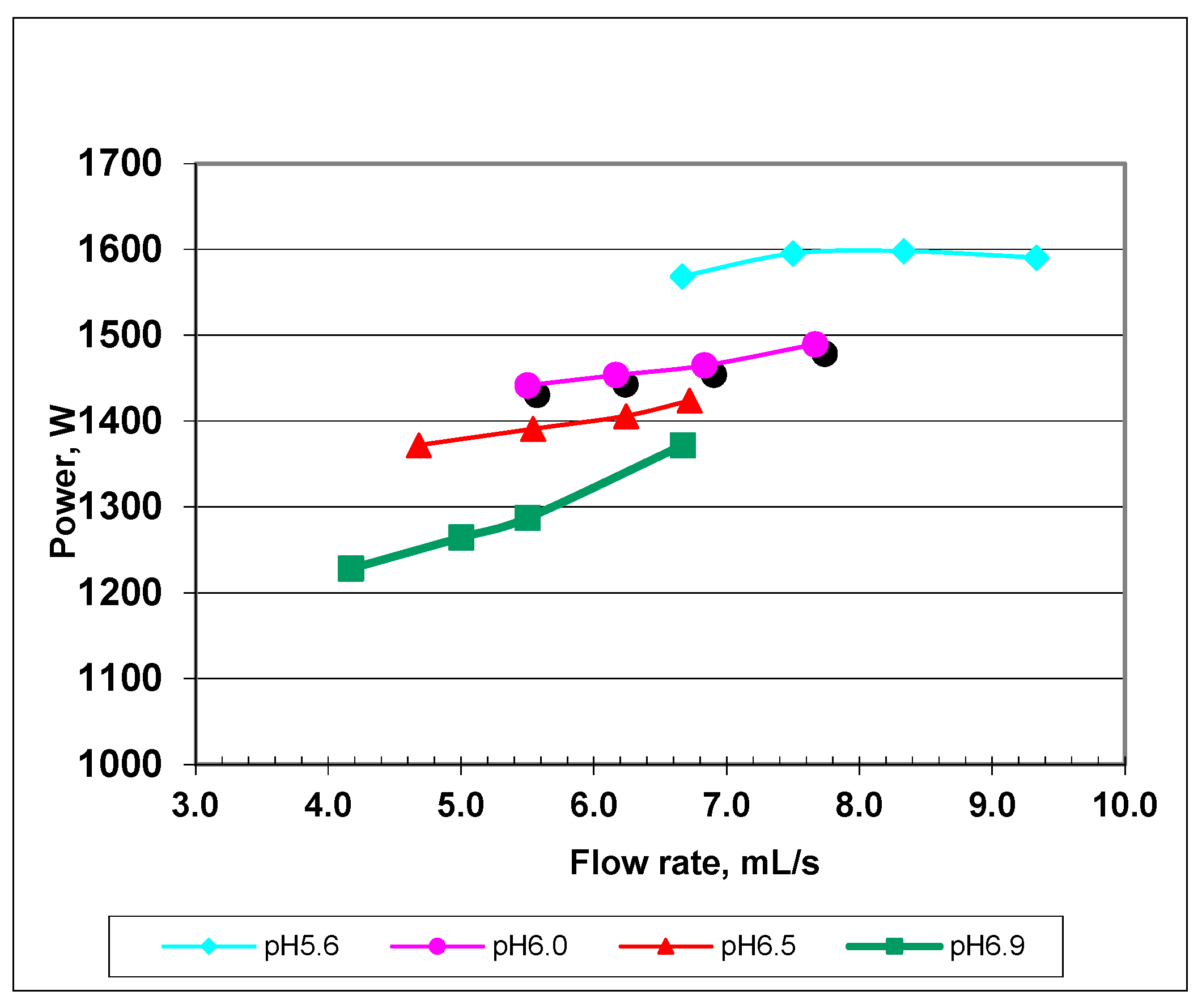
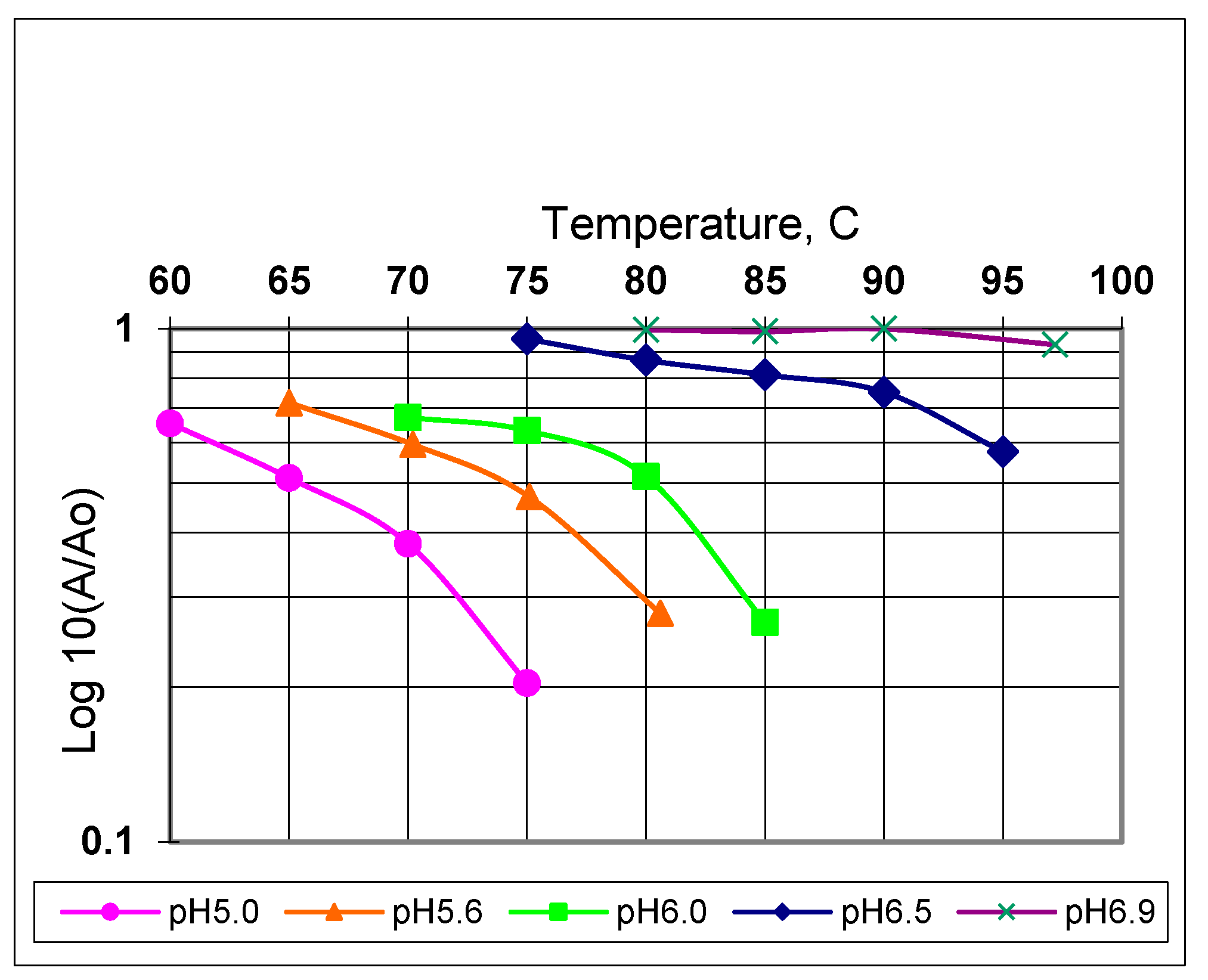
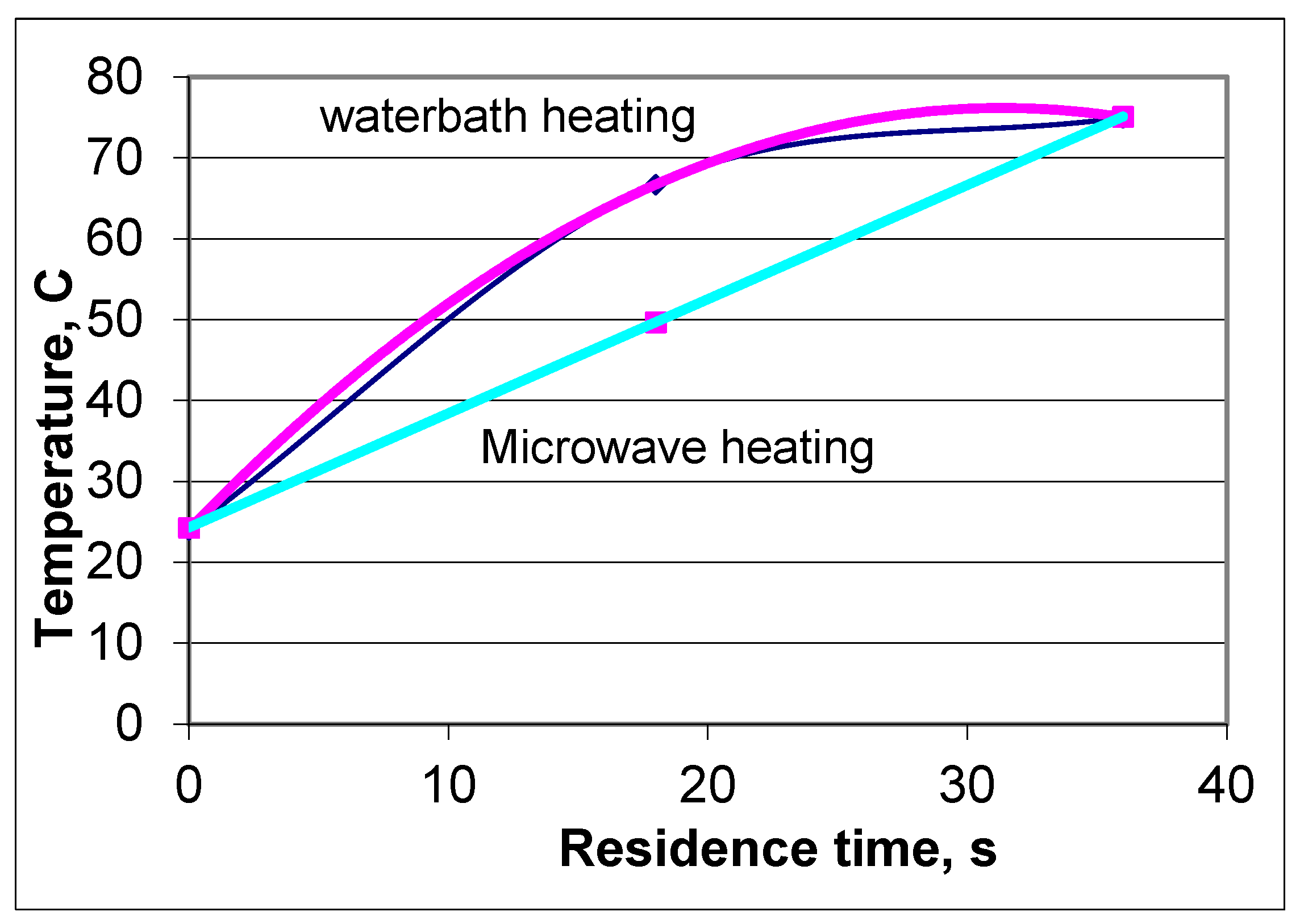
| Exit Temperature, °C | Flow Rate, mL/s | Residence Time, s | Heating Rate, °C/s | Power Absorbed, W | Efficiency, % | Re/De Number |
|---|---|---|---|---|---|---|
| 95 | 4.17 | 64.8 | 1.09 | 1228 | 61 | 600/178 |
| 88 | 5.00 | 54.0 | 1.18 | 1327 | 66 | 720/214 |
| 81 | 5.50 | 49.1 | 1.14 | 1287 | 64 | 792/236 |
| 75 | 6.67 | 40.5 | 1.23 | 1395 | 69 | 960/286 |
| 70 | 7.50 | 36.0 | 1.25 | 1413 | 71 | 1080/322 |
| 66 | 8.33 | 32.4 | 1.27 | 1441 | 72 | 1200/357 |
| Temperature °C | D-Values (s) | |||||
|---|---|---|---|---|---|---|
| at pH 6.0 | at pH 5.6 | at pH 5.0 | ||||
| Microwave continuous flow | Thermal batch | Microwave continuous flow | Thermal batch | Microwave continuous flow | Thermal batch | |
| 65 | N/A | N/A | 27 | 198 | 16 | 102 |
| 70 | 89 | 138 | 15 | 114 | 8 | 66 |
| 75 | 61 | 120 | 12 | 66 | 5 | 24 |
| 80 | 43 | 66 | 6 | 50 | N/A | N/A |
| 85 | 22 | N/A | N/A | N/A | N/A | N/A |
| z-value °C | 25 | 33 | 23 | 30 | 20 | 21 |
| Temperature (°C) | D-Value (s) at pH 5.6 | D-Value (s) at pH 5.6 | D-Value (s) at pH 5.6 |
|---|---|---|---|
| Thermal—continuous flow heating (holding) | Thermal—batch heating | Microwave—continuous flow heating | |
| 65 | 39 | 114 | 27 |
| 70 | 30 | 66 | 15 |
| 75 | 18 | 50 | 118 |
| 80 | N/A | N/A | 6 |
| z-value (°C) | 32 | 30 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Z.; Ramaswamy, H.S. Continuous-Flow Microwave Heating Inactivation Kinetics of α-Amylase from Bacillus subtilis and a Comparison with Conventional Heating Conditions. Appl. Sci. 2023, 13, 9220. https://doi.org/10.3390/app13169220
Tong Z, Ramaswamy HS. Continuous-Flow Microwave Heating Inactivation Kinetics of α-Amylase from Bacillus subtilis and a Comparison with Conventional Heating Conditions. Applied Sciences. 2023; 13(16):9220. https://doi.org/10.3390/app13169220
Chicago/Turabian StyleTong, Zhen, and Hosahalli S. Ramaswamy. 2023. "Continuous-Flow Microwave Heating Inactivation Kinetics of α-Amylase from Bacillus subtilis and a Comparison with Conventional Heating Conditions" Applied Sciences 13, no. 16: 9220. https://doi.org/10.3390/app13169220
APA StyleTong, Z., & Ramaswamy, H. S. (2023). Continuous-Flow Microwave Heating Inactivation Kinetics of α-Amylase from Bacillus subtilis and a Comparison with Conventional Heating Conditions. Applied Sciences, 13(16), 9220. https://doi.org/10.3390/app13169220










