Study of Structure, Morphology and Optical Properties of Cobalt-Doped and Co/Al-co-Doped ZnO Thin Films Deposited by Electrospray Method
Abstract
:1. Introduction
2. Materials and Methods
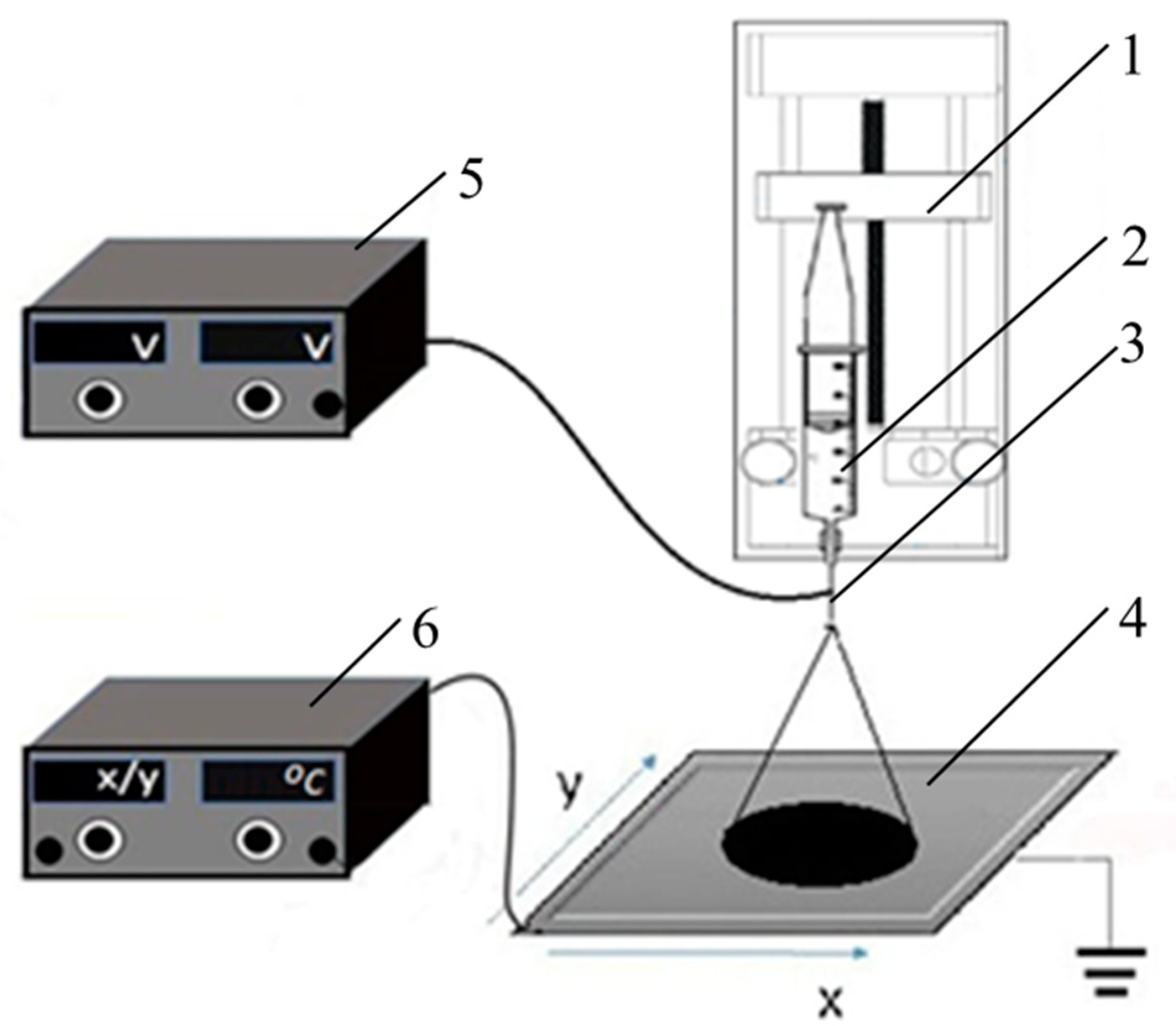
2.1. Thin Film Deposition
2.2. Characterization of Morphology and Structure
2.3. Characterization of Optical Properties
3. Results and Discussions
3.1. Surface Morphology
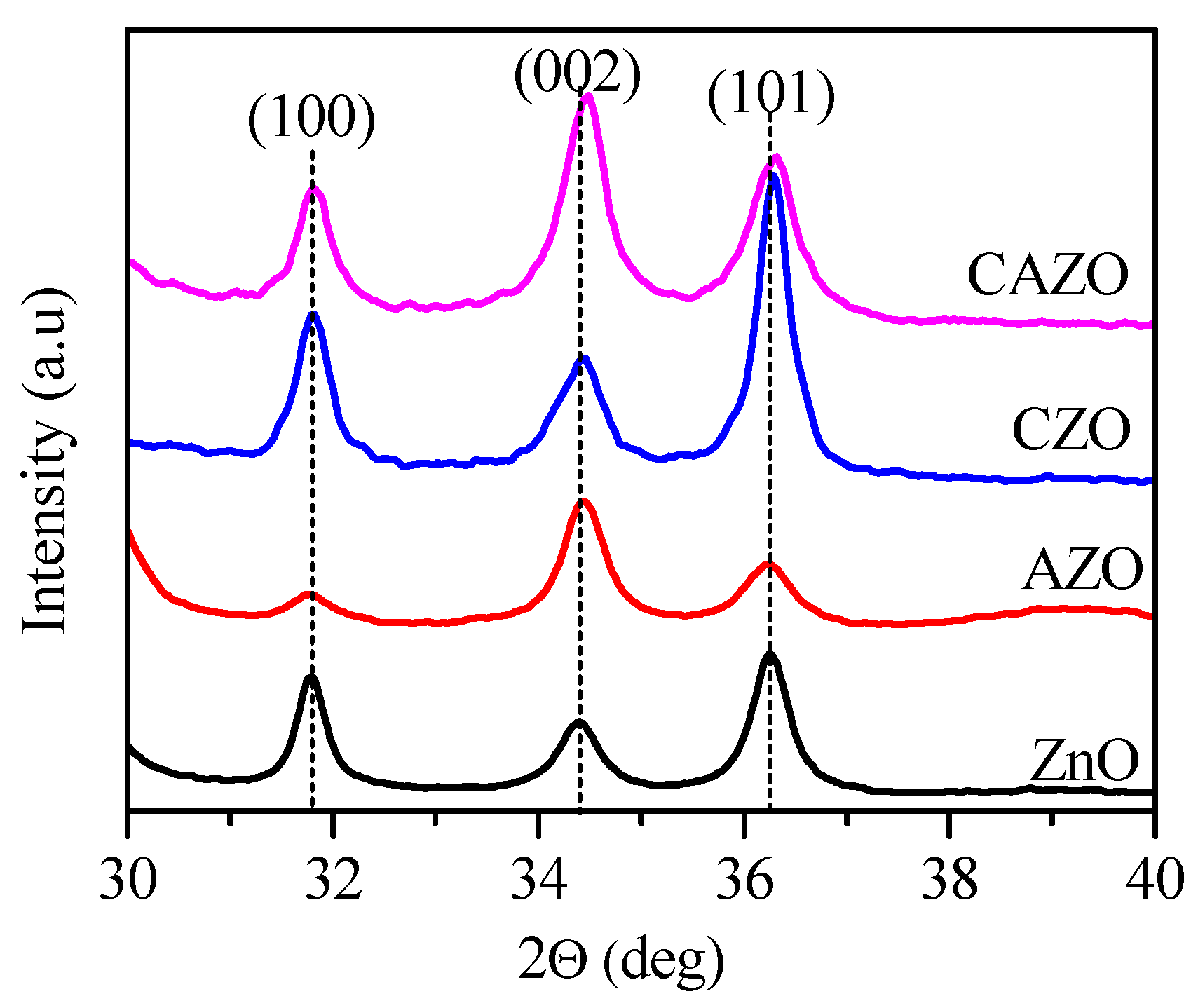
3.2. Structure
3.3. Optical Properties
3.3.1. Transmittance Measurements
3.3.2. Ellipsometric Measurements
3.3.3. Calculation of Optical Band Gap and Urbach Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ozgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Fundamentals of zinc oxide as a semiconductor. Rep. Prog. Phys. 2009, 72, 126501. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Zeng, H. ZnO-based transparent conductive thin films: Doping, performance, and processing. J. Nanomater. 2013, 2013, 196521. [Google Scholar] [CrossRef]
- Poongodi, G.; Anandan, P.; Kumar, R.M.; Jayavel, R. Studies on visible light photocatalytic and antibacterial activities of nanostructured cobalt doped ZnO thin films prepared by sol–gel spin coating method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 148, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Abdelkrim, M.; Guezzoul, M.; Bedrouni, M.; Bouslama, M.; Ouerdane, A.; Kharroubi, B. Effect of slight cobalt incorporation on the chemical, structural, morphological, optoelectronic, and photocatalytic properties of ZnO thin film. J. Alloys Compd. 2022, 920, 165703. [Google Scholar] [CrossRef]
- Ali, D.; Butt, M.Z.; Muneer, I.; Farrukh, M.A.; Aftab, M.; Saleem, M.; Bashir, F.; Khan, A.U. Synthesis and characterization of sol-gel derived La and Sm doped ZnO thin films: A solar light photo catalyst for methylene blue. Thin Solid Film. 2019, 679, 86–98. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Xu, L.; Zhu, S.; Yang, S.; Chen, X.; Dong, B.; Bai, X.; Lu, G.; Song, H. Graphene quantum dot-functionalized three-dimensional ordered mesoporous ZnO for acetone detection toward diagnosis of diabetes. Nanoscale 2019, 11, 11496–11504. [Google Scholar] [CrossRef]
- Alexieva, G.; Lovchinov, K.; Petrov, M.; Gergova, R.; Tyutyundzhiev, N. Influence of Al Doping on the Morphological, Structural and Gas Sensing Properties of Electrochemically Deposited ZnO Films on Quartz Resonators. Coatings 2022, 12, 81. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Sensing behavior to ppm-level gases and synergistic sensing mechanism in metal-functionalized rGO-loaded ZnO nanofibers. Sens. Actuators B Chem. 2018, 255, 1884–1896. [Google Scholar] [CrossRef]
- Rana, S.B.; Singh, R.P.P.; Arya, S. Structural, optical, magnetic and antibacterial study of pure and cobalt doped ZnO nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 2660–2672. [Google Scholar] [CrossRef]
- Mahmood, K.; Swain, B.S.; Jung, H.S. Controlling the surface nanostructure of ZnO and Al-doped ZnO thin films using electrostatic spraying for their application in 12% efficient perovskite solar cells. Nanoscale 2014, 6, 9127–9138. [Google Scholar] [CrossRef] [PubMed]
- Dietl, T.; Ohno, H.; Matsukura, F.; Cibert, J.; Ferrand, D. Zener model description of ferromagnetism in zinc-blende magnetic semiconductors. Science 2000, 287, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.T.; Chin, T.S.; Shih, J.C.; Lin, S.H.; Hong, T.M.; Huang, R.T.; Chen, F.R.; Kai, J.J. Enhancement of ferromagnetic properties in Zn1−xCoxO by additional Cu doping. Appl. Phys. Lett. 2004, 85, 621–623. [Google Scholar] [CrossRef]
- Ueda, K.; Tabota, H.; Kawai, T. Magnetic and electric properties of transition-metal-doped ZnO films. Appl. Phys. Lett. 2001, 79, 988–990. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, S.Y.; Cho, C.R.; Park, C.H. Study of diluted magnetic semiconductor: Co-doped ZnO. Appl. Phys. Lett. 2002, 81, 4020–4022. [Google Scholar] [CrossRef]
- Song, C.; Zeng, F.; Geng, K.W.; Wang, X.B.; Shen, Y.X.; Pan, F. The magnetic properties of Co-doped ZnO diluted magnetic insulator films prepared by direct current reactive magnetron co-sputtering. J. Magn. Magn. Mater. 2007, 309, 25–30. [Google Scholar] [CrossRef]
- Chanda, A.; Gupta, S.; Vasundhara, M.; Joshi, S.R.; Mutta, G.R.; Singh, J. Study of structural, optical and magnetic properties of cobalt doped ZnO nanorods. RSC Adv. 2017, 7, 50527–50536. [Google Scholar] [CrossRef]
- Paskaleva, A.; Buchkov, K.; Galluzzi, A.; Spassov, D.; Blagoev, B.; Ivanov, T.; Mehandzhiev, V.; Avramova, I.A.; Terzyiska, P.; Tzvetkov, P.; et al. Magneto-Optical and Muliferroic Properties of Transition-Metal (Fe, Co, or Ni)-Doped ZnO Layers Deposited by ALD. ACS Omega 2022, 7, 43306–43315. [Google Scholar] [CrossRef]
- Ando, K. Magneto-Optics of Diluted Magnetic Semiconductors: New Materials and Applications. In Magneto-Optics; Sugano, S., Kojima, N., Eds.; Springer Series in Solid-State Sciences; Springer: Berlin/Heidelberg, Germany, 2000; Volume 128, pp. 211–244. [Google Scholar] [CrossRef]
- Sutanto, H.; Wibowo, S.; Hadiyanto, H.; Arifin, M.; Hidayanto, E. Photocatalytic activity of cobalt-doped zinc oxide thin film prepared using the spray coating technique. Mater. Res. Express 2017, 4, 076409. [Google Scholar] [CrossRef]
- Fitzgerald, C.B.; Venkatesan, M.; Lunney, J.G.; Dorneles, L.S.; Coey, J.M.D. Cobalt-doped ZnO—A room temperature dilute magnetic semiconductor. Appl. Surf. Sci. 2005, 247, 493–496. [Google Scholar] [CrossRef]
- Ivill, M.; Pearton, S.J.; Rawal, S.; Leu, L.; Sadik, P.; Das, R.; Hebard, A.F.; Chisholm, M.; Budai, J.D.; Norton, D.P. Structure and magnetism of cobalt-doped ZnO thin films. New J. Phys. 2008, 10, 065002. [Google Scholar] [CrossRef]
- Goktas, A.; Aslan, F.; Mutlu, I.H. Effect of preparation technique on the selected characteristics of Zn1−xCoxO nanocrystalline thin films deposited by sol–gel and magnetron sputtering. J. Alloys Compd. 2014, 615, 765–778. [Google Scholar] [CrossRef]
- Kaphle, A.; Hari, P. Variation of index of refraction in cobalt doped ZnO nanostructures. J. Appl. Phys. 2017, 122, 165304. [Google Scholar] [CrossRef]
- Kaphle, A.; Reed, T.; Apblett, A.; Hari, P. Doping Efficiency in Cobalt-Doped ZnO Nanostructured Materials. J. Nanomater. 2019, 2019, 7034620. [Google Scholar] [CrossRef]
- Nam, G.; Yoon, H.; Kim, B.; Lee, D.Y.; Kim, J.S.; Leem, J.Y. Effect of Co doping concentration on structural properties and optical parameters of Co-doped ZnO thin films by sol-gel dip-coating method. J. Nanosci. Nanotechnol. 2014, 14, 8544–8548. [Google Scholar] [CrossRef]
- Shukla, P.; Tiwari, S.; Joshi, S.R.; Akshay, V.R.; Vasundhara, M.; Varma, S.; Singh, J.; Chanda, A. Investigation on structural, morphological and optical properties of Co-doped ZnO thin films. Phys. B Condens. Matter 2018, 550, 303–310. [Google Scholar] [CrossRef]
- Gungor, E.; Gungor, T.; Caliskan, D.; Ceylan, A.; Ozbay, E. Co doping induced structural and optical properties of sol–gel prepared ZnO thin films. Appl. Surf. Sci. 2014, 318, 309–313. [Google Scholar] [CrossRef]
- Yahmadi, B.; Kamoun, O.; Alhalaili, B.; Alleg, S.; Vidu, R.; Kamoun Turki, N. Physical Investigations of (Co, Mn) Co-Doped ZnO Nanocrystalline Films. Nanomaterials 2020, 10, 1507. [Google Scholar] [CrossRef] [PubMed]
- Marinov, G.; Lovchinov, K.; Madjarova, V.; Strijkova, V.; Vasileva, M.; Malinowski, N.; Babeva, T. Aluminum-doped zinc oxide thin films deposited by electrospray method. Opt. Mater. 2019, 89, 390–395. [Google Scholar] [CrossRef]
- Marinov, G.; Strijkova, V.; Vasileva, M.; Madjarova, V.; Malinowski, N.; Babeva, T. Effect of substrate temperature on the microstructural, morphological, and optical properties of electrosprayed ZnO thin films. Adv. Condens. Matter. Phys. 2018, 2018, 89575077. [Google Scholar] [CrossRef]
- El Mir, L.; Ayadi, Z.B.; Saadoun, M.; Djessas, K.; von Bardeleben, H.J.; Alaya, S. Preparation and characterization of n-type conductive (Al, Co) co-doped ZnO thin films deposited by sputtering from aerogel nanopowders. Appl. Surf. Sci. 2007, 254, 570–573. [Google Scholar] [CrossRef]
- Ghribi, F.; Khalifi, N.; Mrabet, S.; Ghiloufi, I.; Talu, S.; El Mir, L.M.; Da Fonseca Filho, D.H.; Oliveira, R.M.; Matos, R.S. Evaluation of the structure–micromorphology relationship of Co10%–Alx Co-doped Zinc oxide nanostructured thin films deposited by pulsed laser using XRD and AFM. Arab. J. Sci. Eng. 2022, 47, 7717–7728. [Google Scholar] [CrossRef]
- Khan, M.S.; Shi, L.-J.; Zou, B. First principles calculations of optoelectronic and magnetic properties of Co-doped and (Co, Al) co-doped ZnO. J. Appl. Phys. 2020, 127, 065707. [Google Scholar] [CrossRef]
- Vempati, S.; Shetty, A.; Dawson, P.; Nanda, K.K.; Krupanidhi, S.B. Solution-based synthesis of cobalt-doped ZnO thin films. Thin Solid Film. 2012, 524, 137–143. [Google Scholar] [CrossRef]
- Nakrela, A.; Benramdane, N.; Bouzidi, A.; Kebbab, Z.; Medles, M.; Mathieu, C. Site location of Al-dopant in ZnO lattice by exploiting the structural and optical characterisation of ZnO:Al thin films. Results Phys. 2016, 6, 133–138. [Google Scholar] [CrossRef]
- Yoo, Y.-Z.; Fukumura, T.; Jin, Z.; Hasegawa, K.; Kawasaki, M.; Ahmet, P.; Chikyow, T.; Koinuma, H. ZnO–CoO solid solution thin films. J. Appl. Phys. 2001, 90, 4246–4250. [Google Scholar] [CrossRef]
- Forouhi, A.R.; Bloomer, I. Optical properties of crystalline semiconductors and dielectrics. Phys. Rev. B 1988, 38, 1865–1874. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A. States in the gap. J. Non-Cryst. Sol. 1972, 8–10, 569–585. [Google Scholar] [CrossRef]
- Fang, D.; Lin, K.; Xue, T.; Cui, C.; Chen, X.; Yao, P.; Li, H. Influence of Al doping on structural and optical properties of Mg–Al co-doped ZnO thin films prepared by sol–gel method. J. Alloys Compd. 2014, 589, 346–352. [Google Scholar] [CrossRef]




| Sample | Grain Size (nm) | D100 (nm) | D002 (nm) | D101 (nm) |
|---|---|---|---|---|
| ZnO | 143 ± 34 | 26 | 21 | 22 |
| AZO | 128 ± 23 | 25 | 19 | 18 |
| CZO | 62 ± 10 | 22 | 20 | 29 |
| CAZO | 71 ± 10 | 26 | 21 | 22 |
| Sample | rms Roughness (nm) | n @ 600 nm | Eg (eV) | EU (meV) |
|---|---|---|---|---|
| ZnO | 26 | 1.86 | 3.29 | 119 |
| AZO | 34 | 1.94 | 3.35 | 110 |
| CZO | 31 | 2.00 | 3.35 | 236 |
| CAZO | 35 | 1.97 | 3.33 | 138 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinov, G.; Georgieva, B.; Vasileva, M.; Babeva, T. Study of Structure, Morphology and Optical Properties of Cobalt-Doped and Co/Al-co-Doped ZnO Thin Films Deposited by Electrospray Method. Appl. Sci. 2023, 13, 9611. https://doi.org/10.3390/app13179611
Marinov G, Georgieva B, Vasileva M, Babeva T. Study of Structure, Morphology and Optical Properties of Cobalt-Doped and Co/Al-co-Doped ZnO Thin Films Deposited by Electrospray Method. Applied Sciences. 2023; 13(17):9611. https://doi.org/10.3390/app13179611
Chicago/Turabian StyleMarinov, Georgi, Biliana Georgieva, Marina Vasileva, and Tsvetanka Babeva. 2023. "Study of Structure, Morphology and Optical Properties of Cobalt-Doped and Co/Al-co-Doped ZnO Thin Films Deposited by Electrospray Method" Applied Sciences 13, no. 17: 9611. https://doi.org/10.3390/app13179611
APA StyleMarinov, G., Georgieva, B., Vasileva, M., & Babeva, T. (2023). Study of Structure, Morphology and Optical Properties of Cobalt-Doped and Co/Al-co-Doped ZnO Thin Films Deposited by Electrospray Method. Applied Sciences, 13(17), 9611. https://doi.org/10.3390/app13179611






