Abstract
Although there are several studies that have evaluated the bond strength of various adhesives to healthy dentin and caries-affected dentin after traditional caries removal, the objective of this systematic review aimed to assess the bond strength of various adhesives to caries-affected dentin (CAD) after chemo-mechanical caries removal (CMCR) treatment. The review adhered to PROSPERO protocol registration and followed PRISMA guidelines. The research question focused on the bonding effectiveness of dental adhesives to CAD after employing the chemo-mechanical caries removal method. PubMed, the TRIP database, and Scopus were searched, with the last search conducted in February 2023. Two reviewers independently screened and evaluated articles, resulting in 30 articles for full-text analysis out of 434 retrieved from databases. Twelve eligible studies were included in the review. The bond strength of etch-and-rinse (ER) and self-etch (SE) adhesive systems was assessed following CMCR treatment on CAD. SE adhesive systems exhibited higher bond strength to CAD compared to ER adhesive systems. Meta-analysis indicated that the bond strength achieved with self-etching adhesive systems remained consistent, regardless of the CMCR agent (Carisolv or Papacarie) used on dentin. The findings of this systematic review suggest that self-etch adhesive systems show favorable bond strength to caries-affected dentin following chemo-mechanical caries removal, regardless of the specific CMCR agent used. These results support the use of minimally invasive dentistry techniques aimed at preserving healthy tooth structure, dentin in particular.
1. Introduction
Dental caries remains a prevalent global disease, characterized by bacterial infection and mineral loss of hard dental tissues due to oral biofilm formation [1,2]. The major risk factors causing dental caries are enamel hypoplasia, eating habits, poor oral hygiene and trouble brushing, caregiver effect, low income, and low level of education [3,4,5]. Within carious dentin, two distinct layers play a critical role: the irreparable caries-infected dentin, also known as the outer layer, and the repairable caries-affected dentin (CAD), referred to as the inner layer [6]. Infected dentin exhibits significant decalcification, denatured collagen fibers, and disrupted odontoblasts, necessitating removal. In contrast, affected dentin displays minimal demineralization, relatively intact collagen fibers, and lacks bacterial invasion, requiring preservation [6,7,8].
Contemporary adhesive systems strive to achieve robust bonding to various tooth substrates, particularly to caries-affected dentin [9]. In the field of dentistry, there has been a paradigm shift towards minimally invasive and preventive approaches, transforming from G.V. Black’s “extension for prevention” management to a “construction with conservation” mindset [10,11,12]. As a result, the search for less invasive yet effective caries excavation techniques has led to the development of minimally invasive methods that aim to remove only the infected dentin while preserving healthy enamel and dentin [13]. These techniques include air abrasion, sono-abrasion, and chemo-mechanical caries removal (CMCR) methods [12,14,15].
Chemo-mechanical caries removal (CMCR) is a non-aggressive approach involving the application of a chemical gel that selectively removes soft and necrotic infected dentin using a hand instrument while leaving the affected dentin intact [16]. CMCR agents can be categorized into sodium hypochlorite (NaOCl)-based (e.g., Caridex®, Carisolv®) and enzyme-based (e.g., Carie-Care™, Papacarie®, BRIX3000). Carisolv, a pink gel, contains sodium hypochlorite, amino acids (leucine, lysine, glutamic acid), methylcellulose, and erythrosine, acting by chlorinating and facilitating the removal of denatured collagen. On the other hand, Papacarie is an enzyme-based CMCR agent consisting of papain, a proteolytic enzyme with bactericidal and anti-inflammatory properties [17,18,19,20,21].
Bonding agents utilized in clinical practice can be classified into etch-and-rinse (ER), self-etch (SE), universal (U), and resin-modified glass ionomer adhesives (RMGIA) [22,23,24]. However, bonding to caries-affected dentin (CAD) poses challenges during operative treatment. The morphological alterations in CAD, including reduced mineral content, loss of crystallinity, and organic matrix changes, can impede dentin hybridization and compromise the mechanical performance of bonded restorations. High porosity and exposure of collagen fibers, along with a decrease in surface energy, are seen in the inter-tubular CAD. Reduction of these mechanical properties significantly influences a decrease of the mean elastic modulus and nano-hardness in CAD when compared to unaltered tissue. The obliteration of tubules can interfere with resin infiltration, at the same time preventing tags during bonding procedures. On the contrary, the lower mineral content of intertubular dentin in CAD permits deeper etching of this substrate [13,14,15,16].
Self-etch adhesives are the latest generation of adhesives. Adhesive frameworks today are either “etch and rinse” or “self-etch” approaches, which have different mechanisms of action on tooth substrate. Etch and rinse include pre-treatment with phosphoric acid etchant before bonding. Self-etch adhesives are acid-type monomers that perform etching and rinsing at the same time point [25,26]. They are easy to apply and have fewer clinical steps. Both systems structure mixed layers as resin infiltrates the permeable dentin and enamel [22,27]. However still, in clinical practice, etch and rinse system is used more than self-etch adhesives. Reduced bond strength of adhesive systems to CAD has been demonstrated, but little information is available about the bonding and comparison of these two adhesive systems to this clinically relevant substrate.
Therefore, this systematic review aims to evaluate the bond strength of dental adhesives to caries-affected dentin treated with chemo-mechanical caries removal agents, providing valuable insights into optimizing adhesive bonding strategies in caries management.
2. Materials and Methods
This systematic review’s protocol was registered under PROSPERO registration number CRD42021283259. This non-Cochrane in vitro systematic review followed the four-phase flow diagram based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [28]. The following PICO components were established, as presented in Table 1.

Table 1.
PICO components.
The following research question was developed: “What is the bonding effectiveness of different dental adhesives to caries-affected dentin after using chemo-mechanical caries removal method?”
2.1. Search Strategy
Following the PRISMA guidelines, a literature search (P.R.M. and L.M.) was conducted. Studies were identified through PubMed, the TRIP database, and Scopus. The authors established detailed search strategies for each database searched to identify studies for this review (Table 2).

Table 2.
Search strategies.
The final search was performed in February 2023, with English language restrictions. References of each included study were also manually searched.
All the retrieved articles were introduced into a citation manager (EndNote v7.0, Clarivate Analytics, New York, NY, USA) to exclude duplicates.
2.2. Selection of Studies
All the studies were initially screened by titles by two reviewers (P.R.M. and L.M.), followed by the abstract evaluation when the title suggested potential inclusion. After the abstract was evaluated, eligible studies were selected for full-text reading. Complete texts of all the remaining publications were collected and reviewed, and only the articles that completely met the inclusion criteria were considered. In this selection, if there was a difference of opinion, a third reviewer (B.L.) was contacted to reach a consensus. Studies were identified based on eligibility criteria presented in Table 3.

Table 3.
Eligibility criteria.
2.3. Data Extraction
Based on the features of the studies and groups investigated, a standardized outline was utilized for data capture: author details, type of CMCR agent, procedure (caries removal method), sample size, type, name, and brand of adhesive systems, type of bond strength test used. Bond strength mean values and standard deviation were also extracted.
2.4. Assessment of Risk of Bias
The risk of bias (ROB) in the included studies was independently determined by two review authors (P.M. and L.M.). A modified CONSORT checklist of items for reporting in vitro studies of dental materials [29] was used to assess ROB. For each study, the following domains were considered: sample size calculation, samples with similar dimensions, control group, standardization of procedure, statistical analysis, and other risks of bias.
The risk was rated as low, medium, or high for each domain. One study was deemed to have a low risk of bias if the risk was low across the board. The study was classified as having a medium risk of bias if it had an unknown risk for at least two areas. The study was deemed to have a high risk of bias if it had a high risk in more than one domain.
2.5. Data Analysis
The studies’ characteristics were listed in a descriptive summary. When enough information was available, a meta-analysis utilizing a random effects model was performed to determine the pooled mean differences between various dental adhesives after being subjected to Carisolv™ and Papacarie™ treatment. Bond strength data extracted were restricted to those from studies in which similar CMCR treatments were compared under the same conditions and when a pairwise comparison was available. All summary estimates were reported with mean with standard deviation and corresponding 95% confidence intervals (CIs). Statistical heterogeneity was assessed using the Cochrane Q statistic and I2 test (>75% indicates high heterogeneity). The analyses were conducted using Review Manager 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014).
3. Results
The electronic search identified 434 articles from PubMed, the TRIP database, and Scopus. After the removal of duplicated articles, the total number of articles was found to be 333. However, after screening articles based on abstracts and titles, a total of 30 articles could be assessed. Finally, the total number of full-text articles found to be eligible for the study was 12 (Figure 1).
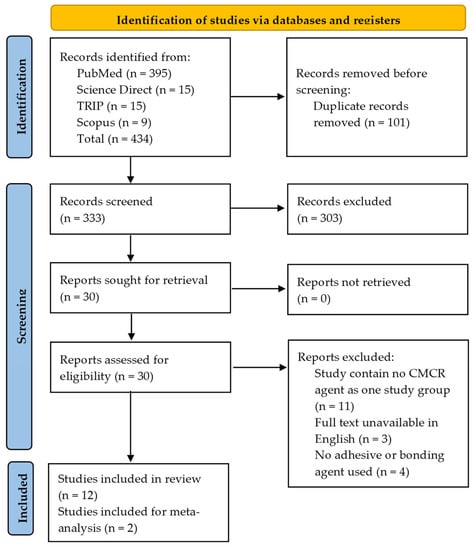
Figure 1.
PRISMA flow diagram of literature search.
3.1. Characteristics of Included Studies
Eleven studies [6,9,30,31,32,33,34,35,36,37,38] used Carisolv as the CMCR agent (Table 4). Another CMCR agent used in four studies [31,32,33,39] was Papacarie. All bonding agents used in the included studies were presented in Table 5.

Table 4.
CMCR agents used in the included studies.

Table 5.
Dental adhesives used in the included studies.
The characteristics of the included studies are summarized in Table 6 and Table 7. Eleven studies [6,9,13,30,31,32,33,34,35,36,39] used the microtensile bond strength test (μTBS), whereas one study [37] used the shear bond strength (SBS) test. Evaluation of μTBS was performed using the universal testing machine at a crosshead speed of 1 mm/min [9,30,31,32,33,35,36,38,39].

Table 6.
Characteristics of the included study for qualitative analysis.

Table 7.
Characteristics of the study for quantitative analysis.
On fracture mode analysis (Table 8) in the self-etch adhesives group, four studies [9,32,33,38] showed cohesive failure in resin composites or within caries-affected dentin, whereas six studies [6,30,35,36,37,39] showed adhesive failure between bonding resin and caries affected dentin. One study [31] showed mixed, partially adhesive, and partially cohesive failure within caries-affected dentin. Meanwhile, in etch-and-rinse adhesives, four studies [30,35,37,39] predominantly showed adhesive failure, and two studies [6,36] showed cohesive failure mode.

Table 8.
Type of failure mode of bonding agents after CMCR application is reported in the included studies.
3.2. Quality Assessment of Included Studies
Figure 2 presents the findings of the risk of bias analysis and Figure 3. Four studies [6,31,38,39] were classified as low risk, seven studies [9,30,32,33,34,35,36] were classified as medium risk, and only one study [37] was classified as high risk of bias.
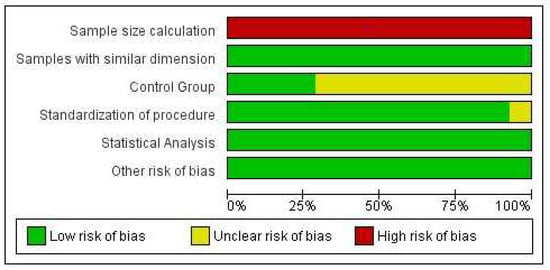
Figure 2.
Risk of bias graph: review authors’ judgments about each risk of bias item, presented as percentages across all included studies.
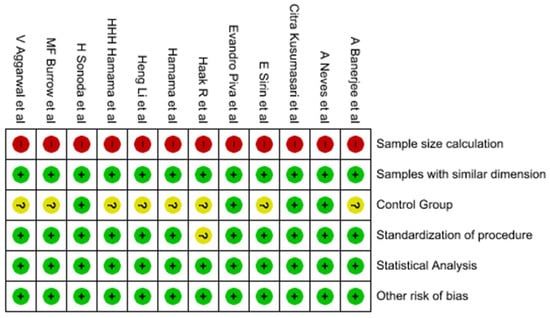
Figure 3.
Summary of risk of bias: evaluations of each study’s included item’s risk of bias [6,9,30,31,32,33,34,35,36,37,38,39].
It was found that none of the studies mentioned sample size calculation. One study [37] did not mention the standardization of the procedure, and seven studies [9,30,32,33,34,35,36,37] did not clearly mention the control group in their respective studies.
3.3. Meta-Analysis
The meta-analysis (Figure 4) included only two studies [31,33] that compared the bond strength of self-etch adhesive (Clearfil SE bond) to caries-affected dentin after it was subjected to Carisolv™ and Papacarie™ treatment. The meta-analysis depicted that SE adhesive allowed to achieve similar bond strength to CAD whether it was treated with Carisolv or Papacarie.
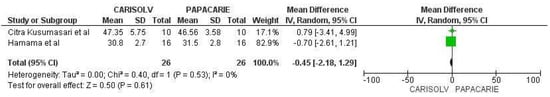
Figure 4.
Meta-analysis comparing the dentin bond strength of Clearfil SE Bond (SE adhesive) in caries-affected dentin after Papacarie and Carisolv application [31,33].
4. Discussion
Compared to enamel, dentin has always posed a challenge for bonding [40,41,42], likely due to its more heterogeneous structure. Dentin is a composite material consisting of approximately 50 vol% mineral phase, 30 vol% collagen, and 20 vol% water [43]. Therefore, the bonding strategy for dentin focuses on two phases: the mineral phase, mainly composed of carbonate-rich hydroxyapatite, and the organic phase, primarily collagen, in a moist environment.
The bonding method for dentin was evaluated in the current investigation. Since it is the most typical substrate in clinical practice, the bonding to carious dentin was assessed. Changes caused by carious lesions result in poorer dentin hybridization [44,45]. In the present study, the bond strength to caries-affected dentin was lower due to low mineral content, which affected dentin hybridization.
Carious dentin is composed of two distinct layers: an outer layer of infected dentin and an inner layer of affected dentin [46]. Fourier-transform infrared imaging (FTIR) analysis of caries-affected dentin (CAD) has revealed lower mineral content and reduced crystallinity in the mineral phase compared to normal dentin. The loss of mineral from the dentin matrix during the carious process is replaced by water, which can account for up to 53% of the volume in CAD [47]. In addition, most clinical substrates are covered with smear layers, which hinder the penetration of adhesive molecules into tooth substances [44,48]. The occlusion of tubules in CAD leads to decreased permeability. Following cavity preparation and removal of CAD, the cavity floor is largely made up of dentin that has been damaged by caries, making it the most common bonding substrate, as opposed to sound dentin. CAD differs from sound dentin in terms of hardness, being twice as soft, even though mineral depositions occlude dentinal tubules. The mineral deposits in CAD, such as plate-like β-octocalcium phosphate (whitlockite), are not as densely packed and are therefore softer than well-packed apatite, although they may be more acid-resistant [49]. Moreover, the ultimate tensile strength of CAD is lower than that of sound dentin, possibly due to the loss of mineral in intertubular dentin. The matrix of demineralized CAD, according to a study, was just as durable as that of healthy dentin. The partial demineralization in CAD may reduce the number of MDP-dentin bonding sites and affect the chemical bonding of adhesives to CAD [50]. The lower bond strength in caries-affected dentin can be attributed to the lower mineral content surrounding and within collagen fibrils, resulting in a softer surface with a higher degree of porosity compared to sound dentin [51].
In this systematic review, which included 12 studies, the bond strength of different adhesives (etch-and-rinse, self-etch, and resin-modified glass ionomer adhesive) on CAD after treatment with chemo-mechanical caries removal (CMCR) agents was assessed. Eleven studies used Carisolv, while four studies used Papacarie. One study used SFC-V (Biosolv), and another used SFC-VIII as CMCR agents compared to Carisolv.
CMCR agent Carisolv has a proteolytic action, using a combination of enzymes to selectively degrade the collagen matrix of carious dentin. The enzymes in Carisolv, such as collagenase and papain, break down the denatured collagen fibrils present in carious dentin, facilitating its removal [52]. This enzymatic action is believed to be less aggressive towards sound dentin compared to mechanical methods, as it selectively targets the demineralized and degraded collagen structure [53,54,55]. The CMCR method did not have any adverse effects on the bonding of adhesives to CAD [6,9,16,30,32,33,34,36,37]. Caries removal with either NaOCl-based or enzyme-based CMCR methods resulted in the partial (Carisolv) or complete (Papacarie) absence of the smear layer and irregular surface characteristics when observed under SEM at 2000× magnification [31,56]. These surface characteristics may affect resin infiltration into the dentin and enhance micromechanical adhesion, leading to good resin infiltration in caries-affected dentin groups [57]. Pre-treatment of dentin with NaOCl derivatives, particularly in caries-affected regions, was found to promote the adhesion of self-etch adhesives to CAD [58]. The predominance of adhesive failures when bonding to carious dentin is likely related to the difficulty of the adhesive to completely infiltrate the exposed and altered collagen mesh [39]. The demineralization and remineralization processes that occur during caries result in the occlusion of dentinal tubules with larger, less soluble calcium phosphate crystals, further affecting adhesion. Additionally, the organic matrix of carious dentin differs from that of the normal substrate due to denatured collagen fibrils, which can also influence the fracture mode [39].
Various techniques are available to evaluate bond strength, with the most commonly used being tension or shear modes [59,60,61,62]. In this systematic review, 13 studies assessed microtensile bond strength (µTBS), while one study evaluated shear bond strength. Tensile bond strength tests require special testing jigs and meticulous procedures [8,62]. Shear bond strength tests, although easier to perform, generate non-uniform stress concentration at the edge of the bonded interface during testing [63,64].
To overcome the limitations of conventional shear or tensile bond strength tests, the µTBS test was introduced. It involves bonding adhesive resins to the entire flat occlusal surface of teeth, which is then covered with a resin composite. Subsequently, the specimen is vertically sectioned into multiple serial sections, creating an hourglass shape for maximum stress development at that region [65,66]. The µTBS test, along with morphological and spectroscopic investigations, has contributed to improving resin/dentin adhesion and has shown greater discriminative power than traditional macro-shear tests [67]. This test is considered a versatile and standard method for bond strength testing, providing better control of regional differences and making more economical use of teeth. Moreover, it allows the exclusive evaluation of adhesive bond failures when the bonded surface area is approximately 1 mm2, leading to better stress distribution at the true interface [66,68,69].
Regarding the failure mode of restorations, they can fail within the restoration itself, at the restoration and bonding adhesive interface, at the restoration and substrate interface, or only at the substrate. Cohesive failure occurs within the resin composite or dentin, adhesive failure refers to failure between dentin and bonding resin, and mixed failure involves a combination of adhesive and cohesive failures.
When bonding to caries-affected dentin, an analysis of failure modes of dental adhesives showed an increased incidence of cohesive failures, possibly due to differences in the nature of the bond. The thickness of the hybrid layer or increased dentin moisture after Carisolv treatment may contribute to a slightly higher number of microscopic defects in the bonded layer as a whole. Additionally, the slight difference in dentin hardness after using Carisolv or other differences in the dentin itself can influence the failure mode [9,16,37,39].
It is essential to keep in mind that the findings of this systematic review indicated that both Carisolv and Papacarie were equally effective in dentinal caries removal and showed similar bond strength to adhesive systems [49,50,70]. However, one group of investigators reported marginally better clinical parameters for Papacarie, particularly about the length of the treatment and the number of dental cavities encountered [49,50,70]. The mode of action of Papacarie involves breaking partially degraded collagen molecules, contributing to the degradation and elimination of the fibrin “mantle” formed during the carious process. Papacarie’s papain enzyme specifically targets dead cells and infected tissues lacking or showing no antitrypsin, which inhibits protein digestion. Therefore, sound collagen fibers in the inner affected and normal dentin are not affected by Papacarie [71].
Despite the promising results of Carisolv and Papacarie in terms of bond strength and clinical parameters, it is important to consider that the success of adhesive restorations depends on various factors beyond caries removal alone. Factors such as proper isolation, effective moisture control, adhesive system properties, and technique sensitivity can significantly influence the longevity and durability of the restoration.
Furthermore, it is worth mentioning that the studies included in this systematic review had certain limitations, including variations in methodology, small sample sizes, and heterogeneity in the adhesive systems and protocols used. In the included articles, no standardized protocols were followed, brief details about the control groups were not mentioned, and the method of randomization into groups was not mentioned in any of the articles. These factors can introduce bias and affect the generalizability of the findings. Therefore, further well-designed studies with standardized protocols are needed to provide more robust evidence on the influence of Carisolv and Papacarie on bond strength to caries-affected dentin.
In conclusion, the selection of a caries removal method can impact the bond strength of adhesive restorations to carious dentin. Carisolv and Papacarie have shown comparable bond strength to adhesive systems when used for caries removal. Their selective removal of carious tissue while preserving sound dentin provides a potentially favorable substrate for bonding. However, it is essential to consider various factors and ensure proper technique and protocol adherence to achieve optimal bonding outcomes.
The present systematic review has some limitations as articles included in the review had low sample sizes, heterogeneity in the adhesive systems, and no proper information about the control group in the studies was provided. In addition, no standardized protocols were mentioned and followed. It is important to note that the quality of the studies included in the systematic review varied, indicating a need for further high-quality research.
Additional research is necessary to confirm the findings of this review and identify optimal strategies for chemo-mechanical caries removal and bonding techniques in order to enhance clinical outcomes. Further research is needed to elucidate the long-term clinical performance and stability of adhesive restorations using these caries removal methods.
Further research on biomimetic dental adhesives and dental restorative materials containing bioactive particles should be conducted as they may exhibit remineralizing potential to enamel and/or dentin-resin interface [72,73,74,75].
5. Conclusions
As a result of the systematic review conducted, the findings that follow can be drawn.
- The use of chemo-mechanical caries removal agents did not significantly affect the bond strength of dental adhesives to caries-affected dentin;
- Two-step self-etch adhesives exhibited higher bond strength compared to etch-and-rinse adhesives when applied on caries-affected dentin. Unlike ER, which destroys collagen fibrils, SE preserves the collagen fibrils and hence superior performance;
- Both Carisolv and Papacarie showed corresponding bond strength to dentin when utilized in conjunction with self-etch dental adhesives.
Hence, chemo-mechanical caries removal agents should be used routinely for caries removal as it is a conservative method and also patient friendly.
Author Contributions
Conceptualization, L.M. and P.R.M.; methodology, L.M.; software, P.R.M.; validation, L.M., P.R.M. and B.L.; formal analysis, P.R.M.; investigation, L.M.; resources, P.R.M. and K.S.; data curation, P.R.M. and K.S.; writing—original draft preparation, P.R.M.; writing—review and editing, L.M. and B.L.; visualization, B.L.; supervision, L.M.; project administration, B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We acknowledge the support given by Saurav Panda for his timely feedback during the study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fejerskov, O. Concepts of Dental Caries and Their Consequences for Understanding the Disease. Community Dent. Oral. Epidemiol. 1997, 25, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Prim. 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Maiorani, C.; Morandini, A.; Simonini, M.; Morittu, S.; Trombini, J.; Scribante, A. Evaluation of Children Caries Risk Factors: A Narrative Review of Nutritional Aspects, Oral Hygiene Habits, and Bacterial Alterations. Children 2022, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Pierce, A.; Singh, S.; Lee, J.; Grant, C.; Cruz de Jesus, V.; Schroth, R.J. The Burden of Early Childhood Caries in Canadian Children and Associated Risk Factors. Front. Public Health 2019, 7, 328. [Google Scholar] [CrossRef] [PubMed]
- Benn, A.M.L.; Heng, N.C.K.; Thomson, W.M.; Broadbent, J.M. Plaque and Dental Caries Risk in Midlife. Caries Res. 2022, 56, 464–476. [Google Scholar] [CrossRef]
- Sonoda, H.; Banerjee, A.; Sherriff, M.; Tagami, J.; Watson, T.F. An in Vitro Investigation of Microtensile Bond Strengths of Two Dentine Adhesives to Caries-Affected Dentine. J. Dent. 2005, 33, 335–342. [Google Scholar] [CrossRef]
- Meraji, N.; Nekoofar, M.H.; Yazdi, K.A.; Sharifian, M.R.; Fakhari, N.; Camilleri, J. Bonding to Caries Affected Dentine. Dent. Mater. 2018, 34, e236–e245. [Google Scholar] [CrossRef]
- Nakajima, M.; Kunawarote, S.; Prasansuttiporn, T.; Tagami, J. Bonding to Caries-Affected Dentin. Jpn. Dent. Sci. Rev. 2011, 47, 102–114. [Google Scholar] [CrossRef]
- Burrow, M.F.; Bokas, J.; Tanumiharja, M.; Tyas, M.J. Microtensile Bond Strengths to Caries-Affected Dentine Treated with Carisolv. Aust. Dent. J. 2003, 48, 110–114. [Google Scholar] [CrossRef]
- Reddy, M.V.; Sai Shankar, A.; Pentakota, V.; Kolli, H.; Ganta, H.; Katari, P. Efficacy of Antimicrobial Property of Two Commercially Available Chemomechanical Caries Removal Agents (Carisolv and Papacarie): An Ex Vivo Study. J. Int. Soc. Prev. Community Dent. 2015, 5, 183. [Google Scholar] [CrossRef]
- Murdoch-Kinch, C.A.; McLEAN, M.E. Minimally Invasive Dentistry. J. Am. Dent. Assoc. 2003, 134, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Jingarwar, M.M.; Bajwa, N.K.; Pathak, A. Minimal Intervention Dentistry—A New Frontier in Clinical Dentistry. J. Clin. Diagn. Res. 2014, 8, ZE04–ZE08. [Google Scholar] [CrossRef]
- Naik, S.V.; Shashikiran, N.D.; Chaitra, N.L.; Syed, G. A Microtensile Bond Strength Evaluation of a Single-Bottle Adhesive to Caries-Affected Dentin in Conventional versus Minimal Invasive Caries Removal Techniques: An in-Vitro Study. Indian J. Dent. 2014, 5, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Showkat, N.; Singh, G.; Singla, K.; Sareen, K.; Chowdhury, C.; Jindal, L. Minimal Invasive Dentistry: Literature Review. J. Curr. Med. Res. Opin. 2020, 3, 631–636. [Google Scholar] [CrossRef]
- Ajai Minimally Invasive Dentistry—A Review. Available online: https://www.ijcommdent.com/article.asp?issn=2589-8388;year=2021;volume=9;issue=2;spage=97;epage=99;aulast=Ajai (accessed on 7 June 2023).
- Çehreli, Z.C.; Yazici, A.R.; Akca, T.; Özgünaltay, G. A Morphological and Micro-Tensile Bond Strength Evaluation of a Single-Bottle Adhesive to Caries-Affected Human Dentine after Four Different Caries Removal Techniques. J. Dent. 2003, 31, 429–435. [Google Scholar] [CrossRef]
- Bussadori, S.K.; de Godoy, C.H.L.; Alfaya, T.A.; Fernandes, K.P.S.; Mesquita-Ferrari, R.A.; Motta, L.J. Chemo-Mechanical Caries Removal with PapacarieTM: Case Series with 84 Reports and 12 Months of Follow-Up. J. Contemp. Dent. Pract. 2014, 15, 250–253. [Google Scholar] [CrossRef]
- Ganesh, M.; Parikh, D. Chemomechanical Caries Removal (CMCR) Agents: Review and Clinical Application in Primary Teeth. J. Dent. Oral Hyg. 2011, 3, 34–45. [Google Scholar]
- Singhal, P.; Vishwanathan, D.; Das, U.; Singhal, A. Carisolv as an Endodontic Irrigant in Deciduous Teeth: An SEM Study. Indian J. Dent. Res. 2012, 23, 120. [Google Scholar] [CrossRef]
- Motta, L.J.; Bussadori, S.K.; Campanelli, A.P.; da Silva, A.L.; Alfaya, T.A.; de Godoy, C.H.L.; de Lima Navarro, M.F. Efficacy of Papacarie® in Reduction of Residual Bacteria in Deciduous Teeth: A Randomized, Controlled Clinical Trial. Clinics 2014, 69, 319–322. [Google Scholar] [CrossRef]
- Maashi, M.S.; Elkhodary, H.M.; Alamoudi, N.M.; Bamashmous, N.O. Chemomechanical Caries Removal Methods: A Literature Review. Saudi Dent. J. 2023, 35, 233–243. [Google Scholar] [CrossRef]
- Perdigão, J.; Araujo, E.; Ramos, R.Q.; Gomes, G.; Pizzolotto, L. Adhesive Dentistry: Current Concepts and Clinical Considerations. J. Esthet. Restor. Dent. 2021, 33, 51–68. [Google Scholar] [CrossRef] [PubMed]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification Review of Dental Adhesive Systems: From the IV Generation to the Universal Type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin Bonding Systems: From Dentin Collagen Structure to Bond Preservation and Clinical Applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Murali, N.; Ganesh.S, B.; Roy, A. Self Etch Adhesives—An Update. Int. J. Res. Pharm. Sci. 2020, 11, 464–468. [Google Scholar] [CrossRef]
- Manihani, A.K.D.S.; Mulay, S.; Beri, L.; Shetty, R.; Gulati, S.; Dalsania, R. Effect of Total-Etch and Self-Etch Adhesives on the Bond Strength of Composite to Glass-Ionomer Cement/Resin-Modified Glass-Ionomer Cement in the Sandwich Technique—A Systematic Review. Dent. Res. J. 2021, 18, 72. [Google Scholar]
- Digole, V.R.; Warhadpande, M.M.; Dua, P.; Dakshindas, D. Comparative Evaluation of Clinical Performance of Two Self-Etch Adhesive Systems with Total-Etch Adhesive System in Noncarious Cervical Lesions: An in Vivo Study. J. Conserv. Dent. 2020, 23, 190–195. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Faggion, C.M. Guidelines for Reporting Pre-Clinical In Vitro Studies on Dental Materials. J. Evid. Based Dent. Pract. 2012, 12, 182–189. [Google Scholar] [CrossRef]
- Li, H.; Wang, W.-M.; Yu, S.-L.; Wen, Q. Morphological and Microtensile Bond Strength Evaluation of Three Adhesive Systems to Caries-Affected Human Dentine with Chemomechanical Caries Removal. J. Dent. 2011, 39, 332–339. [Google Scholar] [CrossRef]
- Kusumasari, C.; Abdou, A.; Nakajima, M.; Tagami, J. Deproteinization of Caries-Affected Dentin with Chemo-Mechanical Caries Removal Agents and Its Effect on Dentin Bonding with Self-Etch Adhesives. J. Dent. 2021, 109, 103665. [Google Scholar] [CrossRef]
- Hamama, H.; Yiu, C.; Burrow, M.F. Effect of Chemomechanical Caries Removal on Bonding of Resin-Modified Glass Ionomer Cement Adhesives to Caries-Affected Dentine. Aust. Dent. J. 2015, 60, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Hamama, H.H.H.; Yiu, C.K.Y.; Burrow, M.F. Effect of Chemomechanical Caries Removal on Bonding of Self-Etching Adhesives to Caries-Affected Dentin. J. Adhes. Dent. 2014, 16, 507–516. [Google Scholar] [CrossRef]
- Aggarwal, V.; Singla, M.; Yadav, S.; Yadav, H. The Effect of Caries Excavation Methods on the Bond Strength of Etch-and-Rinse and Self-Etch Adhesives to Caries Affected Dentine. Aust. Dent. J. 2013, 58, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Sirin Karaarslan, E.; Yildiz, E.; Cebe, M.A.; Yegin, Z.; Ozturk, B. Evaluation of Micro-Tensile Bond Strength of Caries-Affected Human Dentine after Three Different Caries Removal Techniques. J. Dent. 2012, 40, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Kellow, S.; Mannocci, F.; Cook, R.J.; Watson, T.F. An in Vitro Evaluation of Microtensile Bond Strengths of Two Adhesive Bonding Agents to Residual Dentine after Caries Removal Using Three Excavation Techniques. J. Dent. 2010, 38, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Haak, R.; Wicht, M.J.; Noack, M.J. Does Chemomechanical Caries Removal Affect Dentine Adhesion? Eur. J. Oral Sci. 2000, 108, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.d.A.; Coutinho, E.; Cardoso, M.V.; de Munck, J.; Van Meerbeek, B. Micro-Tensile Bond Strength and Interfacial Characterization of an Adhesive Bonded to Dentin Prepared by Contemporary Caries-Excavation Techniques. Dent. Mater. 2011, 27, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Piva, E.; Ogliari, F.A.; de Moraes, R.R.; Corá, F.; Henn, S.; Correr-Sobrinho, L. Papain-Based Gel for Biochemical Caries Removal: Influence on Microtensile Bond Strength to Dentin. Braz. Oral Res. 2008, 22, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Meharry, M.; Moazzami, S.; Li, Y. Comparison of Enamel and Dentin Shear Bond Strengths of Current Dental Bonding Adhesives from Three Bond Generations. Oper. Dent. 2013, 38, E237–E245. [Google Scholar] [CrossRef]
- Reis, A.F.; Giannini, M.; Kavaguchi, A.; Soares, C.J.; Line, S.R.P. Comparison of Microtensile Bond Strength to Enamel and Dentin of Human, Bovine, and Porcine Teeth. J. Adhes. Dent. 2004, 6, 117–121. [Google Scholar]
- Bedran-Russo, A.; Leme-Kraus, A.A.; Vidal, C.M.P.; Teixeira, E.C. An Overview of Dental Adhesive Systems and the Dynamic Tooth–Adhesive Interface. Dent. Clin. N. Am. 2017, 61, 713–731. [Google Scholar] [CrossRef]
- Goldberg, M. Dentin Structure Composition and Mineralization. Front. Biosci. 2011, E3, 281. [Google Scholar] [CrossRef]
- Isolan, C.P.; Sarkis-Onofre, R.; Lima, G.S.; Moraes, R.R. Bonding to Sound and Caries-Affected Dentin: A Systematic Review and Meta-Analysis. J. Adhes. Dent. 2018, 20, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Pinna, R.; Maioli, M.; Eramo, S.; Mura, I.; Milia, E. Carious Affected Dentine: Its Behaviour in Adhesive Bonding. Aust. Dent. J. 2015, 60, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, M.; Yiu, C.K.Y.; Matinlinna, J.P. Bonding of Resin Adhesives to Caries-Affected Dentin—A Systematic Review. Int. J. Adhes. Adhes. 2015, 61, 23–34. [Google Scholar] [CrossRef]
- Pugach, M.K.; Strother, J.; Darling, C.L.; Fried, D.; Gansky, S.A.; Marshall, S.J.; Marshall, G.W. Dentin Caries Zones: Mineral, Structure, and Properties. J. Dent. Res. 2009, 88, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Follak, A.; Miotti, L.; Lenzi, T.; Rocha, R.O.; Maxnuck Soares, F. The Impact of Artificially Caries-Affected Dentin on Bond Strength of Multi-Mode Adhesives. J. Conserv. Dent. 2018, 21, 136. [Google Scholar] [CrossRef]
- Viral, P.; Nagarathna, C.; Shakuntala, B.S. Chemomechanical Caries Removal in Primary Molars: Evaluation of Marginal Leakage and Shear Bond Strength in Bonded Restorations—An in Vitro Study. J. Clin. Pediatr. Dent. 2013, 37, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, S.; Pereira, J.; Rosa, A.; Oliveira, K.; Ghizoni, J.; Oliveira, M. Mineral Content Removal after Papacarie Application in Primary Teeth: A Quantitative Analysis. J. Clin. Pediatr. Dent. 2010, 34, 229–231. [Google Scholar] [CrossRef]
- Bussadori, S.K.; Castro, L.C.; Galvão, A.C. Papain Gel: A New Chemo-Mechanical Caries Removal Agent. J. Clin. Pediatr. Dent. 2006, 30, 115–119. [Google Scholar] [CrossRef]
- Beyth, N.; Mass, A.; Ziskind, D. Carisolv, a Change in the Perception of Caries Treatment—A Chemo-Mechanical Removal of Caries. Refu’at Ha-peh Veha-shinayim (1993) 2003, 20, 23–29, 78. [Google Scholar] [PubMed]
- Zawaideh, F.; Palamara, J.E.A.; Messer, L.B. Bonding of Resin Composite to Caries-Affected Dentin after Carisolv(®) Treatment. Pediatr. Dent. 2011, 33, 213–220. [Google Scholar] [PubMed]
- Hosoya, Y.; Shinkawa, H.; Marshall, G.W. Influence of Carisolv on Resin Adhesion for Two Different Adhesive Systems to Sound Human Primary Dentin and Young Permanent Dentin. J. Dent. 2005, 33, 283–291. [Google Scholar] [CrossRef]
- Mohammed, D.T.I.; Salih, D.S.A. The Effect of Chemomechanical Caries Removal and Different Bonding Systems on Shear Bond Strength of Carious Dentin (In Vitro Study). Mustansiria Dent. J. 2011, 8, 115–126. [Google Scholar] [CrossRef]
- Hamama, H.; Yiu, C.; Burrow, M.; King, N. Chemical, Morphological and Microhardness Changes of Dentine after Chemomechanical Caries Removal. Aust. Dent. J. 2013, 58, 283–292. [Google Scholar] [CrossRef]
- Hossain, M.; Nakamura, Y.; Tamaki, Y.; Yamada, Y.; Jayawardena, J.A.; Matsumoto, K. Dentinal Composition and Knoop Hardness Measurements of Cavity Floor Following Carious Dentin Removal with Carisolv. Oper. Dent. 2003, 28, 346–351. [Google Scholar]
- Taniguchi, G.; Nakajima, M.; Hosaka, K.; Iwamoto, N.; Ikeda, M.; Foxton, R.M.; Tagami, J. Improving the Effect of NaOCl Pretreatment on Bonding to Caries-Affected Dentin Using Self-Etch Adhesives. J. Dent. 2009, 37, 769–775. [Google Scholar] [CrossRef]
- Bowen, R.L. Adhesive Bonding of Various Materials to Hard Tooth Tissues. III. Bonding to Dentin Improved by Pretreatment and the Use of Surface-Active Comonomer. J. Dent. Res. 1965, 44, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Sirisha, K.; Rambabu, T.; Shankar, Y.; Ravikumar, P. Validity of Bond Strength Tests: A Critical Review: Part I. J. Conserv. Dent. 2014, 17, 305. [Google Scholar] [CrossRef] [PubMed]
- El Mourad, A.M. Assessment of Bonding Effectiveness of Adhesive Materials to Tooth Structure Using Bond Strength Test Methods: A Review of Literature. Open Dent. J. 2018, 12, 664–678. [Google Scholar] [CrossRef]
- Braga, R.R.; Meira, J.B.C.; Boaro, L.C.C.; Xavier, T.A. Adhesion to Tooth Structure: A Critical Review of “Macro” Test Methods. Dent. Mater. 2010, 26, e38–e49. [Google Scholar] [CrossRef]
- Zanatta, R.; Lungova, M.; Borges, A.; Torres, C.; Sydow, H.-G.; Wiegand, A. Microleakage and Shear Bond Strength of Composite Restorations Under Cycling Conditions. Oper. Dent. 2017, 42, E71–E80. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.; Loza, M.A.; Elias, A.; Mukhopadhyay, S.; Looney, S.; Rueggeberg, F.A. Tensile Bond Strength of an Adhesive Resin Cement to Different Alloys Having Various Surface Treatments. J. Prosthet. Dent. 2009, 101, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Sinhoreti, M.A.C.; Soares, E.F.; Abuna, G.F.; Correr Sobrinho, L.; Roulet, J.-F.; Geraldeli, S. Microtensile Bond Strength of Adhesive Systems in Different Dentin Regions on a Class II Cavity Configuration. Braz. Dent. J. 2017, 28, 474–481. [Google Scholar] [CrossRef]
- Sano, H.; Chowdhury, A.F.M.A.; Saikaew, P.; Matsumoto, M.; Hoshika, S.; Yamauti, M. The Microtensile Bond Strength Test: Its Historical Background and Application to Bond Testing. Jpn. Dent. Sci. Rev. 2020, 56, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Lula, E.C.d.O.; Leite, T.H.M.; Alves, C.M.C.; Santana, I.L.; Almeida, A.M.L.; Costa, J.F. Parameters That Influence Microtensile Bond Testing of Adhesive Systems. Rev. Gaúcha Odontol. 2014, 62, 65–70. [Google Scholar] [CrossRef]
- Dikmen, B.; Gurbuz, O.; Ozsoy, A.; Eren, M.M.; Cilingir, A.; Yucel, T. Effect of Different Antioxidants on the Microtensile Bond Strength of an Adhesive System to Sodium Hypochlorite-Treated Dentin. J. Adhes. Dent. 2015, 17, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Carvalho, R.M.; Sano, H.; Nakajima, M.; Yoshiyama, M.; Shono, Y.; Fernandes, C.A.; Tay, F. The Microtensile Bond Test: A Review. J. Adhes. Dent. 1999, 1, 299–309. [Google Scholar]
- Costa, A.R.; Garcia-Godoy, F.; Correr-Sobrinho, L.; Naves, L.Z.; Raposo, L.H.A.; de Carvalho, F.G.; Sinhoreti, M.A.C.; Puppin-Rontani, R.M. Influence of Different Dentin Substrate (Caries-Affected, Caries-Infected, Sound) on Long-Term ΜTBS. Braz. Dent. J. 2017, 28, 16–23. [Google Scholar] [CrossRef]
- Kotb, R.M.S.; Abdella, A.A.; El Kateb, M.A.; Ahmed, A.M. Clinical Evaluation of Papacarie in Primary Teeth. J. Clin. Pediatr. Dent. 2009, 34, 117–123. [Google Scholar] [CrossRef]
- Butera, A.; Maiorani, C.; Gallo, S.; Pascadopoli, M.; Quintini, M.; Lelli, M.; Tarterini, F.; Foltran, I.; Scribante, A. Biomimetic Action of Zinc Hydroxyapatite on Remineralization of Enamel and Dentin: A Review. Biomimetics 2023, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Abuna, G.; Feitosa, V.P.; Correr, A.B.; Cama, G.; Giannini, M.; Sinhoreti, M.A.; Pashley, D.H.; Sauro, S. Bonding Performance of Experimental Bioactive/Biomimetic Self-Etch Adhesives Doped with Calcium-Phosphate Fillers and Biomimetic Analogs of Phosphoproteins. J. Dent. 2016, 52, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Ashtijoo, Z.; Pishevar, L.; MalekipourMalekipour, M.-R.; Khodaei, M.; Sabouri, Z. Comparative Evaluation of Incorporation Calcium Silicate and Calcium Phosphate Nanoparticles on Biomimetic Dentin Remineralization and Bioactivity in an Etch-and-Rinse Adhesive System. J. Clin. Exp. Dent. 2022, 14, e903–e910. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Fronza, B.M. The Use of Bioactive Particles and Biomimetic Analogues for Increasing the Longevity of Resin-Dentin Interfaces: A Literature Review. Dent. Mater. J. 2020, 39, 62–68. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).