Ameliorative Effect of Omega-3-Rich Fish Diet on the Neurotoxic Effects of Propionic Acid in a Rodent Model of Autism
Abstract
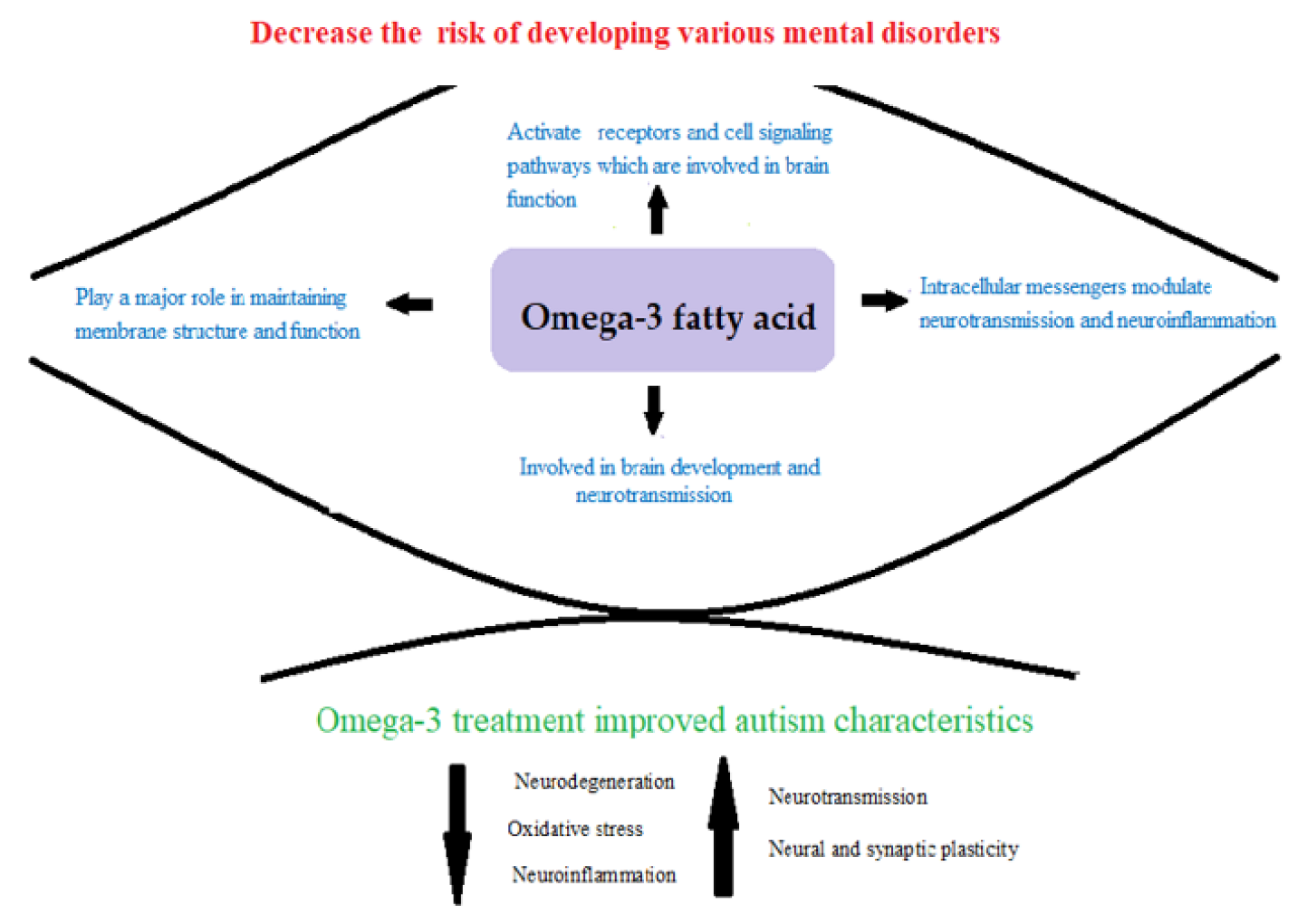
:1. Introduction
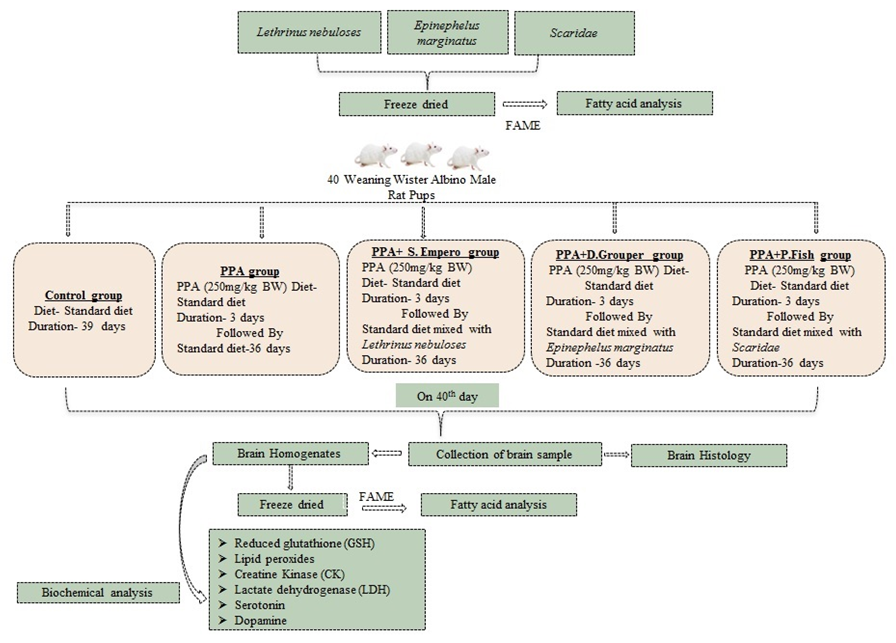
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fish Samples and Its Fatty Acid Composition
2.3. Experimental Design
2.3.1. Animals
2.3.2. Biochemical Analysis
Reduced Glutathione (GSH)
Assay of Lipid Peroxides
Assay of Creatine Kinase (CK)
Assay of Lactate Dehydrogenase (LDH)
Assay of Dopamine
Assay of Serotonin
2.3.3. Fatty Acid Profile of Brain Tissue
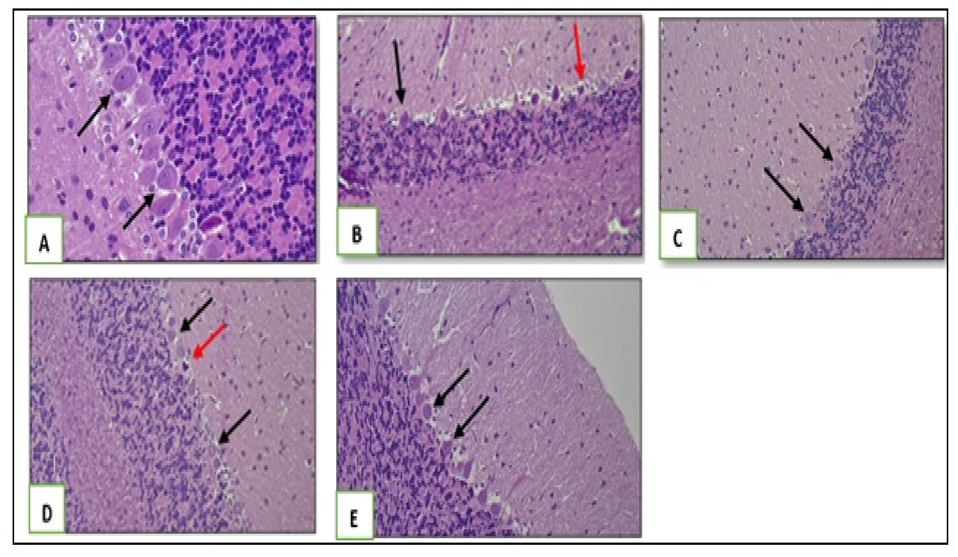
2.3.4. Liver Histology
2.3.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroncke, A.P.; Willard, M.; Huckabee, H. Assessment of Autism Spectrum Disorder: Critical Issues in Clinical, Forensic and School Settings, 1st ed.; Springe: Berlin/Heidelberg, Germany, 2016; 546p. [Google Scholar]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Rylaarsdam, L.; Guemez-Gamboa, A. Genetic Causes and Modifiers of Autism Spectrum Disorder. Front. Cell. Neurosci. 2019, 13, 385. [Google Scholar] [CrossRef] [PubMed]
- Masini, E.; Loi, E.; Vega-Benedetti, A.F.; Carta, M.; Doneddu, G.; Fadda, R.; Zavattari, P. An Overview of the Main Genetic, Epigenetic and Environmental Factors Involved in Autism Spectrum Disorder Focusing on Synaptic Activity. Int. J. Mol. Sci. 2020, 21, 8290. [Google Scholar] [CrossRef]
- Almandil, N.B.; Alkuroud, D.N.; AbdulAzeez, S.; AlSulaiman, A.; Elaissari, A.; Borgio, J.F. Environmental and genetic factors in autism spectrum disorders: Special emphasis on data from Arabian studies. Int. J. Environ. Res. Public Health 2019, 16, 658. [Google Scholar] [CrossRef] [Green Version]
- Hughes, H.K.; Rowland, M.E.; Onore, C.E.; Rogers, S.; Ciernia, A.V.; Ashwood, P. Dysregulated gene expression associated with inflammatory and translation pathways in activated monocytes from children with autism spectrum disorder. Transl. Psychiatry 2022, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A.K. Biomarker-Guided Nutritional Intervention to Treat Autism. Nov. Tech. Nutri. Food Sci. 2017, 1, NTNF.000501. [Google Scholar] [CrossRef]
- Ratnayake, W.M.; Galli, C. Fat and fatty acid terminology, methods of analysis and fat digestion and metabolism: A background review paper. Ann. Nutr. Metab. 2009, 55, 8–43. [Google Scholar] [CrossRef]
- Pawlosky, R.J.; Hibbeln, J.R.; Novotny, J.A.; Salem, N., Jr. Physiological compartmental analysis of alpha-linolenic acid metabolism in adult humans. J. Lipid Res. 2001, 42, 1257–1265. [Google Scholar] [CrossRef]
- Ahmed, A.A.; Balogun, K.A.; Bykova, N.V.; Cheema, S.K. Novel regulatory roles of omega-3 fatty acids in metabolic pathways: A proteomics approach. Nutr. Metab. 2014, 11, 6. [Google Scholar] [CrossRef] [Green Version]
- De la Torre-Aguilar, M.J.; Gomez-Fernandez, A.; Flores-Rojas, K.; Martin-Borreguero, P.; Mesa, M.D.; Perez-Navero, J.L.; Olivares, M.; Gil, A.; Gil-Campos, M. Docosahexaenoic and Eicosapentaenoic intervention modifies plasma and erythrocyte omega-3 fatty acid profiles but not the clinical course of children with autism spectrum disorder: A randomized control trial. Front. Nutr. 2022, 9, 790250. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Satizabal, C.L.; Tintle, N.; Melo van Lent, D.; Vasan, R.S.; Beiser, A.S.; Seshadri, S.; Harris, W.S. Red Blood cell dha is inversely associated with risk of incident alzheimer’s disease and all-cause dementia: Framingham offspring study. Nutrients 2022, 14, 2408. [Google Scholar] [CrossRef] [PubMed]
- Amminger, G.P.; Berger, G.E.; Schafer, M.R.; Klier, C.; Friedrich, M.H.; Feucht, M. Omega-3 fatty acids supplementation in children with autism: A double-blind randomized, placebo-controlled pilot study. Biol. Psychiatry 2007, 61, 551–553. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; O’Keefe, J.H. The importance of marine omega-3s for brain development and the prevention and treatment of behavior, mood, and other brain disorders. Nutrients 2020, 12, 2333. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xiong, J.Y.; Chai, Y.Q.; Huang, L.; Tang, Z.Y.; Zhang, X.F.; Liu, B.; Zhang, J.T. Possible antidepressant mechanisms of omega-3 polyunsaturated fatty acids acting on the central nervous system. Front. Psychiatry 2022, 13, 933704. [Google Scholar] [CrossRef]
- Petermann, A.B.; Reyna-Jeldes, M.; Ortega, L.; Coddou, C.; Yévenes, G.E. Roles of the unsaturated fatty acid docosahexaenoic acid in the central nervous system: Molecular and cellular insights. Int. J. Mol. Sci. 2022, 23, 5390. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Huss, M.; Stauss-Grabo, M.; Hahn, A. significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. Eur. J. Pediatr. 2010, 169, 149–164. [Google Scholar] [CrossRef]
- Agostoni, C.; Nobile, M.; Ciappolino, V.; Delvecchio, G.; Tesei, A.; Turolo, S.; Crippa, A.; Mazzocchi, A.; Altamura, C.A.; Brambilla, P. The role of omega-3 fatty acids in developmental psychopathology: A systematic review on early psychosis, autism, and ADHD. Int. J. Mol. Sci. 2017, 18, 2608. [Google Scholar] [CrossRef] [Green Version]
- Chiang, N.; Serha, C.N. Specialized pro-resolving mediator network: An update on production and actions. Essays Biochem. 2020, 64, 443–462. [Google Scholar]
- Bazan, N.G. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal degenerations, and Alzheimer’s disease. J. Lipid Res. 2009, 50, S400–S405. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Petasis, N.A. Resolvins and protectins in inflammation resolution. Chem. Rev. 2011, 111, 5922–5943. [Google Scholar] [CrossRef] [Green Version]
- Panezai, J.; van Dyke, T. Polyunsaturated fatty acids and their immunomodulatory actions in periodontal disease. Nutrients 2023, 15, 821. [Google Scholar] [CrossRef] [PubMed]
- van Elst, K.; Bruining, H.; Birtoli, B.; Terreaux, C.; Buitelaar, J.K.; Kas, M.J. Food for thought: Dietary changes in essential fatty acid ratios and the increase in autism spectrum disorders. Neurosci. Biobehav. Rev. 2014, 45, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Bazinet, R.P.; Layé, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef]
- Harris, W.S. The Omega-6:Omega-3 ratio: A critical appraisal and possible successor. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Cué, C.; Bartesaghi, R. Fatty Acids: A Safe tool for improving neurodevelopmental alterations in down syndrome? Nutrients 2022, 14, 2880. [Google Scholar] [CrossRef]
- Weiser, M.; Butt, C.; Mohajeri, M. Docosahexaenoic acid and cognition throughout the lifespan. Nutrients 2016, 8, 99. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.; Crivello, F.; Mazoyer, B.; Debette, S.; Tzourio, C.; Samieri, C. Fish Intake and mri burden of cerebrovascular disease in older adults. Neurology 2021, 97, e2213–e2222. [Google Scholar] [CrossRef]
- Avella-Garcia, C.B.; Julvez, J. Seafood intake and neurodevelopment: A systematic review. Curr. Envir. Health Rep. 2014, 1, 46–77. [Google Scholar] [CrossRef] [Green Version]
- Murakami, Y.; Hosomi, R.; Nishimoto, A.; Nishiyama, T.; Yoshida, M.; Fukunaga, K. Protective Effects of Fish (Alaska Pollock) Protein Intake against Short-Term Memory Decline in Senescence-Accelerated Mice. Nutrients 2022, 14, 4618. [Google Scholar] [CrossRef]
- Kirby, A.; Derbyshire, E. Omega-3/6 fatty acids and learning in children and young people: A review of randomised controlled trials published in the last 5 years. J. Nutr. Food Sci. 2018, 8, 1–10. [Google Scholar]
- Reimers, A.; Ljung, H. The emerging role of omega-3 fatty acids as a therapeutic option in neuropsychiatric disorders. Ther. Adv. Psychopharmacol. 2019, 9, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Östlund, S.; Fransson, G.; Kadesjö, B.; Gillberg, C. Omega-3/omega-6 fatty acids for attention deficit hyperactivity disorder: A randomized placebo-controlled trial in children and adolescents. J. Atten. Disord. 2009, 12, 394–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqra; Sughra, K.; Ali, A.; Afzal, F.; Yousaf, M.J.; Khalid, W.; Rasul, H.F.; Aziz, Z.; Aqlan, F.M.; Al-Farga, A.; et al. Wheat-based gluten and its association with pathogenesis of celiac disease: A review. Int. J. Food Prop. 2023, 26, 511–525. [Google Scholar] [CrossRef]
- El-Ansary, A.K.; Bacha, A.B.; Kotb, M. Etiology of autistic features: The persisting neurotoxic Effects of Propionic Acid. J. Neuroinflamm. 2012, 9, 74. [Google Scholar] [CrossRef] [Green Version]
- Abuaish, S.; Al-Otaibi, N.M.; Aabed, K.; Abujamel, T.S.; Alzahrani, S.A.; Alotaibi, S.M.; Bhat, R.S.; Arzoo, S.; El-Ansary, A. The role of sex-differentiated variations in stress hormones, antioxidants, and neuroimmune responses in relation to social interaction impairment in a rodent model of autism. Metab. Brain Dis. 2021, 36, 1369–1379. [Google Scholar] [CrossRef]
- Alsubaiei, S.R.M.; Alfawaz, H.A.; Almubarak, A.Y.; Alabdali, N.A.; Ben Bacha, A.; El-Ansary, A. Independent and combined effects of probiotics and prebiotics as supplements or food-rich diets on a propionic-acid-induced rodent model of autism spectrum disorder. Metabolites 2023, 13, 50. [Google Scholar] [CrossRef]
- Beutler, E. Improved Method for the Determination of Blood Glutathione. J. Lab. Clin. Med. 1963, 61, 882–888. [Google Scholar]
- Ruiz-Larrea, M.B.; Leal, A.M.; Liza, M.; Lacort, M.; de Groot, H. Antioxidant effects of estradiol and 2-hydroxyestradiol on iron-induced lipid peroxidation of rat liver microsomes. Steroids 1994, 59, 383–388. [Google Scholar]
- Todd, J.C.; Sanford, A.H.; Davidsohn, I.; Henry, J.B. Clinical Diagnosis and Management by Laboratory Methods; Saunders: Philadelphia, PA, USA, 1979. [Google Scholar]
- D’Angelo, S.; Motti, M.L.; Meccariello, R. ω-3 and ω-6 Polyunsaturated Fatty Acids, Obesity and Cancer. Nutrients 2020, 12, 2751. [Google Scholar] [CrossRef]
- Numata, N.; Nakagawa, A.; Yoshioka, K.; Isomura, K.; Matsuzawa, D.; Setsu, R.; Nakazato, M.; Shimizu, E. Associations between Autism Spectrum Disorder and Eating Disorders with and without Self-Induced Vomiting: An Empirical Study. J. Eat. Disord. 2021, 9, 1–9. [Google Scholar] [CrossRef]
- Ho, H.H.; Eaves, L.C.; Peabody, D. Nutrient intake and obesity in children with autism. Focus Autism Dev. Disabil. 1997, 12, 187–192. [Google Scholar] [CrossRef]
- Williams, K.E.; Hendy, H.; Knecht, S. Parent feeding practices and child variables associated with childhood feeding problems. J. Dev. Phys. Disabil. 2008, 20, 231–242. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Zhao, Y.; Yang, Y. Spatiotemporal Imaging of cellular energy metabolism with genetically-encoded fluorescent sensors in brain. Neurosci. Bull. 2018, 34, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, F.; Cunnane, S.C.; Guesnet, P. Evidence of the role of omega-3 polyunsaturated fatty acids in brain glucose metabolism. Nutrients 2020, 12, 1382. [Google Scholar] [CrossRef] [PubMed]
- Andres, R.; Meiler, F.; Huber, A.; Perez-Bouza, A.; Seiler, R.; Wallimann, T.; Widmer, H.; Schlattner, U. Human Fetal CNS Tissue Expresses Kreatine Kinases and the Creatine Transporter. FENS 2002, 1, 208. [Google Scholar]
- Feng, S.; Xu, Z.; Yan, Y.B. Blocking creatine kinase refolding by trace amounts of copper ions. J. Inorg. Biochem. 2008, 102, 928–935. [Google Scholar] [CrossRef]
- Al-Mosalem, O.; El-Ansary, A.; Attas, O.; Al-Ayadhi, L. Metabolic biomarkers related to energy metabolism in Saudi autistic children. Clin. Biochem. 2009, 42, 949–957. [Google Scholar] [CrossRef]
- Poling, J.S.; Frye, R.E.; Shoffner, J.; Zimmerman, A.W. Developmental Regression and Mitochondrial Dysfunction in a Child with Autism. J. Child Neurol. 2006, 21, 170–172. [Google Scholar] [CrossRef] [Green Version]
- Frye, R.; Delatorre, R.; Taylor, H.; Slattery, J.; Melnyk, S.; Chowdhury, N.; James, S. Redox Metabolism Abnormalities in Autistic Children Associated with Mitochondrial Disease. Transl. Psychiatry 2013, 3, e273. [Google Scholar] [CrossRef] [Green Version]
- Frye, R.E.; Melnyk, S.; MacFabe, D.F. Unique acyl-carnitine profiles are potential biomarkers for acquired mitochondrial disease in autism spectrum disorder. Transl. Psychiatry 2013, 3, e220. [Google Scholar] [CrossRef] [Green Version]
- Xin, G.; Eshaghi, H. Effect of omega-3 fatty acids supplementation on indirect blood markers of exercise-induced muscle damage: Systematic review and meta-analysis of randomized controlled trials. Food Sci. Nutr. 2021, 9, 6429–6442. [Google Scholar] [CrossRef]
- Rawson, E.S.; Miles, M.P.; Larson-Meyer, D.E. Dietary Supplements for health, adaptation, and recovery in athletes. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 188–199. [Google Scholar] [CrossRef] [Green Version]
- Dentin, R.; Benhamed, F.; Pégorier, J.-P.; Foufelle, F.; Viollet, B.; Vaulont, S.; Girard, J.; Postic, C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J. Clin. Investig. 2005, 115, 2843–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhigbe, R.; Hamed, M.; Odetayo, A.; Akhigbe, T.; Ajayi, A.; Ajibogun, F. Omega-3 fatty acid rescues ischaemia/perfusion-induced testicular and sperm damage via modulation of lactate transport and xanthine oxidase/uric acid signaling. Biomed. Pharmacother. 2021, 142, 111975. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, A.A.; Farooqui, T. Prevention of oxidative stress by omega-3 fatty acids in the brain. In Omega-3 Fatty Acids; Springer: Berlin/Heidelberg, Germany, 2016; pp. 239–249. [Google Scholar]
- Pinot, F.; Kreps, S.E.; Bachelet, M.; Hainaut, P.; Bakonyi, M.; Polla, B.S. Cadmium in the Environment: Sources, Mechanisms of Biotoxicity, and Biomarkers. Rev. Environ. Health 2000, 15, 299–324. [Google Scholar] [CrossRef]
- Avramovic, N.; Dragutinovic, V.; Krstic, D.; Colovic, M.; Trbovic, A.; de Luka, S.; Milovanovic, I.; Popovic, T. The Effects of omega 3 fatty acid supplementation on brain tissue oxidative status in aged wistar rats. Hippokratia 2012, 16, 241. [Google Scholar]
- Lee, H.-J.; Kim, H.-K.; Choi, H. Different sources of omega fatty acids at the fixed ratio of p/s affect glutathione dependent enzymes in rat hepatocarcinogenesis. Korean J. Nutr. 2003, 36, 785–792. [Google Scholar]
- Arnal, E.; Miranda, M.; Barcia, J.; Bosch-Morell, F.; Romero, F. Lutein and docosahexaenoic acid prevent cortex lipid peroxidation in streptozotocin-induced diabetic rat cerebral cortex. Neuroscience 2010, 166, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Shelton, R.J.; Velavan, P.; Nikitin, N.P.; Coletta, A.P.; Clark, A.L.; Rigby, A.S.; Freemantle, N.; Cleland, J.G. Clinical trials update from the American Heart Association Meeting: ACORN-CSD, Primary Care Trial of Chronic Disease Management, PEACE, CREATE, SHIELD, A-HeFT, GEMINI, Vitamin E Meta-analysis, ESCAPE, CARP, and SCD-HeFT Cost-effectiveness Study. Eur. J. Heart Fail. 2005, 7, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Pavăl, D. A Dopamine hypothesis of autism spectrum disorder. Dev. Neurosci. 2017, 39, 355–360. [Google Scholar] [CrossRef]
- Arnsten, A.F. Catecholamine regulation of the prefrontal cortex. J. Psychopharmacol. 1997, 11, 151–162. [Google Scholar] [CrossRef]
- Cools, R.; D’Esposito, M. Inverted-u–shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry 2011, 69, e113–e125. [Google Scholar] [CrossRef] [Green Version]
- Healy-Stoffel, M.; Levant, B. N-3 (omega-3) fatty acids: Effects on brain dopamine systems and potential role in the etiology and treatment of neuropsychiatric disorders. CNS Neurol. Disord. Drug Targets 2018, 17, 216–232. [Google Scholar] [CrossRef]
- Logan, A.C. Neurobehavioral aspects of omega-3 fatty acids: Possible mechanisms and therapeutic value in major depression. Altern. Med. Rev. 2003, 8, 410–425. [Google Scholar] [PubMed]
- Chao, O.Y.; Pathak, S.S.; Zhang, H.; Dunaway, N.; Li, J.-S.; Mattern, C.; Nikolaus, S.; Huston, J.P.; Yang, Y.-M. Altered dopaminergic pathways and therapeutic effects of intranasal dopamine in two distinct mouse models of autism. Mol. Brain 2020, 13, 111. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Halt, A.R.; Realmuto, G.; Earle, J.; Kist, D.A.; Thuras, P.; Merz, A. Purkinje cell size is reduced in cerebellum of patients with autism. Cell. Mol. Neurobiol. 2002, 22, 71–75. [Google Scholar]
- Cupolillo, D.; Hoxha, E.; Faralli, A.; De Luca, A.; Rossi, F.; Tempia, F.; Carulli, D. Autistic-Like Traits and Cerebellar Dysfunction in Purkinje Cell PTEN Knock-Out Mice. Neuropsychopharmacology 2016, 41, 1457–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, P.K.; Singh, M.K.; Yadav, R.S.; Nath, R.; Mehrotra, A.; Rawat, A.; Dixit, R.K. Omega-3 fatty acid attenuates oxidative stress in cerebral cortex, cerebellum, and hippocampus tissue and improves neurobehavioral activity in chronic lead-induced neurotoxicity. Nutr. Neurosci. 2019, 22, 83–97. [Google Scholar] [CrossRef]
- Lobzhanidze, G.; Japaridze, N.; Lordkipanidze, T.; Rzayev, F.; MacFabe, D.; Zhvania, M. Behavioural and brain ultrastructural changes following the systemic administration of propionic acid in adolescent male rats. Further development of a rodent model of autism. Int. J. Dev. Neurosci. 2020, 80, 139–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Fatty Acids | PPA + Lethrinus nebuloses | PPA + Epinephelus marginatus | PPA + Scaridae | p-Value |
|---|---|---|---|---|
| Linoleic acid (C18:2) | 0.414 ± 0.067 | 0.452 ± 0.121 | 0.503 ± 0.121 | 0.428 |
| α-Linolenic acid (C18:3) | 0.362 ± 0.129 | 0.208 ± 0.020 | 0.280 ± 0.116 | 0.243 |
| Arachidonic acid (C20:4) | 0.28 ± 0.047 ac | 2.172 ± 0.101 bc | 0.644 ± 0.141 ab | 0.001 |
| Eicosapentaenoic acid (C20:5) | 0.28 ± 0.047 | 0.415 ± 0.039 | 0.280 ± 0.113 | 0.105 |
| Docosatetraenoic acid (C22:4) | 22.870 ± 0.207 ac | 16.601 ± 0.217 bc | 2.547 ± 0.199 ab | 0.001 |
| Docosahexaenoic acid (C22:6) | 0.155 ± 0.135 a | 24.657 ± 2.839 bc | 0.979 ± 0.195 a | 0.001 |
| Fatty Acids | Control | PPA | PPA + Lethrinus nebuloses | PPA + Epinephelus marginatus | PPA + Scaridae | p-Value |
|---|---|---|---|---|---|---|
| Saturated fatty acids (SFA) | ||||||
| Caprylic acid (C8:0) | 0.007 ± 0.01 | 0.003 ± 0.003 | 0.074 ± 0.18 b | 0.073 ± 0.15 b | 0.039 ± 0.096 | 0.284 |
| Undecanoic acid (C11:0) | 0.015 ± 0.03 | 0.031 ± 0.04 a | 0.002 ± 0.002 b | 0.098 ± 0.15 | 0.051 ± 0.095 | 0.061 |
| Lauric acid (C12:0) | 0.005 ± 0.01 | 0.015 ± 0.02 a | 0.027 ± 0.05 | 0.133 ± 0.14 ab | 0.049 ± 0.096 | 0.037 |
| Tridecanoic acid (C13:0) | 0.023 ± 0.04 | 0.026 ± 0.02 | 0.013 ± 0.008 | 0.047 ± 0.07 | 0.056 ± 0.093 | 0.641 |
| Myristic acid (C14:0) | 1.391 ± 3.06 | 3.942 ± 3.52 a | 2.57 ± 1.62 a | 2.375 ± 2.67 a | 1.062 ± 1.888 b | 0.044 |
| Pentadecanoic acid (C15: 0) | 0.428 ± 0.56 | 0.687 ± 0.46 | 0.602 ± 0.32 | 0.556 ± 0.47 | 0.533 ± 0.483 | 0.904 |
| Palmitic acid (C16:0) | 33.243 ± 9.97 | 39.184 ± 4.26 | 44.471± 4.23 ab | 40.291 ± 6.73 | 42.36 ± 5.241 a | 0.046 |
| Heptadecanoic acid (C17:0) | 0.717 ± 0.31 | 0.749 ± 0.25 | 1.026 ± 0.51 | 0.759 ± 0.24 | 0.644 ± 0.169 | 0.607 |
| Stearic acid (C18:0) | 0.578 ± 0.28 | 0.557 ± 0.13 | 0.613 ± 0.18 | 0.350 ± 0.15 b | 0.755 ± 0.17 b | 0.017 |
| Arachidic acid (C20:0) | 0.369 ± 0.23 | 0.317 ± 0.21 | 0.524 ± 0.54 | 1.781 ± 3.58 | 0.2 ± 0.16 | 0.581 |
| Heneicosanoic acid (C21:0) | 0.162 ± 0.11 | 0.048 ± 0.02 a | 0.104 ± 0.08 b | 0.029 ± 0.02 a | 0.069 ± 0.05 a | 0.023 |
| Behenic acid (C22:0) | 0.256 ± 0.10 | 0.370 ± 0.41 | 0.523 ± 0.47 a | 0.609 ± 0.81 | 0.156 ± 0.11 a | 0.068 |
| Tricosanic acid (C23:0) | 0.126 ± 0.07 | 0.080 ± 0.02 | 0.143 ± 0.10 | 0.167 ± 0.21 | 0.064 ± 0.05 | 0.161 |
| Lignoceric acid (C24:0) | 0.346 ± 0.22 | 0.297 ± 0.13 | 0.317 ± 0.16 | 0.361 ± 0.31 | 0.147 ± 0.14 | 0.275 |
| Monounsaturated fatty acids (MUFA) | ||||||
| Gondoic acid (C20:1) | 0.271 ± 0.16 | 1.867 ± 2.27 a | 1.687 ± 3.37 | 4.074 ± 3.56 a | 0.293 ± 0.334 b | 0.013 |
| Palmitoleic acid (C16: 1) | 1.623 ± 0.67 | 1.348 ± 0.67 | 1.708 ± 1.19 | 8.893± 11.27 ab | 2.997 ± 2.52 | 0.048 |
| cis 10-Heptadecanoic acid (C17: 1) | 10.669 ± 26.77 | 0.573 ± 0.19 | 0.829 ± 0.44 | 1.562 ± 2.37 | 5.023 ± 11.81 | 0.845 |
| Polyunsaturated fatty acids (PUFA) | ||||||
| Linoleic acid (C18:2) | 3.806 ± 2.55 | 10.780 ± 8.07 | 6.035 ± 2.99 | 11.848 ± 14.65 | 5.481 ± 3.64 | 0.517 |
| Methyl8,11,14-eicosatrienoate (C20:3) | 1.206 ± 0.63 | 5.204 ± 7.09 | 3.709 ± 4.88 | 2.067 ± 4.29 | 0.868 ± 0.29 | 0.185 |
| cis 11,14-Eicosadienoic acid (C20:4) | 1.159 ± 0.57 | 0.942 ± 0.53 | 1.227 ± 0.98 | 1.74 ± 1.79 | 1.357 ± 0.64 | 0.851 |
| Linolenic acid (C18:3) | 1.422 ± 0.48 | 4.488 ± 4.78 | 3.731 ± 2.91 | 5.827 ± 7.80 | 2.874 ± 2.17 | 0.300 |
| cis-5,8,11,14,17-Eicosapentaenoic (C20:5) | 19.472 ± 8.09 | 14.018 ± 4.18 | 15.792 ± 4.61 | 7.863 ± 4.29 ab | 17.26 ± 8.08 | 0.023 |
| cis 13,16-Docasadienoic acid (C22:2) | 22.704 ± 10.05 | 14.475 ± 4.53 a | 14.275 ± 7.11 a | 8.497 ± 5.34 a | 18.694 ± 8.82 | 0.020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaqer, N.S.; Al-Nouri, D.M.; Bhat, R.S.; Arzoo, S.; Al-Harbi, L.N.; Bin Obead, M.A.; Almubarak, A.Y.; Alkhalidi, H.; Almotairi, A.; El-Ansary, A.K.E.-D. Ameliorative Effect of Omega-3-Rich Fish Diet on the Neurotoxic Effects of Propionic Acid in a Rodent Model of Autism. Appl. Sci. 2023, 13, 7392. https://doi.org/10.3390/app13137392
Alsaqer NS, Al-Nouri DM, Bhat RS, Arzoo S, Al-Harbi LN, Bin Obead MA, Almubarak AY, Alkhalidi H, Almotairi A, El-Ansary AKE-D. Ameliorative Effect of Omega-3-Rich Fish Diet on the Neurotoxic Effects of Propionic Acid in a Rodent Model of Autism. Applied Sciences. 2023; 13(13):7392. https://doi.org/10.3390/app13137392
Chicago/Turabian StyleAlsaqer, Nouf Saad, Doha M. Al-Nouri, Ramesa Shafi Bhat, Shaista Arzoo, Laila Naif Al-Harbi, Manal Abdulaziz Bin Obead, Abdullah Yaseen Almubarak, Hisham Alkhalidi, Ahmad Almotairi, and Afaf Kamal El-Din El-Ansary. 2023. "Ameliorative Effect of Omega-3-Rich Fish Diet on the Neurotoxic Effects of Propionic Acid in a Rodent Model of Autism" Applied Sciences 13, no. 13: 7392. https://doi.org/10.3390/app13137392
APA StyleAlsaqer, N. S., Al-Nouri, D. M., Bhat, R. S., Arzoo, S., Al-Harbi, L. N., Bin Obead, M. A., Almubarak, A. Y., Alkhalidi, H., Almotairi, A., & El-Ansary, A. K. E.-D. (2023). Ameliorative Effect of Omega-3-Rich Fish Diet on the Neurotoxic Effects of Propionic Acid in a Rodent Model of Autism. Applied Sciences, 13(13), 7392. https://doi.org/10.3390/app13137392








