Abstract
The improved peri-implant bone response demonstrated when utilizing the platform-switching concept may result from the reduced levels of metal ions released from implant–abutment surfaces to the surrounding tissues. These corrosion products may play a major role in crestal bone remodeling around dental implants. This study evaluated the effect of different implant–abutment couplings (platform-matched vs. platform-switched) on osteoblasts’ function. Titanium alloy and cobalt–chrome alloy abutments were coupled with titanium cylinders, forming either platform-switched or platform-matched groups, and were incubated in human osteoblast cultures utilizing a novel direct-exposure technique. Viability was evaluated over 21 days using Alamar Blue assay. Apoptosis was measured after 24 h using flow cytometry. The expression of genes related to bone resorption was analysed over 21 days using a real-time quantitative polymerase chain reaction assay. Cell viability was reduced from day 4 to day 21 (p < 0.05), with higher rates of early apoptosis (p < 0.05) compared to the controls. Apoptosis was higher in the platform-matched groups (p < 0.05). The tested genes’ expression was up-regulated after 1 and 3 days of exposure to implant–abutment couplings (p < 0.05). The upregulation was more pronounced in platform-matched groups (p < 0.05). Exposure of osteoblasts to implant–abutment couplings induced adverse biological responses, which were more pronounced with platform-matched couplings. These reactions might be related to the increased amounts of metal ions released from the platform-matched couplings, highlighting the possible role of corrosion products in the mediation of crestal bone loss around dental implants.
1. Introduction
Dental implants have become an integral part of restorative dental care and the number of implants being placed is steadily increasing. As the use of dental implants increases, it is likely that the complications will also increase if the current systems are not optimized. It has been reported that every fifth inserted implant eventually develops peri-implantitis after masticatory loading for a mean period of time of from 3.4 to 11 years [1,2]. Biological complications involve the mucosa and bone surrounding the dental implant [3,4]. Peri-implant bone complications often involve a loss of bone vertical height and volume. Peri-implant bone loss is of great importance as it has been established as one of the main criteria of implant success [5]. This crestal bone loss seems to be related to the location of the microgap that created between the implant–abutment interface [6]. Decreased peri-implant bone loss was observed previously when utilizing different approaches related to the connection geometry of the dental implant–abutment interface, such as platform-switching [7]. Lazzara and Porter have defined platform-switching as “a protocol that includes smaller diameter restorative components that have been placed onto larger diameter implant restorative platforms—the outer edge of the implant-abutment interface is horizontally repositioned inwardly and away from the outer edge of the implant platform” [7]. The exact aetiology behind this enhanced peri-implant bone response has not been confirmed. Recent studies demonstrated higher levels of metal ions being released through accelerated corrosion from the platform-matched compared to platform-switched implant–abutment couplings (IAC), suggesting that this difference in the amount of corrosion products may be a factor [8,9].
The role of implant corrosion products in peri-prosthetic osteolysis has been extensively discussed in the orthopedic literature, which demonstrated that metal ions/particles in tissues can influence various metabolic pathways leading to cytokine release and interference with the function of various cells, such as osteoblasts, macrophages and fibroblasts, thereby disrupting bone homeostasis and contributing to the development of osteolysis [10,11,12].
The topic of degradation products released from dental implants and their association with peri-implant bone loss has received close attention in recent years [13,14,15,16,17,18,19]. In fact, the earlier work of the current research group [8,9,20] provided some indirect evidence of the possible role of corrosion products in the mediation of crestal bone loss around dental implants. Additionally, this glimpse of the immunological response pattern to the corrosion products that were released, observed in the previous research [20], suggests that there are immunological aspects of peri-implant bone loss, supporting the theory of foreign body response (FBR) to the dental implants introduced by Albrektsson and colleagues [13,14]. This FBR to the implant can be aggravated by various factors related to the implant hardware, patient characteristics or surgical hazards. Regarding implant-related factors, ions and particles released from the implant/prostheses combinations due to corrosion have been suggested to initiate a disbalanced FBR. In fact, the role of peri-implant titanium particles, micromotion and biocorrosion as risk indicators for peri-implantitis has been highlighted but requires further research [21]. Although titanium (Ti) shows a high corrosion resistance, obtained through the formation of a passive oxide layer, certain environmental conditions, such as those experienced in the oral cavity, can breach this protective oxide layer and cause corrosion [22]. Clark and Williams have stated that, even under passive conditions in which metal oxide film breakdown is not observed, metals and alloys will release ions into the surrounding tissue at a finite rate [23], affecting the mechanical integrity of the implant and the health of the surrounding tissue [22].
Therefore, the objective of this study was to evaluate the response of osteoblastic cells incubated with IAC (platform-matched vs. platform-switched) and unconnected implants (UI) using a novel direct-exposure technique. The tested biological responses were cell viability, apoptosis and the expression of genes related to bone resorption.
2. Materials and Methods
2.1. Preparation of Test Specimens
The IAC tested in this experiment were described in an earlier publication [8]. Commercially pure Ti cylinders were fabricated to serve as dental implants. The superstructures were either Ti alloy abutments (Ti-6Al-4V) or cobalt–chrome (CoCr) abutments (Table 1), which were further divided into three groups according to the abutment’s platform diameter (Figure 1).

Table 1.
Materials used in the study.
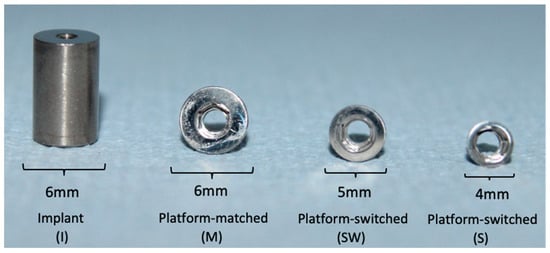
Figure 1.
Titanium cylinders serving as dental implants to be connected to abutments with three different platform diameters (6 mm, 5 mm and 4 mm).
The specimens were subjected to several steps of cleaning and sterilization in a laminar flow hood cabinet prior to testing [24].
2.2. Assembly of Test Specimens
The assembly and fixing of specimens took place under sterile conditions and specimens were handled aseptically with sterile talc-free gloves and sterilised plastic tweezers in the laminar flow hood. Eighteen implants were connected to their assigned abutments using hexed titanium screws and tightened manually (Figure 2a). The implant–abutment couplings and an additional three unconnected implants were securely attached to the sterile plastic cover of a 24-well plate using double-sided adhesive carbon tabs (12 mm diameter) (Elektron Technology UK Ltd., Cambridge, UK) (Figure 2b). The 21 fixed specimens were exposed to UV irradiation for an additional 20 min before direct exposure to cultured cells to insure sterility of the carbon tabs.
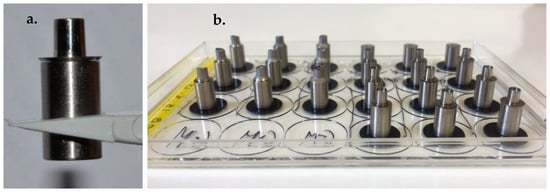
Figure 2.
(a) Implant coupled to abutment (b) Implant–abutment couplings (IAC) and unconnected implants (UI) securely attached to the sterile plastic cover of a 24-well plate using double-sided adhesive carbon tabs.
2.3. Cells and Cell Cultures
Osteoblastic cells were purchased from Lonza (Clonetics™ Normal Human Osteoblast Cell System, NHOst, Lonza, Walkersville, MD, USA). Cells were cultured in monolayer in osteoblast basal medium (Table 1) and incubated at 37 °C in a humidified atmosphere of 5% CO2 and 95% air. Osteoblastic cells of passages 4–6 were used for the experiments. These were seeded in a 24-well plate in triplicate at a density of 3000 cells/well for viability assay and 50,000 cells/well for apoptosis and gene expression, and were allowed to attach for 24 h, after which the culture medium was replaced by fresh media. To directly expose the cells to the specimens, a novel direct-exposure technique was devised and followed in the current experiments using sample-attached lids (SAL) (Figure 3). The SAL technique allowed the plate cover to serve as a vehicle or carrier to hold the specimens directly over the cultured cells. The samples were flipped vertically, with the abutment facing downward, supported by the above implant base and suspended over the attached monolayer of cells without touching the cells. The samples were immersed in the culture media up to the level of the implant platform (Figure 4). Three wells of specimen-free culture medium were used as a reference solution (REF) and served as the control group. Therefore, a total of 24 samples (Table 2) were used for each experiment and all experiments were repeated three times.

Figure 3.
Inverted sample attached lids (SAL) serving as a vehicle or carrier to hold the specimens directly hanging over the culture.
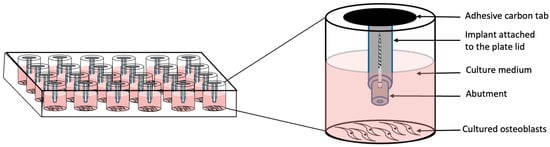
Figure 4.
Schematic diagram of the direct exposure test set-up using SAL technique.

Table 2.
Sample groups and their corresponding codes.
2.4. Cell Viability
Cell viability assay was conducted at time points of 1, 4, 7, 10, 14 and 21 days using Alamar Blue™ bioassay (AbD Serotec, Kidlington, UK). At each time point, the culture medium was replaced with fresh media and the attached specimens were rinsed thoroughly with phosphate-buffered saline. After 21 days, RNA was extracted for later gene expression analysis.
2.5. Apoptosis
After a 24 h exposure period, cells were collected for apoptosis analysis using FITC Annexin V Apoptosis Detection Kit, following the manufacturer’s instructions (BD Pharmingen™, BD Bioscience, Wokingham, UK) and a fluorescence-activated cell sorting (FACS) flow cytometer (EPICS XL®, Coulter Corporation, Brea, CA, USA).
2.6. Gene Expression
RNA levels of interleukin-6 (IL-6), interleukin-8 (IL-8), cyclooxygenase-2 (COX-2), Caspase-8, osteoprotegerin (OPG) and receptor activator of nuclear factor kappa-B ligand (RANKL) expressed by cells were analyzed after 1, 3 and 21 days. For the 21-day exposure period, RNA was extracted from the same cells that were initially seeded for the viability assay after conducting the 21-day time point viability analysis.
After each exposure period, total RNA was extracted from the cells using RNA extraction kit (RNeasy®Plus Mini Kit, QIAGEN GmbH, Hilden, Germany). RNA was reverse-transcribed to cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems™, Thermo Fisher Scientific, Waltham, MA, USA) according to manufacturer’s instructions.
Gene expression was analyzed by means of real-time quantitative polymerase chain reaction (RT-qPCR) using 7300 Real Time PCR System (Applied Biosystems™, ThermoFisher Scientific, USA). GAPDH was chosen as the housekeeping gene. Gene-specific primers and the TaqMan qPCR mastermix were purchased from TaqMan® (TaqMan® Gene Expression Assays, Applied Biosystems™, ThermoFisher Scientific, USA). Values were normalized to GAPDH and the control samples for each incubation time using the 2−∆∆Ct method [25]. A detailed protocol for gene expression analysis was described earlier [20].
2.7. Post-Immersion Observation of Implants and Abutments Interfaces
After the 21-day exposure period, representative specimens from different groups were randomly selected for examinations of the contacting surfaces under scanning electron microscope (SEM) (FEI, Eindhoven, The Netherlands) to observe any corrosion features.
2.8. Statistical Analysis
Statistically significant differences were tested by univariate analysis of variance (ANOVA) using SPSS version 22.0 (IBM SPSS Statistics, IBM, Japan) (p < 0.05). Levene’s test of homogeneity of variance was employed (α = 0.05), following the assumption of equal variances. When equal variances were assumed (p > 0.05), the Bonferroni post hoc test was used to analyze significant differences in mean values between groups. When equal variances were not assumed (p < 0.05), Dunnett’s T3 post hoc test was used to analyze significant differences between the groups.
3. Results
3.1. Cell Viability
The percentages of cell viability are presented in Figure 5. Starting from day 4, all test groups showed a statistically reduced viability compared to the control (p < 0.05). However, the cell viability of the IAC was lower than that of the UI (p < 0.05). The platform-matched groups were significantly lower in cell viability on day 4 compared to their counterparts, CS and TS groups (p < 0.001). On day 7, all the test groups continued to show less viability compared to the control (p < 0.005). The CM and CSW had also lower cell viability than the UI, at (p < 0.001) and (p < 0.01), respectively. On day 10, all the implant–abutment couplings had lower cell viability compared to the control (REF) (p < 0.001), while the UI group was not different from the control but was statistically different from the TM, CM and CSW groups (p < 0.001). At later incubation periods of 14 and 21 days, all IAC continued to show less viability than the control and the UI group (p < 0.001). It was also noted on day 21 that CM and TM had less cell viability than their counterparts, the CS and TS groups (p < 0.05 and p < 0.005 respectively).
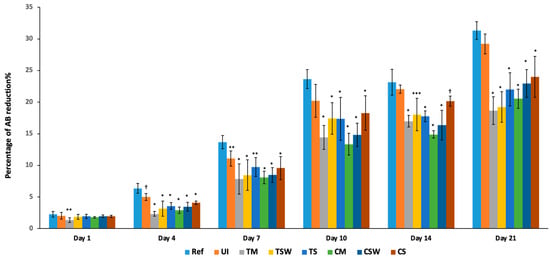
Figure 5.
Osteoblast reduction in Alamar BlueTM (AB) (%) in response to direct exposure to specimens. The results for viability were expressed as mean percentage (%), (* = p ≤ 0.001, ** = p < 0.005, *** = p < 0.01, † = p < 0.05).
3.2. Apoptosis
Cells exposed to IAC had a significantly higher percentage of apoptosis after 24 h compared to the control (p < 0.05) (Figure 6). The TM group had more apoptosis than both platform-switched groups of the same abutment material, the TSW and TS (p < 0.001). Similarly, the CM showed more apoptosis than that of the CSW and CS groups (p < 0.05).
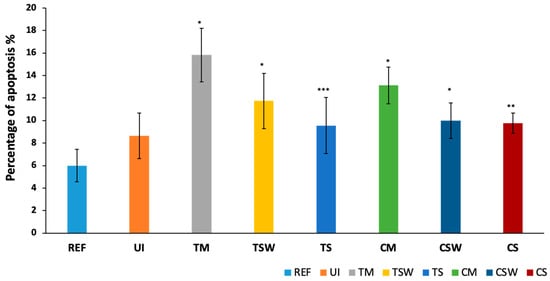
Figure 6.
Osteoblast induction of early apoptosis (%) in response to direct exposure to samples. The results for apoptosis were expressed as mean percentage (%) (* = p ≤ 0.001, ** = p < 0.005, *** = p < 0.01).
3.3. Gene Expression
Figure 7 shows different gene expression profiles during different exposure periods. The exposure of osteoblasts to IAC upregulated the expression of IL-6, IL-8, COX-2, caspase-8 and the RANKL/OPG ratio after 1 day of exposure (p < 0.05). This upregulation was more pronounced in the platform-matched groups (p < 0.05). On day 3, the upregulation of IL-8 and COX-2 continued in the IAC, being significantly high in the TM and CM groups, particularly COX-2 release, which reached up to 5-fold higher than the control (p < 0.001) (Figure 7C,D). As the incubation period increased to 21 days, osteoblasts exposed to IAC showed downregulation in most genes, except for COX-2 (Figure 7D), which was upregulated in all test groups, and IL-6, which was significantly high in the TM group (p < 0.001) (Figure 7B).
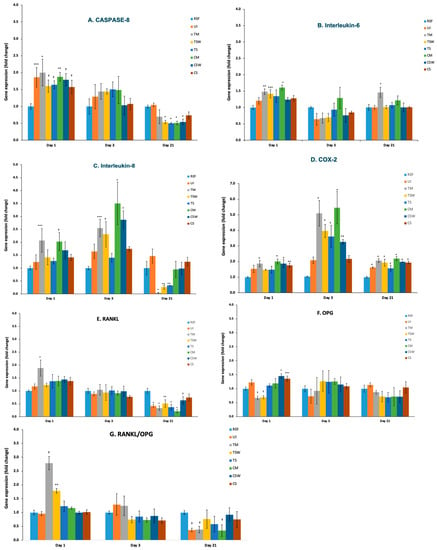
Figure 7.
Expression of genes related to bone resorption by osteoblasts in response to direct exposure to samples (A) Caspase-8, (B) Interlukin-6, (C) Interlukin-8, (D) Cyclooxygenase-2 (COX-2), (E) RANKL, (F) OPG and (G) ratio of RANKL/OPG. The results were expressed as mean fold change, * = p ≤ 0.001, ** = p < 0.005, *** = p < 0.01, † = p < 0.05.
3.4. Post-Immersion SEM Examination
Post-immersion SEM images showed corrosion features in the form of pitting areas and dark spots on the interfacial surfaces of both implants and their opposing abutments in all examined samples (Figure 8).
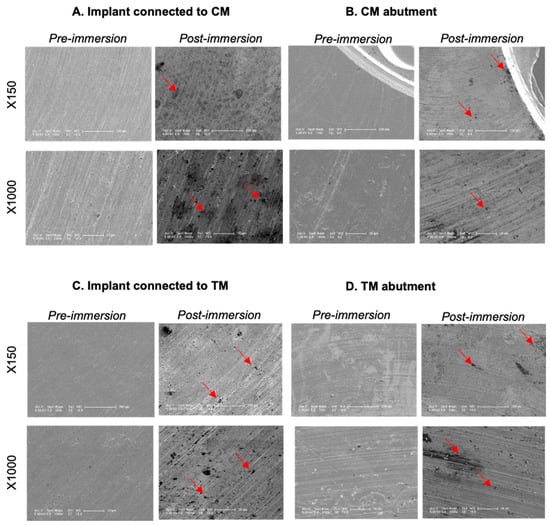
Figure 8.
Pre- and post-immersion SEM images of implants (A,C) and their opposing abutments (B,D) after 21 days of immersion in culture media at ×150 and ×1000 magnification. Pitting areas and black spots were scattered post-immersion on the implant and abutment interfaces at different locations, some denoted with red arrows.
4. Discussion
In this study, the biological responses to different IAC, as well as those directly UI-immersed in osteoblastic cell cultures using a novel direct-exposure technique (SAL), were evaluated. In this technique, the samples did not touch the cultured cells; therefore, the observed adverse cellular reactions were in fact responses to the substances being released from the immersed samples. The current innovative in vitro model might fall into a category between the direct and indirect models described by Wataha [26]. It would be more accurately classified as a modified direct model. This model fits its intended purpose to test the biological responses of cultured cells to the degradation products released from the immersed portion of the hanging solid material. There was no barrier, such as agar or filters, which might influence the results [27] or interrupt the flow of the released products. The culture medium served as a conductive electrolyte, delivering the released substances to the cells from samples that were in the same well and exposed to the same incubation conditions as the cultured cells without direct physical contact with the cultured cells. Therefore, variables related to material surface energy, contact angle, roughness and other material characteristics were avoided [28]. Those variables, however, might have influenced the level of metal ion release and degradation products, which, in turn, might affect the cells. That is the main purpose of this technique: to test the biologic responses to the released substances and not to the material itself.
The release of metal ions suggested the occurrence of corrosion phenomena, which may have developed as result of chemical dissolution in the presence of an electrolyte medium, the galvanic coupling of dissimilar metals of the implant and abutment, and crevices that were formed at the surface contact areas of the implants, abutments and abutment screws [22,29]. The occurrence of corrosion was confirmed by the SEM images, which showed pitting areas and dark spots on the implant and abutment interfaces. The quantification of metal ions released from the IAC into the electrolyte immersion medium has been performed earlier [8], but was not performed using a cell culture medium, which was one of the limitations in the present study. Metal ions released from implant degradation do not exist in unbound ionic or colloidal forms in the serum, but rather specifically bound to proteins, forming metal–protein complexes that would enter the cell through the permeable cell membranes [10,30]. Such metal–protein molecules require advanced digestion and protein-seperation methods, which should be considered in future research.
It was evident that osteoblastic cell viability was influenced by the presence of these specimens, mainly the IAC. This observation was in line with the previous findings of Hjalmarsson et al., who demonstrated that epithelial cells, as well as fibroblasts cultivated with CoCr and Ti cylinders, showed reduced cell viability, with a stronger negative response to CoCr alloy [31]. However, it should be noted that the majority of published in-vitro biologic research has looked into the effect of prepared unconnected discs or cylinders and not IAC, as in the present study, except for the work of Taylor et al. [29], who investigated the effect of the coupling of Ti implants and dissimilar metal abutments on osteoblast differentiation. Therefore, the results of the present study could not be completely compared with other findings. However, it was clear that the UI were less cytotoxic than IAC. This could be due to the galvanic situation present in the IAC, which could have enhanced the metal ion release from the couplings and therefore enhanced the adverse reactions. Another reason for this could be the composition of the IAC vs. UI. The soluble Co and V released from Co- and Ti-based abutment alloys, respectively, are among the most toxic metals, and have been shown to reduce the proliferation and viability of osteoblasts and other periprosthetic cells [32].
Although the influence of the implant–abutment mismatch on cell viability was only statistically significant at some time points, there was an obvious general trend showing that the larger the mismatch, the more favorable the cell viability. In other words, osteoblastic cell viability was inversely related to the abutment size, which means that the groups corresponding to the platform-matched couplings had less viability than the platform-switched groups within the same abutment material. The most logical explanation to this particular cell response could be that less ion is released from platform-switched groups, as was confirmed in earlier publications [8,9]. This could correlate to previously reported findings that the viability of osteoblasts was inversely proportional to the particle and ion concentration [33,34].
Although apoptosis is a normal fate for the majority of osteoblasts [35], the stimulation of osteoblast apoptosis reduces bone formation in the periprosthetic region, which could contribute to the total loss of bone volume around dental implants. It was evident in the present study that stimulated osteoblasts undergo higher rates of apoptosis. This finding was also supported by the upregulation of CASPASE-8 production after 1 day of exposure in most test groups. Similarly, Pioletti et al. found that Ti induced a caspase-dependent apoptosis of osteoblasts [36]. Although the authors used Ti particles rather than actual implants, as in the present study, it is important to bear in mind that metal ions are also released from the particles themselves, as well as directly from implant surfaces [11]. Therefore, it is difficult to anticipate whether the cellular reactions in response to particle stimulation in earlier studies were related to the particles per se, or to the ions released from such particles, as each form acts biologically through different metabolic pathways [10].
Interestingly, the platform-matched groups demonstrated higher levels of apoptosis compared to the platform-switched groups within the same material, and this again could be due to the higher amounts of metal ions released from the platform-matched groups [8].
The results of the present study show that the IAC, mostly the platform-matched groups, have a profound impact on genes that code for inflammatory cytokines and genes involved in bone homeostasis.
Although the upregulation of IL-6 was not remarkable in all test groups, it was noticeable in the platform-matched groups after 24 hrs of exposure, which could be due to the higher ion release, as explained earlier. It has been demonstrated by several investigators [11,27,28,29,37,38] that titanium ions and particles, as well as CoCr, increased the signal levels of IL-6 in a dose-dependent manner. On the other hand, Shida et al. showed that the release of IL-6 from Ti-stimulated osteoblast-like cells occurred in a time-dependent manner, which disagrees with the findings of the present experiment [37]. This apparent contrast could be attributed to the lower cell number caused by higher apoptotic rates and compromised viability after longer exposure periods [33], based on the assumption that only viable cells are capable of releasing IL-6 [32], which could also explain the downregulation observed in some genes after longer incubation periods. However, a statistical regression analysis to correlate cell vitality results with gene expression was not performed in the current study, limiting further anticipation of the data. The production of IL-6 by osteoblasts is significant, as IL-6 has been reported to be important to osteoclast development and bone resorption, both directly and indirectly, via the upregulation of RANKL [12].
Stimulated osteoblasts also produced increased levels of IL-8, which was significant in the platform-matched groups, achieving results up to four-fold higher than the control after 3 days of exposure. The release of IL-8 from osteoblasts in response to implant materials was also confirmed by the work of Kubies [39]. The authors illustrated the distinctively high expression of IL-8 from osteoblasts after cultivation onto a wide range of commercially available implant materials, regardless of the material’s surface roughness. On the other hand, Quabius et al., using actual dental implants, have demonstrated that titanium implants led to a more pronounced increase in IL-8 expression when compared with zirconia implants after incubation in human blood [40]. However, the magnitude of IL-8 expression in the work of Quabius et al. was far higher (>65-fold) than the levels expressed in the current study. This apparent difference could be attributed to either the different cells types used, the use of osteoblasts versus whole blood or the different incubation times [40]. Several investigators have also showed that osteoblasts incubated with Ti and CoCr particles/ions exhibit upregulated IL-8 chemokine expression [32,41,42]. These data indicate that the peri-implant-stimulated osteoblasts, although not the major source of chemotactic factors in the periprosthetic space, may be early and direct contributors to the initiation of the chronic peri-prosthetic inflammatory responses [11,12,41].
Interestingly, the release of COX-2 was upregulated, and the effect lasted for extended periods of exposure in most test groups, being more pronounced in the platform-matched couplings. It has been documented that COX-2 is dramatically upregulated during inflammation and usually increases in parallel with prostaglandin (PG) production and clinical inflammation [43]. Queally et al. [42] also observed an increase in COX-2 secretion from osteoblasts exposed to Co and Cr ions, which corroborates the present results. This suggests that one of the pathogenic mechanisms of peri-implant tissue inflammation in vivo may be the synthesis of COX-2 by osteoblastic cells in response to ion release, in conjunction with PGE2 production [12,42]. Increased levels of PGE2 have been demonstrated in the peri-prosthetic area and have been shown to play a significant role in the pathogenesis of peri-prosthetic osteolysis [11,12].
To our knowledge, this is the first in vitro test that examined the effect of different IAC on the different osteoblast production levels of inflammatory cytokines and chemokines related to bone resorption; therefore, the results could not be precisely compared to previous investigations. However, it appears that the element release from alloys is necessary but not a sufficient condition for all cytotoxicity, as stated by Wataha et al. [44,45]. In other words, elements must be released for cytotoxicity to occur, but not all element releases cause cytotoxicity. Therefore, the observed adverse reactions in osteoblasts were the result of ion release from the surfaces of immersed samples and the pronounced responses in the platform-matched couplings were due to the higher amounts of ion release from those groups. It is obvious then that the proposed theories explaining peri-implant bone loss, the biologic width theory [46], the bacterial theory [47] and the mechanical theory [48], cannot be proposed here to explain the adverse biological responses of the bone cells. Therefore, if we consider the corrosion product theory as explaining the present in vitro situation, it would then be logical to consider applying theory to the in vivo peri-implant bone changes around dental implants.
5. Conclusions
Within the limitations of the present in vitro study, the results indicate the following:
- Osteoblastic cell viability, apoptosis, and regulation of bone-resorbing mediators were significantly altered in the presence of implant–abutment couplings.
- Titanium implants alone did not influence the apoptosis and secretion of the tested cytokines and chemokines, but adversely influenced cell viability up to one week of exposure.
- The adverse biologic responses were more prominent in the platform-matched implant–abutment couplings. Therefore, platform-switching should be considered when restoring dental implants.
- The observed cytotoxic responses in osteoblastic cells could be due to metal ion release from the immersed samples into the surrounding medium as a result of corrosion, suggesting that corrosion products could play a pivotal role in the mediation of crestal bone loss around dental implants.
Author Contributions
Conceptualization, G.A., J.C.K. and H.P.; methodology, G.A. and H.P.; software, G.A.; validation, G.A., J.C.K. and H.P.; formal analysis, G.A.; investigation, G.A.; resources, G.A.; data curation, G.A.; writing—original draft preparation, G.A.; writing—review and editing, G.A., J.C.K. and H.P.; visualization, G.A., J.C.K. and H.P.; supervision, J.C.K. and H.P.; project administration, G.A., J.C.K. and H.P.; funding acquisition, G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by scholarship from King Saud University, Saudi Arabia and the APC was funded by G.A.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request from corresponding author.
Acknowledgments
Special thanks to the statistician from the Eastman Dental Institute for the help with statistical analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42, S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Thanissorn, C.; Guo, J.; Jing Ying Chan, D.; Koyi, B.; Kujan, O.; Khzam, N.; Miranda, L.A. Success rates and complications associated with single immediate implants: A systematic review. Dent. J. 2022, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Bragger, U.; Heitz-Mayfield, L. Biological and hardware complications in implant dentistry. In ITI Treatment Guide; Quintessence Publishing Co., Ltd.: Berlin, Germany, 2015; p. 200. [Google Scholar]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Clin. Periodontol. 2018, 45, S278–S285. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar]
- Hermann, J.S.; Schoolfield, J.D.; Schenk, R.K.; Buser, D.; Cochran, D.L. Influence of the size of the microgap on crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged implants in the canine mandible. J. Periodontol. 2001, 72, 1372–1383. [Google Scholar] [CrossRef]
- Lazzara, R.J.; Porter, S.S. Platform switching: A new concept in implant dentistry for controlling postrestorative crestal bone levels. Int. J. Periodontics Restor. Dent. 2006, 26, 9–17. [Google Scholar]
- Alrabeah, G.O.; Knowles, J.C.; Petridis, H. The effect of platform switching on the levels of metal ion release from different implant–abutment couples. Int. J. Oral Sci. 2016, 8, 117–125. [Google Scholar] [CrossRef]
- Alrabeah, G.O.; Knowles, J.C.; Petridis, H. Reduction of tribocorrosion products when using the platform-switching concept. J. Dent. Res. 2018, 97, 995–1002. [Google Scholar] [CrossRef]
- Hallab, N.J.; Jacobs, J.J. Biologic effects of implant debris. Bull. NYU Hosp. Jt. Dis. 2009, 67, 182–188. [Google Scholar]
- Vermes, C.; Glant, T.T.; Hallab, N.J.; Fritz, E.A.; Roebuck, K.A.; Jacobs, J.J. The potential role of the osteoblast in the development of periprosthetic osteolysis: Review of in vitro osteoblast responses to wear debris, corrosion products, and cytokines and growth factors. J. Arthroplast. 2001, 16, 95–100. [Google Scholar] [CrossRef]
- O’neill, S.C.; Queally, J.M.; Devitt, B.M.; Doran, P.P.; O’Byrne, J.M. The role of osteoblasts in peri-prosthetic osteolysis. Bone Jt. J. 2013, 95, 1022–1026. [Google Scholar] [CrossRef]
- Albrektsson, T.; Dahlin, C.; Jemt, T.; Sennerby, L.; Turri, A.; Wennerberg, A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin. Implant. Dent. Relat. Res. 2014, 16, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Chrcanovic, B.; Mölne, J.; Wennerberg, A. Foreign body reactions, marginal bone loss and allergies in relation to titanium implants. Eur. J. Oral Implantol. 2018, 11 (Suppl. 1), 537–546. [Google Scholar]
- Mombelli, A.; Hashim, D.; Cionca, N. What is the impact of titanium particles and biocorrosion on implant survival and complications? A critical review. Clin. Oral Implants Res. 2018, 29, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Fretwurst, T.; Nelson, K.; Tarnow, D.P.; Wang, H.L.; Giannobile, W.V. Is metal particle release associated with peri-implant bone destruction? An emerging concept. J. Dent. Res. 2018, 97, 259–265. [Google Scholar] [CrossRef]
- Noronha Oliveira, M.; Schunemann, W.V.; Mathew, M.T.; Henriques, B.; Magini, R.S.; Teughels, W.; Souza, J.C. Can degradation products released from dental implants affect peri-implant tissues? J. Periodont. Res. 2018, 53, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.G., Jr. Bone loss around implants—Is it metallosis? J. Periodontol. 2021, 92, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Alhamad, M.; Ampadi Ramachandran, R.; Shukla, A.; Barão, V.A.; Sukotjo, C.; Mathew, M.T. Peri-Implantitis in Relation to Titanium Corrosion: Current Status and Future Perspectives. J. Bio- Tribo-Corros. 2022, 8, 46. [Google Scholar] [CrossRef]
- Alrabeah, G.O.; Brett, P.; Knowles, J.C.; Petridis, H. The effect of metal ions released from different dental implant-abutment couples on osteoblast function and secretion of bone resorbing mediators. J. Dent. 2017, 66, 91–101. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S313–S318. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Tannenbaum, R.; Boyan, B.D.; Schwartz, Z. Electrical implications of corrosion for osseointegration of titanium implants. J. Dent. Res. 2011, 90, 1389–1397. [Google Scholar] [CrossRef]
- Clark, G.C.; Williams, D.F. The effects of proteins on metallic corrosion. J. Biomed. Mater. Res. 1982, 16, 125–134. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5: 2009; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C. Predicting clinical biological responses to dental materials. Dent. Mater. 2012, 28, 23–40. [Google Scholar] [CrossRef]
- Wennberg, A.; Mjör, I.A.; Hensten-Pettersen, A. Biological evaluation of dental restorative materials—A comparison of different test methods. J. Biomed. Mater. Res. 1983, 17, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Özçelik, H.; Stutz, C.; Gegout, P.Y.; Benkirane-Jessel, N.; Petit, C.; Huck, O. Modulation of immune-inflammatory responses through surface modifications of biomaterials to promote bone healing and regeneration. J. Tissue Eng. 2021, 12, 20417314211041428. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.C.; Anderson, G.I.; Sutow, E.J.; Driscoll, C.F.; Mackey, D.C. The effects of the coupling of titanium implants and dissimilar metal abutments on osteoblast differentiation in vitro. Int. J. Oral Maxillofac. Implant. 1999, 14, 785–797. [Google Scholar]
- Hallab, N.J.; Jacobs, J.J.; Skipor, A.; Black, J.; Mikecz, K.; Galante, J.O. Systemic metal–protein binding associated with total joint replacement arthroplasty. J. Biomed. Mater. Res. 2000, 49, 353–361. [Google Scholar] [CrossRef]
- Hjalmarsson, L.; Smedberg, J.I.; Aronsson, G.; Wennerberg, A. Cellular responses to cobalt-chrome and CP titanium--an in vitro comparison of frameworks for implant-retained oral prostheses. Swed. Dent. J. 2011, 35, 177–186. [Google Scholar]
- Hallab, N.J.; Vermes, C.; Messina, C.; Roebuck, K.A.; Glant, T.T.; Jacobs, J.J. Concentration-and composition-dependent effects of metal ions on human MG-63 osteoblasts. J. Biomed. Mater. Res. 2002, 60, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Lochner, K.; Fritsche, A.; Jonitz, A.; Hansmann, D.; Mueller, P.; Mueller-Hilke, B.; Bader, R. The potential role of human osteoblasts for periprosthetic osteolysis following exposure to wear particles. Int. J. Mol. Med. 2011, 28, 1055–1063. [Google Scholar] [CrossRef]
- Dalal, A.; Pawar, V.; McAllister, K.; Weaver, C.; Hallab, N.J. Orthopedic implant cobalt-alloy particles produce greater toxicity and inflammatory cytokines than titanium alloy and zirconium alloy-based particles in vitro, in human osteoblasts, fibroblasts, and macrophages. J. Biomed. Mater. Res. Part A 2012, 100, 2147–2158. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; Weinstein, R.S.; Bellido, T.; Parfitt, A.M.; Manolagas, S.C. Osteoblast programmed cell death (apoptosis): Modulation by growth factors and cytokines. J. Bone Miner. Res. 1998, 13, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Pioletti, D.P.; Leoni, L.; Genini, D.; Takei, H.; Du, P.; Corbeil, J. Gene expression analysis of osteoblastic cells contacted by orthopedic implant particles. J. Biomed. Mater. Res. 2002, 61, 408–420. [Google Scholar] [CrossRef]
- Shida, J.; Trindade, M.C.; Goodman, S.B.; Schurman, D.J.; Smith, R.L. Induction of interleukin-6 release in human osteoblast-like cells exposed to titanium particles in vitro. Calcif. Tissue Int. 2000, 67, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Haddouti, E.M.; Welle, K.; Burger, C.; Wirtz, D.C.; Schildberg, F.A.; Kabir, K. The effects of biomaterial implant wear debris on osteoblasts. Front. Cell Dev. Biol. 2020, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Kubies, D.; Himmlová, L.; Riedel, T.; Chánová, E.; Balík, K.; Douderova, M.; Bártová, J.; Pesakova, V.J. The interaction of osteoblasts with bone-implant materials: 1. The effect of physicochemical surface properties of implant materials. Physiol. Res. 2011, 60, 95. [Google Scholar] [CrossRef]
- Quabius, E.S.; Ossenkop, L.; Harder, S.; Kern, M. Dental implants stimulate expression of Interleukin-8 and its receptor in human blood—An in vitro approach. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1283–1288. [Google Scholar] [CrossRef]
- Fritz, E.A.; Glant, T.T.; Vermes, C.; Jacobs, J.J.; Roebuck, K.A. Chemokine gene activation in human bone marrow-derived osteoblasts following exposure to particulate wear debris. J. Biomed. Mater. Res. Part A 2006, 77, 192–201. [Google Scholar] [CrossRef]
- Queally, J.M.; Devitt, B.M.; Butler, J.S.; Malizia, A.P.; Murray, D.; Doran, P.P.; O’byrne, J.M. Cobalt ions induce chemokine secretion in primary human osteoblasts. J. Orthop. Res. 2009, 27, 855–864. [Google Scholar] [CrossRef]
- Crofford, L.J. COX-1 and COX-2 tissue expression: Implications and predictions. J. Rheumatol. Suppl. 1997, 49, 15–19. [Google Scholar] [PubMed]
- Wataha, J.C.; Craig, R.G.; Hanks, C.T. The release of elements of dental casting alloys into cell-culture medium. J. Dent. Res. 1991, 70, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; Malcolm, C.T.; Hanks, C.T. Correlation between cytotoxicity and the elements released by dental casting alloys. Int. J. Prosthodont. 1995, 8, 9–14. [Google Scholar] [PubMed]
- Hermann, J.S.; Cochran, D.L.; Hermann, J.S.; Buser, D.; Schenk, R.K.; Schoolfield, J.D. Biologic Width around one-and two-piece titanium implants: A histometric evaluation of unloaded nonsubmerged and submerged implants in the canine mandible. Clin. Oral Implant. Res. 2001, 12, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Quaranta, A.; Teles, R.P. The microbiota associated with implants restored with platform switching: A preliminary report. J. Periodontol. 2010, 81, 403–411. [Google Scholar] [CrossRef]
- Maeda, Y.; Miura, J.; Taki, I.; Sogo, M. Biomechanical analysis on platform switching: Is there any biomechanical rationale? Clin. Oral Implant. Res. 2007, 18, 581–584. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).