1. Introduction
The association of vasoconstrictors with local anesthetics has the effect of counteracting the vasodilation caused by these drugs and increasing the permanence of the anesthetic solution in the area of the nerve to be blocked. However, they will be absorbed into the bloodstream and distributed to all tissues. The most used in clinical practice are sympathomimetic drugs such as epinephrine, noradrenaline, levonordephrine, and phenylephrine. Another group, less studied, is the synthetic derivative of vasopressin, felypressin [
1,
2,
3].
In dental practice, tricyclic antidepressants such as amitriptyline have been successfully used in the treatment of chronic orofacial pain, especially in cases of temporomandibular disorders [
4,
5,
6,
7,
8]. On the other hand, vasoconstrictors associated with local anesthetics may have their systemic action potentiated by this pharmacological group, due to the increase in monoaminergic neurotransmission [
9].
Authors have reported a possible interaction, such as protriptyline with noradrenaline and epinephrine [
10], imipramine with phenylephrine, noradrenaline, and epinephrine [
11] and in dogs, desipramine with epinephrine, noradrenaline, and levonordephrine [
12]. Recent literature reviews report the possible interaction, especially with epinephrine, [
13,
14] warning and limiting the dose to 0.054 mg, due to its dose-dependent effects, in addition to the administration of the anesthetic solution being performed slowly, in small volumes [
12].
Epinephrine is certainly the one that promotes more tachycardia, while levonordefrin promotes more intense vasoconstriction, which could cause a great increase in blood pressure [
15,
16]. Sympathomimetic drugs act on alpha and beta-adrenergic receptors, located in virtually all body tissues, with emphasis on alpha-1 receptors in blood vessels and beta-1 receptors in the heart. Their activation leads, respectively, to vasoconstriction and an increase in heart rate. These two effects are directly responsible for the elevation of blood pressure. On the other hand, beta-2 receptors are widely found in resistance vessels located in skeletal muscles and their activation causes them to dilate, diverting blood flow to important areas during the stress response.
Phenylephrine is a selective alpha-1 agonist, causing vasoconstriction without, however, interfering with myocardial contractility. Something similar occurs with felypressin, a vasopressin V1A receptor agonist [
3,
17,
18] which also does not bind to cardiac beta-1 receptors, not promoting tachycardia. This fact could imply a lower hypertensive action, giving these substances a prominent role when used in patients with different heart diseases.
The present study was designed to simulate a situation closer to that experienced by dental professionals, to evaluate cardiovascular parameters after infiltration of different vasoconstrictors in an appropriate animal model. On the other hand, we also tried to simulate an accidental situation, where such vasoconstrictor substances would be injected directly into the bloodstream. Therefore, the present study hypothesizes that the hypertensive action of vasoconstrictors associated with local anesthetics will not be potentiated by amitriptyline, demonstrating the safety of these substances.
2. Materials and Methods
2.1. Animals and Experimental Groups
Forty-two male Wistar rats of 45-day-old, with bodyweight between 300 and 500 g, from the Central Animal Facility of the Medicine School of Ribeirão Preto (FMRP-USP), were randomly allocated into two groups: 21 animals without treatment (control) and 21 animals treated with the tricyclic antidepressant amitriptyline (amitriptyline treated). Each vasoconstrictor used in this study was injected intravenuos and/or by infiltration at the bottom of the vestibular sulcus of the anterior region of the maxilla in all animals from each group (control and amitriptyline treated).
Animals were maintained with free access to food and water and a 12-h light and 12-h light cycle in a dark environment, according to the protocol previously established by the laboratory [
2,
19]. This study was previously approved by the Ethics Committee of the Bauru School of Dentistry-University of São Paulo (protocol 008/2017).
2.2. Treatment
The animals were treated with a daily dose of amitriptyline hydrochloride (Brainfarma Indústria Química e Farmacêutica S.A., Anápolis, Brazil), dissolved in physiological saline solution (0.3 mg/kg/day of body weight), administered by gavage every 24 h for 7 days. The dose used in animals was proportional to that used by humans in the treatment of depressive states, considering the average weight of a human being around 70 kg, receiving 75 mg of amitriptyline daily.
2.3. Catheter Implantation and Blood Pressure Recording
Animals were maintained throughout the experiment under anesthesia with a mixture of ketamine hydrochloride (50 mg/kg of weight, Dopalen®-Sespo Indústria e Comércio Ltda, Vetbrands Saúde Animal Division, Paulínia, Brazil), and xylazine hydrochloride (10 mg/kg of weight, Anasedan®-Sespo Indústria e Comércio Ltda, Vetbrands Animal Health Division).
For direct blood pressure measurement, a PE-50 polyethylene catheter (Clay Adams–Franklin Lakes, NJ, USA), filled with physiological saline and occluded at its distal end, was inserted into her left carotid artery. A similar catheter was inserted into the right jugular vein for intravenous injections. The arterial catheter was connected to the pressure transducer (Physiological Pressure Transducer–ADInstruments Pty. Ltd.–Australia, Bella Vista, Australia) coupled to the PowerLab 4/30 invasive blood pressure recording system (ADInstruments Pty. Ltd., Bella Vista, Australia), using appropriate software (Chart Pro–ADInstruments Pty. Ltd., Bella Vista, Australia) [
2,
19].
Exogenous epinephrine (Adren®-Hipolabor Farmacêutica Ltda-Belo Horizonte, Minas Gerais, Brazil) diluted in 0.1 mL of physiological saline solution was injected into the venous catheter and infiltrated into the sulcus of the anterior region of the maxilla at doses of 160, 640 and 2560 ng. Felipressin (Dentsply Pharmaceutical, Catanduva, São Paulo, Brazil), also diluted in 0.1 mL of physiological saline solution, was used in the experiments at doses of 0.5; 2 and 8 mIU and phenylephrine (Sigma-Aldrich, St. Louis, MA, USA) diluted in the same way, in doses of 6, 25 and 99 µg. During an experiment, the same animal was first injected with all doses of a single vasoconstrictor and then given an intravenous injection of the same vasoconstrictor.
An interval of 30 min between each dose, after infiltration and/or intravenous injection, was necessary to stabilize blood pressure, reducing the cumulative effect of vasoconstrictors. At the end of each experiment, the animals were euthanized by intraperitoneal injection of an excessive dose of sodium thiopental (Thiopentax®, Cristália-Chemicals and Pharmaceuticals, São Paulo, Brazil) (150 mg/kg IP), associated with an intramuscular injection of the local anesthetic lidocaine chloridate, (Xylestesin®, Cristália-Chemicals and Pharmaceuticals) (10 mg/kg IM).
2.4. Statistical Analysis
As more than one response was measured in the same animal, analysis of variance of repeated measures was used (split-plot design), to one or two classification criteria, followed by the Holm-Sidak test, to identify significant differences between the doses used and between the vasoconstrictors used in the groups, experimental and control. The level of significance established was 5%.
3. Results
Considering the mean baseline blood pressure values of the animals used in this study, the results showed that treatment with amitriptyline caused a significant drop in baseline blood pressure in the animals (
Table 1). Baseline values are the mean of those obtained after connecting the arterial cannula to the transduction system, at the beginning of each experiment, during the first 10 min of recording, before infiltration or intravenous injection of any substance.
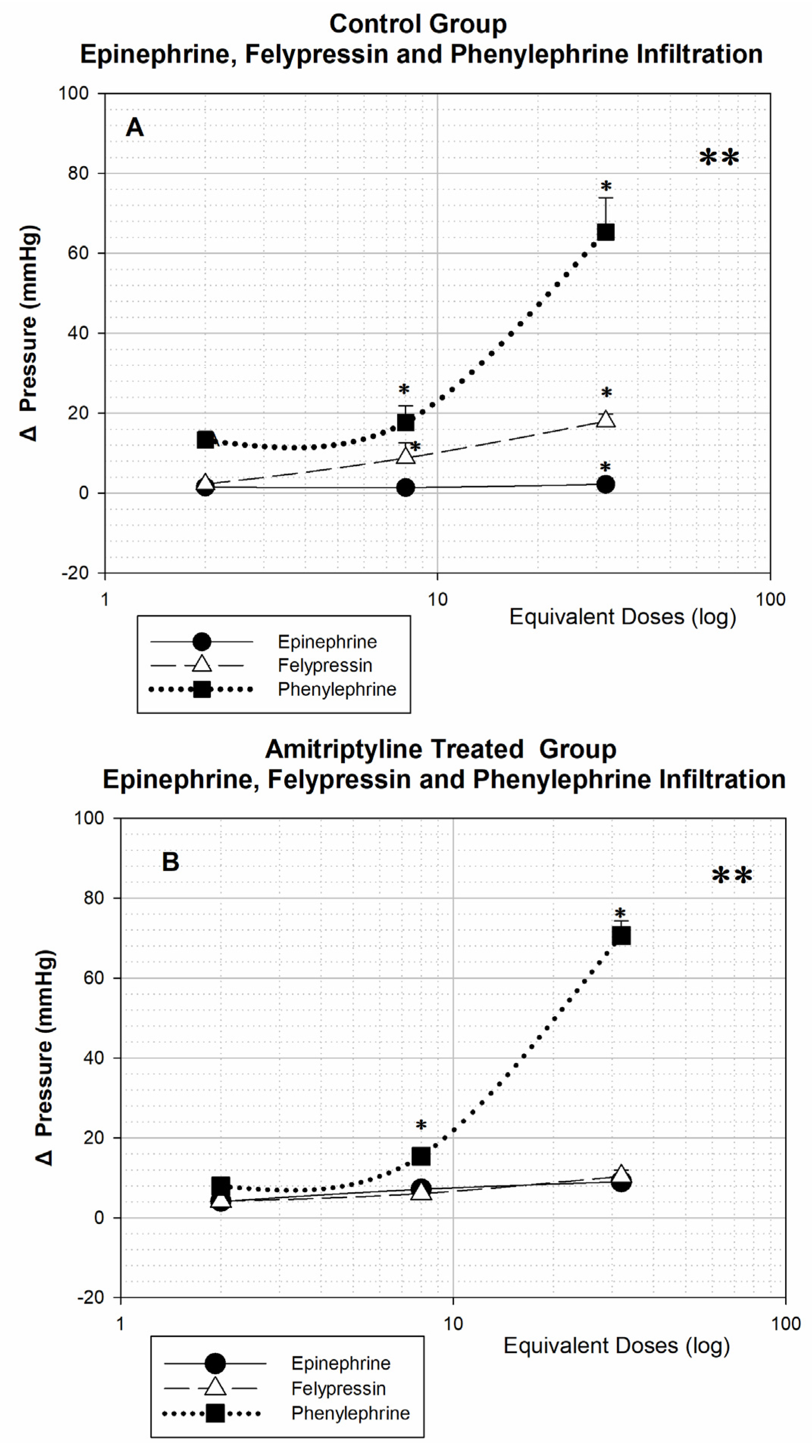
After infiltration of the different doses of the three vasoconstrictors, a significant difference can be seen in the control and amitriptyline treated groups when comparing the three dose-response curves (** p < 0.001). Comparing the dose-by-dose responses, there is no significant difference between the responses for the three vasoconstrictors with the dose equivalent to 2 tubes, in both groups. With a dose equivalent to 8 tubes, in both groups, the response to phenylephrine is significantly higher than the response to epinephrine (* p < 0.05), with the response to felypressin being similar to that of epinephrine in amitriptyline treated animals and greater in the control group (* p < 0.05). With the dose equivalent to 32 tubes, in the control animals the responses to the three vasoconstrictors showed a significant difference (* p < 0.05), while in the amitriptyline treated, only the response to phenylephrine differs from the others (* p < 0.05).
Epinephrine infiltration causes small changes in blood pressure in both groups of animals, even at very high doses (
Figure 1A,B), in contrast to the large pressure effect produced by phenylephrine infiltration.
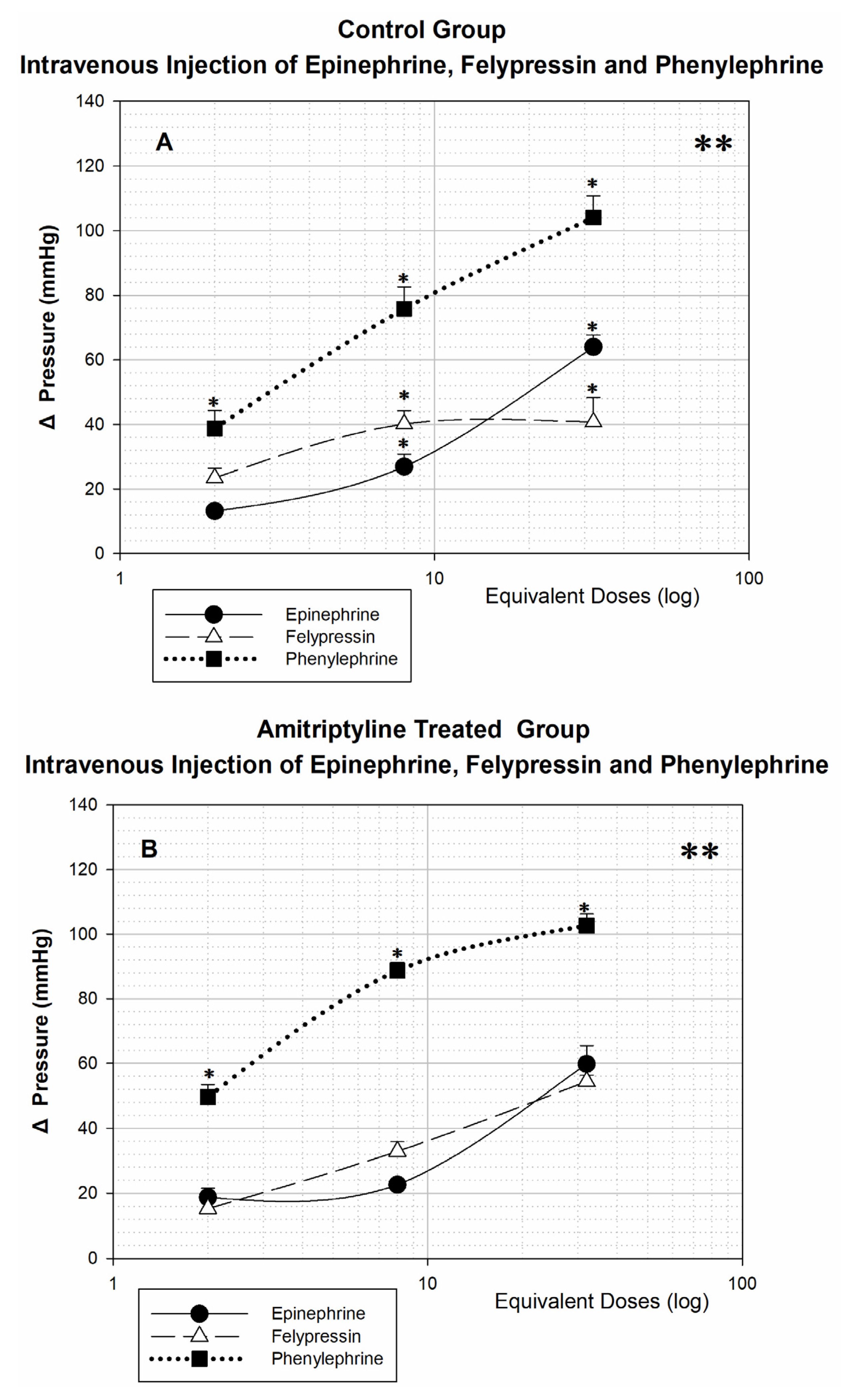
After intravenous injection of vasoconstrictors in the animals of the control group (
Figure 2A) and amitriptyline treated (
Figure 2B), there is a significant difference (**
p < 0.001) when comparing the three dose-response curves, highlighting the hypertensive effect caused by phenylephrine. In both groups, with the lowest equivalent dose (2 tubes), the response produced by phenylephrine is already significantly greater than that produced by epinephrine and felypressin (*
p < 0.05), with no difference between the responses produced by the latter. In the control group, after injection of doses equivalent to 8 and 32 tubes, the responses to the three vasoconstrictors are significantly different from each other (*
p < 0.05), while in the amitriptyline treated group there is no difference when the responses to epinephrine and felypressin are compared; only the responses to phenylephrine differ from the others (*
p < 0.05).
Comparing the responses to the same vasoconstrictor between the control and amitriptyline treated groups, after infiltration into the vestibular sulcus background, it can be seen that all curves follow a dose-response pattern, except for epinephrine in the group of control animals. Even with high doses of epinephrine, blood pressure changes were very small from baseline. (1.45 ± 0.87; 1.4 ± 1.07; 2.2 ± 1.66, respectively, 2, 8 and 32 tubes, p > 0.05).
4. Discussion
The main point of the present study was to evaluate the effect of the administration by different routes of the following vasoconstrictors: epinephrine, most used by dentists and indicated by the American Heart Association (ADA) [
20]; phenylephrine, a substance that acts selectively at adrenergic receptors as an alpha-1 agonist [
21]; and felypressin, which has limited studies on its effects and is analogous to vasopressin, that acts by binding to the V1A type receptor [
3]. In addition, simulated the possible effects of these vasoconstrictors on the cardiovascular system of patients who use amitriptyline, frequently used in patients with temporomandibular disorders.
During a single experiment, the same animal received, firstly, an infiltration and then an intravenous injection of a specific vasoconstrictor. This sequence was chosen to avoid any cumulative cardiovascular effects. The interval of 30 min between doses was established so that the infiltrated or injected substance could be recaptured by nerve or adjacent cells and/or undergo the process of metabolism and destruction, tissue or hepatic, by the enzymes Monoamine-oxidase (MAO) and Catechol–oxymethyltransferase (COMT), thus avoiding the accumulative effect.
In our results, treatment with amitriptyline caused a significant decrease in mean, systolic, and diastolic blood pressure (
Table 1). Tricyclic antidepressants have as their classic mechanism of action the inhibition of noradrenaline and serotonin reuptake, through their binding with the transport proteins responsible for this process [
14,
22,
23,
24,
25]. In addition, this group of drugs also antagonizes several other receptors, such as H1, 5-HT2, alpha-1 adrenergic, and muscarinic in general. This can produce side effects such as blurred vision, dry mouth, constipation, difficulty urinating, as well as hypotension and sedation. The hypotension observed in our animals may have been produced by a set of conditions, including antagonism of alpha-1 adrenergic receptors, produced by amitriptyline and other tricyclic antidepressants, decreased myocardial contractility and peripheral vasodilation [
26].
Amitriptyline treated, (
Figure 1B) have significantly increased pressor responses to the three vasoconstrictors (**
p < 0.001), compared to untreated animals (control group) (
Figure 1A), when epinephrine is infiltrated into the background of the vestibular sulcus. Tricyclic drugs are known to be norepinephrine and serotonin reuptake inhibitors; as the infiltrated epinephrine is also recaptured by the same transporters, which are inhibited by the action of amitriptyline, this substance could remain longer bound to adrenergic receptors, causing a greater increase in blood pressure. Yagiela et al., Saraghi et al. and Ouanounou et al., also agree that epinephrine infiltration in patients treated with tricyclic antidepressants can cause an increase in blood pressure, exactly as observed in our study, where treated animals were more sensitive to epinephrine infiltration [
12,
13,
24]. It is also important to emphasize that, even though these animals treated with amitriptyline are more sensitive to the hypertensive effects of epinephrine infiltration, even with the infiltration of the equivalent dose of 8 tubes, the blood pressure variation was less than 8 mmHg, which clinically does not represent any significant change.
Regarding felypressin infiltration (
Figure 1A), the dose-response curves for blood pressure demonstrate a dose-dependent pattern. The results demonstrate that the amitriptyline treated (
Figure 1B) were less sensitive to the action of felypressin, especially when the highest doses used in this study were infiltrated (equivalent to 32 tubes). Felypressin is a known agonist of V1a receptors for vasopressin and its hypertensive action is practically due to its peripheral vasoconstrictor effects since felypressin acts on V1a receptors widely distributed in the smooth muscle of capillaries, arterioles, and venules [
2,
3]. Our findings coincide with Goldman et al., who observed negligible effects on the cardiovascular system at doses that, based on body weight, would correspond to amounts used clinically [
27].
Felypressin has very similar results to those shown by epinephrine in both groups of animals (
Figure 1B), which places it as an important alternative non-adrenergic vasoconstrictor. According to the literature, felypressin should not increase heart rate, as it does not act on adrenergic receptors. Felypressin acts on a class of receptors called V1a. Under physiological conditions, the activation of this vascular receptor promotes vasoconstriction, which with our results it was possible to confirm [
3,
28].
It is evident the great hypertensive effect of phenylephrine compared to the other vasoconstrictors epinephrine and felypressin, after infiltration of equivalent doses (
Figure 1A). Phenylephrine, being a specific alpha-1 receptor agonist, does not affect vascular beta-2 receptors as epinephrine does; this last agonist action on beta-2 adrenergic leads to great vasodilation in vessels located in skeletal muscles, which would somehow compensate for the agonist action on vascular alpha-1 and cardiac beta-1 receptors [
29]. For this reason, the results of our study showed a great effect of this vasoconstrictor, with a significant increase in blood pressure, especially with the higher doses used in this study. With the lowest dose of 2 tubes, there is no significant difference between the vasoconstrictors, with a significant difference from the dose of 8 tubes between phenylephrine and epinephrine (*
p < 0.05), and only at the high dose of 32 tubes is there a significant difference among the three vasoconstrictors in the control group.
In the present study, the animals were treated with amitriptyline, a drug known to inhibit the membrane transport protein responsible for the reuptake of catecholamines (dopamine, noradrenaline and epinephrine). Phenylephrine differs chemically from noradrenaline in that it does not have a hydroxyl group at position 4 of the benzene ring, which causes this substance to have little affinity for the transporter protein responsible for the reuptake of catecholamines. As in our study, there was no stimulation of sympathetic nerves in the area, there was no release of the neurotransmitter, and phenylephrine does not seem to have an affinity for the transporter protein, it would be expected that its vasoconstrictor action would not be potentiated by treatment with amitriptyline, as shown by our results.
In the amitriptyline group (
Figure 1B), the same effect of phenylephrine can be observed. The significance (*
p < 0.05) is only obtained from the dose of 8 tubes between the infiltration of phenylephrine and the other vasoconstrictors, and between felypressin and epinephrine, there is no significant difference. Tricyclic drugs are known to be norepinephrine and serotonin reuptake inhibitors; as the infiltrated epinephrine is also recaptured by the same transporters, which are inhibited by the action of amitriptyline, this substance could remain longer bound to adrenergic receptors, causing a greater increase in blood pressure. When the three vasoconstrictors were administered intravenous (
Figure 2), the great increase in blood pressure promoted by all is evident, but especially by phenylephrine, even in small doses, highlighting the danger of accidental injection of this vasoconstrictor into a patient’s bloodstream. Corroborating our results with Boakes et al. [
11]. Intravenous or subcutaneous injection of this vasoconstrictor causes an increase in systolic and diastolic pressure, both in men and in other species.
In our results, after intravenous injection of epinephrine (
Figure 2A), treatment with amitriptyline did not influence blood pressure responses when we compared dose-response curves for the two groups of animals. Thus, in the case of intravenous injection of a certain amount of vasoconstrictor, the hypertensive responses would not be potentiated by the previous treatment with amitriptyline, although the injection of the lowest dose used in this study, equivalent to 2 tubes, may cause a variation of almost 20 mmHg, which is clinically quite significant [
10,
11,
16]. On the other hand, Cawson et al. concluded that “there is no clinical evidence of an interaction between tricyclic agents and local anesthesia containing epinephrine” [
30].
As shown in
Figure 2 intravenous injection produces a significantly greater response than infiltration in both amitriptyline treated and control groups. The response after intravenous injection is always greater than the response after infiltration. We did not find articles in the literature from other groups of researchers that compared the infiltrative response and response after intravenous injection of vasoconstrictor drugs. Our intention with this comparison was to highlight the great danger of accidental intravenous injection of vasoconstrictors and to reveal the notable safety of their use by the infiltrative route. Even high doses, infiltrated at the bottom of the vestibular sulcus, caused small changes in blood pressure. In several studies, the duration of treatment with amitriptyline is questioned, most of them performed between 4-5 days. Some authors argued that there would be insufficient time for the regulation of adrenergic receptors [
13]. Our study performed the treatment with amitriptyline for 7 days, providing a more adequate time for the chemical action of tricyclic antidepressants to inhibit the reuptake of adrenergic agonists. However, Brown and Lewis, argued that long-term administration of tricyclic antidepressants may result in desensitization to adrenergic vasoconstrictors, thus decreasing the possible drug interaction [
12,
31].
These results seem to indicate that, when used correctly, by infiltration, two tubes of local anesthetics containing any of the three vasoconstrictors would not cause a significant increase in blood pressure in animals. It is also clear from the analysis of these figures that epinephrine is the vasoconstrictor that leads to smaller changes in blood pressure, both in control animals and in those treated with amitriptyline.
The results obtained in this research cannot be faithfully extrapolated to human beings, but they can contribute and guide future studies in human beings.