Liquid for Fused Deposition Modeling Technique (L-FDM)—A Revolution in Application Chemicals to 3D Printing Technology: Color and Elements
Abstract
Featured Application
Abstract
1. Introduction
1.1. State-of-the-Art
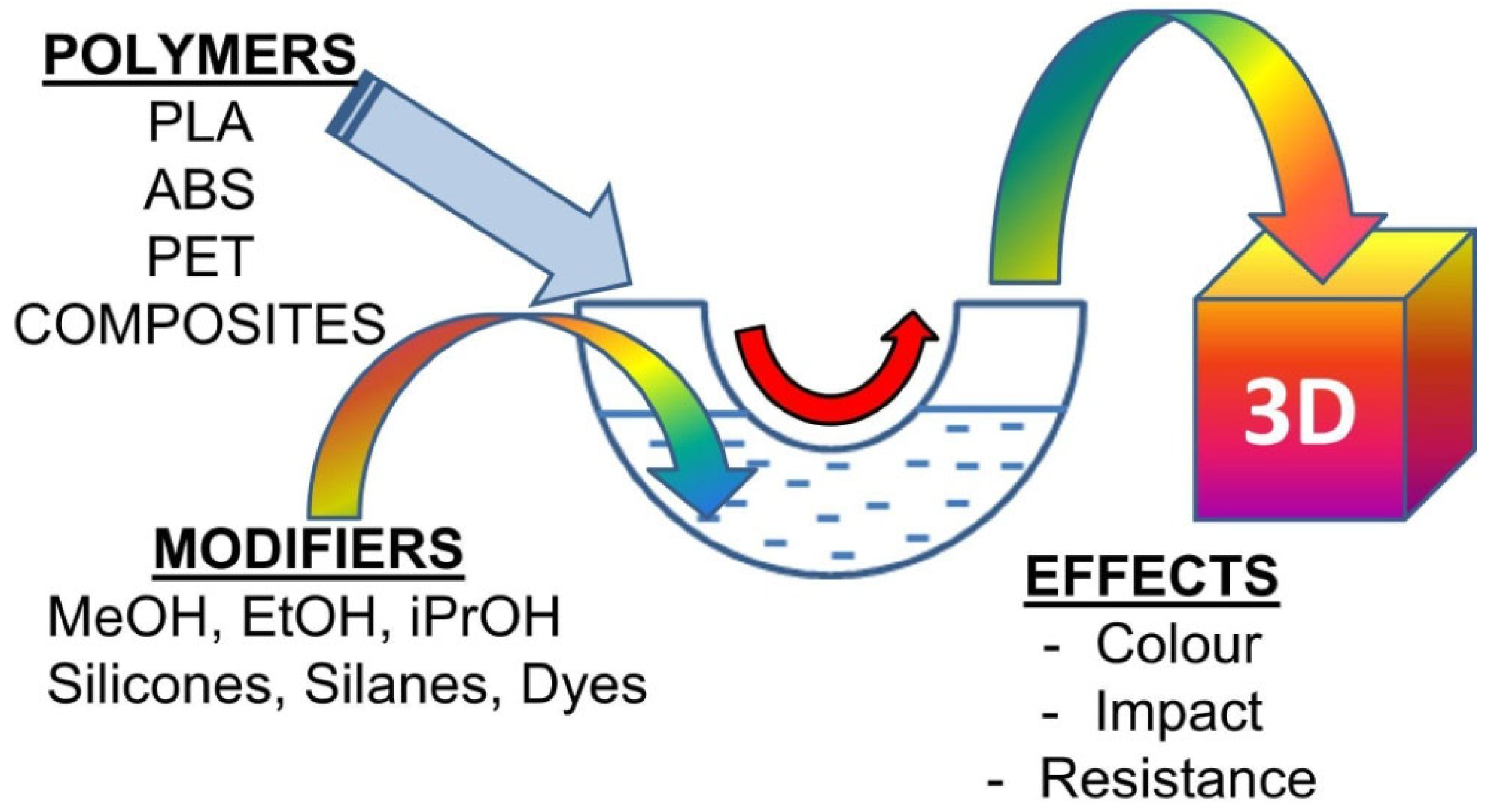
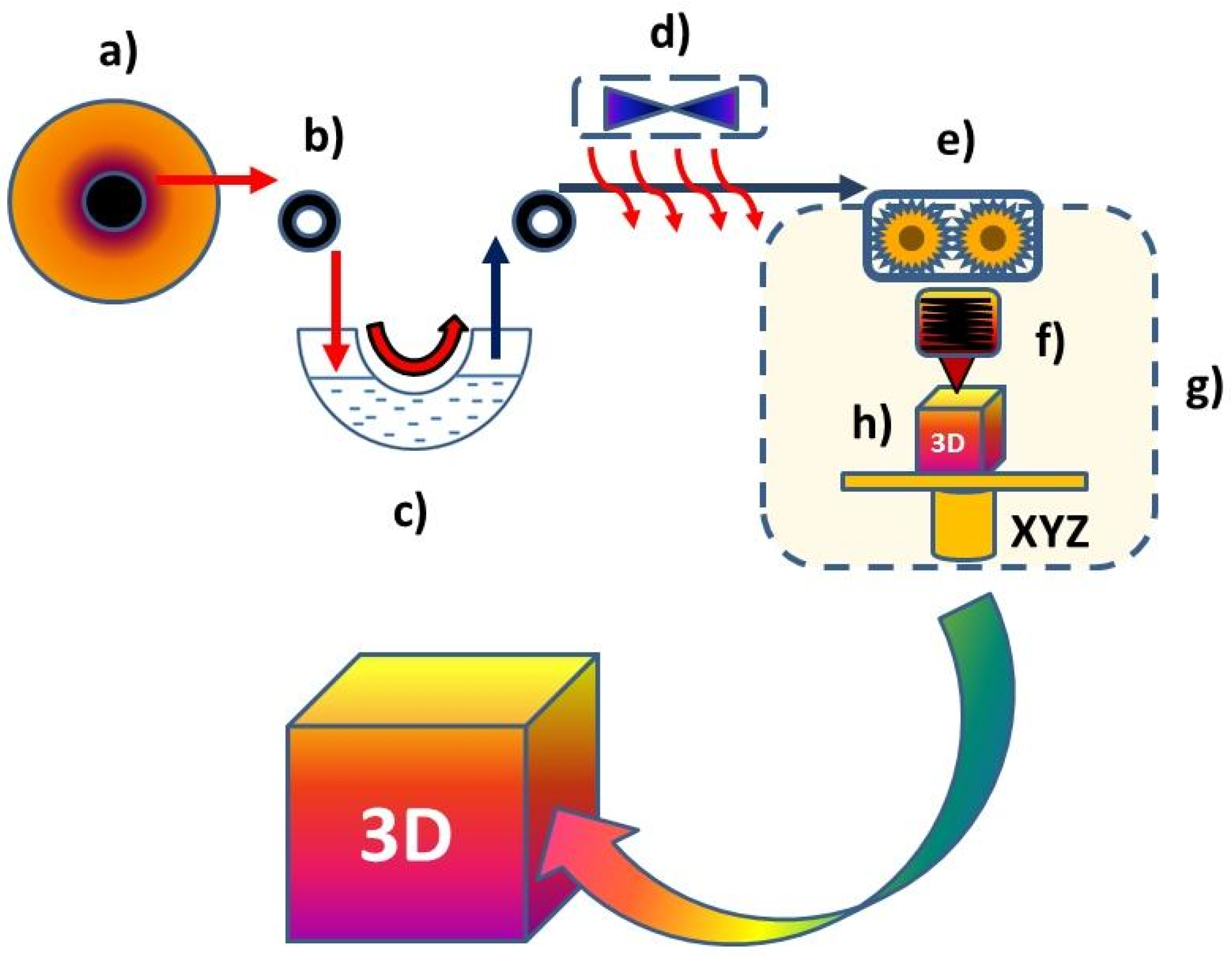
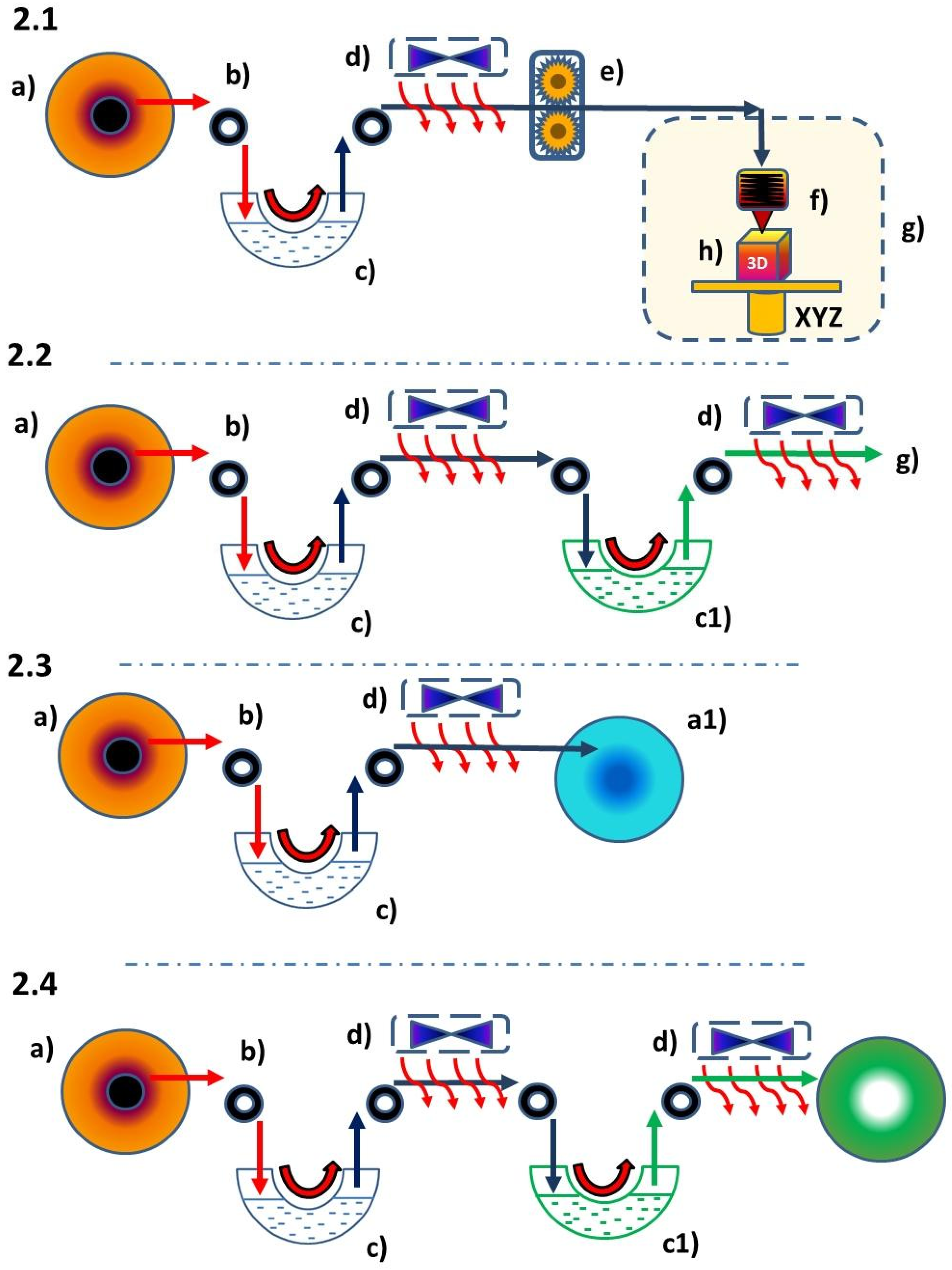
1.2. Liquid for the Fused Deposition Modeling Technique (L-FDM)
2. Materials and Methods
2.1. Materials
2.1.1. Polymers
2.1.2. Solvents and Liquid Chemicals
2.1.3. Solid Chemicals
2.1.4. Dyes and Pigments
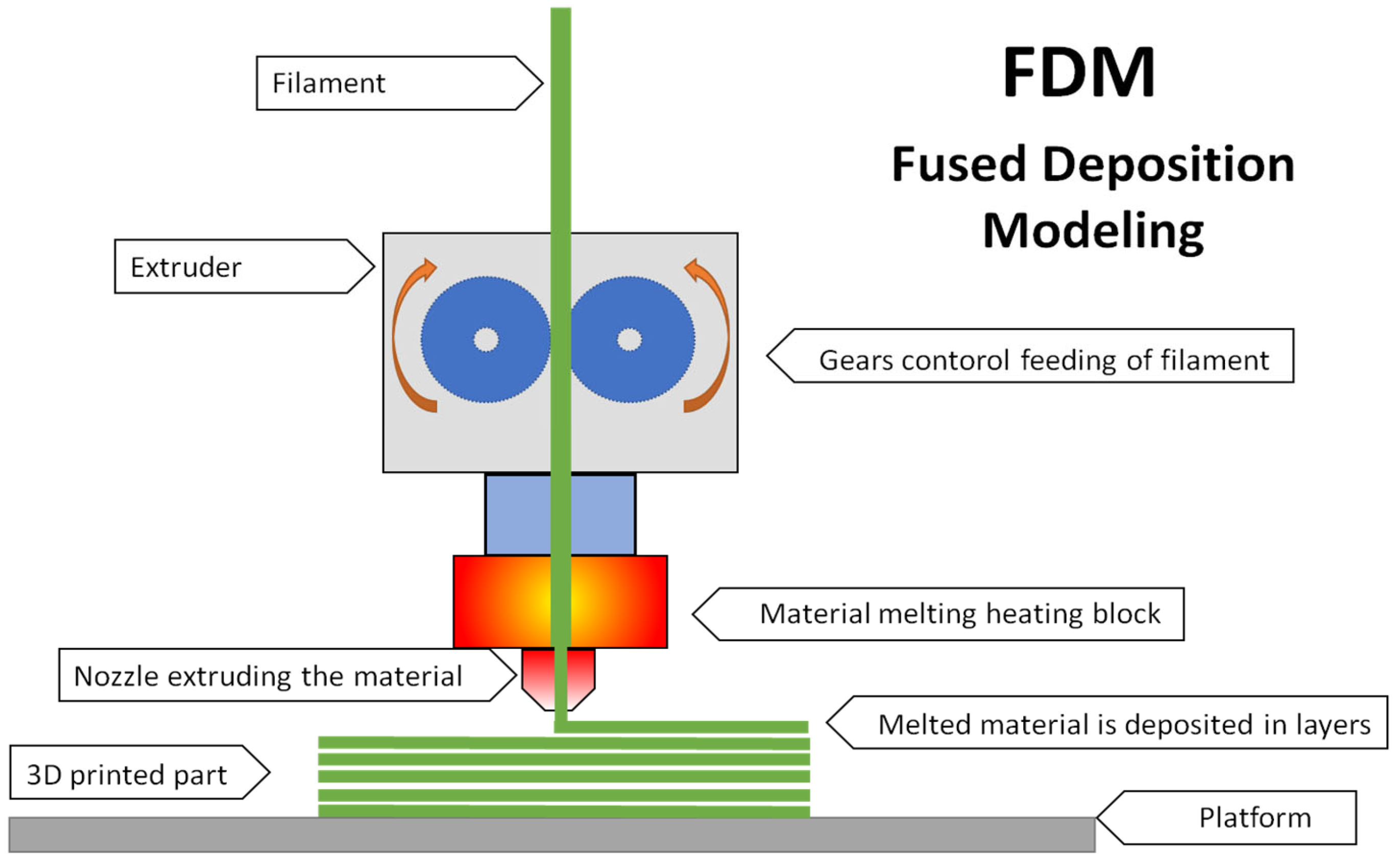
2.2. Devices and Methods
- (1)
- For printing brittle materials, as well as materials with high flexibility, i.e., PLA WOOD, WOODFILL, a TPU Original Prusa i3MK3S+ from Prusa Research a.s. was used. A printer with a direct extruder was used (Figure 5).
- (2)
- For printing samples from materials with high thermal shrinkage, i.e., ABS and ASA, a printer with a thermal chamber FlashForge Guider IIS (from FlashForge) (ABS, ASA) was used.
- (3)
- PLA and PET-G filaments were printed on Creality Ender 5 (Creality 3D) with a Bowden extruder. Table 1 presents the printing parameters.
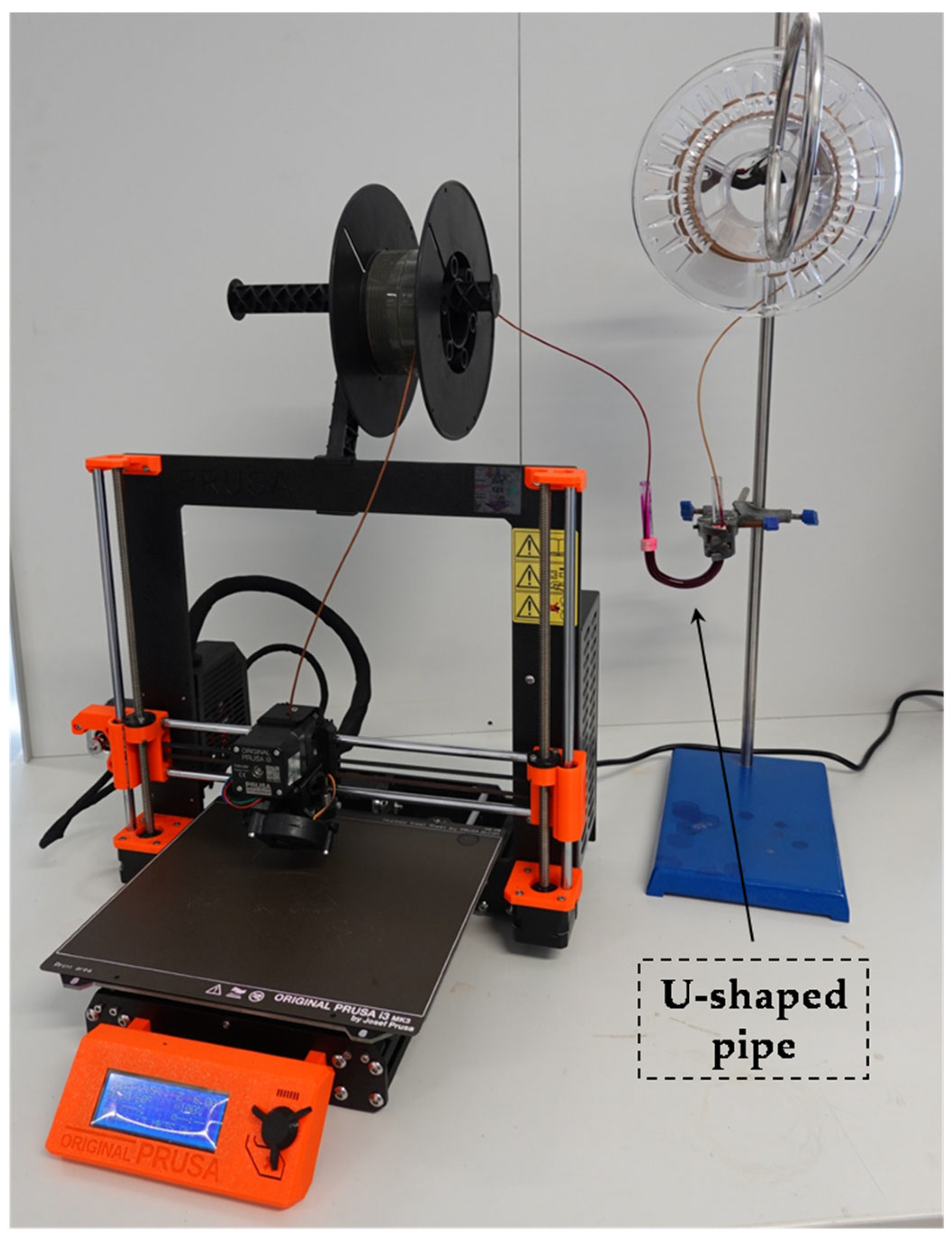
2.3. Analyses—Instrumental Methods and Measurements
3. Results and Discussion
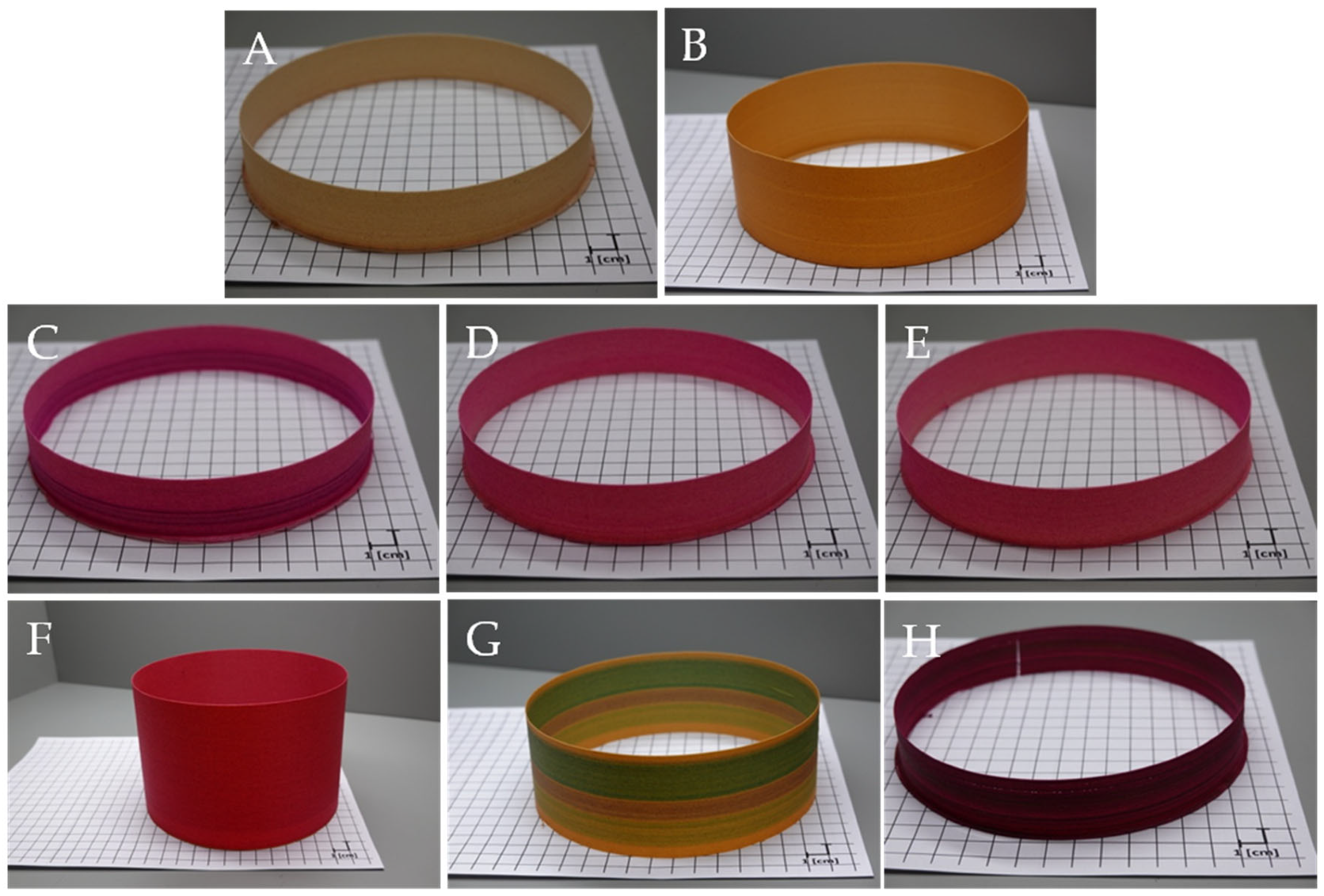
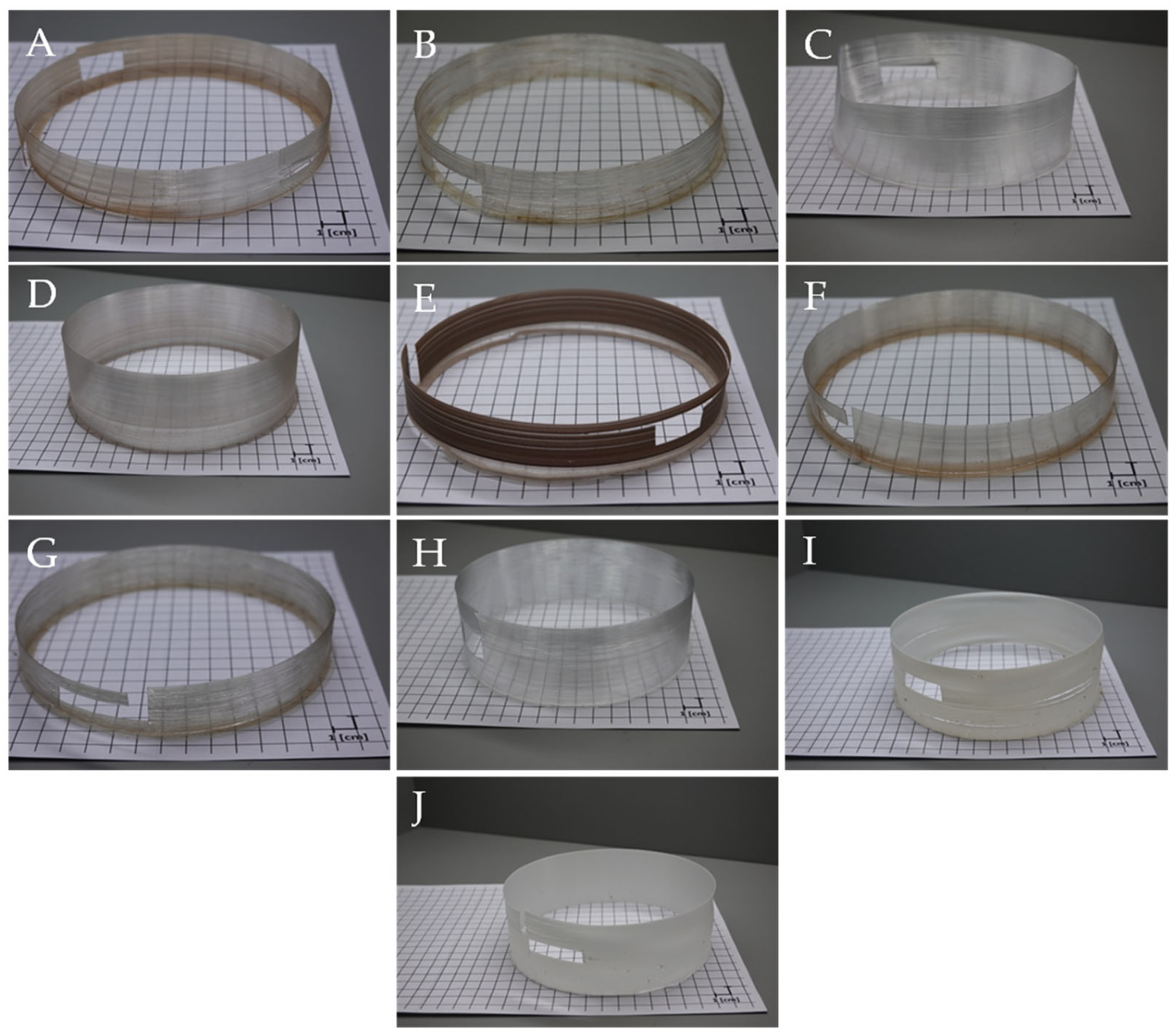
3.1. Colour
3.2. Elements
3.3. Colorimetric Analysis
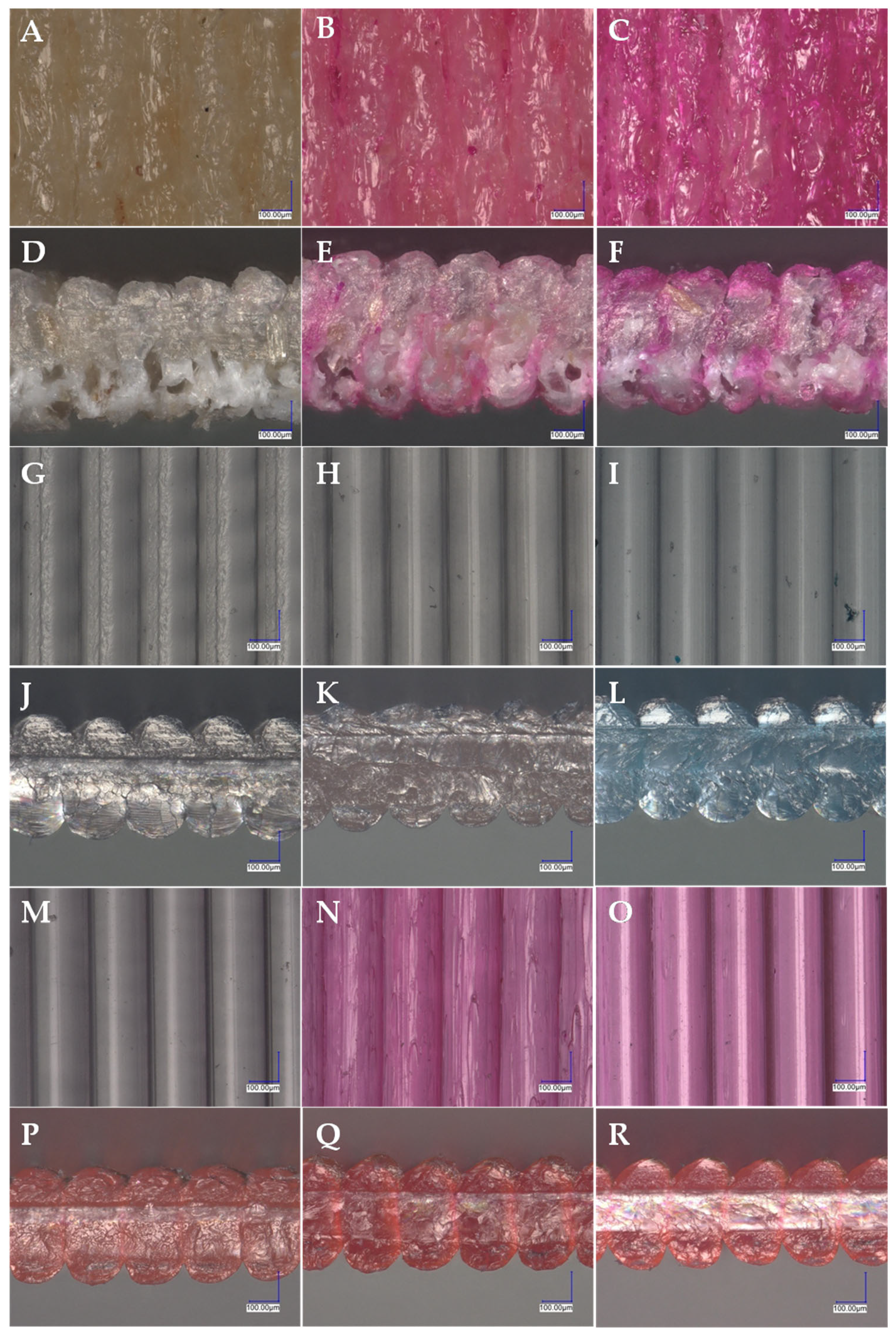
3.4. Microscopic Analysis
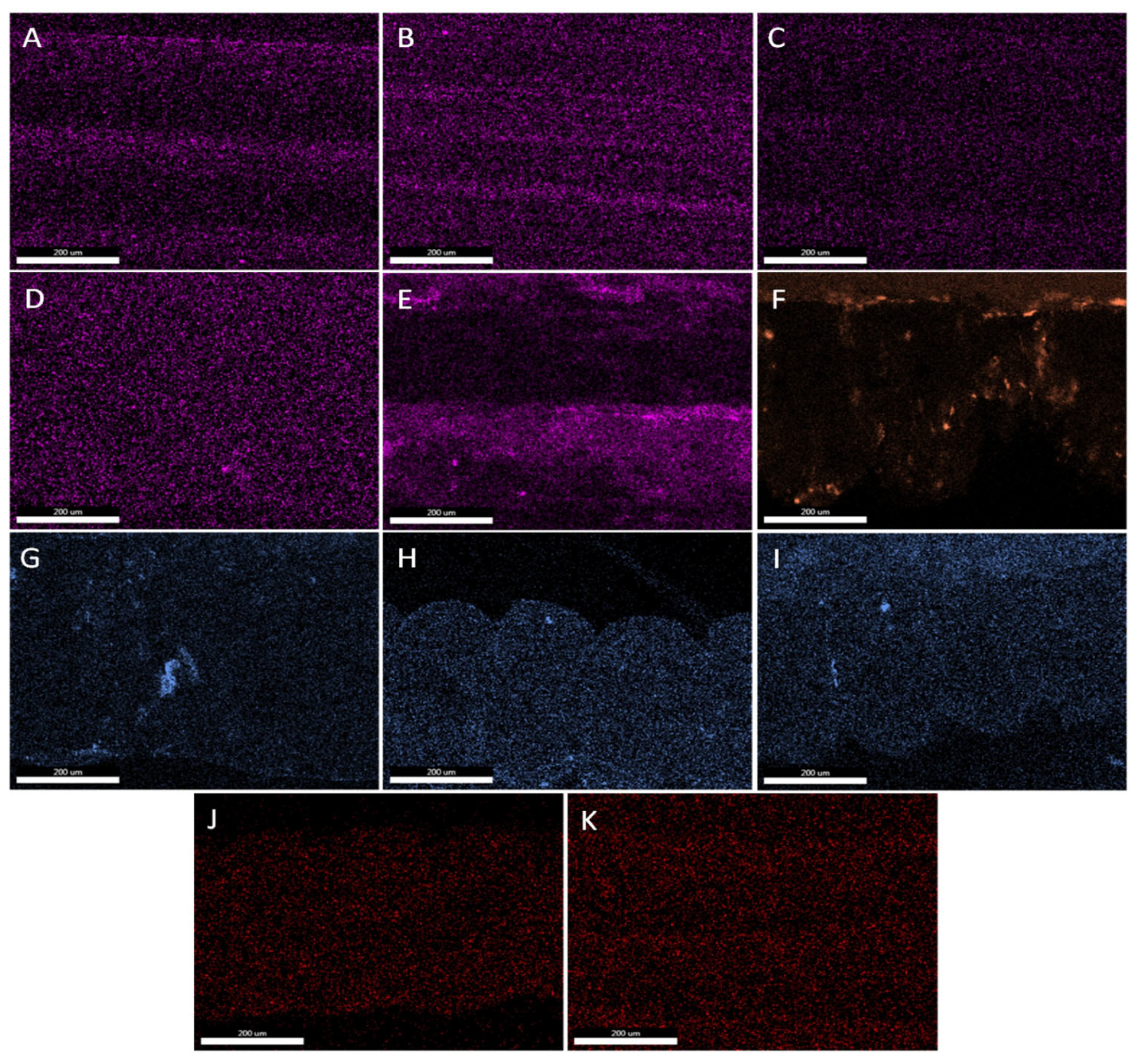
3.5. EDS Analysis—Elements Mapping
3.6. Functional Features, Limitations, and Directions of Potential Applications for the L-FDM Method
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, N.; Ming-Chuan, L. Additive Manufacturing: Technology, Applications and Research Needs. Front. Mech. Eng. 2013, 8, 215–243. [Google Scholar] [CrossRef]
- Samykano, M. Mechanical Property and Prediction Model for FDM-3D Printed Polylactic Acid (PLA). Arab. J. Sci. Eng. 2021, 46, 7875–7892. [Google Scholar] [CrossRef]
- Wang, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Stereolithographic (SLA) 3D Printing of Oral Modified-Release Dosage Forms. Int. J. Pharm. 2016, 503, 207–212. [Google Scholar] [CrossRef]
- Peng, C.; Tran, P. Bioinspired Functionally Graded Gyroid Sandwich Panel Subjected to Impulsive Loadings. Compos. Part B Eng. 2020, 188, 107773. [Google Scholar] [CrossRef]
- Ramya, A.; Vanapalli, S. 3D Printing Technologies in Various Applications. IJMET 2016, 7, 396–409. [Google Scholar]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An Overview on 3D Printing Technology: Technological, Materials, and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Tran, T.Q.; Ng, F.L.; Kai, J.T.Y.; Feih, S.; Nai, M.L.S. Tensile Strength Enhancement of Fused Filament Fabrication Printed Parts: A Review of Process Improvement Approaches and Respective Impact. Addit. Manuf. 2022, 54, 102724. [Google Scholar] [CrossRef]
- Saggiomo, V. A 3D Printer in the Lab: Not Only a Toy. Adv. Sci. 2022, 9, 2202610. [Google Scholar] [CrossRef]
- Salentijn, G.I.; Oomen, P.E.; Grajewski, M.; Verpoorte, E. Fused Deposition Modeling 3D Printing for (Bio)analytical Device Fabrication: Procedures, Materials, and Applications. Anal. Chem. 2017, 89, 7053–7061. [Google Scholar] [CrossRef]
- Capel, A.J.; Rimington, R.P.; Lewis, M.P.; Christie, S.D.R. 3D Printing for Chemical, Pharmaceutical and Biological Applications. Nat. Rev. Chem. 2018, 2, 422–436. [Google Scholar] [CrossRef]
- Mani, M.P.; Sadia, M.; Jaganathan, S.K.; Khudzari, A.Z.; Supriyanto, E.; Saidin, S.; Ramakrishna, S.; Ismail, A.F.; Faudzi, A.A.M. A Review on 3D Printing in Tissue Engineering Applications. J. Polym. Eng. 2022, 42, 243–265. [Google Scholar] [CrossRef]
- Waheed, S.; Cabot, J.M.; Macdonald, N.P.; Lewis, T.; Guijt, R.M.; Paull, B.; Breadmore, M.C. 3D Printed Microfluidic Devices: Enablers and Barriers. Lab Chip 2016, 16, 1993–2013. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Bubliauskas, A.; Kitson, P.J.; Francoia, J.-P.; Powell-Davies, H.; Gutierrez, J.M.P.; Frei, P.; Manzano, J.S.; Cronin, L. Automatic Generation of 3D-Printed Reactionware for Chemical Synthesis Digitization using ChemSCAD. ACS Central Sci. 2021, 7, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Sebai, A.; Łątka, M.; Gadomska-Gajadhur, A. Polilaktyd w Systemach Leków o Kontrolowanym Czasie Uwalniania i Inżynierii Tkankowej; Oficyna Wydawnicza Politechniki Wrocławskiej: Rzeszów, Poland, 2016; pp. 210–213. [Google Scholar]
- Hock, S.; Rein, C.; Rose, M. 3D-Printed Acidic Monolithic Catalysts for Liquid-Phase Catalysis with Enhanced Mass Transfer Properties. Chemcatchem 2022, 14, 202101947. [Google Scholar] [CrossRef]
- Henczykowski, M.; Oleksy, M. Technologia Przetwórstwa Tworzyw Sztucznych; Oficyna Wydawnicza Politechnika Rzeszowska: Rzeszów, Poland, 1999. [Google Scholar]
- Dizon, J.R.C.; Espera, A.H., Jr.; Chen, Q.; Advincula, R.C. Mechanical Characterization of 3D-Printed Polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, S.G.; Kim, H.J.; Ahn, S.H. Measurement of Anisotropic Compressive Strength of Rapid Prototyping Parts. J. Mater. Process. Technol. 2007, 187, 627–630. [Google Scholar] [CrossRef]
- Ryan, K.J.; Lupton, K.E.; Pape, P.G.; John, V.B. Ultra-high-molecular-weight functional siloxane additives in polymers. Effects on processing and properties. J. Vinyl Addit. Technol. 2020, 6, 7–19. [Google Scholar] [CrossRef]
- Abdilla, A.; D’Ambra, C.A.; Geng, Z.; Shin, J.J.; Czuczola, M.; Goldfred, D.J.; Biswas, S.; Mecca, J.M.; Sweir, S.; Bekemeier, T.D.; et al. Silicone Based Polymers Blends: Enhancing Properties Through Compatibilization. Polym. Sci. 2021, 59, 2114. [Google Scholar] [CrossRef]
- Ly, B.C.K.; Dyer, E.B.; Feig, J.L.; Chien, A.L.; Del Bino, S. Research Techniques Made Simple: Cutaneous Colorimetry: A Reliable Technique for Objective Skin Color Measurement. J. Investig. Dermatol. 2020, 140, 3–12.e1. [Google Scholar] [CrossRef]
- Luo, M.R. Encyclopedia of Color Science and Technology; Springer: New York, NY, USA, 2015; pp. 1–7. [Google Scholar]
- Prasad, L.K.; Smyth, H. 3D Printing Technologies for Drug Delivery: A review. Drug Dev. Ind. Pharm. 2015, 42, 1019–1031. [Google Scholar] [CrossRef]
- Jain, V.; Haider, N.; Jain, K. 3D Printing in Personalized Drug Delivery. Curr. Pharm. Des. 2019, 24, 5062–5071. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Almalki, W.H.; Malik, A.; Farhan, M.; Aatif, M.; Rahman, Z.; Alruwaili, N.K.; Alrobaian, M.; Tarique, M.; Rahman, M. 3D Printing for Drug Delivery and Biomedical Applications. Drug Discov. Today 2020, 25, 1668–1681. [Google Scholar] [CrossRef] [PubMed]
- Gear, J.I.; Cummings, C.; Sullivan, J.; Cooper-Rayner, N.; Downs, P.; Murray, I.; Flux, G.D. Radioactive 3D Printing for the Production of Molecular Imaging Phantoms. Phys. Med. Biol. 2020, 65, 175019. [Google Scholar] [CrossRef] [PubMed]
- Tran-Gia, J.; Schlögl, S.; Lassmann, M. Design and Fabrication of Kidney Phantoms for Internal Radiation Dosimetry Using 3D Printing Technology. J. Nucl. Med. 2016, 57, 1998–2005. [Google Scholar] [CrossRef]
- Gear, J.I.; Cummings, C.; Craig, A.J.; Divoli, A.; Long, C.D.C.; Tapner, M.; Flux, G.D. Abdo-Man: A 3D-Printed Anthropomorphic Phantom for Validating Quantitative SIRT. EJNMMI Phys. 2016, 3, 17. [Google Scholar] [CrossRef]
- Jiao, Y.; Stevic, M.; Buanz, A.; Uddin, M.J.; Tamburic, S. Current and Prospective Applications of 3D Printing in Cosmetics: A Literature Review. Cosmetics 2022, 9, 115. [Google Scholar] [CrossRef]
- Islam, H.; Poly, T.S.; Tisha, Z.T.; Rahman, S.; Naveed, A.I.J.; Ahmed, A.; Ahmed, S.N.; Hassan, J.; Uddin, M.J.; Das, D.B. 3D Printed Hollow Microneedles for Treating Skin Wrinkles Using Different Anti-Wrinkle Agents: A Possible Futuristic Approach. Cosmetics 2023, 10, 41. [Google Scholar] [CrossRef]
- De Oliveira, R.S.; Fantaus, S.S.; Guillot, A.J.; Melero, A.; Beck, R.C.R. 3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery. Pharmaceutics 2021, 13, 1946. [Google Scholar] [CrossRef]
- Mohammed, J.S. Applications of 3D Printing Technologies in Oceanography. Methods Oceanogr. 2016, 17, 97–117. [Google Scholar] [CrossRef]
- Parra-Cabrera, C.; Achille, C.; Ameloot, C. 3D Printing in Chemical Engineering and Catalytic Technology: Structured Catalysts, Mixers and Reactors. Chem. Soc. Rev. 2018, 47, 209–230. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer Engineering Based on Reversible Covalent Chemistry: A Promising Innovative Pathway Towards New Materials and New Functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Brząkalski, D.; Sztorch, B.; Frydrych, M.; Pakuła, D.; Dydek, K.; Kozera, R.; Boczkowska, A.; Marciniec, B.; Przekop, R.E. Limonene Derivative of Spherosilicate as a Polylactide Modifier for Applications in 3D Printing Technology. Molecules 2020, 25, 5882. [Google Scholar] [CrossRef] [PubMed]
- Konta, A.A.; García-Piña, M.; Serrano, D.R. Personalised 3D Printed Medicines: Which Techniques and Polymers Are More Successful? Bioengineering 2017, 4, 79. [Google Scholar] [CrossRef]
- Kalita, S. Rapid Prototyping in Biomedical Engineering: Structural Intricacies of Biological Materials. Biointegration of Medical Implant Materials; Woodhead Publishing: Cambridge, UK, 2010; pp. 349–397. [Google Scholar] [CrossRef]
- Vittal, L.V.M.; Rookes, J.; Ben Boyd, B.; Cahill, D. Analysis of Plant Cuticles and Their Interactions with Agrochemical Surfactants Using a 3D Printed Diffusion Chamber. Plant Methods 2023, 19, 37. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Singh, S.K.; Bajpai, J.; Bajpai, A.K. Controlled pesticide release from biodegradable polymers. Open Chem. 2014, 12, 453–469. [Google Scholar] [CrossRef]
- Majeed, Z.; Ramli, N.K.; Mansor, N.; Man, Z. A comprehensive review on biodegradable polymers and their blends used in controlled-release fertilizer processes. Rev. Chem. Eng. 2015, 31, 69–95. [Google Scholar] [CrossRef]
- Podstawczyk, D.; Skrzypczak, D.; Połomska, X.; Stargała, A.; Witek-Krowiak, A.; Guiseppi-Elie, A.; Galewski, Z. Preparation of Antimicrobial 3D Printing Filament: In Situ Thermal Formation of Silver Nanoparticles During the Material Extrusion. Polym. Compos. 2020, 41, 4692–4705. [Google Scholar] [CrossRef]
- Xu, W.; Jambhulkar, S.; Zhu, Y.; Ravichandran, D.; Kakarla, M.; Vernon, B.; Lott, D.G.; Cornella, J.L.; Shefi, O.; Miquelard-Garnier, G.; et al. 3D Printing for Polymer/Particle-Based Processing: A Review. Compos. Part B Eng. 2021, 223, 109102. [Google Scholar] [CrossRef]





| 3D Printer Model | Original Prusa mi3 MK3S+ (by Prusa Research a.s.) | Creality Ender 5 (by Creality 3D) | Flash Froge Guider IIS (by FlashForge) |
|---|---|---|---|
| used filament | PLA WOOD, WOODFILL | PET-G, PLA | ABS+, ASA |
| Slicer | PrusaSlicer-2.3.1 | Creality 1.2.3. | FlashPrint |
| layer height (mm) | 0.2 | 0.2 | 0.2 |
| first layer speed/printing speed (mm/s) | 20/50 | 20/50, vertical strength tests samples printed with lower speed—30 | 10/40 |
| print/bed temp. (°C) | 220/60 | 220/60 for PLA and 230/70 for PET-G | 230/105 for PLA and 230/70 for PET-G |
| number of shells | 1 (cylinders were printed with vase mode) | 2 (cylinders were printed using vase mode) | 1 (cylinders were printed using vase mode) |
| infill (%) | 0 | 100 | 0 |
| infill angle (°) | 0 | 45 | - |
| fan speed (%) | 100 | 100 | 0 |
| Marking of the Sample | Full Sample Name/Filament and Solution Used in Printing L-FDM Samples |
|---|---|
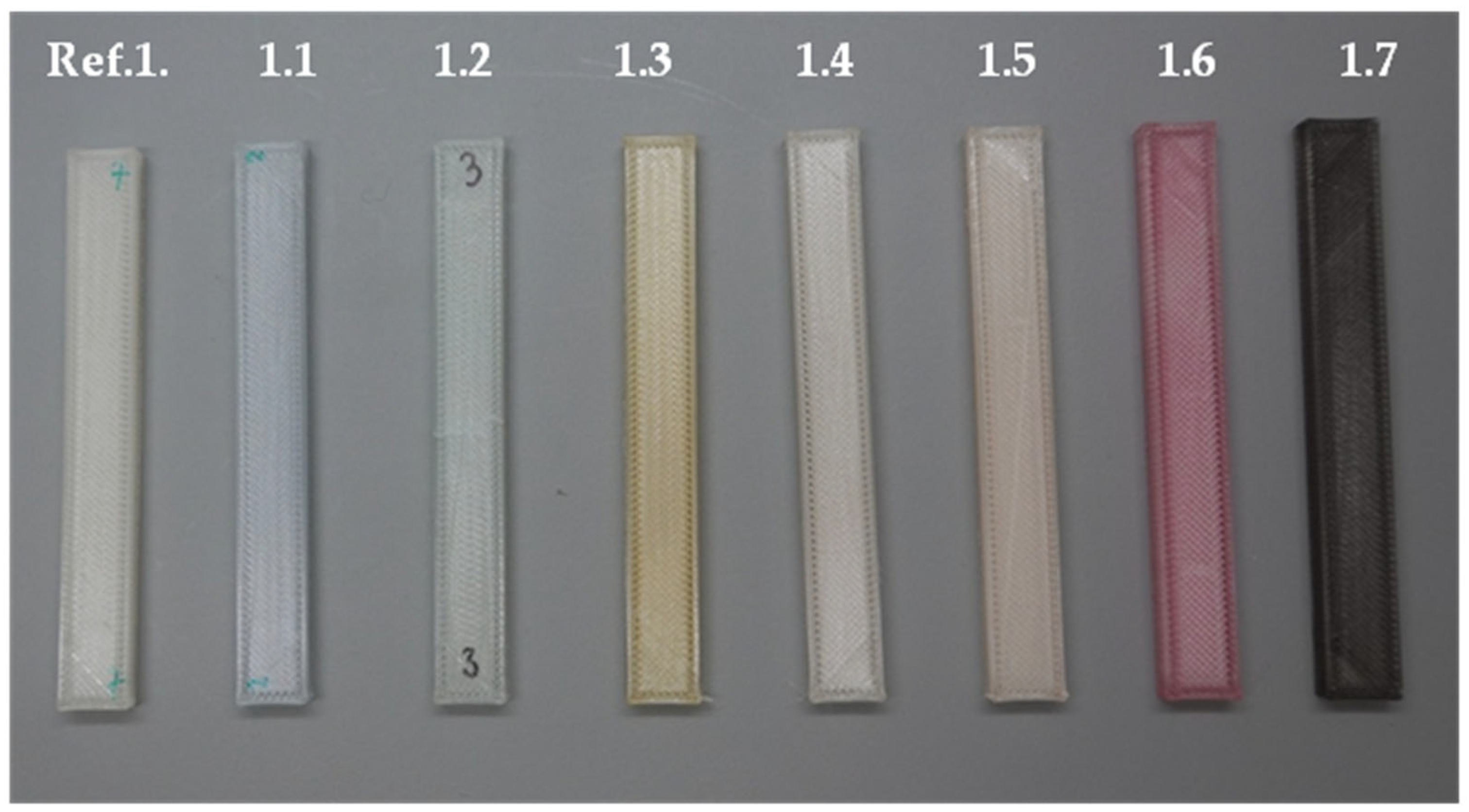
| Ref. 1 | PLA reference |
| 1.1 | PLA + alkaline blue dissolved in methanol |
| 1.2 | PLA + methylene blue dissolved in methanol |
| 1.3 | PLA + methyl orange dissolved in methanol |
| 1.4 | PLA + eosin dissolved in methanol |
| 1.5 | PLA + fluorexone dissolved in methanol |
| 1.6 | PLA + transparent red dye by Moldoo |
| 1.7 | PLA + printer ink 545 Black PIXMA 545 by CANON |
| Marking of the Sample | Full Sample Name Filament and Solution Used in Printing L-FDM Samples |
|---|---|
| Ref. 2 | PLA reference |
| 2.1 | PLA + transparent green dye by Ag-Bet |
| 2.2 | PLA rhodamine B dissolved in water and isopropanol mixed in a 1:1 ratio |
| 2.3 | PLA + transparent yellow dye by Ag-Bet |
| 2.4 | PLA + navy blue fabric dye dissolved in methanol (saturated solution) |
| 2.5 | PLA + Transparent blue turquoise resin by Ag-Bet |
| 2.6 | PLA + rhodamine B dissolved in water (proportion of 50 mg per 1 mL) |
| 2.7 | PLA + rhodamine B dissolved in water and isopropanol mixed in a 1:1 ratio (proportion of 50 mg per 1 mL of solvent) |
| 2.8 | PLA + rhodamine B dissolved in water, isopropanol, and glycerine mixed in a 1:1:1 ratio (proportion of 50 mg per 1 mL of solvent) |
| 2.9 | PLA + rhodamine B dissolved in water (saturated solution) |
| 2.10 | PLA + rhodamine B dissolved in water and isopropanol mixed in a 1:1 ratio (saturated solution) |
| 2.11 | PLA + rhodamine B dissolved in water, isopropanol, and glycerine mixed in a 1:1:1 ratio (saturated solution) |
| Marking of the Sample | Full Sample Name |
|---|---|
| Ref. 3 | PET-G reference |
| 3.1 | PET-G + rhodamine B dissolved in water and isopropanol mixed in a 1:1 ratio (saturated solution) |
| 3.2 | PET-G + rhodamine B dissolved in water, isopropanol, and glycerine mixed in a 1:1:1 ratio (saturated solution) |
| 3.3 | PET-G + rhodamine B dissolved in water (proportion of 50 mg per 1 mL) |
| 3.4 | PET-G + rhodamine B dissolved in water and isopropanol mixed in a 1:1 ratio (proportion of 50 mg per 1 mL of solvent) |
| 3.5 | PET-G + rhodamine B dissolved in water, isopropanol, and glycerine mixed in a 1:1:1 ratio (proportion of 50 mg per 1 mL of solvent) |
| 3.6 | PET-G + Transparent yellow dye by Ag-Bet |
| 3.7 | PET-G + navy blue fabric dye dissolved in methanol (saturated solution) |
| 3.8 | PET-G + Transparent blue turquoise dye by Ag-Bet |
| Ref. 4 | ABS reference |
| 4.1 | ABS + Transparent green dye by Ag-Bet |
| 4.2 | ABS + rhodamine B dissolved in water, isopropanol, and glycerine mixed in a 1:1:1 ratio (saturated solution) |
| Marking of the Sample | Full Sample Name |
|---|---|
| Ref. 5 | WOODFILL reference |
| 5.1 | WOODFILL + rhodamine B dissolved in water (proportion of 50 mg per 1 mL) |
| 5.2 | WOODFILL + rhodamine B dissolved in water and isopropanol mixed in a 1:1 ratio (proportion of 50 mg per 1 mL of solvent) |
| 5.3 | WOODFILL + rhodamine B dissolved in water, isopropanol, and glycerine mixed in a 1:1:1 ratio (proportion of 50 mg per 1 mL of solvent) |
| Ref. 6 | PLA WOOD reference |
| 6.1 | PLA WOOD + rhodamine B dissolved in methanol (unsaturated solution) |
| 6.2 | PLA WOOD + Transparent dye by Ag-Bet in order starting from the bottom: green, purple, and blue turquoise. |
| 6.3 | PLA WOOD + rhodamine B dissolved in water and dissolved in water and isopropanol mixed in a 1:1 ratio |
| Marking of the Sample | Full Sample Name |
|---|---|
| 7.1 | PLA + Silver nitrate dissolved in water, isopropanol, and glycerine mixed in a 1:1:1 ratio (saturated solution) |
| 7.2 | PLA + Iron (III) chloride dissolved in water, isopropanol, and glycerine mixed in a 1:1:1 ratio (saturated solution) |
| 8.1 | PET-G + rubidium sulfate dissolved in water and methanol mixed in a 1:9 ratio (saturated solution) |
| 8.2 | PET-G + Silver nitrate dissolved in water (saturated solution) |
| 8.3 | PET-G + Silver nitrate dissolved in water and isopropanol mixed in a 1:1 ratio (saturated solution) |
| 8.4 | PET-G + Silver nitrate dissolved in water, isopropanol, and glycerine mixed in a 1:1:1 ratio (saturated solution) |
| 8.5 | PET-G + Iron (III) chloride dissolved in water, isopropanol, and glycerine mixed in a 1:1:1 ratio (saturated solution) |
| 8.6 | PET-G + lead acetate dissolved in methanol (saturated solution) |
| 9.1 | ASA + lead acetate dissolved in methanol (saturated solution) |
| 9.2 | ABS + lead acetate dissolved in methanol (saturated solution) |
| Marking of the Sample | Full Sample Name |
|---|---|
| Ref. 6 | PLA WOOD reference |
| 6.4 | PLA WOOD + silver nitrate dissolved in water and isopropanol mixed in a 1:1 ratio (saturated solution) |
| 6.5 | PLA WOOD + iron (III) chloride dissolved in water, isopropanol, and glycerine mixed in a 1:1:1 ratio (saturated solution) |
| ΔE Value | Meaning |
|---|---|
| 0 < ΔE < 1 | A normally invisible difference |
| 1 < ΔE < 2 | Very small difference, only obvious to a trained eye |
| 2 < ΔE < 3.5 | Medium difference, also obvious to an untrained eye |
| 3.5 < ΔE < 5 | An obvious difference |
| ΔE > 5 | A very obvious difference |
| Field of Application | Potential Application | Refs. |
|---|---|---|
| pharmaceutical | drugs, customized drugs, and therapy | [23,24,25] |
| isotope technologies | introducing radioactive isotopes into 3D prints | [26] |
| nuclear medicine | manufacturing individualized anthropomorphic phantoms in many clinical applications and radiopharmaceuticals | [27,28] |
| personal care | skin delivery platforms | [29,30,31] |
| oceanography | coral restoration; slow-release materials, maintaining an alkaline pH level | [32] |
| chemical engineering | chemical reactors, the introduction of some elements of catalyst (e.g., Pt and Rh) | [33] |
| material engineering | imparting new properties to polymer materials and testing new modifiers | [34] |
| chemistry | testing of newly obtained chemical compounds directly in the laboratory after synthesis | [35] |
| bioengineering | implants, tissue engineering, and regenerative medicine | [36,37] |
| agrochemistry | controlled release of micronutrients and pesticides | [38,39,40] |
| nanotechnology | formation nanoparticles from chemical precursors in thermal decomposition directly during 3D printing | [41] |
| polymerization of materials | polymerization of monomers during printing, e.g., methacrylates | [42] |
| Feature | Limitations | Solution |
|---|---|---|
| modifier content in the final product measurable value (% w/w) | limited absorption |
|
| precise dosing of the ingredient measurable value (% w/w) | difficulty with precise determination of the amount of the modifier introduced |
|
| compatibility of the polymer with the modifier (low solubility, inertness, lack of reactivity) of the solvent with the printing material used, value measure (filament solubility < 0.1 g/L solution) | the solubility of the filament material in the solvent used |
|
| print uniformity measurable value (% w/w modifier) | wetting of the filament with the solution, uneven coverage of the filament surface with the solution |
|
| viscosity of the dispersion medium (solvent or modifier) measure (viscosity > 100 cSt) | difficult transfer from the cartridge to the filament | use of diluter |
| mechanical strength of the filament (impact strength > 1 (J/m2)) | brittleness of the filament after passing through the reservoir, increase in stiffness |
|
| process problems | clogging of the extruder system and uneven extrusion |
|
| incompatibility of the system with the feeding system | printer specifications | changing the design of the feeding system |
| no miscibility of the modifier with the polymer matrix | no homogeneous dispersion of the modifier within a single layer |
|
| Interaction time (filament feed rate) between the modifier and the warp in a ‘U tube’ | the duration of interaction can influence the physicochemical properties of composites | reduction/increase of the printing speed |
| duration of the printing process | amount of solution in the container and concentration of the chemical substance in the solvent |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przekop, R.E.; Gabriel, E.; Pakuła, D.; Sztorch, B. Liquid for Fused Deposition Modeling Technique (L-FDM)—A Revolution in Application Chemicals to 3D Printing Technology: Color and Elements. Appl. Sci. 2023, 13, 7393. https://doi.org/10.3390/app13137393
Przekop RE, Gabriel E, Pakuła D, Sztorch B. Liquid for Fused Deposition Modeling Technique (L-FDM)—A Revolution in Application Chemicals to 3D Printing Technology: Color and Elements. Applied Sciences. 2023; 13(13):7393. https://doi.org/10.3390/app13137393
Chicago/Turabian StylePrzekop, Robert E., Ewa Gabriel, Daria Pakuła, and Bogna Sztorch. 2023. "Liquid for Fused Deposition Modeling Technique (L-FDM)—A Revolution in Application Chemicals to 3D Printing Technology: Color and Elements" Applied Sciences 13, no. 13: 7393. https://doi.org/10.3390/app13137393
APA StylePrzekop, R. E., Gabriel, E., Pakuła, D., & Sztorch, B. (2023). Liquid for Fused Deposition Modeling Technique (L-FDM)—A Revolution in Application Chemicals to 3D Printing Technology: Color and Elements. Applied Sciences, 13(13), 7393. https://doi.org/10.3390/app13137393







