Polycyclic Aromatic Hydrocarbon Occurrence and Formation in Processed Meat, Edible Oils, and Cereal-Derived Products: A Review
Abstract
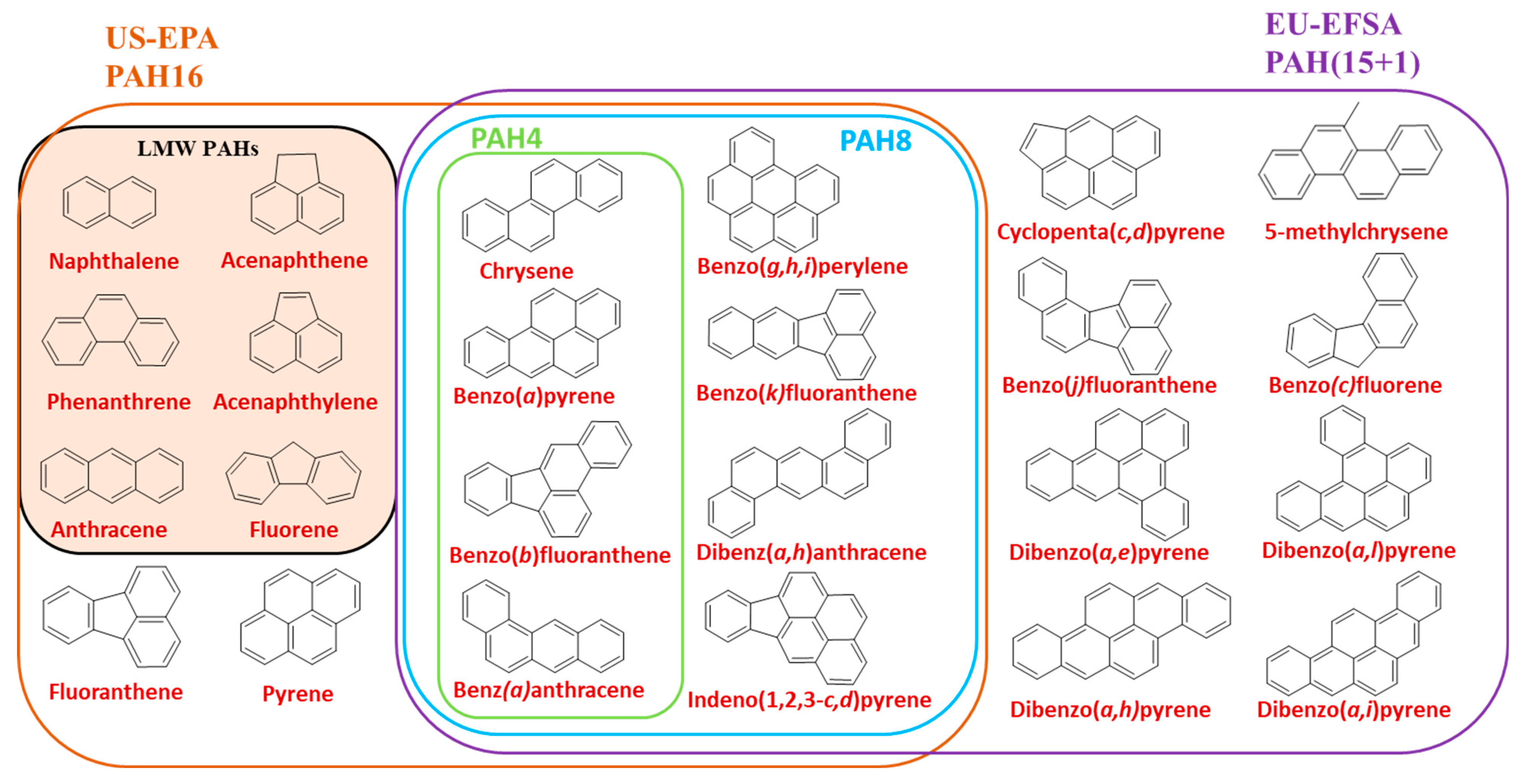
:1. Introduction
2. PAH Sample Pretreatment in Food and Quantitative Analysis
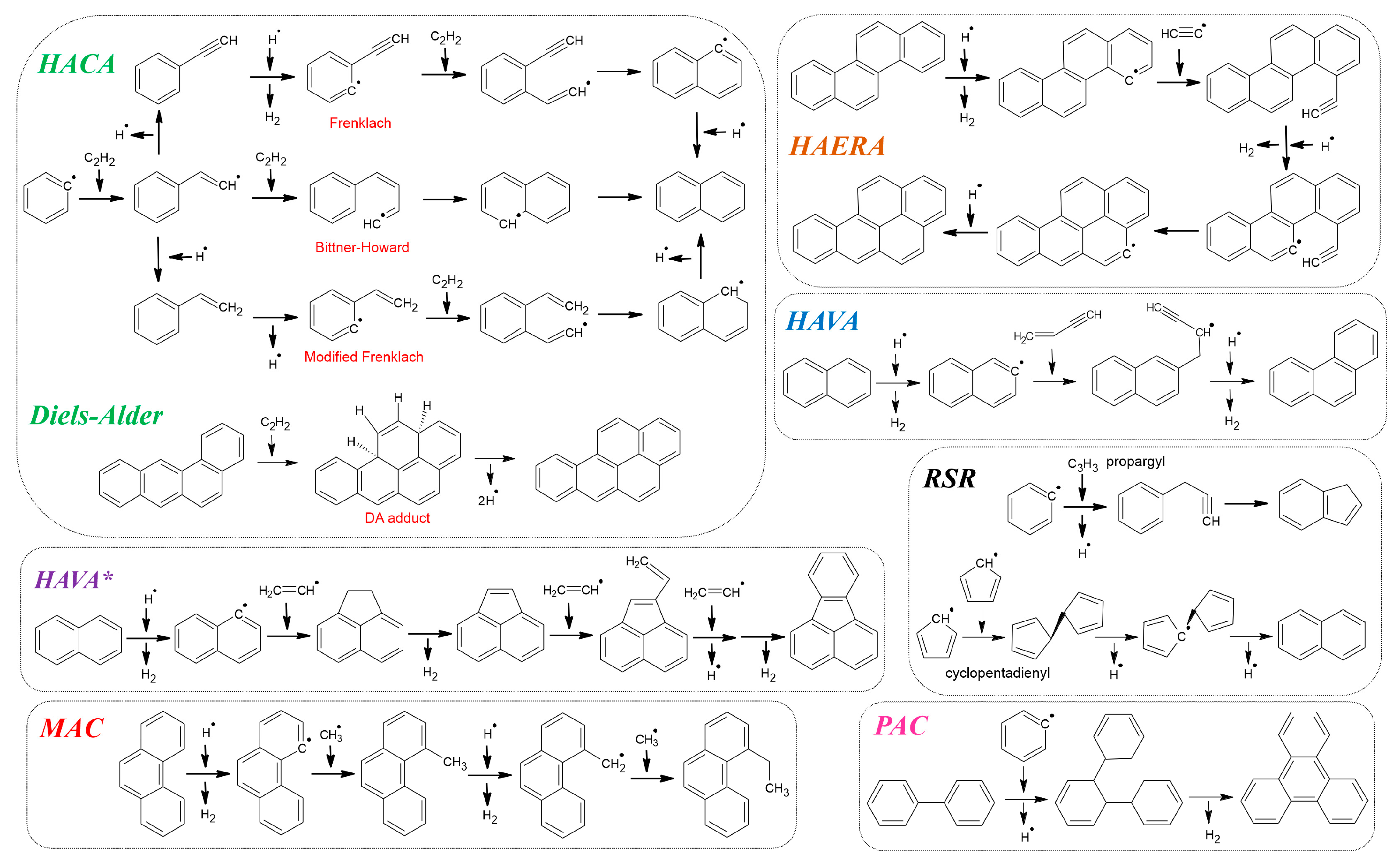
3. PAH Formation—Mechanistic Features
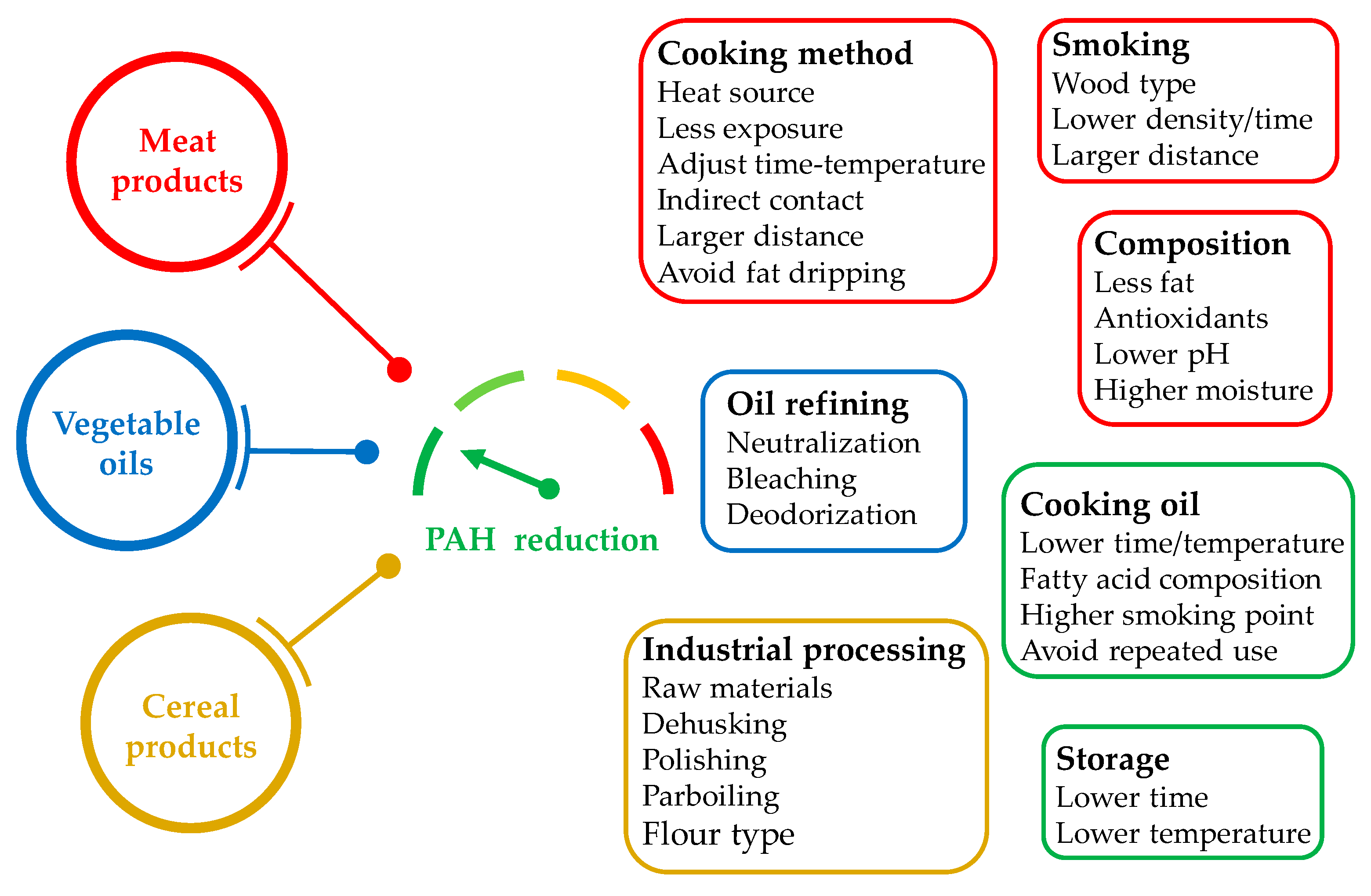
4. PAH Occurrence and Formation in Processed Meat, Edible Oils, and Cereal-Derived Products
4.1. Features Underlying PAH Formation in Meat and Meat-Derived Products
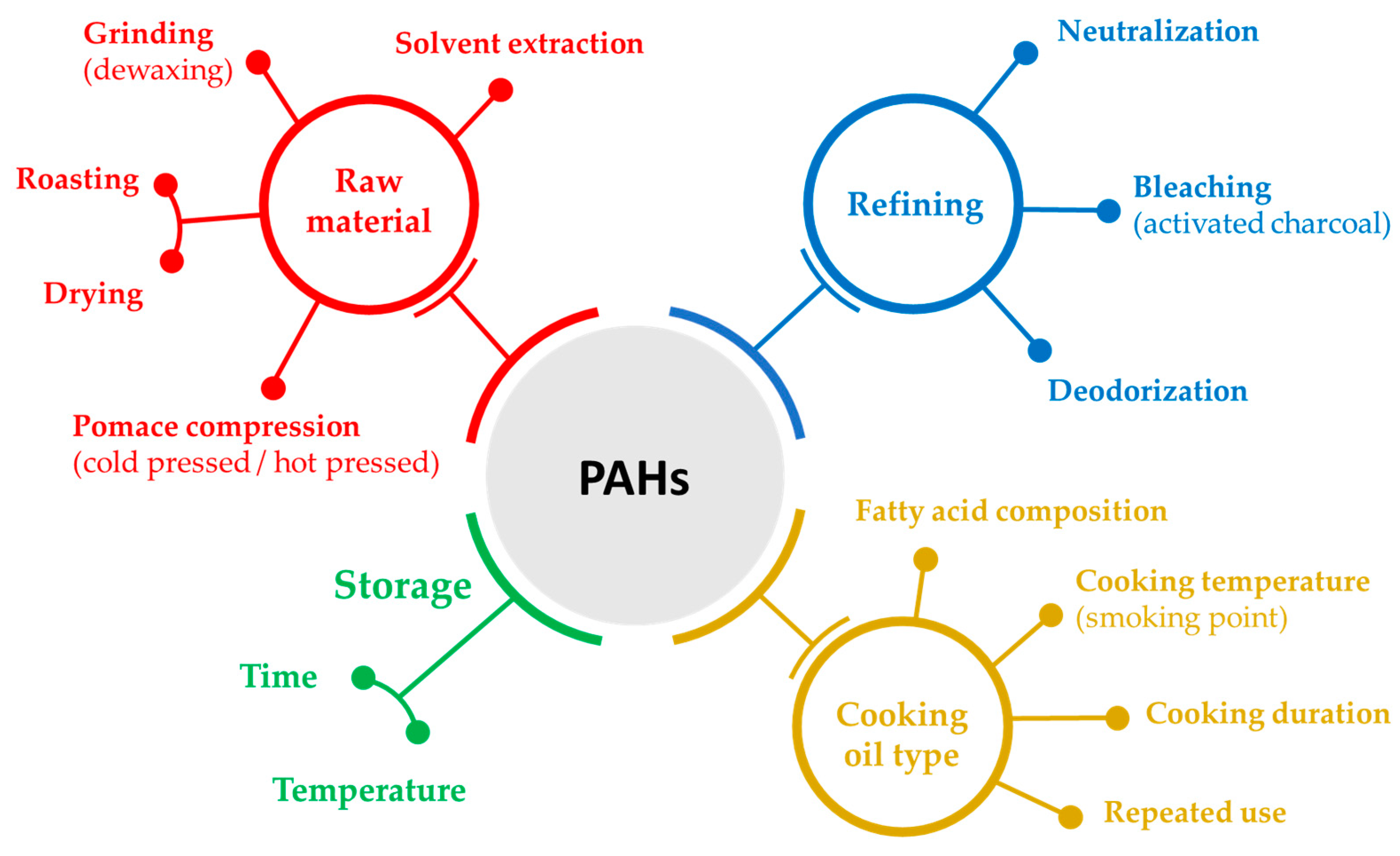
4.2. Features Underlying PAH Formation in Edible Oils
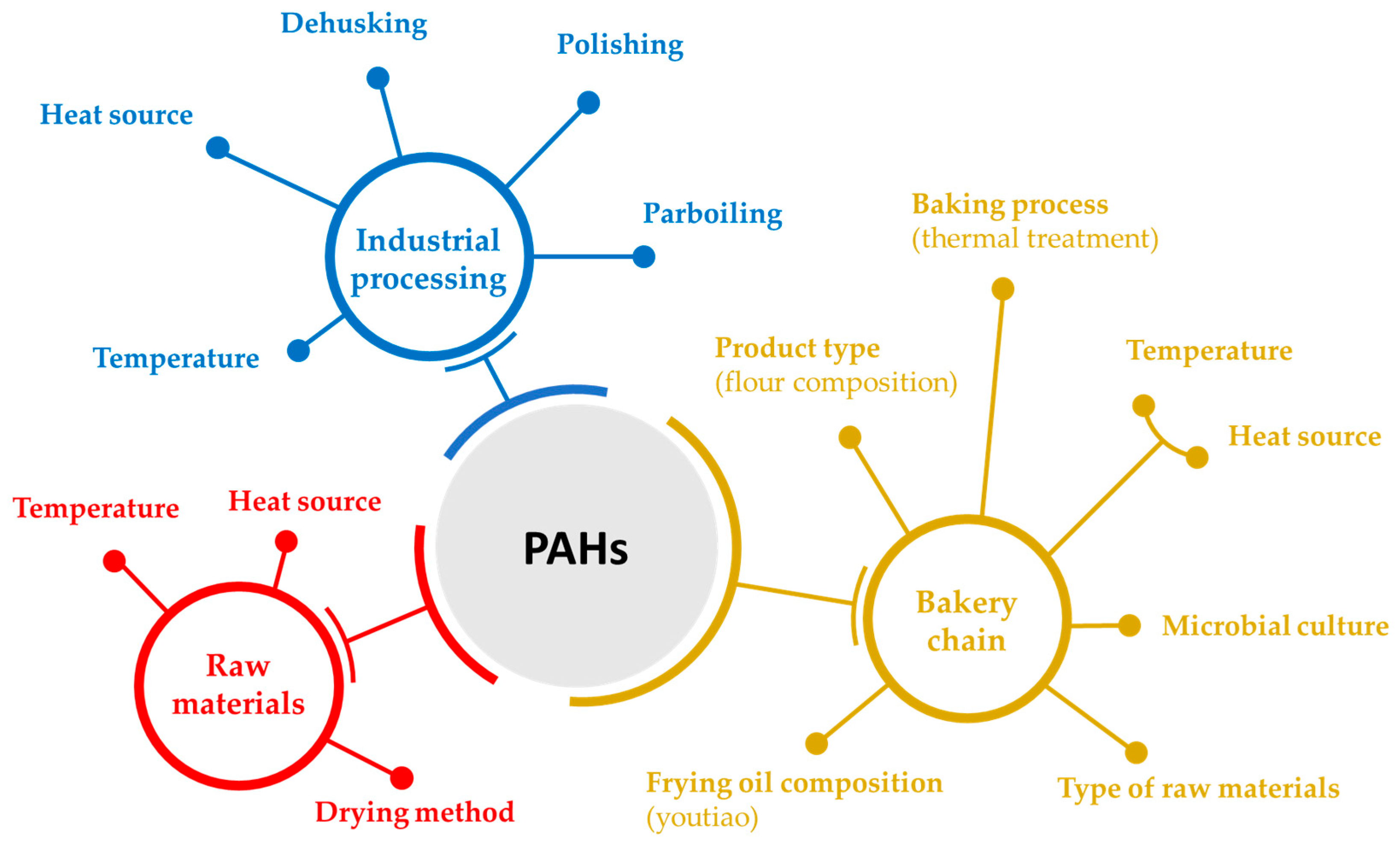
4.3. Features Underlying PAH Formation in Cereal and Cereal-Derived Products
4.4. Outlook on Associated Knowledge Gaps and Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, L.; Varshney, J.G.; Agarwal, T. Polycyclic aromatic hydrocarbons’ formation and occurrence in processed food. Food Chem. 2016, 199, 768–781. [Google Scholar] [CrossRef]
- Wu, S.; Gong, G.; Yan, K.; Sun, Y.; Zhang, L. Chapter Two—Polycyclic aromatic hydrocarbons in edible oils and fatty foods: Occurrence, formation, analysis, change and control. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 93, pp. 59–112. ISBN 1043-4526. [Google Scholar]
- Gachanja, A.N.; Maritim, P.K. Polycyclic Aromatic Hydrocarbons|Determination☆. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Poole, C., Townshend, A., Miró, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 328–340. ISBN 978-0-08-101984-9. [Google Scholar]
- Achten, C.; Andersson, J.T. Overview of Polycyclic Aromatic Compounds (PAC). Polycycl. Aromat. Compd. 2015, 35, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Wu, S.; Zeng, J.; Wang, L.; Yu, W. Effect of frying and aluminium on the levels and migration of parent and oxygenated PAHs in a popular Chinese fried bread youtiao. Food Chem. 2016, 209, 123–130. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, S.; Gong, G. Trends of research on polycyclic aromatic hydrocarbons in food: A 20-year perspective from 1997 to 2017. Trends Food Sci. Technol. 2019, 83, 86–98. [Google Scholar] [CrossRef]
- Wang, Z.; Ng, K.; Warner, R.D.; Stockmann, R.; Fang, Z. Reduction strategies for polycyclic aromatic hydrocarbons in processed foods. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1598–1626. [Google Scholar] [CrossRef]
- Andersson, J.T.; Achten, C. Time to Say Goodbye to the 16 EPA PAHs? Toward an Up-to-Date Use of PACs for Environmental Purposes. Polycycl. Aromat. Compd. 2015, 35, 330–354. [Google Scholar] [CrossRef] [Green Version]
- Nowakowski, M.; Rykowska, I.; Wolski, R.; Andrzejewski, P. Polycyclic Aromatic Hydrocarbons (PAHs) and their Derivatives (O-PAHs, N-PAHs, OH-PAHs): Determination in Suspended Particulate Matter (SPM)—A Review. Environ. Process. 2021, 9, 2. [Google Scholar] [CrossRef]
- Xie, J.; Tao, L.; Wu, Q.; Lei, S.; Lin, T. Environmental profile, distributions and potential sources of halogenated polycyclic aromatic hydrocarbons. J. Hazard. Mater. 2021, 419, 126164. [Google Scholar] [CrossRef]
- Suzuki, S.; Kiuchi, S.; Kinoshita, K.; Takeda, Y.; Tanaka, K.; Oguma, M. Formation of Polycyclic Aromatic Hydrocarbons (PAHs) and Oxygenated PAHs in the Oxidation of Ethylene Using a Flow Reactor. Combust. Sci. Technol. 2022, 194, 464–490. [Google Scholar] [CrossRef]
- Li, W.; Wu, S. Challenges of halogenated polycyclic aromatic hydrocarbons in foods: Occurrence, risk, and formation. Trends Food Sci. Technol. 2023, 131, 1–13. [Google Scholar] [CrossRef]
- Sampaio, G.R.; Guizellini, G.M.; da Silva, S.A.; de Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; de Camargo, A.C.; Torres, E.A.F.S. Polycyclic aromatic hydrocarbons in foods: Biological effects, legislation, occurrence, analytical methods, and strategies to reduce their formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- EFSA. Findings of the EFSA Data Collection on Polycyclic Aromatic Hydrocarbons in Food; EFSA: Parma, Italy, 2008; Volume 5.
- Onopiuk, A.; Kołodziejczak, K.; Szpicer, A.; Wojtasik-Kalinowska, I.; Wierzbicka, A.; Półtorak, A. Analysis of factors that influence the PAH profile and amount in meat products subjected to thermal processing. Trends Food Sci. Technol. 2021, 115, 366–379. [Google Scholar] [CrossRef]
- Anyanwu, I.N.; Semple, K.T. Fate and behaviour of nitrogen-containing polycyclic aromatic hydrocarbons in soil. Environ. Technol. Innov. 2015, 3, 108–120. [Google Scholar] [CrossRef]
- Hrdina, A.I.H.; Kohale, I.N.; Kaushal, S.; Kelly, J.; Selin, N.E.; Engelward, B.P.; Kroll, J.H. The Parallel Transformations of Polycyclic Aromatic Hydrocarbons in the Body and in the Atmosphere. Environ. Health Perspect. 2022, 130, 25004. [Google Scholar] [CrossRef]
- Lammel, G.; Kitanovski, Z.; Kukučka, P.; Novák, J.; Arangio, A.M.; Codling, G.P.; Filippi, A.; Hovorka, J.; Kuta, J.; Leoni, C.; et al. Oxygenated and Nitrated Polycyclic Aromatic Hydrocarbons in Ambient Air—Levels, Phase Partitioning, Mass Size Distributions, and Inhalation Bioaccessibility. Environ. Sci. Technol. 2020, 54, 2615–2625. [Google Scholar] [CrossRef] [Green Version]
- Qiao, M.; Qi, W.; Liu, H.; Qu, J. Oxygenated polycyclic aromatic hydrocarbons in the surface water environment: Occurrence, ecotoxicity, and sources. Environ. Int. 2022, 163, 107232. [Google Scholar] [CrossRef]
- Haynes, J.P.; Miller, K.E.; Majestic, B.J. Investigation into Photoinduced Auto-Oxidation of Polycyclic Aromatic Hydrocarbons Resulting in Brown Carbon Production. Environ. Sci. Technol. 2019, 53, 682–691. [Google Scholar] [CrossRef]
- Masuda, M.; Wang, Q.; Tokumura, M.; Miyake, Y.; Amagai, T. Simultaneous determination of polycyclic aromatic hydrocarbons and their chlorinated derivatives in grilled foods. Ecotoxicol. Environ. Saf. 2019, 178, 188–194. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Ionas, A.C. Formation and mitigation of PAHs in barbecued meat—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 3553–3568. [Google Scholar] [CrossRef]
- Zelinkova, Z.; Wenzl, T. The Occurrence of 16 EPA PAHs in Food—A Review. Polycycl. Aromat. Compd. 2015, 35, 248–284. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Jeong, J.H.; Park, S.; Lee, K.G. Monitoring and risk assessment of polycyclic aromatic hydrocarbons (PAHs) in processed foods and their raw materials. Food Control 2018, 92, 286–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Zhang, Y. Analytical chemistry, formation, mitigation, and risk assessment of polycyclic aromatic hydrocarbons: From food processing to in vivo metabolic transformation. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1422–1456. [Google Scholar] [CrossRef]
- Duedahl-Olesen, L.; Iversen, N.M.; Kelmo, C.; Jensen, L.K. Validation of QuEChERS for screening of 4 marker polycyclic aromatic hydrocarbons in fish and malt. Food Control 2020, 108, 106434. [Google Scholar] [CrossRef]
- Ledesma, E.; Rendueles, M.; Díaz, M. Contamination of meat products during smoking by polycyclic aromatic hydrocarbons: Processes and prevention. Food Control 2016, 60, 64–87. [Google Scholar] [CrossRef]
- Hokkanen, M. Polycyclic Aromatic Hydrocarbons in Foods and Their Mitigation, Food Mutagenicity and Children’s Dietary Exposure in Finland. Ph.D. Disertation, University of Helsinki, Finnish Food Authority (Ruokavirasto) Laboratory and Research Division Chemistry Unit, Helsinki, Finland, 2021. [Google Scholar]
- Lau, E.V.; Gan, S.; Ng, H.K. Extraction Techniques for Polycyclic Aromatic Hydrocarbons in Soils. Int. J. Anal. Chem. 2010, 2010, 398381. [Google Scholar] [CrossRef]
- Jinadasa, B.K.K.K.; Monteau, F.; Morais, S. Critical review of micro-extraction techniques used in the determination of polycyclic aromatic hydrocarbons in biological, environmental and food samples. Food Addit. Contam. Part A 2020, 37, 1004–1026. [Google Scholar] [CrossRef]
- Andreu, V.; Picó, Y. Pressurized liquid extraction of organic contaminants in environmental and food samples. TrAC Trends Anal. Chem. 2019, 118, 709–721. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef] [Green Version]
- Kim, L.; Lee, D.; Cho, H.-K.; Choi, S.-D. Review of the QuEChERS method for the analysis of organic pollutants: Persistent organic pollutants, polycyclic aromatic hydrocarbons, and pharmaceuticals. Trends Environ. Anal. Chem. 2019, 22, e00063. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Câmara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Anastassiades, M.; Maštovská, K.; Lehotay, S.J. Evaluation of analyte protectants to improve gas chromatographic analysis of pesticides. J. Chromatogr. A 2003, 1015, 163–184. [Google Scholar] [CrossRef]
- BS EN 15662:2018; Foods of Plant Origin. Multimethod for the Determination of Pesticide Residues Using GC- and LC-Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE. Modular QuEChERS-method. British Standards Institution (BSI): London, UK, 2018.
- Lehotay, S.J. Determination of Pesticide Residues in Foods by Acetonitrile Extraction and Partitioning with Magnesium Sulfate: Collaborative Study. J. AOAC Int. 2007, 90, 485–520. [Google Scholar] [CrossRef] [Green Version]
- Santana-Mayor, Á.; Socas-Rodríguez, B.; Herrera-Herrera, A.V.; Rodríguez-Delgado, M.Á. Current trends in QuEChERS method. A versatile procedure for food, environmental and biological analysis. TrAC Trends Anal. Chem. 2019, 116, 214–235. [Google Scholar] [CrossRef]
- Zachara, A.; Gałkowska, D.; Juszczak, L. Contamination of smoked meat and fish products from Polish market with polycyclic aromatic hydrocarbons. Food Control 2017, 80, 45–51. [Google Scholar] [CrossRef]
- ISO 15302:2017; Animal and Vegetable Fats and Oils—Determination of Benzo[a]pyrene—Reverse-Phase High Performance Liquid Chromatography Method. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- ISO 15753:2016; Animal and Vegetable Fats and Oils—Determination of Polycyclic Aromatic Hydrocarbons. International Organization for Standardization (ISO): Geneva, Switzerland, 2016.
- ISO 22959:2009; Animal and Vegetable Fats and Oils—Determination of Polycyclic Aromatic Hydrocarbons by On-Line Donor-Acceptor Complex Chromatography and HPLC with Fluorescence Detection. International Organization for Standardization (ISO): Geneva, Switzerland, 2009.
- CEN/TS16621:2014; Food Analysis—Determination of Benzo[a]pyrene, benz[a]Anthracene, Chrysene and benzo[b]Fluoranthene in Foodstuffs by High Performance Liquid Chromatography with Fluorescence Detection (HPLC-FD). British Standards Institution (BSI): London, UK, 2014.
- EN 16619:2015; Food Analysis—Determination of benzo[a]pyrene, benz[a]anthracene, chrysene and benzo[b]fluoranthene in foodstuffs by gas chromatography mass spectrometry (GC-MS). European Standards: Brussels, Belgium, 2015.
- PD ISO/TR 24054:2019; Animal and Vegetable Fats and Oils—Determination of Polycyclic Aromatic Hydrocarbons (PAH)—Method Using Gas Chromatography/Mass Spectrometry (GC/MS). British Standards Institution (BSI): London, UK, 2019.
- Slámová, T.; Sadowska-Rociek, A.; Fraňková, A.; Surma, M.; Banout, J. Application of QuEChERS-EMR-Lipid-DLLME method for the determination of polycyclic aromatic hydrocarbons in smoked food of animal origin. J. Food Compos. Anal. 2020, 87, 103420. [Google Scholar] [CrossRef]
- Reizer, E.; Viskolcz, B.; Fiser, B. Formation and growth mechanisms of polycyclic aromatic hydrocarbons: A mini-review. Chemosphere 2022, 291, 132793. [Google Scholar] [CrossRef]
- Kislov, V.V.; Sadovnikov, A.I.; Mebel, A.M. Formation Mechanism of Polycyclic Aromatic Hydrocarbons beyond the Second Aromatic Ring. J. Phys. Chem. A 2013, 117, 4794–4816. [Google Scholar] [CrossRef]
- Lemmens, A.K.; Rap, D.B.; Thunnissen, J.M.M.; Willemsen, B.; Rijs, A.M. Polycyclic aromatic hydrocarbon formation chemistry in a plasma jet revealed by IR-UV action spectroscopy. Nat. Commun. 2020, 11, 269. [Google Scholar] [CrossRef] [Green Version]
- Frenklach, M.; Clary, D.W.; Gardiner, W.C.; Stein, S.E. Detailed kinetic modeling of soot formation in shock-tube pyrolysis of acetylene. Symp. Combust. 1985, 20, 887–901. [Google Scholar] [CrossRef]
- Frenklach, M.; Wang, H. Detailed modeling of soot particle nucleation and growth. Symp. Combust. 1991, 23, 1559–1566. [Google Scholar] [CrossRef]
- Frenklach, M. Reaction mechanism of soot formation in flames. Phys. Chem. Chem. Phys. 2002, 4, 2028–2037. [Google Scholar] [CrossRef]
- Raj, A.; Prada, I.D.C.; Amer, A.A.; Chung, S.H. A reaction mechanism for gasoline surrogate fuels for large polycyclic aromatic hydrocarbons. Combust. Flame 2012, 159, 500–515. [Google Scholar] [CrossRef]
- Bittner, J.D.; Howard, J.B. Composition profiles and reaction mechanisms in a near-sooting premixed benzene/oxygen/argon flame. Symp. Combust. 1981, 18, 1105–1116. [Google Scholar] [CrossRef]
- Mebel, A.M.; Georgievskii, Y.; Jasper, A.W.; Klippenstein, S.J. Temperature- and pressure-dependent rate coefficients for the HACA pathways from benzene to naphthalene. Proc. Combust. Inst. 2017, 36, 919–926. [Google Scholar] [CrossRef]
- Froese, R.D.J.; Coxon, J.M.; West, S.C.; Morokuma, K. Theoretical Studies of Diels−Alder Reactions of Acetylenic Compounds. J. Org. Chem. 1997, 62, 6991–6996. [Google Scholar] [CrossRef]
- Siegmann, K.; Sattler, K. Formation mechanism for polycyclic aromatic hydrocarbons in methane flames. J. Chem. Phys. 2000, 112, 698–709. [Google Scholar] [CrossRef]
- Kislov, V.V.; Islamova, N.I.; Kolker, A.M.; Lin, S.H.; Mebel, A.M. Hydrogen Abstraction Acetylene Addition and Diels−Alder Mechanisms of PAH Formation: A Detailed Study Using First Principles Calculations. J. Chem. Theory Comput. 2005, 1, 908–924. [Google Scholar] [CrossRef]
- Mebel, A.M.; Landera, A.; Kaiser, R.I. Formation Mechanisms of Naphthalene and Indene: From the Interstellar Medium to Combustion Flames. J. Phys. Chem. A 2017, 121, 901–926. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, L.; Yuan, W.; Qi, F.; Li, Y. Experimental and kinetic modeling investigation on laminar premixed benzene flames with various equivalence ratios. Proc. Combust. Inst. 2015, 35, 855–862. [Google Scholar] [CrossRef]
- Yang, X.J.; Glaser, R.; Li, A.; Zhong, J.X. The carriers of the unidentified infrared emission features: Clues from polycyclic aromatic hydrocarbons with aliphatic sidegroups. New Astron. Rev. 2017, 77, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Wang, Q.; Violi, A. Reaction pathways for the formation of five-membered rings onto polyaromatic hydrocarbon framework. Fuel 2021, 283, 119023. [Google Scholar] [CrossRef]
- Shukla, B.; Miyoshi, A.; Koshi, M. Role of Methyl Radicals in the Growth of PAHs. J. Am. Soc. Mass Spectrom. 2010, 21, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Georganta, E.; Rahman, R.K.; Raj, A.; Sinha, S. Growth of polycyclic aromatic hydrocarbons (PAHs) by methyl radicals: Pyrene formation from phenanthrene. Combust. Flame 2017, 185, 129–141. [Google Scholar] [CrossRef]
- Jones, B.M.; Zhang, F.; Kaiser, R.I.; Jamal, A.; Mebel, A.M.; Cordiner, M.A.; Charnley, S.B. Formation of benzene in the interstellar medium. Proc. Natl. Acad. Sci. USA 2011, 108, 452–457. [Google Scholar] [CrossRef]
- Mebel, A.M.; Kislov, V.V.; Kaiser, R.I. Photoinduced Mechanism of Formation and Growth of Polycyclic Aromatic Hydrocarbons in Low-Temperature Environments via Successive Ethynyl Radical Additions. J. Am. Chem. Soc. 2008, 130, 13618–13629. [Google Scholar] [CrossRef] [PubMed]
- Reizer, E.; Csizmadia, I.G.; Nehéz, K.; Viskolcz, B.; Fiser, B. Theoretical investigation of benzo(a)pyrene formation. Chem. Phys. Lett. 2021, 772, 138564. [Google Scholar] [CrossRef]
- Shukla, B.; Koshi, M. A novel route for PAH growth in HACA based mechanisms. Combust. Flame 2012, 159, 3589–3596. [Google Scholar] [CrossRef]
- Abdel-Shafy, H.I.; Mansour, M.S.M. A review on polycyclic aromatic hydrocarbons: Source, environmental impact, effect on human health and remediation. Egypt. J. Pet. 2016, 25, 107–123. [Google Scholar] [CrossRef] [Green Version]
- Shukla, B.; Susa, A.; Miyoshi, A.; Koshi, M. Role of Phenyl Radicals in the Growth of Polycyclic Aromatic Hydrocarbons. J. Phys. Chem. A 2008, 112, 2362–2369. [Google Scholar] [CrossRef]
- Raj, A.; Man, P.L.W.; Totton, T.S.; Sander, M.; Shirley, R.A.; Kraft, M. New polycyclic aromatic hydrocarbon (PAH) surface processes to improve the model prediction of the composition of combustion-generated PAHs and soot. Carbon N. Y. 2010, 48, 319–332. [Google Scholar] [CrossRef]
- Shukla, B.; Koshi, M. Comparative study on the growth mechanisms of PAHs. Combust. Flame 2011, 158, 369–375. [Google Scholar] [CrossRef]
- Zhao, L.; Prendergast, M.B.; Kaiser, R.I.; Xu, B.; Ablikim, U.; Ahmed, M.; Sun, B.-J.; Chen, Y.-L.; Chang, A.H.H.; Mohamed, R.K.; et al. Synthesis of Polycyclic Aromatic Hydrocarbons by Phenyl Addition–Dehydrocyclization: The Third Way. Angew. Chem. Int. Ed. 2019, 58, 17442–17450. [Google Scholar] [CrossRef]
- Raj, A.; Al Rashidi, M.J.; Chung, S.H.; Sarathy, S.M. PAH Growth Initiated by Propargyl Addition: Mechanism Development and Computational Kinetics. J. Phys. Chem. A 2014, 118, 2865–2885. [Google Scholar] [CrossRef]
- Matsugi, A.; Miyoshi, A. Modeling of two- and three-ring aromatics formation in the pyrolysis of toluene. Proc. Combust. Inst. 2013, 34, 269–277. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, W.; Ahmed, M.; Zagidullin, M.V.; Azyazov, V.N.; Morozov, A.N.; Mebel, A.M.; Kaiser, R.I. Gas-phase synthesis of benzene via the propargyl radical self-reaction. Sci. Adv. 2023, 7, eabf0360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, X.; Sun, Y.; Zhang, Q.; Wang, W. The growth mechanism of polycyclic aromatic hydrocarbons from the reactions of anthracene and phenanthrene with cyclopentadienyl and indenyl. Chemosphere 2017, 189, 265–276. [Google Scholar] [CrossRef]
- Long, A.E.; Merchant, S.S.; Vandeputte, A.G.; Carstensen, H.-H.; Vervust, A.J.; Marin, G.B.; Van Geem, K.M.; Green, W.H. Pressure dependent kinetic analysis of pathways to naphthalene from cyclopentadienyl recombination. Combust. Flame 2018, 187, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.K.; Lindstedt, R.P. On the chemical kinetics of cyclopentadiene oxidation. Combust. Flame 2011, 158, 666–686. [Google Scholar] [CrossRef]
- Sharma, S.; Green, W.H. Computed Rate Coefficients and Product Yields for c-C5H5 + CH3 → Products. J. Phys. Chem. A 2009, 113, 8871–8882. [Google Scholar] [CrossRef]
- Ghildina, A.R.; Porfiriev, D.P.; Azyazov, V.N.; Mebel, A.M. The mechanism and rate constants for oxidation of indenyl radical C9H7 with molecular oxygen O2: A theoretical study. Phys. Chem. Chem. Phys. 2019, 21, 8915–8924. [Google Scholar] [CrossRef]
- Ghildina, A.R.; Porfiriev, D.P.; Azyazov, V.N.; Mebel, A.M. Scission of the Five-Membered Ring in 1-H-Inden-1-one C9H6O and Indenyl C9H7 in the Reactions with H and O Atoms. J. Phys. Chem. A 2019, 123, 5741–5752. [Google Scholar] [CrossRef]
- Sinha, S.; Rahman, R.K.; Raj, A. On the role of resonantly stabilized radicals in polycyclic aromatic hydrocarbon (PAH) formation: Pyrene and fluoranthene formation from benzyl–indenyl addition. Phys. Chem. Chem. Phys. 2017, 19, 19262–19278. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Nadal, M. Human dietary exposure to polycyclic aromatic hydrocarbons: A review of the scientific literature. Food Chem. Toxicol. 2015, 86, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, E.N.; Hajeb, P.; Selamat, J.; Razis, A.F.A. Polycyclic aromatic hydrocarbons (PAHs) and their bioaccessibility in meat: A tool for assessing human cancer risk. Asian Pac. J. Cancer Prev. 2016, 17, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Scientific Committee on Food (SCF). Opinion of the Scientific Committee on Food on the Risks to Human Health of Polycyclic Aromatic Hydrocarbons in Food; European Commission: Brussels, Belgium, 2002.
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs; European Commission: Brussels, Belgium, 2006; OJ L364/5.
- European Commission. Commission Regulation (EU) No 835/2011 of 19 August 2011 amending Regulation (EC) No 1881/2006 as regards maximum levels for polycyclic aromatic hydrocarbons in foodstuffs. Off. J. Eur. Union 2011, 215, 4–8. [Google Scholar] [CrossRef]
- EFSA European Food Safety Authority. Polycyclic Aromatic Hydrocarbons in Food—Scientific Opinion of the Panel on Contaminants in the Food Chain. EFSA J. 2008, 724, 1–114. [Google Scholar] [CrossRef]
- Yebra-Pimentel, I.; Fernández-González, R.; Martínez-Carballo, E.; Simal-Gándara, J. A Critical Review about the Health Risk Assessment of PAHs and Their Metabolites in Foods. Crit. Rev. Food Sci. Nutr. 2015, 55, 1383–1405. [Google Scholar] [CrossRef]
- Ma, J.-K.; Li, K.; Li, X.; Elbadry, S.; Raslan, A.A.; Li, Y.; Mulla, Z.S.; Tahoun, A.B.M.B.; El-Ghareeb, W.R.; Huang, X.-C. Levels of polycyclic aromatic hydrocarbons in edible and fried vegetable oil: A health risk assessment study. Environ. Sci. Pollut. Res. 2021, 28, 59784–59791. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Li, C.; Xu, X.; Zhou, G. Effects of Phenolic Acid Marinades on the Formation of Polycyclic Aromatic Hydrocarbons in Charcoal-Grilled Chicken Wings. J. Food Prot. 2019, 82, 684–690. [Google Scholar] [CrossRef]
- Kim, H.-J.; Cho, J.; Jang, A. Effect of charcoal type on the formation of polycyclic aromatic hydrocarbons in grilled meats. Food Chem. 2021, 343, 128453. [Google Scholar] [CrossRef]
- Deng, K.; Wong, T.-Y.; Wang, Y.; Leung, E.M.K.; Chan, W. Combination of Precolumn Nitro-reduction and Ultraperformance Liquid Chromatography with Fluorescence Detection for the Sensitive Quantification of 1-Nitronaphthalene, 2-Nitrofluorene, and 1-Nitropyrene in Meat Products. J. Agric. Food Chem. 2015, 63, 3161–3167. [Google Scholar] [CrossRef]
- Wongmaneepratip, W.; Vangnai, K. Effects of oil types and pH on carcinogenic polycyclic aromatic hydrocarbons (PAHs) in grilled chicken. Food Control 2017, 79, 119–125. [Google Scholar] [CrossRef]
- Qu, L.; Yu, H.; Yin, S.; Li, Y.; Sun, C. Solid-Phase Extraction Combined with Ultra-High-Performance Liquid Chromatography-Tandem Mass Spectrometry for the Determination of 5 Trace Nitro-Polycyclic Aromatic Hydrocarbons in Barbecued Foods. J. AOAC Int. 2020, 103, 1512–1520. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Kuhnle, G.K.; Cheng, Q. The effect of common spices and meat type on the formation of heterocyclic amines and polycyclic aromatic hydrocarbons in deep-fried meatballs. Food Control 2018, 92, 399–411. [Google Scholar] [CrossRef]
- Oz, F.; Yuzer, M.O. The effects of cooking on wire and stone barbecue at different cooking levels on the formation of heterocyclic aromatic amines and polycyclic aromatic hydrocarbons in beef steak. Food Chem. 2016, 203, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-G.; Kim, S.-Y.; Moon, J.-S.; Kim, S.-H.; Kang, D.-H.; Yoon, H.-J. Effects of grilling procedures on levels of polycyclic aromatic hydrocarbons in grilled meats. Food Chem. 2016, 199, 632–638. [Google Scholar] [CrossRef]
- Nor Hasyimah, A.K.; Jinap, S.; Sanny, M.; Ainaatul, A.I.; Sukor, R.; Jambari, N.N.; Nordin, N.; Jahurul, M.H.A. Effects of Honey-Spices Marination on Polycyclic Aromatic Hydrocarbons and Heterocyclic Amines Formation in Gas-Grilled Beef Satay. Polycycl. Aromat. Compd. 2020, 42, 1620–1648. [Google Scholar] [CrossRef]
- Zastrow, L.; Speer, K.; Schwind, K.-H.; Jira, W. A sensitive GC–HRMS method for the simultaneous determination of parent and oxygenated polycyclic aromatic hydrocarbons in barbecued meat and meat substitutes. Food Chem. 2021, 365, 130625. [Google Scholar] [CrossRef] [PubMed]
- García-Lomillo, J.; Viegas, O.; Gonzalez-SanJose, M.L.; Ferreira, I.M. Influence of red wine pomace seasoning and high-oxygen atmosphere storage on carcinogens formation in barbecued beef patties. Meat Sci. 2017, 125, 10–15. [Google Scholar] [CrossRef]
- Park, K.-C.; Pyo, H.; Kim, W.; Yoon, K.S. Effects of cooking methods and tea marinades on the formation of benzo[a]pyrene in grilled pork belly (Samgyeopsal). Meat Sci. 2017, 129, 1–8. [Google Scholar] [CrossRef]
- Jia, Y.; Zhao, Y.; Zhao, M.; Wang, Z.; Chen, X.; Wang, M. Core–shell indium (III) sulfide@metal-organic framework nanocomposite as an adsorbent for the dispersive solid-phase extraction of nitro-polycyclic aromatic hydrocarbons. J. Chromatogr. A 2018, 1551, 21–28. [Google Scholar] [CrossRef]
- Kim, S.Y.; Shin, H.W.; Kim, G.H.; Kim, Y.-Y.; Kang, M.-J.; Shin, H.-S. Risk Assessment and Evaluation of Analytical Method of Polycyclic Aromatic Hydrocarbons (PAHs) for Deep-Fat Fried Pork Products in Korea. Foods 2022, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, T.; Pleadin, J.; Petričević, S.; Listeš, E.; Sokolić, D.; Marković, K.; Ozogul, F.; Šimat, V. The occurrence of polycyclic aromatic hydrocarbons in fish and meat products of Croatia and dietary exposure. J. Food Compos. Anal. 2019, 75, 49–60. [Google Scholar] [CrossRef]
- Racovita, R.C.; Secuianu, C.; Ciuca, M.D.; Israel-Roming, F. Effects of Smoking Temperature, Smoking Time, and Type of Wood Sawdust on Polycyclic Aromatic Hydrocarbon Accumulation Levels in Directly Smoked Pork Sausages. J. Agric. Food Chem. 2020, 68, 9530–9536. [Google Scholar] [CrossRef]
- Mastanjević, K.; Kartalović, B.; Petrović, J.; Novakov, N.; Puljić, L.; Kovačević, D.; Jukić, M.; Lukinac, J.; Mastanjević, K. Polycyclic aromatic hydrocarbons in the traditional smoked sausage Slavonska kobasica. J. Food Compos. Anal. 2019, 83, 103282. [Google Scholar] [CrossRef]
- Malarut, J.; Vangnai, K. Influence of wood types on quality and carcinogenic polycyclic aromatic hydrocarbons (PAHs) of smoked sausages. Food Control 2018, 85, 98–106. [Google Scholar] [CrossRef]
- da Silva, S.A.; Sampaio, G.R.; Torres, E.A.F.d.S. Optimization and validation of a method using UHPLC-fluorescence for the analysis of polycyclic aromatic hydrocarbons in cold-pressed vegetable oils. Food Chem. 2017, 221, 809–814. [Google Scholar] [CrossRef]
- Gong, G.; Wu, S.; Wu, X. Effects of storage time and temperature on toxic aldehydes and polycyclic aromatic hydrocarbons in flavouring oil gravy during storage. LWT 2019, 116, 108510. [Google Scholar] [CrossRef]
- Krajian, H.; Odeh, A. Levels of 15 + 1 EU Priority Polycyclic Aromatic Hydrocarbons in Different Edible Oils Available in the Syrian Market. Polycycl. Aromat. Compd. 2018, 38, 369–378. [Google Scholar] [CrossRef]
- Zhao, X.; Gong, G.; Wu, S. Effect of storage time and temperature on parent and oxygenated polycyclic aromatic hydrocarbons in crude and refined vegetable oils. Food Chem. 2018, 239, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhao, X.; Wu, S.; Li, G. Impact of refining on the levels of 4-hydroxy-trans-alkenals, parent and oxygenated polycyclic aromatic hydrocarbons in soybean and rapeseed oils. Food Control 2016, 67, 82–89. [Google Scholar] [CrossRef]
- Teng, C.; Wu, S.; Sun, Y.; Gong, G. Determination of Parent and Oxygenated Polycyclic Aromatic Hydrocarbons (PAHs) in Waste Cooking Oil and Oil Deodorizer Distillate by GC–QQQ–MS. J. AOAC Int. 2019, 102, 1884–1891. [Google Scholar] [CrossRef]
- Yan, K.; Wu, S.; Gong, G.; Sun, Y. A new approach of specific determination for 6-chlorobenzo[a]pyrene and 7-chlorobenzo[a]anthracene in six different oils. Food Chem. 2020, 316, 126344. [Google Scholar] [CrossRef]
- Stenerson, K.K.; Shimelis, O.; Halpenny, M.R.; Espenschied, K.; Ye, M.M. Analysis of Polynuclear Aromatic Hydrocarbons in Olive Oil after Solid-Phase Extraction Using a Dual-Layer Sorbent Cartridge Followed by High-Performance Liquid Chromatography with Fluorescence Detection. J. Agric. Food Chem. 2015, 63, 4933–4939. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, I.; Moret, S.; Chaari, O.; Issaoui, M.; Conte, L.S.; Lucci, P.; Hammami, M. Evaluation of hydrocarbon contaminants in olives and virgin olive oils from Tunisia. Food Control 2017, 75, 160–166. [Google Scholar] [CrossRef]
- Rascón, A.J.; Azzouz, A.; Ballesteros, E. Multiresidue determination of polycyclic aromatic hydrocarbons in edible oils by liquid-liquid extraction–solid-phase extraction–gas chromatography–mass spectrometry. Food Control 2018, 94, 268–275. [Google Scholar] [CrossRef]
- Shi, L.-K.; Zhang, D.-D.; Liu, Y.-L. Incidence and survey of polycyclic aromatic hydrocarbons in edible vegetable oils in China. Food Control 2016, 62, 165–170. [Google Scholar] [CrossRef]
- Roszko, M.Ł.; Juszczyk, K.; Szczepańska, M.; Świder, O.; Szymczyk, K. Background levels of polycyclic aromatic hydrocarbons and legacy organochlorine pesticides in wheat sampled in 2017 and 2018 in Poland. Environ. Monit. Assess. 2020, 192, 142. [Google Scholar] [CrossRef] [Green Version]
- Deng, K.; Chan, W. Development of a QuEChERS-Based Method for Determination of Carcinogenic 2-Nitrofluorene and 1-Nitropyrene in Rice Grains and Vegetables: A Comparative Study with Benzo[a]pyrene. J. Agric. Food Chem. 2017, 65, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Khalili, F.; Shariatifar, N.; Dehghani, M.H.; Yaghmaeian, K.; Nodehi, R.N.; Yaseri, M.; Arabameri, M. The analysis and probabilistic health risk assessment of polycyclic aromatic hydrocarbons in cereal products. Environ. Sci. Pollut. Res. 2022, 29, 31099–31109. [Google Scholar] [CrossRef]
- Kacmaz, S.; Zelinkova, Z.; Wenzl, T. Rapid and sensitive method for the determination of four EU marker polycyclic aromatic hydrocarbons in cereal-based foods using isotope-dilution GC/MS. Food Addit. Contam. Part A 2016, 33, 631–638. [Google Scholar] [CrossRef]
- Rascón, A.J.; Azzouz, A.; Ballesteros, E. Assessing polycyclic aromatic hydrocarbons in cereal-based foodstuffs by using a continuous solid-phase extraction system and gas chromatography–mass spectrometry. Food Control 2018, 92, 92–100. [Google Scholar] [CrossRef]
- Kamalabadi, M.; Kamankesh, M.; Mohammadi, A.; Hadian, Z.; Ferdowsi, R. Contamination and Daily Intake of Polycyclic Aromatic Hydrocarbons in Iranian Bread Samples. Polycycl. Aromat. Compd. 2020, 40, 1187–1195. [Google Scholar] [CrossRef]
- Chawda, S.; Tarafdar, A.; Sinha, A.; Mishra, B.K. Profiling and Health Risk Assessment of PAHs Content in Tandoori and Tawa Bread from India. Polycycl. Aromat. Compd. 2020, 40, 21–32. [Google Scholar] [CrossRef]
- Rostampour, R.; Kamalabadi, M.; Kamankesh, M.; Hadian, Z.; Jazaeri, S.; Mohammadi, A.; Zolgharnein, J. An efficient, sensitive and fast microextraction method followed by gas chromatography-mass spectrometry for the determination of polycyclic aromatic hydrocarbons in bread samples. Anal. Methods 2017, 9, 6246–6253. [Google Scholar] [CrossRef]
- Kacmaz, S. Polycyclic aromatic hydrocarbons in cereal products on the Turkish market. Food Addit. Contam. Part B 2016, 9, 191–197. [Google Scholar] [CrossRef]
- Gong, G.; Zhao, X.; Wu, S. Effect of natural antioxidants on inhibition of parent and oxygenated polycyclic aromatic hydrocarbons in Chinese fried bread youtiao. Food Control 2018, 87, 117–125. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Li, F.; Li, R.; Zhu, J.; Chen, J.; Li, W.; Jiang, D. Occurrence of polycyclic aromatic hydrocarbons in youtiao and exposure assessment from Shandong Province, China. Food Control 2020, 111, 107049. [Google Scholar] [CrossRef]
- Tran-Lam, T.-T.; Hai Dao, Y.; Kim Thi Nguyen, L.; Kim Ma, H.; Nguyen Tran, H.; Truong Le, G. Simultaneous Determination of 18 Polycyclic Aromatic Hydrocarbons in Daily Foods (Hanoi Metropolitan Area) by Gas Chromatography–Tandem Mass Spectrometry. Foods 2018, 7, 201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihedioha, J.N.; Okali, E.E.; Ekere, N.R.; Ezeofor, C.C. Risk Assessment of Polycyclic Aromatic Hydrocarbons in Pasta Products Consumed in Nigeria. Iran. J. Toxicol. 2019, 13, 19–26. [Google Scholar] [CrossRef]
- Charles, I.A.; Ogbolosingha, A.J.; Afia, I.U. Health risk assessment of instant noodles commonly consumed in Port Harcourt, Nigeria. Environ. Sci. Pollut. Res. 2018, 25, 2580–2587. [Google Scholar] [CrossRef] [Green Version]
- Suleman, R.; Wang, Z.; Aadil, R.M.; Hui, T.; Hopkins, D.L.; Zhang, D. Effect of cooking on the nutritive quality, sensory properties and safety of lamb meat: Current challenges and future prospects. Meat Sci. 2020, 167, 108172. [Google Scholar] [CrossRef]
- Mejborn, H.; Hansen, M.; Biltoft-Jensen, A.; Christensen, T.; Ygil, K.H.; Olesen, P.T. Suggestion for a subdivision of processed meat products on the Danish market based on their content of carcinogenic compounds. Meat Sci. 2019, 147, 91–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molognoni, L.; Daguer, H.; Motta, G.E.; Merlo, T.C.; Lindner, J.D.D. Interactions of preservatives in meat processing: Formation of carcinogenic compounds, analytical methods, and inhibitory agents. Food Res. Int. 2019, 125, 108608. [Google Scholar] [CrossRef]
- Abrajano, T.A.; Yan, B.; O’Malley, V. 11.13—High Molecular Weight Petrogenic and Pyrogenic Hydrocarbons in Aquatic Environments. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 481–509. ISBN 978-0-08-098300-4. [Google Scholar]
- Ledesma, E.; Rendueles, M.; Díaz, M. Benzo(a)pyrene penetration on a smoked meat product during smoking time. Food Addit. Contam. Part A 2014, 31, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Olatunji, O.S.; Fatoki, O.S.; Opeolu, B.O.; Ximba, B.J. Determination of polycyclic aromatic hydrocarbons [PAHs] in processed meat products using gas chromatography—Flame ionization detector. Food Chem. 2014, 156, 296–300. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Mozuriene, E.; Krungleviciute, V.; Novoslavskij, A.; Santini, A.; Rozentale, I.; Juodeikiene, G.; Cizeikiene, D. The impact of lactic acid bacteria with antimicrobial properties on biodegradation of polycyclic aromatic hydrocarbons and biogenic amines in cold smoked pork sausages. Food Control 2017, 71, 285–292. [Google Scholar] [CrossRef]
- Pöhlmann, M.; Hitzel, A.; Schwägele, F.; Speer, K.; Jira, W. Influence of different smoke generation methods on the contents of polycyclic aromatic hydrocarbons (PAH) and phenolic substances in Frankfurter-type sausages. Food Control 2013, 34, 347–355. [Google Scholar] [CrossRef]
- Gomes, A.; Santos, C.; Almeida, J.; Elias, M.; Roseiro, L.C. Effect of fat content, casing type and smoking procedures on PAHs contents of Portuguese traditional dry fermented sausages. Food Chem. Toxicol. 2013, 58, 369–374. [Google Scholar] [CrossRef]
- Škaljac, S.; Jokanović, M.; Tomović, V.; Ivić, M.; Tasić, T.; Ikonić, P.; Šojić, B.; Džinić, N.; Petrović, L. Influence of smoking in traditional and industrial conditions on colour and content of polycyclic aromatic hydrocarbons in dry fermented sausage “Petrovská klobása”. LWT 2018, 87, 158–162. [Google Scholar] [CrossRef]
- Lin, G.; Weigel, S.; Tang, B.; Schulz, C.; Shen, J. The occurrence of polycyclic aromatic hydrocarbons in Peking duck: Relevance to food safety assessment. Food Chem. 2011, 129, 524–527. [Google Scholar] [CrossRef]
- Djinovic, J.; Popovic, A.; Jira, W. Polycyclic aromatic hydrocarbons (PAHs) in different types of smoked meat products from Serbia. Meat Sci. 2008, 80, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Essumang, D.K.; Dodoo, D.K.; Adjei, J.K. Effect of smoke generation sources and smoke curing duration on the levels of polycyclic aromatic hydrocarbon (PAH) in different suites of fish. Food Chem. Toxicol. 2013, 58, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Patra, J.K.; Shin, H.-S. Factors influencing inhibition of eight polycyclic aromatic hydrocarbons in heated meat model system. Food Chem. 2018, 239, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Essumang, D.K.; Dodoo, D.K.; Adjei, J.K. Effective reduction of PAH contamination in smoke cured fish products using charcoal filters in a modified traditional kiln. Food Control 2014, 35, 85–93. [Google Scholar] [CrossRef]
- Haiba, N.S.; Asaal, A.M.; El Massry, A.M.; Ismail, I.; Basahi, J.; Hassan, I.A. Effects of “Doneness” Level on PAH Concentrations in Charcoal-Grilled Beef and Chicken: An Egyptian Study Case. Polycycl. Aromat. Compd. 2021, 41, 553–563. [Google Scholar] [CrossRef]
- Rose, M.; Holland, J.; Dowding, A.; Petch, S.R.G.; White, S.; Fernandes, A.; Mortimer, D. Investigation into the formation of PAHs in foods prepared in the home to determine the effects of frying, grilling, barbecuing, toasting and roasting. Food Chem. Toxicol. 2015, 78, 1–9. [Google Scholar] [CrossRef]
- Stumpe-Vīksna, I.; Bartkevičs, V.; Kukāre, A.; Morozovs, A. Polycyclic aromatic hydrocarbons in meat smoked with different types of wood. Food Chem. 2008, 110, 794–797. [Google Scholar] [CrossRef]
- Pöhlmann, M.; Hitzel, A.; Schwägele, F.; Speer, K.; Jira, W. Contents of polycyclic aromatic hydrocarbons (PAH) and phenolic substances in Frankfurter-type sausages depending on smoking conditions using glow smoke. Meat Sci. 2012, 90, 176–184. [Google Scholar] [CrossRef]
- Hitzel, A.; Pöhlmann, M.; Schwägele, F.; Speer, K.; Jira, W. Polycyclic aromatic hydrocarbons (PAH) and phenolic substances in meat products smoked with different types of wood and smoking spices. Food Chem. 2013, 139, 955–962. [Google Scholar] [CrossRef]
- Viegas, O.; Novo, P.; Pinto, E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Effect of charcoal types and grilling conditions on formation of heterocyclic aromatic amines (HAs) and polycyclic aromatic hydrocarbons (PAHs) in grilled muscle foods. Food Chem. Toxicol. 2012, 50, 2128–2134. [Google Scholar] [CrossRef] [PubMed]
- Rey-Salgueiro, L.; García-Falcón, M.S.; Martínez-Carballo, E.; Simal-Gándara, J. Effects of toasting procedures on the levels of polycyclic aromatic hydrocarbons in toasted bread. Food Chem. 2008, 108, 607–615. [Google Scholar] [CrossRef]
- Ghorbani, M.; Najafi Saleh, H.; Barjasteh-Askari, F.; Nasseri, S.; Davoudi, M. The effect of gas versus charcoal open flames on the induction of polycyclic aromatic hydrocarbons in cooked meat: A systematic review and meta-analysis. J. Environ. Health Sci. Eng. 2020, 18, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, C.; Ye, K.; Bai, Y.; Yu, X.; Li, C.; Zhou, G. Effect of fatty acid on the formation of polycyclic aromatic hydrocarbons (PAHs) and the proposed formation mechanism during electric roasting. Br. Food J. 2019, 121, 3193–3207. [Google Scholar] [CrossRef]
- Lu, F.; Kuhnle, G.K.; Cheng, Q. Vegetable oil as fat replacer inhibits formation of heterocyclic amines and polycyclic aromatic hydrocarbons in reduced fat pork patties. Food Control 2017, 81, 113–125. [Google Scholar] [CrossRef]
- Saito, E.; Tanaka, N.; Miyazaki, A.; Tsuzaki, M. Concentration and particle size distribution of polycyclic aromatic hydrocarbons formed by thermal cooking. Food Chem. 2014, 153, 285–291. [Google Scholar] [CrossRef]
- Nie, W.; Cai, K.; Li, Y.; Zhang, S.; Wang, Y.; Guo, J.; Chen, C.; Xu, B. Small Molecular Weight Aldose (d-Glucose) and Basic Amino Acids (l-Lysine, l-Arginine) Increase the Occurrence of PAHs in Grilled Pork Sausages. Molecules 2018, 23, 3377. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Wu, S.; Gong, G.; Li, G.; Zhuang, L. TBHQ and peanut skin inhibit accumulation of PAHs and oxygenated PAHs in peanuts during frying. Food Control 2017, 75, 99–107. [Google Scholar] [CrossRef]
- Janoszka, B. HPLC-fluorescence analysis of polycyclic aromatic hydrocarbons (PAHs) in pork meat and its gravy fried without additives and in the presence of onion and garlic. Food Chem. 2011, 126, 1344–1353. [Google Scholar] [CrossRef]
- Nie, W.; Cai, K.; Li, Y.; Hu, G.; Xing, W.; Wang, X.; Wang, Y.; Chen, C. Application of grape seed extract lead to a higher formation of polycyclic aromatic hydrocarbons in roasted pork sausage at the end of storage. J. Food Process. Preserv. 2020, 44, e14532. [Google Scholar] [CrossRef]
- Lee, J.-S.; Han, J.-W.; Jung, M.; Lee, K.-W.; Chung, M.-S. Effects of Thawing and Frying Methods on the Formation of Acrylamide and Polycyclic Aromatic Hydrocarbons in Chicken Meat. Foods 2020, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-W.; Lee, Y.-T.; Inbaraj, B.S.; Chen, B.-H. Formation and Inhibition of Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Ground Pork during Marinating. Foods 2022, 11, 3080. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Gomes, A.; Roseiro, L.C. Polycyclic aromatic hydrocarbons incidence in Portuguese traditional smoked meat products. Food Chem. Toxicol. 2011, 49, 2343–2347. [Google Scholar] [CrossRef]
- Youssef, M.K.E.; Abou-EL-Hawa, S.H.; Hussein, S.M.; Mahmoud, A.R. Influence of Casing Types and Cold Storage on Polycyclic Aromatic Hydro Carbons (PAHs) in Smoked Beef Sausage. Assiut J. Agric. Sci. 2016, 47, 1–12. [Google Scholar] [CrossRef]
- Ledesma, E.; Rendueles, M.; Díaz, M. Characterization of natural and synthetic casings and mechanism of BaP penetration in smoked meat products. Food Control 2015, 51, 195–205. [Google Scholar] [CrossRef]
- Farhadian, A.; Jinap, S.; Hanifah, H.N.; Zaidul, I.S. Effects of meat preheating and wrapping on the levels of polycyclic aromatic hydrocarbons in charcoal-grilled meat. Food Chem. 2011, 124, 141–146. [Google Scholar] [CrossRef]
- Škaljac, S.; Petrović, L.; Tasić, T.; Ikonić, P.; Jokanović, M.; Tomović, V.; Džinić, N.; Šojić, B.; Tjapkin, A.; Škrbić, B. Influence of smoking in traditional and industrial conditions on polycyclic aromatic hydrocarbons content in dry fermented sausages (Petrovská klobása) from Serbia. Food Control 2014, 40, 12–18. [Google Scholar] [CrossRef]
- Esfahani Mehr, A.; Hosseini, S.E.; Seyadain Ardebili, S.M. Effects of nutmeg and ginger essential oils and their nanoemulsions on the formation of heterocyclic aromatic amines and polycyclic aromatic hydrocarbons in beef patties during 90 days freezing storage. J. Food Meas. Charact. 2019, 13, 2041–2050. [Google Scholar] [CrossRef]
- Pöhlmann, M.; Hitzel, A.; Schwägele, F.; Speer, K.; Jira, W. Polycyclic aromatic hydrocarbons (PAH) and phenolic substances in smoked Frankfurter-type sausages depending on type of casing and fat content. Food Control 2013, 31, 136–144. [Google Scholar] [CrossRef]
- Veyrand, B.; Sirot, V.; Durand, S.; Pollono, C.; Marchand, P.; Dervilly-Pinel, G.; Tard, A.; Leblanc, J.C.; Le Bizec, B. Human dietary exposure to polycyclic aromatic hydrocarbons: Results of the second French Total Diet Study. Environ. Int. 2013, 54, 11–17. [Google Scholar] [CrossRef]
- Singh, L.; Agarwal, T.; Simal-Gandara, J. PAHs, diet and cancer prevention: Cooking process driven-strategies. Trends Food Sci. Technol. 2020, 99, 487–506. [Google Scholar] [CrossRef]
- Bansal, V.; Kim, K.-H. Review of PAH contamination in food products and their health hazards. Environ. Int. 2015, 84, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Moretti, S.; Dusi, G.; Giusepponi, D.; Pellicciotti, S.; Rossi, R.; Saluti, G.; Cruciani, G.; Galarini, R. Screening and confirmatory method for multiclass determination of 62 antibiotics in meat. J. Chromatogr. A 2016, 1429, 175–188. [Google Scholar] [CrossRef]
- Ji, J.; Liu, Y.; Wang, D. Comparison of de-skin pretreatment and oil extraction on aflatoxins, phthalate esters, and polycyclic aromatic hydrocarbons in peanut oil. Food Control 2020, 118, 107365. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Farssi, M.; Ait Taleb, A.; Guillaume, D.; Laknifli, A. Influence of roasting olive fruit on the chemical composition and polycyclic aromatic hydrocarbon content of olive oil. OCL 2018, 25, A303. [Google Scholar] [CrossRef] [Green Version]
- Bertoz, V.; Purcaro, G.; Conchione, C.; Moret, S. A review on the occurrence and analytical determination of pahs in olive oils. Foods 2021, 10, 324. [Google Scholar] [CrossRef]
- Rojo Camargo, M.C.; Antoniolli, P.R.; Vicente, E. Evaluation of polycyclic aromatic hydrocarbons content in different stages of soybean oils processing. Food Chem. 2012, 135, 937–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, V.H.; Casal, S.; Oliveira, M.B.P.P. PAHs content in sunflower, soybean and virgin olive oils: Evaluation in commercial samples and during refining process. Food Chem. 2007, 104, 106–112. [Google Scholar] [CrossRef]
- Kiralan, S.S.; Toptancı, İ.; Tekin, A. Further Evidence on the Removal of Polycyclic Aromatic Hydrocarbons (PAHs) During Refining of Olive Pomace Oil. Eur. J. Lipid Sci. Technol. 2019, 121, 1800381. [Google Scholar] [CrossRef]
- Guillén, M.D.; Goicoechea, E.; Palencia, G.; Cosmes, N. Evidence of the Formation of Light Polycyclic Aromatic Hydrocarbons during the Oxidation of Edible Oils in Closed Containers at Room Temperature. J. Agric. Food Chem. 2008, 56, 2028–2033. [Google Scholar] [CrossRef]
- Chiang, K.-M.; Xiu, L.; Peng, C.-Y.; Lung, S.-C.C.; Chen, Y.-C.; Pan, W.-H. Particulate matters, aldehydes, and polycyclic aromatic hydrocarbons produced from deep-frying emissions: Comparisons of three cooking oils with distinct fatty acid profiles. NPJ Sci. Food 2022, 6, 28. [Google Scholar] [CrossRef]
- Chung, S.Y.; Yettella, R.R.; Kim, J.S.; Kwon, K.; Kim, M.C.; Min, D.B. Effects of grilling and roasting on the levels of polycyclic aromatic hydrocarbons in beef and pork. Food Chem. 2011, 129, 1420–1426. [Google Scholar] [CrossRef]
- Pei, W.; Wang, J.; Zhang, L.; Guo, Y.; Cao, M.; Liu, R.; Chang, M.; Wang, X. Effect of Catechin on the Formation of Polycyclic Aromatic Hydrocarbons in Camellia oleifera Oil during Thermal Processing. Foods 2023, 12, 980. [Google Scholar] [CrossRef] [PubMed]
- Choe, E.; Min, D.B. Chemistry of Deep-Fat Frying Oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Andrés, A.; Arguelles, Á.; Castelló, M.L.; Heredia, A. Mass Transfer and Volume Changes in French Fries During Air Frying. Food Bioprocess Technol. 2013, 6, 1917–1924. [Google Scholar] [CrossRef]
- Kang, S.-J.; Yang, S.-Y.; Lee, J.-W.; Lee, K.-W. Polycyclic aromatic hydrocarbons in seasoned-roasted laver and their reduction according to the mixing ratio of seasoning oil and heat treatment in a model system. Food Sci. Biotechnol. 2019, 28, 1247–1255. [Google Scholar] [CrossRef]
- An, K.-J.; Liu, Y.-L.; Liu, H.-L. Relationship between total polar components and polycyclic aromatic hydrocarbons in fried edible oil. Food Addit. Contam. Part A 2017, 34, 1596–1605. [Google Scholar] [CrossRef]
- Hao, X.; Li, J.; Yao, Z. Changes in PAHs levels in edible oils during deep-frying process. Food Control 2016, 66, 233–240. [Google Scholar] [CrossRef]
- Olatunji, O.S.; Fatoki, O.S.; Ximba, B.J.; Opeolu, B.O. Polycyclic aromatic hydrocarbons (PAHs) in edible oil: Temperature effect on recovery from base hydrolysis product and health risk factor. Food Public Health 2014, 4, 23–30. [Google Scholar]
- Mocek, K.; Ciemniak, A. Influence of physical factors on polycyclic aromatic hydrocarbons (PAHs) content in vegetable oils. J. Environ. Sci. Health Part B 2016, 51, 96–102. [Google Scholar] [CrossRef]
- Mafra, J.B.; Santos, R.F.; Venturelli, G.M.; Bassegio, D.; Coelho, S.R.M.; Simonetti, A.P.M.M.; Branco, T.M.; Branco, K.C. Evaluation of polycyclic aromatic hydrocarbons (PAHs) in the corn drying process. Res. Soc. Dev. 2021, 10, e403101622444. [Google Scholar] [CrossRef]
- Rey-Salgueiro, L.; Martínez-Carballo, E.; García-Falcón, M.S.; Simal-Gándara, J. Effects of a chemical company fire on the occurrence of polycyclic aromatic hydrocarbons in plant foods. Food Chem. 2008, 108, 347–353. [Google Scholar] [CrossRef]
- de Lima, R.F.; Dionello, R.G.; Peralba, M.d.C.R.; Barrionuevo, S.; Radunz, L.L.; Reichert Júnior, F.W. PAHs in corn grains submitted to drying with firewood. Food Chem. 2017, 215, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Ciecierska, M.; Obiedziński, M.W. Polycyclic aromatic hydrocarbons in the bakery chain. Food Chem. 2013, 141, 1–9. [Google Scholar] [CrossRef]
- Rozentale, I.; Zacs, D.; Perkons, I.; Bartkevics, V. A comparison of gas chromatography coupled to tandem quadrupole mass spectrometry and high-resolution sector mass spectrometry for sensitive determination of polycyclic aromatic hydrocarbons (PAHs) in cereal products. Food Chem. 2017, 221, 1291–1297. [Google Scholar] [CrossRef]
- Aamir, M.; Yin, S.; Liu, Y.; Ullah, H.; Khan, S.; Liu, W. Dietary exposure and cancer risk assessment of the Pakistani population exposed to polycyclic aromatic hydrocarbons. Sci. Total Environ. 2021, 757, 143828. [Google Scholar] [CrossRef]
- Veiga, L.L.A.; Amorim, H.; Moraes, J.; Silva, M.C.; Raices, R.S.L.; Quiterio, S.L. Quantification of polycyclic aromatic hydrocarbons in toasted guaraná (Paullinia cupana) by high-performance liquid chromatography with a fluorescence detector. Food Chem. 2014, 152, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Einolghozati, M.; Talebi-Ghane, E.; Amirsadeghi, S. Fereshteh mehri Evaluation of polycyclic aromatic hydrocarbons (PAHs) in processed cereals: A meta-analysis study, systematic review, and health risk assessment. Heliyon 2022, 8, e12168. [Google Scholar] [CrossRef]
- Bertinetti, I.A.; Ferreira, C.D.; Monks, J.L.F.; Sanches-Filho, P.J.; Elias, M.C. Accumulation of polycyclic aromatic hydrocarbons (PAHs) in rice subjected to drying with different fuels plus temperature, industrial processes and cooking. J. Food Compos. Anal. 2018, 66, 109–115. [Google Scholar] [CrossRef]
- Li, G.; Wu, S.; Wang, L.; Akoh, C.C. Concentration, dietary exposure and health risk estimation of polycyclic aromatic hydrocarbons (PAHs) in youtiao, a Chinese traditional fried food. Food Control 2016, 59, 328–336. [Google Scholar] [CrossRef]
- Iwegbue, C.M.A.; Onyonyewoma, U.A.; Bassey, F.I.; Nwajei, G.E.; Martincigh, B.S. Concentrations and Health Risk of Polycyclic Aromatic Hydrocarbons in Some Brands of Biscuits in the Nigerian Market. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 338–357. [Google Scholar] [CrossRef]
- Kacmaz, S. Chapter—Polycyclic Aromatic Hydrocarbons (PAHs) in Flour, Bread, and Breakfast Cereals. In Flour and Breads and Their Fortification in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 13–20. ISBN 978-0-12-814639-2. [Google Scholar]
- Aydın, Ö.Ş.; Şahan, Y. Influence of Different Cooking Methods on Polycyclic Aromatic Hydrocarbons Formation in Various Meat Types. Akad. Gıda 2018, 16, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Sahin, S.; Ulusoy, H.I.; Alemdar, S.; Erdogan, S.; Agaoglu, S. The Presence of Polycyclic Aromatic Hydrocarbons (PAHs) in Grilled Beef, Chicken and Fish by Considering Dietary Exposure and Risk Assessment. Food Sci. Anim. Resour. 2020, 40, 675–688. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, G.; Su, S.; Shen, H.; Huang, Y.; Li, T.; Li, W.; Zhang, Y.; Lu, Y.; Chen, H.; et al. Contamination and distribution of parent, nitrated, and oxygenated polycyclic aromatic hydrocarbons in smoked meat. Environ. Sci. Pollut. Res. 2014, 21, 11521–11530. [Google Scholar] [CrossRef]
- Gong, G.; Wu, S.; Wu, X. Influences of Light Intensity and β-Carotene on Polycyclic Aromatic Hydrocarbons and Aldehydes in Vegetable Oil: A Case Study Using Palm Oil. J. Agric. Food Chem. 2018, 66, 11124–11132. [Google Scholar] [CrossRef]
- Munir, M.A.; Badri, K.H. The Importance of Derivatizing Reagent in Chromatography Applications for Biogenic Amine Detection in Food and Beverages. J. Anal. Methods Chem. 2020, 2020, 5814389. [Google Scholar] [CrossRef]


| Product | Processing | PAHs (µg/kg) | PAH Derivatives (µg/kg) | Reference | |||
|---|---|---|---|---|---|---|---|
| BaP | Total Sum | OPAHs | NPAHs | XPAHs | |||
| Meat and meat-derived products | |||||||
| Chicken | Grilling | 1.38–3.27 | 10.10–12.83 (8) | - | - | - | [92] |
| Grilling | <LOD-22.09 | 118.10–473.78 (16) | - | - | - | [93] | |
| Boiling; Grilling | - | - | - | <LOD-195.4 (3) | - | [94] | |
| Grilling | <LOD-86.4 | 190.1–1781.4 (16) | - | - | - | [95] | |
| Smoking | <LOD-2.30 | 0.21–8.30 (4) | - | - | - | [39] | |
| Frying | - | - | - | <LOD-2.13 (5) | - | [96] | |
| Grilling | - | - | - | <LOD-14.43 (5) | - | [96] | |
| Meatballs | Frying | 0.10–1.64 | 0.10–3.66 (2) | [97] | |||
| Beef | Grilling | <LOD-43.63 | 328.78–817.18 (16) | - | - | - | [93] |
| Boiling; Grilling | - | - | - | 2.1–2.6 (3) | - | [94] | |
| Barbequing | <LOD-0.29 | <LOD-2.63 (8) | - | - | - | [98] | |
| Grilling | 2.20–5.07 | 9.20–23.81 (4) | - | - | - | [99] | |
| Frying | - | - | - | <LOD-2.19 (5) | - | [96] | |
| Grilling | - | - | - | <LOD-7.92 (5) | - | [96] | |
| Satay | Grilling | 0.95–32.60 | 69.14–350.38 (15) | - | - | - | [100] |
| Meatballs | Frying | <LOD-1.96 | 0.08–3.87 (2) | - | - | - | [97] |
| Patties | Barbequing | - | 12.8–33.4 (6) | 26.0–62.4 (7) | - | - | [101] |
| Patties | Barbequing | 0.20–0.34 | 8.79–16.63 (11) | - | - | - | [102] |
| Pork | Grilling | <LOD-163.31 | 304.42–2290.31 (16) | - | - | - | [93] |
| Boiling; Grilling | - | - | - | <LOD-4.2 (3) | - | [94] | |
| Grilling | <LOD-8.04 | - | - | - | - | [103] | |
| Grilling | 0.68–5.99 | 4.38–33.17 (4) | - | - | - | [99] | |
| Grilling | - | - | - | 5.62 (16) | - | [104] | |
| Frying | - | - | - | <LOD-2.15 (5) | - | [96] | |
| Grilling | - | - | - | <LOD-11.46 (5) | - | [96] | |
| Grilling | - | 3.0–570 (12) | - | - | 0.02–0.08 (20) | [21] | |
| Frying | 0.38–6.83 | 0.38–7.90 (4) | - | - | - | [105] | |
| Mutton | Frying | - | - | - | <LOD-2.26 (5) | - | [96] |
| Grilling | - | - | - | <LOD-7.63 (5) | - | [96] | |
| Sausages | Commercial | <LOQ-1.05 | <0.40–3.31 (4) | - | - | - | [106] |
| Commercial | - | - | - | 46 (16) | - | [104] | |
| Frying | - | - | - | <LOD-<LOQ (5) | - | [96] | |
| Grilling | - | - | - | <LOD-6.48 (5) | - | [96] | |
| Smoking | 1.6–32.7 | 10.2–271.0 (4) | - | - | - | [107] | |
| Smoking | <LOD-6.20 | 1.21–35.90 (4) | - | - | - | [39] | |
| Smoking | <LOD-<LOQ | 114–679 (16) | - | - | - | [108] | |
| Smoking | 0.43–0.47 | 24.42–34.07 (16) | - | - | - | [109] | |
| Edible oils | |||||||
| Coconut | Cold-pressed | <LOQ-1.01 | <LOQ-4.95 (4) | - | - | - | [110] |
| Safflower | Cold-pressed | <LOD-0.90 | 1.43–3.16 (4) | - | - | - | [110] |
| Linseed | Cold-pressed | <LOD-<LOQ | <LOQ-2.44 (4) | - | - | - | [110] |
| Canola | Commercial | 0.98–4.23 | 39.43–47.41 (15) | - | - | - | [91] |
| Palm | Storage | - | 0.16–8.98 (8) | 0.67–18 (5) | - | - | [111] |
| Corn | Commercial | 0.32–4.39 | 1.83–47.0 (16) | - | - | - | [112] |
| Commercial | 0.23–0.69 | 12.41–20.64 (15) | - | - | - | [91] | |
| Sunflower | Commercial | <LOD-5.29 | 30.6–75.9 (16) | - | - | - | [112] |
| Commercial | 0.71–1.56 | 21.68–26.76 (15) | - | - | - | [91] | |
| Soybean | Refining | 0.59–1.82 | 16.52–58.04 (16) | 2.04–11.26 (5) | - | - | [113] |
| Refining | 0.32–0.76 | 19.71–48.72 (16) | 2.04–20.04 (5) | - | - | [114] | |
| Commercial | <LOD-3.74 | 1.83–51.1 (16) | - | - | - | [112] | |
| Cooking waste | 0.17–3.33 | 18.34–239.01 (16) | 1.34–39.60 (4) | - | - | [115] | |
| Cooking waste | - | - | - | - | 0.27–0.49 (2) | [116] | |
| Rapeseed | Refining | 0.03–0.94 | 20.02–52.03 (16) | 0.93–10.88 (5) | - | - | [113] |
| Refining | 0.03–0.31 | 17.25–38.02 (16) | 2.03–6.56 (5) | - | - | [114] | |
| Cooking waste | - | - | - | - | <LOD-0.27 (2) | [116] | |
| EVOO | Commercial | <LOD | 9.9–48.3 (16) | - | - | - | [117] |
| Commercial | 0.08–5.79 | 33.4–82.4 (16) | - | - | - | [112] | |
| Commercial | <LOQ | 11.4–45.8 (16) | - | - | - | [118] | |
| Commercial | 0.02–0.07 | 0.07–4.32 (16) | - | - | - | [119] | |
| VOO | Commercial | 0.26–6.71 | 39.4–96.7 (16) | - | - | - | [112] |
| Commercial | 0.04–0.06 | 4.42–6.36 (16) | - | - | - | [119] | |
| OO | Commercial | 0.27–0.89 | 17.90–55.55 (16) | - | - | - | [120] |
| Commercial | 0.01–0.44 | 11.55–16.65 (15) | - | - | - | [91] | |
| OPO | Commercial | 0.04–0.15 | 1.21–2.85 (16) | - | - | - | [119] |
| Cereal and cereal-derived products | |||||||
| Wheat | Commercial | 0.08–0.20 | 9.74–23.87 (28) | - | - | - | [121] |
| Rice | Commercial | <LOD-0.97 | - | - | <LOD-4.19 (2) | - | [122] |
| Cooked | <LOD | 248.3 (16) | - | - | - | [123] | |
| Corn | Frying | - | - | - | <LOD-<LOQ (5) | - | [96] |
| Grilling | - | - | - | <LOD-3.26 (5) | - | [96] | |
| Bread | Commercial | <LOQ–0.20 | 0.11–0.22 (4) | - | - | - | [124] |
| Commercial | ≤LOD | 1.29–4.80 (16) | - | - | - | [125] | |
| Baking | - | 9.46–228.98 (13) | - | - | - | [126] | |
| Commercial | 0.19–17.41 | 59.64–211.19 (16) | - | - | - | [127] | |
| Commercial | <LOD-0.95 | 1.60–16.91 (16) | - | - | - | [128] | |
| Commercial | 0.11–0.25 | 0.16–0.46 (4) | - | - | - | [129] | |
| Frying | - | - | - | <LOD-<LOQ (5) | - | [96] | |
| Grilling | - | - | - | <LOD-1.96 (5) | - | [96] | |
| Commercial | <LOD | 98.2–176.3 (16) | - | - | - | [123] | |
| Youtiao | Frying | 0.40–1.38 | 13.81–18.00 (16) | 0.54–9.42 (5) | - | - | [5] |
| Youtiao | Frying | - | 9.67–12.48 (16) | 3.46–6.10 (5) | - | - | [130] |
| Youtiao | Frying | <LOQ-1.18 | <LOQ-195 (15) | - | - | - | [131] |
| Breakfast cereals | Commercial | <LOQ–0.30 | 0.23–0.87 (4) | - | - | - | [124] |
| Commercial | 0.09–0.30 | 0.07–0.87 (4) | - | - | - | [129] | |
| Cookies | Frying | <LOD-1.33 | <LOD-26.92 (18) | - | - | - | [132] |
| Pasta | Commercial | ≤LOD | 0.16–1.98 (16) | - | - | - | [125] |
| Spaghetti | Commercial | 0.4–2.0 | 9.0–200 (16) | - | - | - | [133] |
| Macaroni | Commercial | 0.2–0.7 | 30–60 (16) | - | - | - | [133] |
| Commercial | <LOD | 176.2 (16) | - | - | - | [123] | |
| Noodles | Frying | <LOD-11.9 | <LOD-182.8 (18) | - | - | - | [132] |
| Commercial | 350–830 | 560–7889 (16) | - | - | - | [134] | |
| Commercial | 0.3–3.0 | 300–800 (16) | - | - | - | [133] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palade, L.M.; Negoiță, M.; Adascălului, A.C.; Mihai, A.L. Polycyclic Aromatic Hydrocarbon Occurrence and Formation in Processed Meat, Edible Oils, and Cereal-Derived Products: A Review. Appl. Sci. 2023, 13, 7877. https://doi.org/10.3390/app13137877
Palade LM, Negoiță M, Adascălului AC, Mihai AL. Polycyclic Aromatic Hydrocarbon Occurrence and Formation in Processed Meat, Edible Oils, and Cereal-Derived Products: A Review. Applied Sciences. 2023; 13(13):7877. https://doi.org/10.3390/app13137877
Chicago/Turabian StylePalade, Laurentiu Mihai, Mioara Negoiță, Alina Cristina Adascălului, and Adriana Laura Mihai. 2023. "Polycyclic Aromatic Hydrocarbon Occurrence and Formation in Processed Meat, Edible Oils, and Cereal-Derived Products: A Review" Applied Sciences 13, no. 13: 7877. https://doi.org/10.3390/app13137877
APA StylePalade, L. M., Negoiță, M., Adascălului, A. C., & Mihai, A. L. (2023). Polycyclic Aromatic Hydrocarbon Occurrence and Formation in Processed Meat, Edible Oils, and Cereal-Derived Products: A Review. Applied Sciences, 13(13), 7877. https://doi.org/10.3390/app13137877








