Abstract
The present study identifies the phytochemical profile of a hydroalcoholic extract derived from Smilax aspera leaves and stems, estimates its antioxidant capacity and evaluates its cytotoxic activity against glioblastoma (A172 cell line) and rhabdomyosarcoma (TE671 cell line). Chemical analysis of leaves and stems was performed with liquid chromatography analysis combined with a quadrupole time-of-flight high-resolution mass spectrometry (LC/Q-TOF/HRMS). The antioxidant activity of the extract was evaluated with the 2,2-Diphenyl-1-picrylhydrazyl (DPPH) and the 2,2′-azinobis[3-ethylbenzthiazoline-6-acid)] (ABTS) assays. Cell viability was examined using the alamar blue assay. Most of the compounds tentatively identified belonged to the flavonoids family, with rutin being the most abundant, followed by luteolin. The extract showed potent antioxidant activity which corresponded to 13.9 ± 1.91 μg/mL (DPPH assay) and 6.27 ± 1.7 μg/mL (ABTS assay), expressed as IC50 values. The extract inhibited the proliferation of cancer cells. The lowest IC50 value for A172 cells was observed 48 h after treatment and was calculated at 0.482 ± 0.98 mg/mL while for the TE671 cell line the lowest IC50 value was 0.629 ± 1.31 mg/mL, calculated 72 h after treatment. Considering the high biological value of flavonoids as health defense promoters, S. aspera leaves and stems can be an important natural source to consider as they may provide important health benefits.
1. Introduction
The family Smilacaceae consists of two genera, namely Smilax L. and Heterosmilax Kunth, and is mainly distributed in the tropics, but also extends into temperate regions of both hemispheres. Smilax, the core genus of Smilacaceae, contains c. 200 species and shows the highest species diversity in East Asia, but only two species are distributed in southern Europe [1,2]. Smilax aspera L. is considered a Tertiary relict and it is widespread throughout the circum-Mediterranean region, including North Africa and Macaronesia (Canary Islands and Madeira), but also occurs with scattered populations in the East African highlands (Kenya, Ethiopia, Tanzania) and South Asia (Nepal, Sri Lanka, India, Bhutan, Kashmir, Southwestern China) [2].
In traditional and folk medicine, Smilax spp. have been used in the treatment of numerous ailments, such as cancer, syphilis, acute bacterial dysentery, inflammation, rheumatism and hypoglycemia [3,4,5,6]. Specifically, regarding the anticancer potential of Smilax spp., an ethanolic extract of Smilax glabra rhizomes, demonstrated its anticancer activity by inhibiting cell adhesion of HepG2, MDA–MB–231 and T24 cancer cell lines [5] while a methanolic extract of Smilax spinosa rhizomes exhibited antineoplastic activity against HL–60, MCF–7 and MDA231 cancer cell lines [7].
Gliomas are common neoplasms, stemming from glial cells of the central nervous system (CNS). The most common types of glioma are astrocytomas, with glioblastoma multiforme (grade IV astrocytoma) being the most devastating primary brain tumor, as it is a highly invasive and aggressive tumor. Despite advances in surgical techniques and adjuvant treatment, the average survival is only 1–2 years, which has not improved significantly during the last decades. Fewer than 5–10% of patients survive up to 5 years and only 25% of glioblastoma patients survive more than one year.
Sarcomas develop in the connective tissue, e.g., bones, muscles, fat, ligaments, and blood vessels. Rhabdomyosarcoma (RMS) is a highly aggressive soft sarcoma, originating from the mesenchymal cells of skeletal muscles. It is a metastatic tumor with the most common sites of metastasis being the lungs and the lymph nodes [8,9]. The prognosis of non-metastatic RMS is good and survival rates are high if treated early. Therapy and treatment include surgery, chemotherapy and radiotherapy. Nevertheless, recurrence cases are common and are accompanied by poor survival rates [10].
Phytochemicals are intensively studied against various chronic diseases including cancer. It is discussed that their efficacy is mainly due to their antioxidant activity. Though treatment with natural products is yet preliminary and much information is needed regarding their mechanism of action, data from many in vitro, in vivo and clinical trial studies evidence that these substances are of great therapeutic importance and may consist a new era of treatment [11,12].
Secondary metabolites isolated from various plant materials are complex mixtures. Therefore, in order to understand their activity, the chemical characterization of these mixtures is necessary. Mass spectrometry is a powerful tool for the screening of various types of samples including plant-derived extracts [13,14]. A common type of this analytical technique is quadrupole time of flight mass spectrometry. By coupling Q-TOF/MS with liquid chromatography, a challenging method that gains popularity is generated. High resolution, high detection sensitivity in full scan acquisition mode and fragmentation of the molecular ions formed make the Q-TOF mass spectrometer an advantageous tool for qualitative analysis.
Within this context, our study aims: (a) to explore the phytochemical profile of S. aspera leaves and stems, originating from Greece, with the use of liquid chromatography combined with a quadrupole time-of-flight high-resolution mass spectrometry (LC/Q-TOF/HRMS), (b) to estimate their antioxidant capacity with both DPPH and ABTS assays and, (c) to evaluate their cytotoxic activity against glioblastoma and rhabdomyosarcoma cell lines.
2. Materials and Methods
2.1. Plant Material
In the present study, plant material of Smilax aspera was collected from northwestern Peloponnese, Greece. Vouchers have been deposited at the Herbarium of the Agricultural University of Athens (ACA), with the following label: Greece, Peloponnisos, prefecture of Achaia, ca. 0.4 km S of Achaiko village, garrigue with Quercus coccifera and roadsides, alt. 50 m, 38° 07.09′ N, 21°35.52′ E, 20.09.2016, Trigas 6331, ACA (voucher number: 008842). Plant identification was based on morphological features, using the identification key provided by [15]. Berries were separated from the stems and the plant was kept in the dark, at a low temperature for 15 days.
2.2. Sample Preparation
Two grams (2.0 g) of powdered plant material were extracted with 30 mL of a hydromethanolic solution (70% v/v) with the assistance of an ultrasonic water bath, as previously described by [13].
2.3. LC/Q-TOF-HRMS Analysis
The crude extract of the S. aspera aerial part was dissolved in methanol (LC-MS grade, Fischer Scientific, Madrid, Spain) (70% v/v). Standard solutions of rutin trihydrate, catechin, luteolin and luteolin glucoside, were similarly prepared. Reference compounds were all purchased from Extrasynthese (Genay, France). All the tested solutions were prepared on the day of the analysis.
LC/Q-TOF/HRMS analysis was performed on an HPLC system (Agilent Series 1260-Agilent Technologies, Santa Clara, CA, USA) coupled to a 6530 Q-TOF mass spectrometer (Agilent Technologies, Singapore). The HPLC system consists of a degasser, autosampler, quaternary pump, diode array detector and a thermostatically controlled column oven. Chromatographic separation was performed at 40 °C on a Zorbax Extend-C18 Rapid Resolution HT 2.1 mm × 50 mm, 1.8 μm reversed-phase column. The solvent system consisted of water LC-MS (solvent A) and acetonitrile LC-MS (solvent B) both acidified with 0.1% formic acid. The elution program was as follows: 5–95% solvent B from 0 to 33 min and maintained at 95% up to 38 min. Experimental conditions were adjusted as in the study of [13]. The extract was analyzed under the positive and negative ionization modes. The parameters set for the Q-TOF mass analysis follow those described in our previous analysis [13]. CID-ms/ms spectra were recorded on the auto MS/MS mode. The mass range was set to 50–1000 and the collision energy was set at 40 V. The results were analyzed using the Agilent MassHunter Workstation software LC-MS Data Acquisition for 6530 series Q-TOF.
Molecular formulas were automatically generated using the ‘Generate formulas’ of the Agilent Masshunter Qualitative Analysis. The identification of the compounds was based on the available standard references for rutin trihydrate, luteolin, catechin and luteolin glucoside and data from the available literature [16,17]. Accurate mass ≤ 5 ppm was used to generate molecular formulas.
Quantification of Flavonoids by LC/Q-TOF-HRMS
Identified flavonoids of S. aspera leaves and stems were quantified with the single-point external standard method [18]. Standard solutions of known concentration were analyzed as aforementioned. Peak areas were recorded and the respective response factor was calculated according to the following formula:
Response factor = (peak area of the standard solution)/(concentration of the standard solution)
Afterward, the analytes sample of unknown concentration was treated under the same conditions and the peak area was also recorded. Then the amount of each analyte was calculated using the following equation:
Amount of analyte = (peak area of the analyte)/(response factor)
Analyte quantification when no standard solution was available, was performed by using the standard solutions of similar molecular structures.
2.4. Determination of Total Phenolic Content
Total phenolic content was estimated using Folin–Ciocalteu reagent (Sigma-Aldrich, Darmstadt, Germany) and according to [19]. The final concentration of the extract used was 0.25 mg/mL. The absorbance was measured at 765 nm. A standard curve was constructed using gallic acid (Riedel-de Haën AG, Seelze, Germany).
Total phenolic content was calculated according to the following equation derived from a gallic acid standard curve:
where y stands for the absorbance of gallic acid measured at 765 nm and x represents the concentration of the standard solution in μg/mL. Threefold measurements were performed and results were expressed as mg of gallic acid equivalents (GAE) per g of dry material. The regression obtained an r = 0.999.
y = 0.0012x − 0.0016 (r = 0.999, n = 3)
2.5. Estimation of Antioxidant Activity
2.5.1. DPPH• (2,2-Diphenyl-1-picrylhydrazyl) Radical Scavenging Assay
A DPPH• hydromethanolic solution (70% v/v) (Sigma-Aldrich, Darmstadt, Germany), at the concentration of 60 × 10−6 M was prepared and kept in the dark during the experiment.
The range of the final concentrations tested as far as the extract is concerned was 3.6 to 19.80 μg/mL. The experiment took place as previously described by [13]. Measurements were performed in triplicate at 517 nm. The following equation was used to calculate the inhibition percentage for DPPH scavenging:
where Acontrol is the absorbance of the control solution and Asample is the absorbance of the tested extract. The experiment took place in triplicates.
% radical scavenging activity= [(Acontrol − Asample)/Acontrol] × 100
A scatter plot of % inhibition versus concentration (μg/mL) was constructed and results were expressed as IC50 value which was calculated using the equation derived from the plot. The antioxidant activity of Trolox (Acrós organics, Waltham, MA, USA) and caffeic acid (Sigma-Aldrich, Darmstadt, Germany) was also calculated under the same conditions.
2.5.2. ABTS•+ (2,2′-Azinobis[3-ethylbenzthiazoline-6-acid]) Radical Scavenging Assay
For the ABTS assay, 7 mM of ABTS+ reagent (Sigma-Aldrich, Germany) and 2.45 mM of potassium persulfate (Sigma-Aldrich, Germany) were mixed. The solution was left at room temperature, in the dark for 18 h. The ABTS+ stock solution was then diluted with 70% methanol, so the absorbance of the final working solution was 0.700 ± 0.01 at 734 nm. The range of the final concentrations used, as far as the extract is concerned, was 1.98 to 12.87 μg/mL The experiment took place as described by [14], in triplicates. Control solution and calculation of the results were carried out as previously described for the DPPH assay.
2.6. Evaluation of Cytotoxic Activity
2.6.1. Cell Treatment and Exposure to the Extract
Two cell lines were used to evaluate the cytotoxicity of the S. aspera extract. The TE671 rhabdomyosarcoma cancer cell line was obtained from the European Collection of cell cultures (ECACC). The A172 glioblastoma cell line was obtained from a male patient of 53 years (ECACC, London, UK, Cat. Nr 88062428). Cells were grown in a cell culture flask (75 cm2 surface area) in Dulbecco’s modified Eagle’s Medium (DMEM) (ThermoFisher Scientific Inc. (Gibco), Waltham, MA, USA Cat. Nr. 10566016) with a high concentration of glucose (4500 mg/mL), enriched with 15% fetal bovine serum (FBS) (ThermoFisher Scientific Inc. (Gibco), Waltham, MA, USA Cat. Nr. 26140-079), L-glutamine (2 mM) (ThermoFisher Scientific Inc. (Gibco), Waltham, MA, USA Cat. Nr. A12860-01). In order to avoid pathogen contamination a dual antibiotic solution of penicillin G (100IU) and streptomycin (100 μg/mL), was added (ThermoFisher Scientific Inc. (Gibco), Waltham, MA, USA Cat. Nr. 15140-122), in addition to an amphotericin B solution (ThermoFisher Scientific Inc. (Gibco), Waltham, MA, USA Cat. Nr. 14140-122). The cell population was measured with the use of a Coulter counter (CellTaq α, Nihon Kodhen Inc., Tokyo, Japan), in order to determine the absolute initial number of cells inoculated in each respective experimental setup. Cells were inoculated in 96 well plates (1.5 × 103 cells/mL) and were allowed to grow for 24 h until ~80% confluence. After 24 h cells were exposed to successively diluted concentrations of the extract (t = 0 h), ranging from 0.02 to 3.125 mg/mL for 24, 48 and 72 h. Extracts were diluted in DMSO. Control experiments included both cells with no further treatment (no extract), as well as cells with vehicle only (DMSO at a final concentration of 4% (data not shown)). Vehicle had no effect on cell proliferation and viability.
Experiments were performed in 96-well plates (CellStar® Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany DE Cat. Nr. M3687-60EA) as described previously by [14].
2.6.2. Cell Viability Assessment Protocol (Alamar Blue Assay)
The assessment of cell viability after incubation with testing agents was performed with resazurin reduction experiments, using the Alamar Blue viability assay. Cell viability at each time point (24, 48, 72 h) was quantified by adding Alamar Blue (Gibco, Invitrogen Inc.) to each well, to a final concentration of 10%. The experiment took place as previously described by [14].
The percentage of viable cells was calculated according to the following formula:
where OD1 stands for the optical density in nm for cells treated with the chemical, OD2 stands for the optical density in nm for wells containing nutrient medium only and no chemical and OD3 stands for cells that were not exposed to the chemical. Optical density was measured at 570 nm and 600 nm. Results were expressed as IC50 values, the concentration of the chemical that causes 50% inhibition with respect to the untreated cells [14].
2.6.3. Giemsa Staining
Cells were microscopically observed at 24, 48 and 72 h of incubation. Cells were colored with the Giemsa stain as we describe in our previous study [14].
2.6.4. Data Analysis
IC50 was calculated using GraphPad Prism (ver. 8.4.2) and according to a four-parameter logistic model. The data were presented as means ± standard error of the mean (SEM). The dose and time-dependent effect of the tested drugs with respect to the control group were also calculated with GraphPad Prism (ver, 8.4.2). Statistical differences between untreated and treated cells were evaluated with the student t-test. p values < 0.05 were considered statistically significant and confidence intervals were at ±95% (±95% CI). Results were expressed as μg/mL of the extract. Normalized results are presented as log10 concentration [14].
3. Results and Discussion
3.1. Identification of Metabolites by LC/Q-TOF/HRMS Analysis
The extract was analyzed under positive and negative ionization modes for complementary information. Tentatively identified metabolites of the hydroalcoholic extract of S. aspera leaves and stems are summarized in Table 1, along with their retention time (tR), molecular formula, m/z ratio, UVmax, mass error (ppm) and ms/ms product ions in the MS(+) and MS(−) scan, respectively. The fragmentation process is described in detail in the supporting supplementary material (part I). Low energy CID MS/MS spectra and suggested fragmentation patterns of compounds at the positive and negative ionization modes are also presented as supplementary material (part II).

Table 1.
Tentatively identified compounds for Smilax aspera leaves and stems at positive and negative ionization mode.
Phenolic compounds such as chlorogenic acid, caffeoyl shikimic acid, kaempferol and its glycosides, quercetin, isorhamnetin, rutin, catechin, are among the compounds identified in Smilax spp. extracts [16]. Our results confirm previous studies [16,20] highlighting S. aspera as an important source of bioactive compounds, namely phenolic compounds and steroidal saponins. Regarding both positive and negative ionization modes, flavonoid glycosides and aglycons were identified as major metabolites of S. aspera aerial parts, and rutin was the major compound detected. Compounds isoshaftoside, isorhamnetin pentoside-hexoside, luteolin glucoside and isorhamnetin hexoside have not been previously reported in the genus Smilax.
In addition, types of steroidal saponins belonging to the furostane, spirostane and isospirostane classes were detected. Spirostane and isosprirostane saponins are six-ring stereoisomers and differ at the R or S configuration of the methyl group at the position C–27 of the F ring. The sugar moiety is directly attached to the steroid nucleus, contrary to the five-ring furostane saponin that is adhered to the position C–26, separated from the saponin aglycon skeleton by a hemiacetal hydroxyl group. The position C–3 of the steroidal saponins is occupied by a group of sugar moieties most common of which are glucopyranosyl, rhamnopyranosyl, galactopyranosyl, fructopyranosyl and arabinopyranosyl residues [16]. Position C–26 is of crucial importance for the identification of the type of saponin presented. When this position is occupied by a sugar moiety, as in the case of furostane saponins, then the cleavage of the E-ring from which the characteristic neutral loss of 144 D arises, occurs after the elimination of the attached sugar moiety. In our study, the detected saponins presented a main peak at m/z 253 (when a double bond at position C5–C6 was presented) or at m/z 255, arising from the main aglycon skeleton.
The chemical profile of various Smilax species has been elucidated and many pharmacological properties are related to the presence of steroidal saponins and flavonoids [20,21,22]. Regarding S. aspera, the presence of steroidal saponins in the underground plant organs which is also the most well-studied part of the plant and the presence of carotenoids at the berries, has been reported [16,23,24]. Nevertheless, uncertainty still exists about the phytochemical profile and the antioxidant activity of leaves and stems. Natural antioxidants are considered more effective and less harmful than synthetic ones; therefore, the search for new natural antioxidant sources has been intensified [25,26]. The especially rich Mediterranean flora offer remarkable research opportunities for the discovery of new natural antioxidants.
3.2. Quantification of Flavonoids, Total Phenolic Content and Antioxidant Activity
Notably, our results revealed the dominance of rutin (31.17 μg/mL) compared to the other flavonoids, followed by luteolin (14.46 μg/mL), isorhamnetin hexoside-pentoside (2.68 μg/mL), quercetin hexoside (2.33 μg/mL), kaempferol hexoside-pentoside (0.609 μg/mL), catechin (1.73 μg/mL), quercitrin (0.082 μg/mL) and naringenin (0.024 μg/mL). The estimated value of the total phenolic content of the extract expressed as mg of GAE per g of dry material was 120.56 ± 2.25 GAE/g. The antioxidant activity of the extract was evaluated by DPPH and ABTS assays and the results were expressed as IC50 values. S. aspera aerial parts exhibited a notable antioxidant activity which corresponds to 13.9 ± 1.91 μg/mL for the DPPH method and 6.27 ± 1.7 μg/mL for the ABTS method.
Various studies dealing with total phenolic content and the antioxidant activity of several Smilax spp. have been published in the last decades. Underground plant organs, i.e., roots and rhizomes, have been intensively studied [16], while the antioxidant activity of leaves and stems of certain species has also been examined [16,27,28]. All studied species have shown a notable antioxidant activity which in some cases was comparable to that of strong synthetic scavengers and was strongly correlated to their phenolic content [29,30]. In our study, S. aspera exhibited a high phenolic content and significant antioxidant activity. By comparing the calculated IC50 values to known antioxidants [trolox (the IC50 value corresponds to 4.53 ± 1.89 μg/mL for the DPPH assay and to 2.66 ± 0.90 μg/mL for the ABTS assay) and caffeic acid (the IC50 value is equal to 2.36 ± 0.61 μg/mL and 1.72 ± 1.29 μg/mL for DPPH and ABTS assay, respectively)], it is concluded that the plant under study can be classified among those with potent antioxidant capacity. Only a few studies have investigated the antioxidant activity of S. aspera. The study of [31], evaluated the non-polar fraction of the leaves, in terms of tocopherol content. Furthermore, [32], examined the antioxidant activity of leaves and fruits from S. aspera from different regions with the FRAP assay. The calculated antioxidant activity regarding leaves ranges from 62.97 to 64.57 mmol Fe+2/kg while for the fruits of the plant, this value is from 64.51 to 66.31 mmol Fe+2/kg. Both studies concluded that S. aspera is a good source of antioxidants. Our results confirm the previous findings and add novel knowledge to the field of natural antioxidants.
Phenolic compounds belong to a class of chemicals consisting of one or more hydroxyl groups (-OH), bonded to an aromatic ring. Therefore, they can donate an electron or a hydrogen atom to a free radical, interrupting in this way the free radical chain reaction. As a result, there is a strong correlation between the total phenolic content of a plant and its antioxidant activity [33]. Indeed, in our study, the estimated values of the total phenolic content and the antioxidant capacity of S. aspera leaves and stems confirmed this statement. Although phenolic compounds are endowed with many pharmacological properties, they are mostly known for their radical scavenging activity. As numerous diseases are strongly correlated with high concentrations of free radicals in cells and tissues, phenolic compounds may play a beneficial key role in the prevention of different pathological conditions. However, there is a strong rationale between flavonoid structure and their antioxidant activity. In the study of [34], it is reported that the antioxidant activity of a flavonoid increases when a hydroxyl group is attached to the position C–3 of the C ring, simultaneously to the presence of a 2,3 double bond conjugated to a 4-keto function at the same ring. In addition, the authors also demonstrated that the ortho 3′-4′-dihydroxy moiety at the B ring is important as it enhances the antioxidant capacity of a flavonoid. Among the flavonoids detected in our study, kaempherol hexoside-pentoside, isorhamnetin hexoside-pentoside, luteolin and its glycoside, catechin, quercetin hexoside and rutin are in accordance with this hypothesis.
3.3. Evaluation of Cytotoxicity
A dose and time-dependent effect of S. aspera extract was observed for both cell lines. Interestingly, for TE671 cells, the cytotoxic effect was more prominent at 72 h of treatment, at the range of concentrations between 0.04–3.125 mg/mL. At 24 h, inhibition was observed at 0.78–3.12 mg/mL whereas after 48 h of treatment, the effect was observed at 0.39–3.12 mg/mL (Figure 1A). On the other hand, the range of the concentrations (0.39–3.12 mg/mL) that significantly inhibited the proliferation of A172 cells with respect to the control group after treatment with the extract of S. aspera, was the same for all time points. At the concentrations from 0.19 mg/mL to 0.02 mg/mL, no statistically significant effect was observed, while cells recovered at 0.04 mg/mL (Figure 1B). This effect was also evident through microscopical investigation. TE671 cells were confluent without any treatment (Figure 2A), while a similar behavior was observed for the 0.39 mg/mL concentration (Figure 2C). On the other hand, at 0.78 mg/mL cells were significantly reduced (Figure 2B). The same behavior was observed for the A172 cell line, where cells were confluent without any treatment (Figure 3A), while cells were also near-confluence at the 0.39 mg/mL concentration (Figure 3C). Finally, at 0.78 mg/mL cells were significantly reduced (Figure 3B), as in the case of TE671 cells.
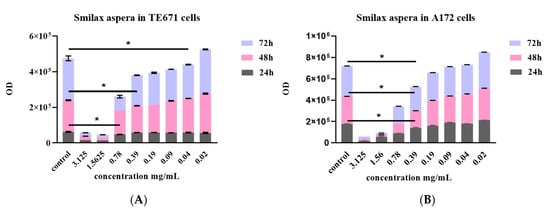
Figure 1.
Dose dependent and time dependent effect after treatment with S. aspera extract on TE671 (A) and A172 (B) cells. Data are presented as the mean ± standard error of the mean (SEM) (with n = 8). The asterisk (*) indicates significant differences between untreated and treated cells. The grey color corresponds to 24 h of treatment, the pink to 48 h and the light blue to 72 h.
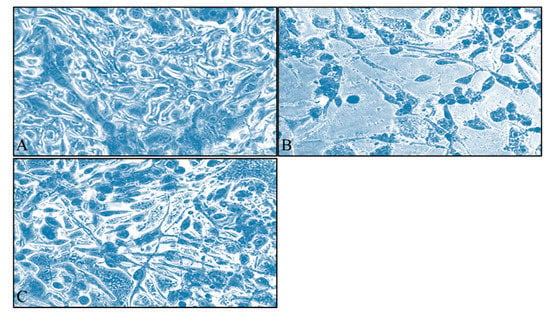
Figure 2.
Microscopy inspection of the TE671 rhabdomyosarcoma cells, grown for 72 h in DMEM with no other treatment (A), cells treated with 0.78 mg/mL of the extract (B) and cells treated with 0.39 mg/mL of the extract (C). Images were captured at ×200 magnification.
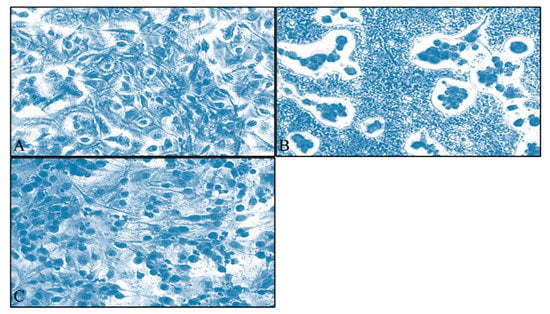
Figure 3.
Microscopy inspection of the A172 glioblastoma cells, grown for 72 h in DMEM with no other treatment (A), cells treated with 0.78 mg/mL of the extract (B) and cells treated with 0.39 mg/mL of the extract (C). Images were captured at ×200 magnification.
In the case of TE671 cells, the lowest IC50 was observed at the time point of 72 h and was calculated at 0.629 ± 1.31 mg/mL. The IC50 values for 24 and 48 h were calculated at 0.820 ± 1.38 mg/mL and 1.044 ± 1.22 mg/mL, respectively (Figure 4A). For the A172 cell line, the lowest IC50 was calculated at 0.482 ± 0.98 mg/mL 48 h after treatment. The IC50 values for 24 and 72 h were calculated at 1.061 ± 1.09 mg/mL and 0.749 ± 1.18 mg/mL, respectively (Figure 4B).
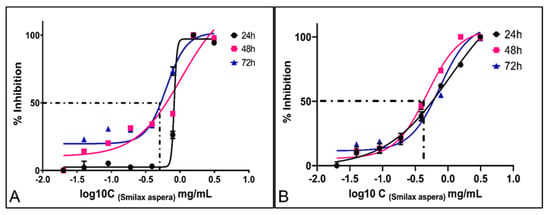
Figure 4.
IC50 of S. aspera extract on TE671 cells (A) and A172 cells at 24, 48 and 72 h (B). The lowest IC50 value for TE671 cell line was 0.629 mg/mL, calculated at 72 h and 0.482 mg/mL for A172 cell line, calculated at 48 h. Cancer cell viability increases as concentration of the drug decreases.
Various chemotherapeutic agents are available and are currently used to combat cancer [35,36]. However, due to their high cost and the often-severe associated side effects their partial or even full replacement is a compelling research objective. Natural products are promising substitutes and they are intensively studied for their anticancer activity. Cases of in vitro experiments in which cells were co-treated with a chemotherapeutic drug and a natural product have shown that natural products are able to decrease the dose of the first agent but also to counteract cell damage caused by chemotherapeutics due to increased reactive oxygen species (ROS) production [37]. In addition, crude extracts of various plants have been tested as monotherapy in both in vitro and in vivo experiments, and their cytotoxic activity has been proved [38,39]. These extracts contain a vast number of active compounds endowered with strong antioxidant activity. Nevertheless, there is still a controversial debate to clarify the actual role of antioxidants in human cancer progression. Some studies support the idea that antioxidants desensitize cancer cells against chemotherapy. On the other hand, many studies acknowledge the beneficial, protective role of these compounds against cancer and also relate them to a better survival rate [40].
S. aspera cytotoxic activity on glioblastoma has not been previously reported. Thus, we tried to explain the cytotoxic effect of the extract based on the compounds presented in abundance, namely rutin and luteolin. Rutin, a glycoside of the flavonoid quercetin, is of great pharmacological importance as apart its antioxidant activity it possesses among others also anticancer activity [37,41,42,43]. For example, rutin, isolated from Dimorphandra mollis seeds, was studied for its cytotoxic activity against GL–15 glioblastoma cell line. Rutin when tested at the range of concentrations between 50 and 100 μM caused a dose-dependent inhibition of growth and proliferation of the treated cells, while the maximum inhibitory effect was observed at 72 h. Apoptosis and cell cycle arrest at the G2/M phase was proposed by the authors as the mechanism of action by which rutin exerts its cytotoxic activity [44]. Similarly, [37], examined the effect of rutin alone or in combination with temozolomide (TMZ) on U87–MG, DS4–MG, and U251–MG glioblastoma cells (concentration range 25–400 μM). Rutin reduced the survival of cancer cells. When cells were treated with a combination of rutin and TMZ, rutin at a low dose managed to increase the cytotoxic activity of TMZ. Apart from the in vitro experiments, the same combination of drugs reduced the volume of the tumor in xenograft mice. The possible suggested mechanism of action of rutin was that the flavonoid inhibited autophagy and c-Jun N-terminal kinase (JNK) activity. Moreover, the cytotoxicity of rutin was also examined on CHME cells. The maximum concentration of the flavonoid used was 20 μM and its cytotoxic activity was exerted via upregulation of the p53 gene [43].
On the other hand, luteolin, a flavone, also plays an important role in cancer progression since it influences several signaling pathways. Types of glioblastoma cell lines including LN229, U251, U251MG and U87MG, have been examined for their sensitivity against treatment with luteolin. A dose and time-dependent effect of cell viability were observed after treatment with luteolin at the concentration of 0–80 μM of the flavonoid, via activation of apoptosis, autophagy, death receptor (FADD) expression, stimulation of MAPK signaling pathway [45] and interference with cell cycle and EGFR protein [46]. A similar effect of luteolin was also observed for A172 and U373MG cancer cell lines, in which 200 μM of the flavonoid were needed to decrease cell viability for the first 24 h which was further decreased at 50 μM for the next 48 and 72 h of treatment [47].
In this study, the extract of S. aspera compounds that do not belong to the flavonoid family was also identified. Taking into account that the concentration of both rutin and luteolin in the extract used to estimate cytotoxicity of cancer cells were calculated at μM levels, which means much lower than those used in the literature, we deduce that a synergistic effect of all the compounds that the extract contains is responsible for its cytotoxic activity. Hence, we mention here that apart from flavonoids also, steroidal saponins have been evaluated for their cytotoxic activity [48]. For example, the cytotoxic effect of dioscin on C6 rat glioma cells was investigated and it was found that dioscin enhances the cytotoxicity of treated cells in a dose and time-dependent manner. It also promotes apoptosis by interfering with the S phase of the cell cycle and blocks the activity of topoisomerase I due to its antioxidant activity [49].
Natural products have also been studied for their efficacy against rhabdomyosarcoma and they have been found to inhibit cancer cell proliferation via various mechanisms [50,51]. However, as regards the extract examined in this study, literature data are limited and besides neither data of only rutin activity are available. Nevertheless, as it is previously mentioned, rutin is a quercetin glucoside bearing a rhamnose and a glucose moiety attached to position 3 of the aglycon. Studies of rutin metabolism demonstrated that the flavonoid is metabolized by various microorganisms and enzymes of the gut microbiota to quercetin and quercetin 3-O-glucoside [52,53]. Thus, in the present study, we discuss the effect of quercetin, a compound used against various types of sarcomas. Quercetin is a flavonol and its cytotoxic effect has been examined against various osteosarcoma cell lines, followed by different mechanisms. For example, in the study of [54], quercetin was examined for its capacity to inhibit the proliferation of a highly metastatic cancer cell line, the 143B osteosarcoma cell line. It was observed that quercetin at a dose of 10 μM decreases cell proliferation while at higher doses induces apoptosis, disrupts the cell cycle at the G2/M phase and inhibits the migration of cancer cells. Similarly, another osteosarcoma cell line, i.e., HOS and MG63, was evaluated for their sensitivity against quercetin. Similar results were observed regarding quercetin activity on cancer cells viability and cell cycle arrest [55]. Methotrexate at high doses is a basic treatment approach for children suffering from osteosarcoma. However multi-drug resistance in cancer chemotherapy is a quite common phenomenon and some patients come across methotrexate resistance. For this reason, [56] examined the effect of quercetin against U2-OS and U2-OS/MTX300 cell lines, sensitive and resistant respectively to methotrexate treatment. According to their results, quercetin inhibited cancer cell viability via apoptosis as it diminishes mitochondria activity. Similar to quercetin, luteolin has also been proven as a potential candidate against sarcomas. Uterine sarcoma cells, MES-SA/Dx5, were susceptible to luteolin as the flavonoid inhibited their proliferation in a dose-dependent manner.
Taken together, these data demonstrate the important role of natural compounds in cancer-fighting. In our study, an A172 glioblastoma and a TE671 rhabdomyosarcoma cell line were used to investigate the cytotoxic effect of S. aspera extract. Since the presence of rutin and luteolin was dominant, emphasis was given to the above-mentioned flavonoids or their metabolites. For both compounds, the concentrations that inhibited cancer cells growth and proliferation were calculated at 3.46 μM for rutin and 3.39 μM for luteolin. Considering that both flavonoids can cross the blood-brain barrier, it is important to extensively study these compounds. Our results are in accordance with the literature data presented herein and highlight de novo the very important role of natural products for cancer research. Although the exact mechanism by which the extract induces cytotoxicity is to be clarified, its strong antioxidant activity as estimated by the DPPH and ABTS assays, is possibly the key to the extract activity.
4. Conclusions
Chemical analysis of leaves and stems of Smilax aspera was conducted by the LC/Q-TOF/HRMS analysis. A high number of phenolic compounds and steroidal saponins were identified. Compounds isoshaftoside, isorhamnetin pentoside-hexoside, luteolin glucoside and isorhamnetin hexoside are reported for the first time in the genus Smilax. Rutin was the compound presented in abundance, followed by luteolin. Furthermore, the extract also showed a high phenolic content and potent antioxidant activity when examined either with the DPPH or the ABTS assay. The extract also presented a promising antiproliferative activity against A172 and TE671 cancer cell lines, in a dose and time-dependent manner. Considering the significance of natural antioxidants and the pharmacologic activity of rutin and luteolin, S. aspera aerial parts can be used as a defense against free radicals, compounds that are implicated in numerous diseases, including cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app13084784/s1, Part I: Fragmentation process of the identified compounds; Part II: Low energy CID MS/MS spectra and suggested fragmentation patterns of compounds detected at the ESI(+).
Author Contributions
Conceptualization, E.K., G.I.L. and P.A.T.; methodology, E.K., K.H., O.N., and C.K.; investigation, E.K., C.K., G.I.L., P.T., C.K.-G. and P.A.T.; data curation, E.K., G.I.L. and C.K.; writing—original draft preparation, E.K.; writing—review and editing, E.K., O.N., C.K., K.H., G.I.L., P.T., C.K.-G. and P.A.T.; supervision, P.A.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cameron, K.M.; Fu, C. A nuclear rDNA phylogeny of Smilax (Smilacaceae). Aliso A J. Syst. Florist. Bot. 2006, 22, 598–605. [Google Scholar] [CrossRef]
- Chen, C.; Qi, Z.C.; Xu, X.H.; Comes, H.P.; Koch, M.A.; Jin, X.J.; Fu, C.X.; Qiu, Y.X. Understanding the formation of Mediterranean–African–Asian disjunctions: Evidence for Miocene climate-driven vicariance and recent long-distance dispersal in the tertiary relict Smilax aspera (Smilacaceae). New Phytol. 2014, 204, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Ageel, A.M.; Mossa, J.S.; Al-Yahya, M.A.; Al-Said, M.S.; Tariq, M. Experimental studies on antirheumatic crude drugs used in Saudi traditional medicine. Drugs. Exp. Clin. Res. 1989, 15, 369–372. [Google Scholar] [PubMed]
- Fukunaga, T.; Miura, T.; Furuta, K.; Kato, A. Hypoglycemic effect of the rhizome of Smilax glabra in normal and diabetic mice. Biol. Pharm. Bull. 1997, 20, 44–46. [Google Scholar] [CrossRef]
- She, T.; Zhao, C.; Feng, J.; Wang, L.; Qu, L.; Fang, K.; Cai, S.; Shou, C. Sarsaparilla (Smilax glabra Rhizome) extract inhibits migration and invasion of cancer cells by suppressing TGF-β1 pathway. PLoS ONE 2015, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Yeşilada, E.; Sezik, E.; Honda, G.; Takaishi, Y.; Takeda, Y.; Tanaka, T. Traditional medicine in Turkey IX: Folk medicine in north-west Anatolia. J. Ethnopharmacol. 1999, 64, 195–210. [Google Scholar] [CrossRef]
- Seelinger, M.; Popescu, R.; Giessrigl, B.; Jarukamjorn, K.; Unger, C.; Wallnöfer, B.; Fritzer-Szekeres, M.; Szekeres, T.; Diaz, R.; Jäger, W.; et al. Methanol extract of the ethnopharmaceutical remedy Smilax spinosa exhibits anti-neoplastic activity. Int. J. Oncol. 2012, 41, 1164–1172. [Google Scholar] [CrossRef]
- Shern, J.F.; Yohe, M.E.; Khan, J. Pediatric Rhabdomyosarcoma. Crit. Rev. Oncog. 2015, 20, 227–243. [Google Scholar] [CrossRef]
- Huang, C.; Jian, B.; Su, Y.; Xu, N.; Yu, T.; He, L.; Zhang, X.; Liu, Y.; Jin, M.; Ma, X. Clinical features and prognosis of paediatric rhabdomyosarcoma with bone marrow metastasis: A single Centre experiences in China. BMC Pediatr. 2021, 21, 463. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Bisogno, G.; Garaventa, A.; Cecchetto, G.; Ferrari, A.; Sotti, G.; Donfrancesco, A.; Madon, E.; Casula, L.; Carli, M. Outcomes and prognostic factors after recurrence in children and adolescents with nonmetastatic rhabdomyosarcoma. Cancer 2005, 104, 183–190. [Google Scholar] [CrossRef]
- Kakouri, E.; Hatziagapiou, K.; Bethanis, K.; Nikola, O.A.; Lambrou, G.I.; Tarantilis, P.A. Tumor-Suppressing Properties of Crocus sativus L.: Nature as an Anti-Cancer Agent. Crit. Rev. Oncog. 2017, 22, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Ozleyen, A.; Boyunegmez Tumer, T.; Oluwaseun Adetunji, C.; El Omari, N.; Balahbib, A.; Taheri, Y.; Bouyahya, A.; Martorell, M.; Martins, N.; et al. Natural Products and Synthetic Analogs as a Source of Antitumor Drugs. Biomolecules 2019, 9, 679. [Google Scholar] [CrossRef] [PubMed]
- Kakouri, E.; Kanakis, C.; Trigas, P.; Tarantilis, P.A. Characterization of the chemical composition of Drimia numidica plant parts using high-resolution mass spectrometry: Study of their total phenolic content and antioxidant activity. Anal. Bioanal. Chem. 2019, 411, 3135–3150. [Google Scholar] [CrossRef] [PubMed]
- Kakouri, E.; Nikola, O.; Kanakis, C.; Hatziagapiou, K.; Lambrou, G.I.; Trigas, P.; Kanaka-Gantenbein, C.; Tarantilis, P.A. Cytotoxic Effect of Rosmarinus officinalis Extract on Glioblastoma and Rhabdomyosarcoma Cell Lines. Molecules 2022, 27, 6348. [Google Scholar] [CrossRef]
- DeFilipps, R.A. Smilax L. In Flora Europaea; Tutin, T.G., Burges, N.A., Chater, A.O., Edmondson, J.R., Heywood, V.H., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1980; Volume 5, p. 74. [Google Scholar]
- Raúl, S.C.; Beatriz, H.C.; Joseoziel, L.G.; Santos-Sánchez Norma Francenia, S.S. Phenolic Compounds in Genus Smilax (Sarsaparilla). In Phenolic Compounds-Natural Sources, Importance and Applications, 1st ed.; Soto-Hernández, M., Palma-Tenango, M., Garcia-Mateos, M.R., Eds.; Intech: Wellington, New Zealand, 2017; pp. 233–260. [Google Scholar]
- Tian, L.W.; Zhang, Z.; Long, H.L.; Zhang, Y.J. Steroidal Saponins from the Genus Smilax and Their Biological Activities. Nat. Prod. Bioprospecting 2017, 7, 283–298. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Petrakis, E.A.; Kimbaris, A.C.; Pappas, C.; Tarantilis, P.A.; Polissiou, M.G. Classification of Greek Mentha pulegium L. (Pennyroyal) samples, according to geographical location by Fourier transform infrared spectroscopy. Phytochem. Anal. 2012, 23, 34–43. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]
- Longo, L.; Vasapollo, G. Extraction and identification of anthocyanins from Smilax aspera L. berries. Food Chem. 2006, 94, 226–231. [Google Scholar] [CrossRef]
- Ivanova, A.; Mikhova, B.; Batsalova, T.; Dzhambazov, B.; Kostova, I. New furostanol saponins from Smilax aspera L. and their in vitro cytotoxicity. Fitoterapia 2011, 82, 282–287. [Google Scholar] [CrossRef]
- Challinor, V.L.; Parsons, P.G.; Chap, S.; White, E.F.; Blanchfield, J.T.; Lehmann, R.P.; De Voss, J.J. Steroidal saponins from the roots of Smilax sp.: Structure and bioactivity. Steroids 2012, 77, 504–511. [Google Scholar] [CrossRef]
- Belhouchet, Z.; Sautour, M.; Miyamoto, T.; Lacaille-Dubois, M.A. Steroidal saponins from the roots of Smilax aspera subsp. mauritanica. Chem. Pharm. Bull. 2008, 56, 1324–1327. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Hornero-Mendez, D. Identification and quantitative analysis of carotenoids and their esters from sarsaparilla (Smilax aspera L.) berries. J. Agric. Food Chem. 2012, 60, 8225–8232. [Google Scholar] [CrossRef]
- Akbarirad, H.; Ardabili, A.G.; Kazemeini, S.M.; Khaneghah, A.M. An overview on some of important sources of natural antioxidants. Int. Food Res. J. 2016, 23, 928–933. [Google Scholar]
- Nemzer, B.V.; Yashin, A.Y.; Vedenin, A.N.; Yashin, Y.I.; Yashunsky, D.V.; Nifantiev, N.E.; Kalita, D. Selected powerful natural antioxidants: Structure, food sources, antioxidant activities, and important health benefits. J. Food Res. 2019, 8, 60. [Google Scholar] [CrossRef]
- Chevolleau, S.; Mallet, J.F.; Ucciani, E.; Gamisans, J.; Gruber, M. Antioxidant Activity in Leaves of Some Mediterranean Plants. J. Am. Oil Chem. Soc. 1992, 69, 1269–1271. [Google Scholar] [CrossRef]
- Ozsoy, N.; Can, A.; Yanardag, R.; Akev, N. Antioxidant activity of Smilax excelsa L. leaf extracts. Food Chem. 2008, 110, 571–583. [Google Scholar] [CrossRef]
- Piluzza, G.; Bullitta, S. Correlations between phenolic content and antioxidant properties in twenty-four plant species of traditional ethnoveterinary use in the Mediterranean area. Pharm. Biol. 2011, 49, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Morais, M.I.; Pinto, M.E.; Araujo, S.G.; Castro, A.H.; Duarte-Almeida, J.M.; Rosa, L.H.; Rosa, C.A.; Johann, S.; Lima, L.A. Antioxidant and antifungal activities of Smilax campestris Griseb. (Smilacaceae). Nat. Prod. Res. 2014, 28, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Demo, A.; Petrakis, C.; Kefalas, P.; Boskou, D. Nutrient antioxidants in some herbs and Mediterranean plant leaves. Food Res. Int. 1998, 31, 351–354. [Google Scholar] [CrossRef]
- Yildiz, Ö.Ş.; Ayanoglu, F.; Bahadirli, N.P. Some morphological and chemical characteristics of Sarsaparilla (Smilax aspera L., Smilax excelsa L.). JAFES 2018, 23, 254–261. [Google Scholar]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Hatziagapiou, K.; Braoudaki, M.; Karpusas, M.; Tzortzatou-Stathopoulou, F. Evaluation of antitumor activity of gefitinib in pediatric glioblastoma and neuroblastoma cells. Clin. Lab. 2011, 57, 781–784. [Google Scholar]
- Brouwer, T.P.; van der Zanden, S.Y.; van der Ploeg, M.; van Eendenburg, J.D.H.; Bonsing, B.A.; de Miranda, N.F.C.C.; Neefjes, J.J.; Vahrmeijer, A.L. The identification of the anthracycline aclarubicin as an effective cytotoxic agent for pancreatic cancer. Anticancer Drugs. 2022, 33, 614–621. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, S.; Li, N.; Ho, A.; Kiang, K.; Zhang, X.; Cheng, Y.S.; Poon, M.W.; Lee, D.; Pu, J.; et al. Rutin increases the cytotoxicity of temozolomide in glioblastoma via autophagy inhibition. J. Neurooncol. 2017, 132, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Uddin, S.J.; Grice, I.D.; Tiralongo, E. Cytotoxic activity screening of Bangladeshi medicinal plant extracts. J. Nat. Med. 2014, 68, 246–252. [Google Scholar] [CrossRef]
- Lombardi, V.R.; Carrera, I.; Cacabelos, R. In Vitro Screening for Cytotoxic Activity of Herbal Extracts. Evid. Based Complement. Altern. Med. 2017, 2017, 2675631. [Google Scholar] [CrossRef]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity—Exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef]
- Farha, A.K.; Gan, R.Y.; Li, H.B.; Wu, D.T.; Atanasov, A.G.; Gul, K.; Zhang, J.R.; Yang, Q.Q.; Corke, H. The anticancer potential of the dietary polyphenol rutin: Current status, challenges, and perspectives. Crit. Rev. Food Sci. Nutr. 2020, 62, 832–859. [Google Scholar] [CrossRef]
- Satari, A.; Ghasemi, S.; Habtemariam, S.; Asgharian, S.; Lorigooini, Z. Rutin: A Flavonoid as an Effective Sensitizer for Anticancer Therapy; Insights into Multifaceted Mechanisms and Applicability for Combination Therapy. Evid. Based Complement. Altern. 2021, 2021, 9913179. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.P.; Hao, Y.L.; Chen, S.L.; Jia, G.J.; Guo, Y.H.; Zhang, G.L.; Wang, C.H.; Cheng, R.; Hu, T.; Zhang, X.; et al. Rutin induces apoptosis via P53 up-regulation in human glioma CHME cells. Transl. Cancer Res. 2019, 8, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Santos, B.L.; Silva, A.R.; Pitanga, B.P.; Sousa, C.S.; Grangeiro, M.S.; Fragomeni, B.O.; Coelho, P.L.; Oliveira, M.N.; Menezes-Filho, N.J.; Costa, M.F.; et al. Antiproliferative, proapoptotic and morphogenic effects of the flavonoid rutin on human glioblastoma cells. Food Chem. 2011, 127, 404–411. [Google Scholar] [CrossRef]
- You, Y.; Wang, R.; Shao, N.; Zhi, F.; Yang, Y. Luteolin suppresses tumor proliferation through inducing apoptosis and autophagy via MAPK activation in glioma. Onco. Targets Ther. 2019, 12, 2383–2396. [Google Scholar] [CrossRef]
- Anson, D.M.; Wilcox, R.M.; Huseman, E.D.; Stump, T.A.; Paris, R.L.; Darkwah, B.O.; Lin, S.; Adegoke, A.O.; Gryka, R.J.; Jean-Louis, D.S.; et al. Luteolin Decreases Epidermal Growth Factor Receptor-Mediated Cell Proliferation and Induces Apoptosis in Glioblastoma Cell Lines. Basic Clin. Pharmacol. Toxicol. 2018, 123, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Park, B.S.; Kang, H.M.; Kim, J.H.; Shin, S.H.; Kim, I.R. Role of Luteolin-Induced Apoptosis and Autophagy in Human Glioblastoma Cell Lines. Medicina 2021, 57, 879. [Google Scholar] [CrossRef] [PubMed]
- Sobolewska, D.; Galanty, A.; Grabowska, K.; Makowska-Was, J.; Wrobel-Biedrawa, D.; Podolak, I. Saponins as cytotoxic agents: An update (2010-2018). Part I-steroidal saponins. Phytochem Rev. 2020, 19, 139–189. [Google Scholar] [CrossRef]
- Lv, L.; Zheng, L.; Dong, D.; Xu, L.; Yin, L.; Xu, Y.; Qi, Y.; Han, X.; Peng, J. Dioscin, a natural steroid saponin, induces apoptosis and DNA damage through reactive oxygen species: A potential new drug for treatment of glioblastoma multiforme. Food Chem. Toxicol. 2013, 59, 657–669. [Google Scholar] [CrossRef]
- Cai, S.; Risinger, A.L.; Petersen, C.L.; Grkovic, T.; O’Keefe, B.R.; Mooberry, S.L.; Cichewicz, R.H. Anacolosins A-F and Corymbulosins X and Y, Clerodane Diterpenes from Anacolosa clarkii Exhibiting Cytotoxicity toward Pediatric Cancer Cell Lines. J. Nat. Prod. 2019, 82, 928–936. [Google Scholar] [CrossRef]
- Menke, K.; Schwermer, M.; Schramm, A.; Zuzak, T.J. Preclinical Evaluation of Antitumoral and Cytotoxic Properties of Viscum album Fraxini Extract on Pediatric Tumor Cells. Planta Med. 2019, 85, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Kolimar, D.; Spittler, A.; Wisgrill, L.; Herbold, C.W.; Abranko, L.; Berry, D. Conversion of Rutin, a Prevalent Dietary Flavonol, by the Human Gut Microbiota. Front. Microbiol. 2020, 11, 585428. [Google Scholar] [CrossRef]
- Weiz, G.; Breccia, J.D.; Mazzaferro, L.S. Screening and quantification of the enzymatic deglycosylation of the plant flavonoid rutin by UV-visible spectrometry. Food Chem. 2017, 229, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Berndt, K.; Campanile, C.; Muff, R.; Strehler, E.; Born, W.; Fuchs, B. Evaluation of quercetin as a potential drug in osteosarcoma treatment. Anticancer Res. 2013, 33, 1297–1306. [Google Scholar] [PubMed]
- Lan, H.; Hong, W.; Fan, P.; Qian, D.; Zhu, J.; Bai, B. Quercetin Inhibits Cell Migration and Invasion in Human Osteosarcoma. Cells. Cell. Physiol. Biochem. 2017, 43, 553–567. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yin, J.; Jia, Q.; Wang, J.; Zou, C.; Brewer, K.J.; Colombo, C.; Wang, Y.; Huang, G.; Shen, J. Quercetin induces apoptosis in the methotrexate-resistant osteosarcoma cell line U2-OS/MTX300 via mitochondrial dysfunction and dephosphorylation of Akt. Oncol. Rep. 2011, 26, 687–693. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).