Featured Application
The use of microwaves in drying has been the subject of studies in the post-harvest sector. Some authors have identified damage to the seeds because of microwaves, while others have found promising results for the technique. This study verified that the use of microwaves can be safe if the appropriate temperature and power conditions are applied. In this work, readers will also be able to verify that the use of electromagnetic energy can also be more economical and efficient, as it greatly reduces the drying time, in addition to being possible to increase germination during the process, which is highly desirable. In addition to the temperature x power binomial, water content is another variable that can affect the process. This should be a stimulus for further research and studies in the area that address a broader spectrum of varieties and water content to that the technique can be safer to the point that its use reaches the public.
Abstract
The use of electromagnetic energy for drying seeds has been shown to be a promising alternative. However, studies show that the technique still lacks careful evaluation due to the sensitivity of soybean seeds to damage caused using microwaves. Studies have shown that the use of microwaves during drying can be safe in seeds of soybeans, corn, and beans, among others. These studies recognize that drying using microwaves has a great potential for savings in the drying process, as it considerably reduces the drying time. This work aimed to evaluate the immediate damage caused in soybean seeds subjected to drying at temperatures of 40, 50, and 60 °C, with power of 0, 0.5, and 1 W/g at a frequency of 2.45 GHz. The results of the vigor and germination tests showed that the action of microwaves on seeds was not only safe and more efficient but also brought about increases in seed germination and vigor. However, the process must be judicious and obey precise indices of power and temperature to not cause damage to the seeds.
1. Introduction
Drying is a technology whose main function is to reduce the water content and can be applied to different materials. The procedure limits the chemical reactions of microorganisms, conserving the physical and biological structure of the stored material and its applicability of extreme contribution in sustainable processes for different segments [1,2,3,4]. The electromagnetic radiation emitted by the microwave is concentrated in the heating of water molecules, generating friction and collision between them quickly [1], focusing only on the matter, increasing the combined interior and exterior temperature, providing faster than traditional heating [5,6,7].
The microwave has been used in different agricultural products [8,9,10], optimizing the drying time, and it has been verified that the power levels, together with the time variable, cause different effects in different treated products. Evaluating the effect of drying with MW on the nutritional quality of persimmon slices, it was noticed that the increase in power of content of sugar, vitamin C, and tannin increased; however, the opposite occurred with the content of protein and vitamin E [1]; the preservation of vitamins A, C and E in addition to color has been observed in apricots at different maturation [11]. The application of waves can also be carried out intermittently, allowing the redistribution of moisture throughout the product and controlling high temperatures [12]. In pepper samples, drying resulted in a bright pepper powder with an intense red color [13]. The removal of water from bioproducts, in general, is essential for further processing and safe storage of these products. The microwave drying process can be used to intensify the drying rate, maintaining the nutritional compounds of these products [14].
In the wood industry, the properties of the material suffer from variations in dimensions due to the circumstances of ambient humidity and can suffer mechanical damage, such as cracking and warping during the process of removing water, with the use of microwaves (MW) being an option fast that can be applied after the sawing. The maritime pine (Pinus pinaster) and eucalyptus (Eucalyptus globulus) are two Portuguese wood species widely used for furniture, fences, and structural purposes. Analyzing small cores of wood treated in the MW, observed that eucalyptus heartwood takes longer to dry compared to pine heartwood, and the absorption of water after using MW obtained better impregnability than the control heartwood, being a viable possibility in the wood industry to work the raw material [15,16,17,18]. This example, and others related to the humidity of woods, shows all the versatile proposes the MW drying could have.
So, in all these studies [19,20,21], microwave drying showed better performance, improving the quality of various vegetal products. In this study, the objective was to evaluate the immediate effect of microwave drying on the quality of soybean seeds, aiming to improve the performance of seed germination and have greater efficiency in the drying process.
2. Materials and Methods
2.1. Preparation of Soybean Seeds
Samples of soybean seeds were produced and collected in 2018, on a farm in Brazil, located in southeastern Goiás Province, at coordinates 17.48–48.20. The samples of cultivated soybean were prepared to increase their moisture value. For this, the seeds were placed in a glass bottle in a refrigerated chamber at 10 °C, for 72 h, with an amount of water to increase the humidity to a level of 21% (w.b.). After 72 h, the moisture content was verified by the oven test, with 105 °C for 24 h, using three repetitions with six seeds each [22].
2.2. Drying Process
Samples with 21% water contents were withdrawn from the cold room and were kept at room temperature for 30 min before drying. After this period, they were subjected to microwave drying with intermittent control of 30 s off and 30 s on at the powers of 0, 0.5, and 1 W/g. In this study, the microwave apparatus could automatically and continuously adjust the microwave power, control the drying temperature, measure the mass of the product, and record the data in real-time (Figure 1 [9]).
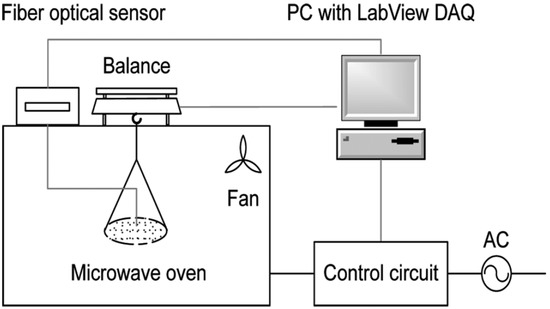
Figure 1.
Dryer equipment (microwave generator, circulator, power meters, adjusting controls, weight balance, microwave chamber, sample support, and fan).
The experiment was conducted with constant temperature and intermittent power. The microwave used for the study has a maximum power output of 600 W. The control circuits were modified to allow the power to be controlled. The temperature of the soybean seeds was measured by inserting an optical fiber sensor into a hole made in the soybean seed.
2.3. Constant Temperature
The temperature of the soybean samples was kept constant throughout the experiment. When the temperature crossed the desired level, the microwave energy supply was cut off automatically by the control circuit. The temperature levels used for the experiments were 40, 50, and 60 °C.
2.4. Intermittent Power
This method of microwave drying was conducted with an intermittent supply of power on a cycle of 30 s. A hundred grams of soybean seeds with an average moisture content of 21% (w.b.) were taken for microwave drying. The microwave energy generator was adjusted in such a way that the power shut off for 30 s and switched on for 30 s. The power levels used for these experiments were 0, 0.5, and 1.0 W/g. Drying was carried out until reaching final weight stability, represented by the same measurement on the scale for three consecutive readings.
Conventional drying was carried out in the same microwave equipment, without activating the power, that is, with 0 W/g, at a temperature of 40 °C.
2.5. Vigor and Germination Tests
To carry out the “first count” vigor test and the germination test, the soybean seeds subjected to drying were placed on moistened germination paper. The paper rolls were closed in plastic bags and kept in a germination chamber for 5 and 8 days at 24 °C. After the first 5 days, the germinated seeds that produced strong seedlings were counted, and the germinated percentage represented the “First Count” vigor test. The seeds that did not germinate were kept on the germination paper and returned to the germination chamber until the 8 days were completed. After this period, the paper rolls were again removed to count germination (normal seedlings) and protrusion (seeds that emitted at least 2 mm of radicle). Abnormal seedlings were those that were missing some part, such as hypocotyl, root, and leaves, or those that showed some abnormality in their development, such as hypocotyl and twisted roots. The germination rate was expressed as a percentage based on the initial number of seeds used. The seed germination and vigor tests were carried out in four repetitions with 50 seeds each, according to the International Seed Testing Association (ISTA) International Rules for Seed Analysis [23,24,25,26,27,28,29].
The water absorption curve [23,30], was performed by measuring the individual weight of 30 seeds, in three repetitions. The seeds were placed in Petri dishes, on two sheets of germination paper. Each seed had its weight previously measured on a precision scale of 0.0001 g. In each plate, 20 mL of distilled water were added. During the test, the seeds were kept in germination chambers at 25 °C for a period of 8 days. However, the weight of each seed was measured again at intervals ranging from 3 to 24 h during the incubation period. At each weighing, each seed was placed on absorbent paper, making sure that there was no excess free water around the seed coat. The water absorption curve was constructed from the percentage values of the weight of water absorbed by each seed. The adjustment of the water intake curve models was performed using the Curve Expert® 2.6 software.
2.6. Experimental Design
Variation effects in germination time and temperature were analyzed using the response surface methodology (RSM). The effect of two independent variables, namely, temperature (A) and power (B), on the quality of the seed was investigated using a two-factor CCD-RSM experimental, and the results are shown in Table 1.

Table 1.
Coded levels for independent variables used in experimental design for response surfaces to predict soybean seed germination after drying.
2.7. Statistical Analysis
The program RStudio R 3.3.0+ was used to determine the effects of the independent variables, calculate regression coefficients (R2), carry out an analysis of variance (ANOVA), and build the response surface, at a 5% significance level.
The following second-order polynomial model (Equation (1)) was fitted to the data:
where Y is the response variable, X1 and X2, are the coded process variables and β0, β1, β2, β11, β22, β12 are the regression coefficients. A stepwise methodology was followed to determine the significant terms in Equation (1).
The Minitab 21.4 program was used to analyze the average obtained by the vigor and germination test, using a box plot, with a confidence level of 95% for the applied treatments.
Principal component analysis (PCA) was used for analyzing the vigor and germination test and was performed using the software R version 4.3.0 [31], packages (devtools and ggbiplot) functions (prcomp, screeplot ggbiplot2) (Table 2).

Table 2.
Treatments identification for PCA analysis.
3. Results
After drying the seeds, the samples of each treatment were submitted to the vigor and germination test. Test results were subjected to response surface analysis. The contour graphs and the generated surfaces showed an unexpected behavior in the region between 40 and 50 °C and between 0 and 0.5 W/g in this region, and the total germination obtained an increment, which may indicate a biostimulant effect coming from the effect of microwave exposure, as shown in Figure 2.
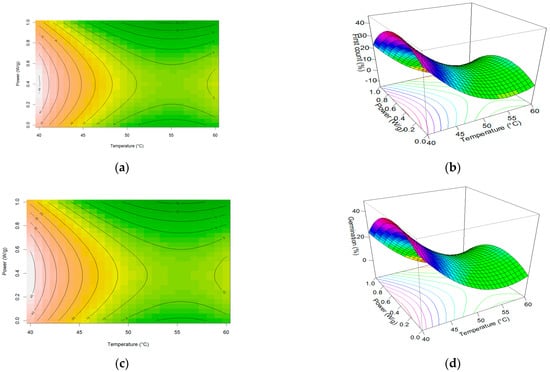
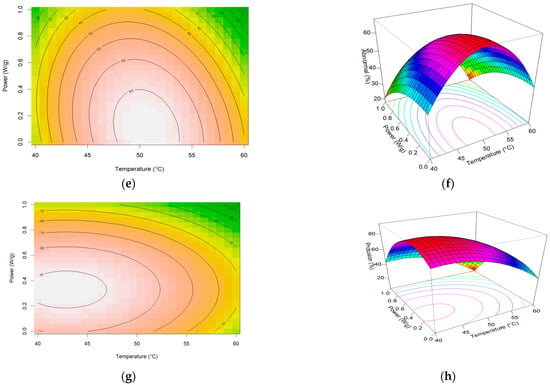
Figure 2.
Graphic plots showing the effects of temperature and microwave power on vigor test First Count of germination: (a) Contour of First Count test; (b) Response surface of First Count test; (c) Contour of germination of normal plants; (d) Response surface of germination of normal plants; (e) Contour of total abnormal plants; (f) Response surface of total abnormal plants; (g) Contour of total protrusion of seeds; (h) Response surface of total protrusion of seeds.
The behavior of seed drying, with the increase in germination indicated on the response surface, was repeated in the first count (vigor test) and seed germination. The contour plot shows us more clearly that this region comprises, more specifically, a small area where power was applied at lower levels, that is, between 0 and 0.5 W/g. Similar behavior was found for corn seeds [32]. For temperatures above 50 °C, the damage to seeds was drastic for any power level.
The seeds start to be drying with 21% (w.b.) water content. This is considered a high moisture content level for drying soybean seed. Another study using microwaves as a biostimulant effect identified that drying could safely take place at a level of 0.2 W/g, with seeds having a water content of around 10% [33,34].
The analysis of variance and the coefficients of the models generated by the response surface are described in Table 3.

Table 3.
The ANOVA table shows the independent variables in linear, quadratic, or interaction terms on each of the responses for soybean germination.
The protrusion model indicates as a stationary point in original units the temperature of 42.5 °C and a power of 0.31 W/g. These points represent the maximum gain in germination rates for drying using microwave energy.
The averages obtained in the germination and vigor test to determine seed quality are represented in Figure 3 through box plot analysis. Through the figure, one can identify the best treatments obtained through the vigor and germination tests right after drying the soybean seeds.
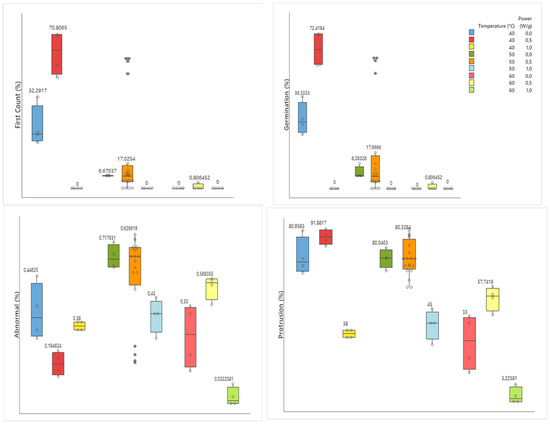
Figure 3.
Box plot analyses of germination and vigor tests showing the percentage results for soybean seeds after drying under temperatures of 40, 50, and 60 °C and microwave power of 0, 0.5, and 1.0 W/g. The numbers represent the media, and the box represents the interquartile interval at a 95% level of confidence. The data, and outliers are represented in the figures for the dots, and  , respectively.
, respectively.
 , respectively.
, respectively.
Figure 3 presents the result of the germination and vigor test as a function of temperature and power. The treatments that used the temperature of 40 °C showed the best averages compared to the temperature of 50 and 60 °C. Treatments submitted to a power of 1.0 W/g had a drastic reduction in germination rates and an increase in abnormal seedlings.
The best treatment obtained in the vigor and germination test was the one that used a temperature of 40 °C, associated with a potency of 0.5 W/g. This treatment showed the highest rates of germination with protrusion of up to 91.88%, and the lowest rates of abnormal seedlings, surpassing the control treatment.
The quality of seeds can also be discussed by PCA analysis, as shown in Figure 4 below.
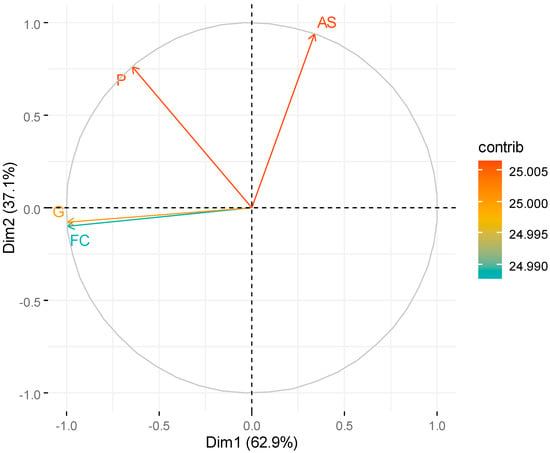
Figure 4.
Biplot Autovectors in principal components 1 and 2: First Count (FC), Germination (G), Abnormal Seedlings (AS), Protrusion (P) conformable treatment of Table 2.
The first two components explained the totality of approximately 99% of all variability in the data set (62.9% explained by Principal Component 1 (CP1), 37.1% by CP2) according to [35,36], this proportion of the variance explained by (CP) must correspond to at least 70%.
We can observe in Figure 5, CP1 showed great negative associations with the variables germination and first count. In CP2, it is observed that the variables and abnormal seedlings and protrusion presented negative associations.
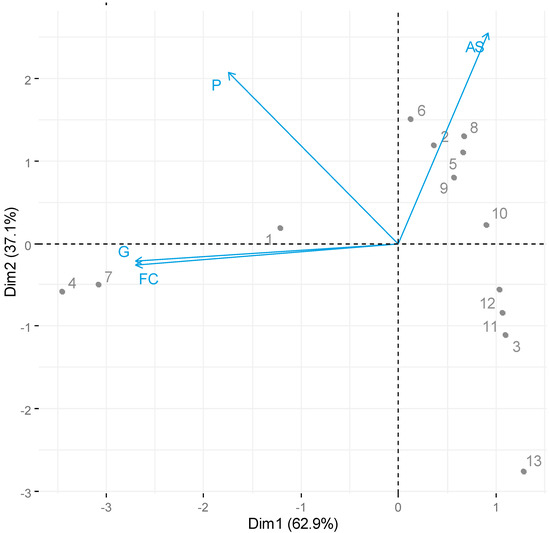
Figure 5.
Biplot Principal components Scores 1 and 2 to an average of: First Count (FC), Germination (G), Abnormal Seedlings (AS), Protrusion (P) conformable treatment of Table 2.
We can observe that the samples were united in agreement with the powers used as a factor within the treatments.
Treatment 1, with a temperature equal to 40 °C and power 0, proved to be efficient for germination and first count. It is noteworthy that treatments 4 and 7 are closer to germination and first count, indicating better responses to the corresponding treatments; these are 40 °C and 50 °C with a power of 0.5, respectively, which suggest that the use of this power is stimulating for the germination and vigor of seedlings associated with the first count, more than just the temperature without using power.
Treatments 2, 5, 6, 8, 9, and 10 indicate that there were stimuli in cellular elongation but that these seedlings were not able to become normal, a fact that can be suggested by the temperature.
Treatments 3, 11, and 12 showed a distancing from the other groups evaluated, a fact that can be observed by the use of high power, 1. As well as the treatment that most distanced itself from the others was the 13 at 60 °C and power of 1, being opposed to the protrusion, indicating very low values for all the variables observed, being unfeasible for application in seeds.
The behavior of water loss during seed drying in the two main treatments observed by the germination and vigor test is shown in Figure 6. The seed drying rate was higher at the beginning of the process, and after a period, it stabilized until the end.
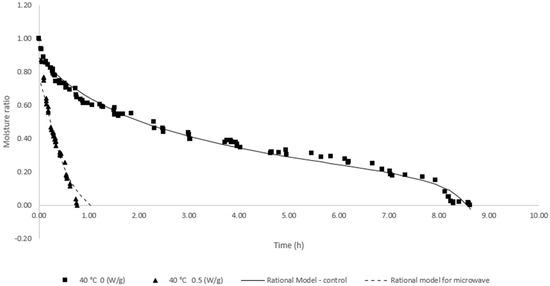
Figure 6.
Graphic plots showing the effects of moisture content versus time during conventional and microwave drying.
The values of the water content of the seeds at the end of the drying process in each treatment were 6 and 4% for conventional drying and with the use of microwaves, respectively. At the end of the drying process, the water content for conventional drying at 40 °C and 0 W/g was 15.15% (w.b.), and for drying using microwaves at a temperature of 40 °C and power of 0.5 W/g was 17.29%. For storage purposes, both processes were inefficient since drying should occur up to a content of 12% (w.b.) of water. However, the conditions of the drying air did not allow the removal of a higher value, which could be reached at a temperature of 42 °C, with 0.3 W/g, as identified by the RSM model, as the ideal condition for maximizing gains in biostimulation of applied energy. Table 4 shows the parameters adjusted for the models applied for fit in the drying behavior.

Table 4.
The rational model parameters adjusted for the reduction in the water content in soybean seeds after microwave drying.
The behavior of the temperature of the soybean seeds and the insufflated air during microwave drying can be seen in Figure 7. In the image, we can identify that there is a slight increase in the internal temperature of the seeds during conventional drying, which did not happen in the same intensity for dry seeds using microwaves.
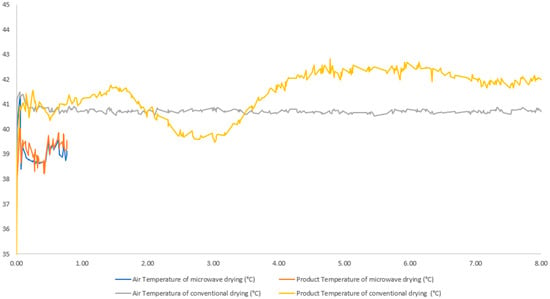
Figure 7.
Drying time vs. drying air temperature and product temperature in conventional drying (40 °C and 0.0 W/g) and microwave drying (40 °C and 0.5 W/g).
Figure 7 may have the key to begin to understand the reasons why seed damage was considerably higher in conventional drying. In the microwave drying process, the temperature did not exceed the recommended index for most soybean seeds of 42 °C. In addition, the seeds had an exposure time to temperature a little more than eight times longer in the conventional process, which was approximately 8 h 37 min against 46 min for the microwave process.
In Figure 8, the water absorption test was performed, showing a similar behavior between the two main treatments studied. However, in the first 10 h, the seeds that were treated by microwave drying showed greater water absorption.
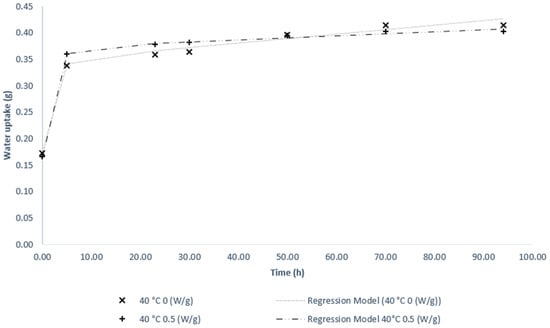
Figure 8.
Water absorption curve of soybean seeds for conventional drying and microwave drying.
The ability to absorb more water, especially in the first hours of germination, is an indication of good quality in seeds [33,37,38]. The result of this test corroborates the performance of the microwave treatment vigor test using a power of 0.5 W/g, which surpassed conventional drying in its germination percentage. In a combined study of microwaves and ultrasound (US-MW) in slices of green olives, the authors noticed that the power of 180 W provided a higher rate of rehydration for dry olives compared to higher powers, determining that the US pre-treatment -MW is promising [39]. Table 5 are presented the adjusted models for the water up take curve.

Table 5.
Parameters of the exponential plus linear model given by y = a + brx + cx and adjusted for the water absorption curve in soybean seeds for conventional drying and microwave drying.
In okra samples, the rehydration rate had a better performance at 540 W of microwave power; the pre-treatment maintained the characteristics of the product compared to the fresh product [40]. Again, we have a result that indicates the biostimulant effect of microwave drying, which has an advantage over conventional drying.
4. Discussion
Soybean seeds, when subjected to microwave drying at temperatures of 40 to 50 °C and microwave power above 0 to 0.5 W/g, may have their drying time reduced by up to eight times. The process is safe for water contents below 21%, as shown by the results of this experiment.
Several studies report gains in reducing drying time or other advantages using microwaves and other electromagnetic energies [2,3,41,42]. However, studies that report gains in seed quality are still incipient. Recently, it was proved in a study on the use of magneto priming in soybean seeds they can promote biostimulation and carry out the removal of water from the seeds [33].
In this study, soybean seeds were submitted to different drying conditions, which led to a region of exploration of potential gains in bio stimulus by microwaves. The gain in seed vigor observed in the physiological tests indicates a large area of study to be explored as new post-harvest technologies for seeds. Similar results have already been shown in previous studies [43,44,45,46,47], but little is known about the biological or biophysical reasons related to this phenomenon.
In other types of food products, the MW power can minimize the nutritional degradation influencing the oxidation effect of different products; in samples of sugarcane juice, it was verified that the lower power of the lesser antioxidant activity verified through the elimination of free radicals (DPPH), being the device an efficient technique for concentrate juice [48]. The same antioxidant effect was observed in peas treated at a power of 100 W; there was a decrease in DPPH and an increase in flavonoid levels [49]. In jelly processing, the microwave had an advantage, such as higher DPPH, phenolic content, and ascorbic acid content over the conventional use of the open pan [50]. Despite this, these products are no longer like soybean seeds—the antioxidant effect shown by these studies could explain a possible similar effect on seeds during drying.
This study corroborates the data presented in [51], which using a power of 0.13 W/G, identified that it is possible to dry soybean seeds without damage to germination with greater speed and efficiency of the process. Microwave drying is still a field of study that needs many exploratory studies to certify, over a wide range of drying conditions, the safety and benefits of this process. This study demonstrated that we have a reach region for drying, considering the binomial temperature and power that can not only be safe but also promising for the performance of seeds in the field.
5. Conclusions
The results showed that microwave drying could not only safely dry soybean seeds but also increase their quality level. There is a germinal biostimulation zone for soybean seeds located between the temperature of 40 to 45 °C and the potency of 0 to 0.5 W/g.
The power of 1 W/g is not recommended for drying seeds, according to this study, at any of the temperatures studied. The use of a temperature of 40 °C is indicated for drying; when associated with a power of 0.5 W/g, it becomes a biostimulant to increase seed quality and increase water absorption during the first moments of germination.
The drying carried out on soybean seeds with 21% water content at 40 °C and a power of 0.5 W/g is safe and drastically reduces drying time.
Author Contributions
Conceptualization, R.Q.d.F. and A.R.P.d.S.; methodology, R.Q.d.F., A.R.P.d.S. and Y.G.; software, Y.G.; validation, R.Q.d.F. and A.R.P.d.S.; formal analysis, R.Q.d.F., A.R.P.d.S. and L.C.P.d.S.V.; investigation, R.Q.d.F.; resources, V.R. and M.M.P.S.; data curation, Y.G., R.Q.d.F. and A.R.P.d.S.; writing—original draft preparation, R.Q.d.F., A.R.P.d.S. and L.C.P.d.S.V.; writing—review and editing, A.R.P.d.S., L.V, V.R., Y.G. and M.M.P.S.; visualization, R.Q.d.F., A.R.P.d.S. and L.C.P.d.S.V.; supervision, V.R. and M.M.P.S.; project administration, R.Q.d.F.; funding acquisition, V.R. and M.M.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by FAPESP (Foundation for Research Support of the State of São Paulo) (grant number 2018/25698-4).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Goiano (Instituto Federal Goiano) (PORTARIA 514 de 11 de julho de 2016). Government of Canada, by Global Affairs Canada (IRCC special program code 509) through McGill University—Campus McDonald.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Qin, Y.; Duan, Z.; Zhou, S.; Wei, Z. Effect of Intermittent Microwave Drying on Nutritional Quality and Drying Characteristics of Persimmon Slices. Food Sci. Technol. 2022, 42, 1–10. [Google Scholar] [CrossRef]
- Martins, C.P.C.; Cavalcanti, R.N.; Rocha, R.S.; Esmerino, E.A.; Freitas, M.Q.; Pimentel, T.C.; Silva, M.C.; Cruz, A.G. Microwave Heating Impacts Positively on the Physical Properties of Orange Juice-milk Beverage. Int. J. Dairy Technol. 2022, 75, 129–138. [Google Scholar] [CrossRef]
- Motasemi, F.; Salema, A.A.; Afzal, M.T. Dielectric Characterization of Corn Stover for Microwave Processing Technology. Fuel. Process. Technol. 2015, 131, 370–375. [Google Scholar] [CrossRef]
- Luan, D.; Wang, Y.; Tang, J.; Jain, D. Frequency Distribution in Domestic Microwave Ovens and Its Influence on Heating Pattern. J. Food Sci. 2017, 82, 429–436. [Google Scholar] [CrossRef]
- Martins, C.P.C.; Ramos, G.L.P.A.; Pimentel, T.C.; Freitas, M.Q.; Duarte, M.C.K.H.; Azeredo, D.P.R.; Silva, M.C.; Cavalcanti, R.N.; Esmerino, E.A.; Cruz, A.G. How Microwave Technology Is Perceived? A Food Safety Cross-Cultural Study between Brazil and Portugal. Food Control. 2022, 134, 108763. [Google Scholar] [CrossRef]
- International Microwave Power Institute. The Journal of Microwave Power and Electromagnetic Energy: A Publication of the International Microwave Power Institute; International Microwave Power Institute: Mechanicsvlle, VA, USA, 1985. [Google Scholar]
- Song, G.; Choudhary, R.; Watson, D.G. Microwave Drying Kinetics and Quality Characteristics of Corn. Int. J. Agric. Biol. Eng. 2013, 6, 90–99. [Google Scholar] [CrossRef]
- Đurović, S.; Nikolić, B.; Luković, N.; Jovanović, J.; Stefanović, A.; Šekuljica, N.; Mijin, D.; Knežević-Jugović, Z. The Impact of High-Power Ultrasound and Microwave on the Phenolic Acid Profile and Antioxidant Activity of the Extract from Yellow Soybean Seeds. Ind. Crops. Prod. 2018, 122, 223–231. [Google Scholar] [CrossRef]
- Nair, G.R.; Li, Z.; Gariepy, Y.; Raghavan, V. Microwave Drying of Corn (Zea mays L. sp.) for the Seed Industry. Dry. Technol. 2011, 29, 1291–1296. [Google Scholar] [CrossRef]
- Arion, M.; Şoproni, D.; Vicaş, S.; Leuca, T.; Hathazi, F.I.; Molnar, C.; Bandici, L. Microwave Drying Process of Corn Seeds. J. Electr. Electron. Eng. 2011, 4, 11–14. [Google Scholar]
- Karatas, F.; Kamışlı, F. Variations of Vitamins (A, C and E) and MDA in Apricots Dried in IR and Microwave. J. Food Eng. 2007, 78, 662–668. [Google Scholar] [CrossRef]
- Duc Pham, N.; Khan, M.I.H.; Joardder, M.U.H.; Rahman, M.M.; Mahiuddin, M.d.; Abesinghe, A.M.N.; Karim, M.A. Quality of Plant-Based Food Materials and Its Prediction during Intermittent Drying. Crit. Rev. Food Sci. Nutr. 2019, 59, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Soysal, Y.; Keskin, M. Mathematical Modeling, Moisture Diffusion and Color Quality in Intermittent Microwave Drying of Organic and Conventional Sweet Red Peppers. AgriEngineering 2020, 2, 393–407. [Google Scholar] [CrossRef]
- Hii, C.L.; Ong, S.P.; Yap, J.Y.; Putranto, A.; Mangindaan, D. Hybrid Drying of Food and Bioproducts: A Review. Dry. Technol. 2021, 39, 1554–1576. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Cao, L.-K.; Yi, S.-J.; Che, G.; Wang, W.-H.; Liu, W.; Jia, X.-Y.; Wei, C.-H.; Wang, Y.-F.; Wu, Y.-J.; et al. Proteomic Analysis of Japonica Sorghum Following Microwave Intermittent Drying Based on Label-Free Technology. Food Sci. Technol. 2022, 42, 1–10. [Google Scholar] [CrossRef]
- Aksenov, A.A.; Malyukov, S. V Microwave Modification of Wood: Determination of Mechanical Properties of Softwood. IOP Conf. Ser. Earth Environ. Sci. 2020, 595, 012012. [Google Scholar] [CrossRef]
- Ganguly, S.; Balzano, A.; Petrič, M.; Kržišnik, D.; Tripathi, S.; Žigon, J.; Merela, M. Effects of Different Energy Intensities of Microwave Treatment on Heartwood and Sapwood Microstructures in Norway Spruce. Forests 2021, 12, 598. [Google Scholar] [CrossRef]
- Mascarenhas, F.J.R.; Geraldes Dias, A.M.P.; Christoforo, A.L.; dos Santos Simões, R.M.; Cunha, A.E.P. Effect of Microwave Treatment on Drying and Water Impregnability of Pinus pinaster and Eucalyptus globulus. Maderas. Cienc. Y Tecnol. 2022, 25. [Google Scholar] [CrossRef]
- Qu, Y.; Xie, D.; Hu, C.; Deng, H.; Meng, Y. Direct Steam Injection Pretreatment Improves Microwave-Assisted Extraction Yield for Total Flavonoids and Myricetin from Hovenia dulcis thunb. Food Sci. Technol. 2021, 41, 334–342. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Prieto, M.A.; Vazquez, J.A.; Barreiro, M.F.; Barros, L.; Ferreira, I.C.F.R. Recovery of Bioactive Compounds from Arbutus unedo L. Fruits: Comparative Optimization Study of Maceration/Microwave/Ultrasound Extraction Techniques. Food Res. Int. 2018, 109, 455–471. [Google Scholar] [CrossRef]
- ElKhori, S.; Paré, J.R.J.; Bélanger, J.M.R.; Pérez, E. The Microwave-Assisted Process (MAPTM1): Extraction and Determination of Fat from Cocoa Powder and Cocoa Nibs. J. Food Eng. 2007, 79, 1110–1114. [Google Scholar] [CrossRef]
- ISTA Standard. International Rules for Seed Testing; ISTA: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.M.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 9781461446927. [Google Scholar]
- Ratnayake, S.; Shaw, D.R. Influence of Seed Vigor, Herbicide Rates, and Incorporation Depths on Emergence and Seedling Development of Soybean (Glycine max). Weed Sci. 2018, 6, 801–806. [Google Scholar] [CrossRef]
- International Seed Testing Association. Seed Science and Technology. Seed Sci. Technol. 1985, 13, 299–513. [Google Scholar]
- De Vitis, M.; Hay, F.R.; Dickie, J.B.; Trivedi, C.; Choi, J.; Fiegener, R. Seed storage: Maintaining seed viability and vigor for restoration use. Restor. Ecol. 2020, 28, S249–S255. [Google Scholar]
- Faria, R.Q.D.; Teixeira, I.R.; Cunha, D.A.D.; Honorato, J.M.; Devilla, I.A. Physiological Quality of Crambe Seeds Submitted to Drying. Rev. Ciência Agronôomica 2014, 45, 453–460. [Google Scholar] [CrossRef]
- Milošević, M.; Vujaković, M.; Karagić, D. Vigour Tests as Indicators of Seed Viability. Genetika 2010, 42, 103–118. [Google Scholar] [CrossRef]
- Kaya, M.D.; Kulan, E.G.; Daghan, H.; Ileri, O.; Avci, S. Efficiency of Vigor Tests and Seed Elemental Concentrations to Estimate Field Emergence in Soybean (Glycine max). Int. J. Agric. Biol. 2016, 18, 1075–1080. [Google Scholar] [CrossRef]
- Min, T.-G.; Yoon, H.-J. Effect of Controlled Hydration on Germination of Tobacco Seeds. Korean J. Crop Sci. 2003, 48, 322–325. [Google Scholar]
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- De Faria, R.Q.; dos Santos, A.R.P.; Gariepy, Y.; da Silva, E.A.A.; Sartori, M.M.P.; Raghavan, V. Optimization of the Process of Drying of Corn Seeds with the Use of Microwaves. Dry. Technol. 2020, 38, 676–684. [Google Scholar] [CrossRef]
- De Faria, R.Q.; dos Santos, A.R.P.; Batista, T.B.; Gariepy, Y.; da Silva, E.A.A.; Sartori, M.M.P.; Raghavan, V. The Effect of Magneto-Priming on the Physiological Quality of Soybean Seeds. Plants 2023, 12, 1477. [Google Scholar] [CrossRef]
- Momenzadeh, L.; Zomorodian, A.; Mowla, D. Experimental and Theoretical Investigation of Shelled Corn Drying in a Microwave-Assisted Fluidized Bed Dryer Using Artificial Neural Network. Food Bioprod. Process. 2011, 89, 15–21. [Google Scholar] [CrossRef]
- Rencher, A.C. Methods of Multivariate Analysis. In Wiley Series in Probability and Statistics; John Wiley & Sons, Inc.: New York, NY, USA, 2002; ISBN 0471418897. [Google Scholar]
- Varmuza, K.; Filzmoser, P. Introduction to Multivariate Statistical Analysis in Chemometrics; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Zhang, M.; He, S.; Qin, B.; Jin, X.; Wang, M.; Ren, C.; Cao, L.; Zhang, Y. Exogenous Melatonin Reduces the Inhibitory Effect of Osmotic Stress on Antioxidant Properties and Cell Ultrastructure at Germination Stage of Soybean. PLoS ONE 2020, 15, e0243537. [Google Scholar] [CrossRef]
- Meyer, C.J.; Steudle, E.; Peterson, C.A. Patterns and Kinetics of Water Uptake by Soybean Seeds. J. Exp. Bot. 2007, 58, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Aydar, A.Y. Investigation of Ultrasound Pretreatment Time and Microwave Power Level on Drying and Rehydration Kinetics of Green Olives. Food Sci. Technol. 2021, 41, 238–244. [Google Scholar] [CrossRef]
- Sunil, C.K.; Kamalapreetha, B.; Sharathchandra, J.; Aravind, K.S.; Rawson, A. Effect of Ultrasound Pre-Treatment on Microwave Drying of Okra. J. Appl. Hortic. 2017, 19, 58–62. [Google Scholar] [CrossRef]
- Hemwimon, S.; Pavasant, P.; Shotipruk, A. Microwave-Assisted Extraction of Antioxidative Anthraquinones from Roots of Morinda Citrifolia. Sep. Purif. Technol. 2007, 54, 44–50. [Google Scholar] [CrossRef]
- Yoshida, H.; Kajimoto, G. Effects of Microwave Energy on the Tocopherols of Soybean Seeds. J. Food Sci. 1989, 54, 1596–1600. [Google Scholar] [CrossRef]
- Rękas, A.; Ścibisz, I.; Siger, A.; Wroniak, M. The Effect of Microwave Pretreatment of Seeds on the Stability and Degradation Kinetics of Phenolic Compounds in Rapeseed Oil during Long-Term Storage. Food Chem. 2017, 222, 43–52. [Google Scholar] [CrossRef]
- Hemis, M.; Gariépy, Y.; Choudhary, R.; Raghavan, V. New Coupling Model of Microwave Assisted Hot-Air Drying of a Capillary Porous Agricultural Product: Application on Soybeans and Canola Seeds. Appl. Therm. Eng. 2017, 114, 931–937. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, W.; Xie, J.; Zheng, B.; Miao, S.; Lo, Y.M.; Zheng, Y.; Zhuang, W.; Tian, Y. Microwave Vacuum Drying of Lotus Seeds: Effect of a Single-Stage Tempering Treatment on Drying Characteristics, Moisture Distribution, and Product Quality. Dry. Technol. 2017, 35, 1561–1570. [Google Scholar] [CrossRef]
- Balboni, B.M.; Ozarska, B.; Garcia, J.N.; Torgovnikov, G. Microwave Treatment of Eucalyptus Macrorhyncha Timber for Reducing Drying Defects and Its Impact on Physical and Mechanical Wood Properties. Eur. J. Wood Wood Prod. 2018, 76, 861–870. [Google Scholar] [CrossRef]
- Hernández Maqueda, R.; Ballesteros Redondo, I.; Jiménez Jácome, S.; Moreno, Á.H. Microwave Drying of Amaranth and Quinoa Seeds: Effects of the Power Density on the Drying Time, Germination Rate and Seedling Vigour. J. Microw. Power Electromagn. Energy 2018, 52, 299–311. [Google Scholar] [CrossRef]
- Alvi, T.; Khan, M.K.I.; Maan, A.A.; Nazir, A.; Ahmad, M.H.; Khan, M.I.; Sharif, M.; Khan, A.U.; Afzal, M.I.; Umer, M.; et al. Modelling and Kinetic Study of Novel and Sustainable Microwave-Assisted Dehydration of Sugarcane Juice. Processes 2019, 7, 712. [Google Scholar] [CrossRef]
- Chahbani, A.; Fakhfakh, N.; Balti, M.A.; Mabrouk, M.; El-Hatmi, H.; Zouari, N.; Kechaou, N. Microwave Drying Effects on Drying Kinetics, Bioactive Compounds and Antioxidant Activity of Green Peas (Pisum sativum L.). Food Biosci. 2018, 25, 32–38. [Google Scholar] [CrossRef]
- Curi, P.N.; Salgado, D.L.; Mendonça, K.; Pio, R.; Ferreira, J.L.G.; Souza, V.R. de Influence of Microwave Processing on the Bioactive Compounds, Antioxidant Activity and Sensory Acceptance of Blackberry Jelly. Food Sci. Technol. 2019, 39, 386–391. [Google Scholar] [CrossRef]
- Shivhare, U.; Raghavan, V.; Bosisio, R.; Giroux, M. Microwave Drying of Soybean at 2.45 GHz. J. Microw. Power Electromagn. Energy 1993, 28, 11–17. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).